Summary
Objectives
The knowledge about outcomes of infant vaccination against HBV infections using the DPT-HepB-Hib vaccine in Ghana is limited. This study therefore investigated the levels of immunity to HBV among children who received the DPT-HepB-Hib vaccine and HBsAg carriage in non-responders. Correlates for non-response or poor response were also investigated.
Methods
Cross-sectional study. A major paediatric hospital in Accra. Four hundred and twenty four children between the ages of 5 to 32 months who had completed the full vaccination schedule for the DPT-HepB-Hib vaccine.
Results
Of the 424 children, 358 (84.4%) developed anti-HBs while 340 (80.2%) developed ≥10 mIU/ml anti-HBs (sero-protection) and 3 had HBsAg. A binary logistic regression analysis showed that younger children were associated with sero-conversion (p=.022) and sero-protection (p=.021). For anti-HBs titres ≥100 mIU/ml age was a weaker but significant contributor (p=.041), as compared to the number of vaccines from different manufacturers the child used (p=.028). The mean age of those who used a single type of vaccine was higher (14.75 ± 6.056 months; n=268) than those who used vaccines from two or more manufacturers (11.96 ± 4.645 months; n=156), p= <.001 (CI: −3.897 − 1.688), an indication that efforts to procure vaccine from same source when it was initially introduced are waning.
Conclusions
There is still a residual possibility of infection with HBV in spite of infant vaccination. In the light of possible loss of anamnestic response over time, there is the need to consider a birth dose for HBV vaccination for all neonates or booster dose for infants who may not have received the vaccine at birth. Using vaccines from a single manufacturer is recommended.
Funding
None declared
Keywords: Infant, hepatitis B virus, vaccination, surface antigen, surface antibody
Introduction
There is evidence that mass infant vaccinations can reduce hepatitis B virus (HBV) infections in highly endemic environments resulting in drastic reduction of HBV transmission.1–4 Some of these programmes may not be limited to infants but may be extended to other age groups.2 The complete elimination of hepatitis B surface antigen (HBsAg) carriage in infants under 5 years in South Africa 4, could therefore be a model for countries who are over a decade into HBV vaccination through the Expanded Programme of Immunization (EPI).
Infant vaccination may not always achieve the desired short and long term results as hepatitis B antibody (anti-HBs) levels may wane over time5, immunity to HBV antigens may not be sufficient in significant proportions of children6, a delayed second dose in an infant vaccination schedule in a national EPI programme may lead to the increased risk of infection7, and a loss to immune memory may occur resulting in the absence of an anamnestic response after encounter with HBV antigens.8 There is therefore the need for a clearer understanding of the dynamics of immune responses in infants after they have received all three doses of hepatitis B vaccine during the first fourteen weeks of life.
Even though data on the evaluation of the effectiveness of infant vaccination in the West African sub-region is still emerging5,6,9–12, the factors that may determine responses to vaccination in resource-limited settings are still unclear. Such gaps may lead to reduced adherence to protocols that provide maximum efficiency and will in such instances reduce the frequency of sero-protection.13
A systematic approach to the evaluation of ongoing infant HBV vaccination programmes will ensure that maximum benefits are derived. This is emphasized by the findings that infants in Cameroon did not respond well to the same HBV vaccination regimen as compared to those in the Gambia 6, and also that genetic factors may account for non-response.14 In countries where vaccines were administered at birth and subsequently using the EPI protocol, there has been residual infections in young adults in spite of the reduction in prevalence of HBV infections. 15,16
It also seems that in the Gambia including a birth dose may help to give long lasting protection in adolescence.17 Apart from providing early protection against the establishment of HBV infection in infants, the use of a birth dose has been associated with increased rates of individuals who actually complete the vaccination schedule.18,19 Furthermore, an early booster dose between 4–5 years may increase the number of children with sero-protection. 16
Since the introduction of HBV vaccination in the EPI programme in 2002 in Ghana, few studies to evaluate the development of sero-protection against HBV infections in infants have been done11,20, and the manufacturers of the supply of vaccines have been changed a number of times. Furthermore, limited information on HBV infections in children in Ghana suggests a very high prevalence in some parts of the country21, with no effective vaccination programmes at birth. In the absence of an institutionalized birth dose, there is the need to monitor current vaccination programs for sero-protection rates and HBsAg carriage to estimate residual infections in our hyper endemic environment where prevalence rates are around 10% in different populations.22–25
This study therefore reports the levels of immunity to HBV among children who received the DPT-HepB-Hib vaccine and HBsAg carriage in those who did not sero-convert to anti-HBs (non-responders). Since immune recognition can wane 8, the factors that determine high (100 mIU/ml) anti-HBs titres were also determined. Correlates for non-response are also discussed.
Methods
Study site and population
The study was conducted at the Princess Marie Louis Children's Hospital (PML) located in the central business district of Accra, the capital city of Ghana. This hospital is a major paediatric hospital, which caters for children from different parts of the capital city. A cross-section of 424 children between the ages of 5–32 months who had completed their DPT-HepB-Hib vaccination schedules for at least one month was selected using a convenient sampling approach. These children were visiting the facility for varying health conditions from March 2012 to January 2013. The study was approved by the Ethical and Review Committee of the School of Medicine and Dentistry, University of Ghana, and written informed consent was sought from all parents on behalf of their children after the nature and consequences of the study was fully explained.
A structured questionnaire was used to obtain demographic data and information for the following variables; timeliness (T6, 10, 14), whether the study participant received the vaccine at scheduled times on the 6th, 10th and 14th weeks after birth; type of vaccines (V1/>1), whether the vaccines received by the child were from one manufacturer or two or more manufacturers (GSK, Belgium; Berna Biotech, Korea; Panacea Biotech, India); and exclusive breast feeding (EBF). Attempts were made to retrieve antenatal records for the mothers to determine their HBsAg status prior to parturition but this was unsuccessful.
Blood collection and laboratory testing
After informed written consent had been sought from parents or guardians, blood from the children which had already been collected with EDTA anticoagulant tubes for other diagnostic purposes were retrieved from the PML laboratory and plasma obtained by centrifuging at 4500 RPM for 3 minutes using the EBA 20 table-top centrifuge (Hettich Zentrifugen, Tuttlingen, Germany). Plasma was kept at −20°C for short-term storage until serological testing was done. Plasma was analysed qualitatively and quantitatively for anti-HBs using a sandwich EIA principle (Antisurase B-96 ELISA, General Biologicals, Corp., Taiwan).
A 1000 mIU/ml standard was diluted with normal human plasma which had tested negative for anti-HBs, anti-HBc IgM, anti-HBc IgG, and HBsAg, to make 1/2, 1/4, 1/8, 1/16, 1/32, 1/64 dilutions and included in ELISA runs to facilitate quantitative analysis. The cut-off value for each ELISA plate was used to determine the children who had any level of anti-HBs, and these were further categorized into those with ≥10 mIU/m and ≥100 mIU/ml of anti-HBs using appropriate standards.
All standards were from General Biologicals, Corp., Taiwan, and anti-HBs determination was done in duplicates. In order to identify those with HBsAg carriage, all children who had not developed anti-HBs were screened for HBsAg with the Surase B-96 ELISA (General Biologicals Corp., Taiwan) and supplemental testing done with the Wondfo rapid test (Guangzhou Wonfo Biotech Inc., China).
Qualitative screening for anti-HBc IgG was done for the 354 children with detectable anti-HBs with an ELISA (Foresight, Acon Laboratories Inc., USA), in order to have presumptive idea of the children who may have had natural infections with HBV. Supplemental testing for anti-HBc IgG was done with Anticorase B-96 ELISA (General Biologicals, Corp., Taiwan) for 21 of the 45 plasma samples that were reactive for anti-HBc IgG with the Foresight ELISA.
Data analysis
The primary study outcomes determined were children who had anti-HBs of ≥10 mIU/ml (sero-protection) 26, and those who had HBsAg. The independent t-test and chi squared test were used to determine the correlates for seroconversion, sero-protection and having high anti-HBs.
A stepwise bivariate logistic regression was also done to determine the most important determinants for seroconversion, sero-protection and having high anti-HBs (≥100 mIU/ml). A spearman rank correlation was done to determine the correlation between actual anti-HBs titres and age. The SPSS V17 software (SPSS for Windows, SPSS Inc., Chicago) was used for all statistical analysis and p values of <0.05 considered statistically significant for all analysis.
Results
Description of population
Of the total number of participants tested, 215 (50.7%) and 209 (49.3%) were males and females respectively. The mean and median age of the study participants were 13.7 and 13.0 months respectively. The highest frequency was seen among children who were 11 months old (34 children) while those 28 and 31 months old had the lowest frequencies.
Majority of the children, 260 (63.2%), received vaccines from the same manufacturer, while 155 (36.6%) and 1 (0.23%) had two and three doses from two and three different manufacturers (GSK, Belgium; Berna Biotech, Korea; Panacea Biotech, India) respectively. The majority of children, 333 (78.5%), took the vaccines at schedule times as compared to 91 (21.5%) who did not.
Two hundred and ninety-two (68.9%) of the children were reported as having been EBF for the first 6 months of life while 132 (31.1%) had supplementary feeding. Details of the characteristics of the children have been summarized in Table 1.
Table 1.
Characteristics of children with completed DTP-HBV-Hib vaccination (n=424)
| Characteristics | Description |
| Mean Age | 13.73±5.72 |
| [5–32months] | |
| Male | 209 (49.3%) |
| Female | 215 (50.7%) |
| T6,10,14 (yes) | 333 (78.5%) |
| V1/>1 (only one type) | 260 (63.2%) |
T6, 10, 14, if the child received the vaccine on the schedule time of life at 6, 10, 14 weeks of age; V1/>1, the number of vaccines the child took from different manufacturers; EBF, exclusive breast feeding during the first six months of life.
Hepatitis B viral markers
The prevalence of anti-HBs among the children was 358 (84.4%), while 3 (0.05%) of the 66 non-responders were positive for HBsAg. These 3 were 10, 17 and 23 months of age. Of the 358 who had anti-HBs, 340 (95.0%), 205 (57.3%) and 16 (4.5%) had titres ≥10 mIU/ml, ≥100 mIU/ml and ≥1000 mIU/ml respectively. Only one HBsAg positive child had the mother show evidence of HBsAg reactivity during pregnancy in her antenatal record book. No record was available to ascertain if the child had received prophylaxis at birth.
After initial screening for anti-HBc IgG in 354 children who were reactive for anti-HBs, 308 (87.0%) were non-reactive while 45 (12.7%) were reactive. Supplemental testing of 21 reactive samples from the initial anti-HBc IgG screening using a second ELISA (Anti Corase, General Bioilogicals corp., Taiwan), showed 6 positives which all had Optical Density/cut-off ratio of the ELISA being >2. It is therefore unlikely that the 24 (53.3%) of the 45 initially reactive plasma samples with low OD/cut-off ratios (1.01 to 1.44) in the first anti-HBc IgG screening were positive.
Determination of correlates for seroconversion and sero-protection
The ages of the children affected the seroconversion rate and sero-protective levels for anti-HBs (≥10 mIU/ml) (Table 2). EBF, gender, T6, 10, 14, and V1/>1 did not determine if a child would have the minimum protective levels of anti-HBs or not (Table 2). When considering a minimum titre of 100 mIU/ml. the age of children and V1/>1 significantly influenced outcomes (Table 2).
Table 2.
Correlates for anti-HBs responses in vaccinated infants
| Reponses after completion of 3 vaccine doses (N=424) | |||||||||
| Seroconversion | Sero-protection (≥10 mIU/ml) | ≥100 mIU/ml | |||||||
| Variables | Anti-HBs | No response | p value | ≥10 mIU/ml | <10 mIU/ml | p value | ≥100 mIU/ml | <100 mIU/ml | p value |
| Mean Age (Months) | 13.45 | 15.23 | .020 | 13.41 | 15.02 | 0.020 | 12.95 | 14.46 | .007 |
| [CI] | [12.86–14.04] | [13.84–16.62] | [12.80–14.02] | [13.80–16.25] | [12.15–13.75] | [13.71–15.20] | |||
| Sex/Male (%) | 86.05 | 13.95 | .353 | 82.79 | 17.21 | .173 | 53.02 | 46.98 | .051 |
| [CI] | [81.42–90.68] | [9.32–18.58] | [77.74–87.84] | [12.16–22.26] | [46.35–59.69] | [40.31–53.65] | |||
| EBF/Yes (%) | 83.56 | 16.44 | .461 | 80.14 | 19.86 | .968 | 50.00 | 50.00 | .312 |
| [CI] | [79.31–87.81] | [12.19–20.69] | [75.56–84.72] | [15.28–24.44] | [44.27–55.73] | [44.27–55.73] | |||
| Single Vaccine/Yes | 82.84 | 17.16 | .234 | 77.99 | 22.01 | .136 | 43.28 | 56.72 | .006 |
| (%) | [78.33–87.35] | [12.68–21.72] | [73.03–82.95] | [12.68–21.72] | [37.35–49.21] | [50.79–62.65] | |||
| [CI] | |||||||||
| T6, 10, 14/Yes (%) | 85.59 | 14.41 | .211 | 81.08 | 18.92 | .378 | 48.95 | 51.05 | .636 |
| [CI] | [81.82–89.36] | [10.64–18.18] | [76.87–85.29] | [14.71–23.13] | [43.58–54.32] | [45.68–56.42] | |||
EBF, exclusive breast feeding; Single Vaccine, infants vaccinated with vaccine from only one pharmaceutical company; T6, 10, 14, infants received vaccine doses in a timely manner at 6, 10 and 14 weeks after birth.
A scatter plot of the actual anti-HBs titres for 230 children (r=−0.155; p=.019) who had anti-HBs titres available, and 171 (r=−0.190; p=.013) whose anti-HBc IgG were confirmed as non-reactive, established that age was an important factor determining anti-HBs response (Figure 1).
Figure 1.
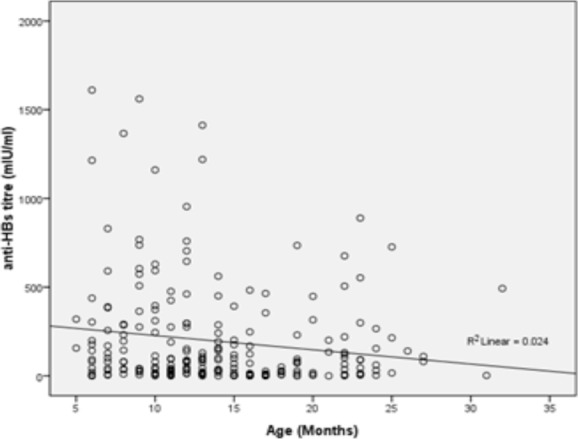
A scatter-plot showing the relationship between the ages of the children and the actual anti-HBs titres.
After regression analysis, age had the tendency to be the most important determinant for seroconversion (OR.908, CI .908–.992) and sero-protection (OR .953, CI .915–.993).
Even though this tendency was still relevant when considering anti-HBs titres ≥100 mIU/ml (OR .964, CI .931–.999), V1/>1 (OR 1.588, CI 1.052 – 2.397) was the most important determinant (Table 3).
Table 3.
Significant factors affecting anti-HBs responses in vaccinated infants
| Anti-HBs levels | Significant contributors |
OR | CI | p value |
| Seroconversion | Age | .949 | .908 – .992 | .022 |
| Sero-protection | Age | .953 | .915 – .993 | .021 |
| (≥10 mIU/ml) | ||||
| ≥100 mIU/ml | Age | .964 | .931 – .999 | .041 |
| V1/>1 | 1.588 | 1.052 – 2.397 | .028 | |
| Sex | .691 | .469 – 1.020 | .063 |
Relationships between variables
Children who received vaccinations in a timely manner were more likely to have used vaccines from a single rather than 2 or more manufacturers (χ2=5.452, p=.020).
Furthermore, the mean age of those who used a single type of vaccines was higher (14.75 ± 6.056 months; n=268) than those who used vaccines from two or more manufacturers (11.96 ± 4.645 months; n=156), <.001 (CI: −3.897 − 1.688).
Discussion
Sero-protection rates due to HBV vaccination during the EPI have been shown to be high in children within the West African sub-region 6,10,11, even though some evidence suggests that this may not be universal 6. Several reasons may have been adduced for the low protective levels in Senegalese children in a previous study 6, but it is also possible that the results may reflect a genetic problem 14. Admittedly, different assays and slightly modified vaccination schedules may have been used for the various studies.
However, the use of different assays in them may not be problematic when comparing results from different studies. The standardization of the different assays to international units may reduce the differences between assays and therefore make results comparable. Perhaps the use of a different vaccine or genome wide association studies, may confirm the actual reasons for the low response in Senegalese as compared to Cameroonian, and by extension, Ghanaian children 6.
Since the prevalence of anti-HBc in vaccinated infants and children is likely to be low 4, it suggests that majority of the study subjects with low ELISA optical density/cut-off ratios were unlikely to have anti-HBc. Thus the findings that the age of the children was of significant importance for sero-protection is of concern. It seems that the older children in this study had taken vaccines from particular manufacturers and this may reflect on the procurement processes which occurred at the time.
This is because the children whose T6, 10, 14 were not on schedule were more likely to have used vaccines from different manufacturers. Irrespective of the fact that a delayed second dose may have some effects on vaccination outcomes 27, there was no significant lack of protection for those who did not take the vaccine in a timely manner. Unfortunately, this study did not collate the kind of data to deeply analyse this assertion. The issue related to the procurement of the pentavalent DTP-HBV-Hib vaccine has also been brought to the fore by this study. In resource limited countries where procurement issues may not be stable, different vaccines from several companies may be combined during the regular schedule of the EPI. In this study however, the results suggest that those with the combination of vaccines were more likely to have better responses and this may be because the younger children were the ones who used the mixture of vaccines.
In the face of the possibility of declining titres with age irrespective of the vaccination schedule of infants5,17, and the loss of immune memory8, 28, it may be important to consider 100 mIU/ml as the desired response after vaccination since the use of either one or multiple vaccines from different manufacturers significantly contributed to that level of protection.
Though maternal HBsAg testing is the most effective way to identify infants exposed to HBV who need post exposure immunoprophylaxis, the use of a birth dose to infants will also serve to prevent perinatal infection among infants born to HBsAg-positive pregnant women who are not identified or have limited resources to purchase the Hepatitis B immune globulin (HBIG). A birth dose for all neonates may therefore be useful to prevent loss of immune recognition at a much older age as seen elsewhere 29.
However, for those who did not receive the birth dose, an early booster dose may be helpful 16. It is noteworthy to mention that in developing countries where there are also hard to reach populations, majority of infants who benefit from the vaccination program may never have the opportunity to take boosters. The need to evoke high enough anti-HBs during infancy is therefore of paramount importance.
In Ghana, the use of vitamin A has been shown not to contribute significantly to response to HBV vaccination 11. However, malnutrition may be responsible for the low response to vaccine 6. In this study, there was no statistical difference in EBF for those with or without sero-protection suggesting that the relationship between nutrition or nutrition supplements and immune response to HBV vaccination needs further study.
Even though limited data on HBV infections in children is available 21,30, the prevalence rates observed in these studies were fairly high. The anti-HBs sero-protection in this study may be similar to others done in Ghana with different primary foci 11,20, however none of these studies considered the prevalence of HBsAg carriage in vaccinated children. Considering the hyper endemic environment in which this study was done, and HBsAg prevalence in vaccinated children in other West African countries 10,31, it may be conclusive to say that the immediate results of the vaccination programme covering the study period are desirable. The three children who had HBsAg carriage may therefore have had mothers who were also carriers.
The main challenge here is to ensure that protocols developed for pregnant women who are HBsAg carriers are adhered to13, and the universal screening of pregnant women for HBsAg should be aggressively pursued and vaccination of all infants at birth considered.32 There is also the possibility that these children may have been infected within the first 6 months of life as documented in Senegal.27
Conclusion
There is still a residual possibility of infection with HBV in spite of infant vaccination but it is unclear if these infections may have occurred before or after completion of vaccination. It is therefore important to acquire pre- and post-vaccination outcomes in children so as to understand the transmission patterns causing this residual infection. National data and urgent protocols are therefore needed to ensure that the progress in reducing or elimination of HBV infections in Ghana can be effectively monitored. The responses to the HBV component of the pentavalent DTP-HBV-Hib vaccine are encouraging but the introduction of a birth dose for all neonates may be desirable. A booster dose during early childhood may also be helpful for young children.
Acknowledgement
Thomas Apiung was partly supported by the College of Health Sciences, University of Ghana, students' research grant. We acknowledge the support of the Head and Staff of the Princess Marie Louis Children's Hospital. Isaac Boamah, Anna Aba Hayford, Makafui Seshie and Kwabena Obeng Duedu of the Clinical Virology Laboratory, School of Biomedical and Allied Health Sciences, are acknowledged for their technical support. Thomas Apiung was partly supported by the College of Health Sciences, University of Ghana, students research grant.
References
- 1.Liang X, Bi S, Yang W, et al. Epidemiological serosurvey of hepatitis B in China—declining HBV prevalence due to hepatitis B vaccination. Vaccine. 2009;27(47):6550–6557. doi: 10.1016/j.vaccine.2009.08.048. [DOI] [PubMed] [Google Scholar]
- 2.Yang S-g, Wang B, Chen P, et al. Effectiveness of HBV vaccination in infants and prediction of HBV prevalence trend under new vaccination plan: findings of a large-scale investigation. PloS one. 2012;7(10):e47808. doi: 10.1371/journal.pone.0047808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Viviani S, Jack A, Hall AJ, et al. Hepatitis B vaccination in infancy in The Gambia: protection against carriage at 9 years of age. Vaccine. 1999;17(23–24):2946–2950. doi: 10.1016/s0264-410x(99)00178-4. [DOI] [PubMed] [Google Scholar]
- 4.Tsebe KV, Burnett RJ, Hlungwani NP, Sibara MM, Venter PA, Mphahlele MJ. The first five years of universal hepatitis B vaccination in South Africa: evidence for elimination of HBsAg carriage in under 5-year-olds. Vaccine. 2001;19(28–29):3919–3926. doi: 10.1016/s0264-410x(01)00120-7. [DOI] [PubMed] [Google Scholar]
- 5.van der Sande MAB, Waight PA, Mendy M, et al. Long-term protection against HBV chronic carriage of Gambian adolescents vaccinated in infancy and immune response in HBV booster trial in adolescence. PloS one. 2007;2(8):e753. doi: 10.1371/journal.pone.0000753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rey-Cuille M-A, Seck A, Njouom R, et al. Low immune response to hepatitis B vaccine among children in Dakar, Senegal. PloS one. 2012;7(5):e38153. doi: 10.1371/journal.pone.0038153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tharmaphornpilas P, Rasdjarmrearnsook A-o, Plianpanich S, Sa-nguanmoo P, Poovorawan Y. Increased risk of developing chronic HBV infection in infants born to chronically HBV infected mothers as a result of delayed second dose of hepatitis B vaccination. Vaccine. 2009;27(44):6110–6115. doi: 10.1016/j.vaccine.2009.08.034. [DOI] [PubMed] [Google Scholar]
- 8.Lu CY, Ni YH, Chiang BL, et al. Humoral and cellular immune responses to a hepatitis B vaccine booster 15–18 years after neonatal immunization. J Infect Dis. 2008;197(10):1419–1426. doi: 10.1086/587695. [DOI] [PubMed] [Google Scholar]
- 9.Ekra D, Herbinger K-H, Konate S, et al. A non-randomized vaccine effectiveness trial of accelerated infant hepatitis B immunization schedules with a first dose at birth or age 6 weeks in Côte d'Ivoire. Vaccine. 2008;26(22):2753–2761. doi: 10.1016/j.vaccine.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 10.Magoni M, Ekra KD, Aka LN, Sita KS, Kanga K. Effectiveness of hepatitis-B vaccination in Ivory Coast: the case of the Grand Bassam health district. Annals of tropical medicine and parasitology. 2009;103(6):519–527. doi: 10.1179/136485909X451816. [DOI] [PubMed] [Google Scholar]
- 11.Newton S, Owusu-Agyei S, Ampofo W, et al. Vitamin A supplementation enhances infants' immune responses to hepatitis B vaccine but does not affect responses to Haemophilus influenzae type b vaccine. The Journal of nutrition. 2007;137(5):1272–1277. doi: 10.1093/jn/137.5.1272. [DOI] [PubMed] [Google Scholar]
- 12.Newton S, Filteau S, Owusu-Agyei S, Ampofo W, Kirkwood BR. Seroprotection associated with infant vitamin A supplementation given with vaccines is not related to antibody affinity to Hepatitis B and Haemophilus influenzae type b vaccines. Vaccine. 2010;28(30):4738–4741. doi: 10.1016/j.vaccine.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Hu Y, Zhang S, Luo C, Liu Q, Zhou Y-H. Gaps in the prevention of perinatal transmission of hepatitis B virus between recommendations and routine practices in a highly endemic region: a provincial population-based study in China. BMC infectious diseases. 2012;12:221. doi: 10.1186/1471-2334-12-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan K, Cai W, Cao F, et al. Genetic effects have a dominant role on poor responses to infant vaccination to hepatitis B virus. Journal of human genetics. 2013;58(5):293–297. doi: 10.1038/jhg.2013.18. [DOI] [PubMed] [Google Scholar]
- 15.Peto TJ, Mendy ME, Lowe Y, Webb EL, Whittle HC, Hall AJ. Efficacy and effectiveness of infant vaccination against chronic hepatitis B in the Gambia Hepatitis Intervention Study (1986–90) and in the nationwide immunisation program. BMC Infect Dis. 2014;14:7. doi: 10.1186/1471-2334-14-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steiner M, Ramakrishnan G, Gartner B, Van Der Meeren O, Jacquet J-M, Schuster V. Lasting immune memory against hepatitis B in children after primary immunization with 4 doses of DTPa-HBV-IPV/Hib in the first and 2nd year of life. BMC Infect Dis. 2010;10:9. doi: 10.1186/1471-2334-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mendy M, Peterson I, Hossin S, et al. Observational study of vaccine efficacy 24 years after the start of hepatitis B vaccination in two Gambian villages: no need for a booster dose. PloS one. 2013;8(3):e58029. doi: 10.1371/journal.pone.0058029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yusuf HR, Daniels D, Smith P, Coronado V, Rodewald L. Association between administration of hepatitis B vaccine at birth and completion of the hepatitis B and 4:3:1:3 vaccine series. JAMA. 2000;284(8):978–983. doi: 10.1001/jama.284.8.978. [DOI] [PubMed] [Google Scholar]
- 19.Luman ET, Fiore AE, Strine TW, Barker LE. Impact of thimerosal-related changes in hepatitis B vaccine birth-dose recommendations on childhood vaccination coverage. JAMA. 2004;291(19):2351–2358. doi: 10.1001/jama.291.19.2351. [DOI] [PubMed] [Google Scholar]
- 20.Hodgson A, Forgor AA, Chandramohan D, et al. A phase II, randomized study on an investigational DTPw-HBV/Hib-MenAC conjugate vaccine administered to infants in Northern Ghana. PloS one. 2008;3(5):e2159. doi: 10.1371/journal.pone.0002159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinson FE, Weigle KA, Mushahwar IK, Weber DJ, Royce R, Lemon SM. Seroepidemiological survey of hepatitis B and C virus infections in Ghanaian children. Journal of medical virology. 1996;48(3):278–283. doi: 10.1002/(SICI)1096-9071(199603)48:3<278::AID-JMV11>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 22.Cho Y, Bonsu G, Akoto-Ampaw A, et al. The prevalence and risk factors for hepatitis B surface ag positivity in pregnant women in eastern region of Ghana. Gut Liver. 2012;6(2):235–240. doi: 10.5009/gnl.2012.6.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dongdem JT, Kampo S, Soyiri IN, Asebga PN, Ziem JB, Sagoe K. Prevalence of hepatitis B virus infection among blood donors at the Tamale Teaching Hospital, Ghana (2009) BMC Res Notes. 2012;5:115. doi: 10.1186/1756-0500-5-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nkrumah B, Owusu M, Frempong HO, Averu P. Hepatitis B and C viral infections among blood donors from rural Ghana. Ghana Med J. 2011;45(3):97–100. [PMC free article] [PubMed] [Google Scholar]
- 25.Sarkodie F, Adarkwa M, Adu-Sarkodie Y, Candotti D, Acheampong JW, Allain JP. Screening for viral markers in volunteer and replacement blood donors in West Africa. Vox sanguinis. 2001;80(3):142–147. doi: 10.1046/j.1423-0410.2001.00023.x. [DOI] [PubMed] [Google Scholar]
- 26.Jack AD, Hall AJ, Maine N, Mendy M, Whittle HC. What level of hepatitis B antibody is protective? J Infect Dis. 1999;179(2):489–492. doi: 10.1086/314578. [DOI] [PubMed] [Google Scholar]
- 27.Roingeard P, Diouf A, Sankale JL, et al. Perinatal transmission of hepatitis B virus in Senegal, west Africa. Viral immunology. 1993;6(1):65–73. doi: 10.1089/vim.1993.6.65. [DOI] [PubMed] [Google Scholar]
- 28.Chang YC, Wang JH, Chen YS, Lin JS, Cheng CF, Chu CH. Hepatitis B virus vaccination booster does not provide additional protection in adolescents: a cross-sectional school-based study. BMC public health. 2014;14:991. doi: 10.1186/1471-2458-14-991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McMahon BJ, Bruden DL, Petersen KM, et al. Antibody levels and protection after hepatitis B vaccination: results of a 15-year follow-up. Annals of internal medicine. 2005;142(5):333–341. doi: 10.7326/0003-4819-142-5-200503010-00008. [DOI] [PubMed] [Google Scholar]
- 30.Otatume S, Afoakwa SN, Mingle JAA, Derban IKA, Laryea EO. Sero-epidemiological survey of hepatitis B-virus infection in school children around Senchi area (Preliminary Report) Ghana Med J. 1976;15(4):297–299. [Google Scholar]
- 31.Whittle H, Jaffar S, Wansbrough M, et al. Observational study of vaccine efficacy 14 years after trial of hepatitis B vaccination in Gambian children. BMJ (Clinical research ed) 2002;325(7364):569. doi: 10.1136/bmj.325.7364.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qureshi H, Javaid N, Alam SE, Bile KM. The evidence of mother to child transmission of hepatitis B virus infection in Pakistan and the need for hepatitis B immunization policy change. JPMA The Journal of the Pakistan Medical Association. 2014;64(4):403–408. [PubMed] [Google Scholar]


