ABSTRACT
Nematode–insect interactions are ubiquitous, complex and constantly changing as the host and nematode coevolve. The entomophilic nematode Pristionchus pacificus is found on a myriad beetle species worldwide, although the molecular dynamics of this relationship are largely unknown. To better understand how host cues affect P. pacificus embryogenesis, we characterized the threshold of sensitivity to the pheromone (Z)-7-tetradecen-2-one (ZTDO) by determining the minimum exposure duration and developmental window that results in P. pacificus embryonic lethality. We found early-stage embryos exposed to volatile ZTDO for as few as 4 h all display terminal embryogenesis, characterized by punctuated development up to 48 h later, with abnormal morphology and limited cavity formation. To determine if the pheromone arrests pre-hatching development by suffocating or permeabilizing the eggshells, we raised embryos under anoxic conditions and also examined eggshell permeability using the lipophilic dye FM4-64. We found that asphyxiating the embryos arrested embryogenesis in a reversible manner but did not phenocopy the effects of ZTDO exposure, whereas the ZTDO-induced disruption of embryogenesis did correlate with increased eggshell permeability. The effects of ZTDO are also highly specific, as other lipid insect compounds do not produce any detectable embryocidal effect. The high specificity and unusual teratogenic effect of ZTDO may be important in mediating the host–nematode relationship by regulating P. pacificus development.
KEY WORDS: Nematode, Pristionchus pacificus, Eggshell, Pheromone, Embryogenesis
Summary: Insect-associated nematodes coordinate their development using host cues to ‘walk the line’ between mutualism and pathogenesis. A host pheromone causes terminal embryogenesis by permeabilizing the nematode eggshell.
INTRODUCTION
Many parasites regulate their development to coordinate with that of their host species. In particular, the life cycle of parasitic nematodes includes one or more infective stages that respond to host cues, such as the dauer and pre-hatch juveniles. In most plant parasitic nematodes, the egg protects the pre-hatch juveniles but can still respond to the presence of hosts. For example, the hatching rate in plant parasitic nematodes Heterodera spp. and Globodera spp. is significantly enhanced in the presence of root extracts (Tefft and Bone, 1985; Ibrahim and Haydock, 1999; Byrne et al., 2001). However, the coordination between the development of insect parasitic nematodes and their insect hosts is not well documented. In particular, the entomophilic nematodes that remain non-pathogenic on specific hosts may reveal novel signaling mechanisms necessary to regulate developmental progress between nematodes and their insect hosts.
In a type of association known as necromeny, the nematode Pristionchus pacificus and related nematodes live on various species of beetles and wait until their hosts die before resuming reproductive development to feed on the microorganisms growing on the host carcasses (Manegold and Kiontke, 2001; Herrmann et al., 2006a,b; Sudhaus, 2009). However, as entomophilic nematodes can continue to evolve new relationships with potential pathogenic bacteria to infect insect hosts (Zhang et al., 2008; Ye et al., 2010; Dillman et al., 2012), it is not known how Pristionchus species and related nematodes maintain their strictly necromenic and non-pathogenic interaction with their hosts. It is possible that the more species-specific relationship found in certain P. pacificus populations have mechanisms that prevent reproductive development on living hosts, such as the association between P. pacificus and the oriental beetle Exomala orientalis (Herrmann et al., 2007). Such mechanisms may depend on specific host cues that can regulate a durable but non-pathogenic necromenic relationship. In particular, the sex pheromone of the oriental beetle, (Z)-7-tetradecen-2-ol (ZTDO) (Zhang et al., 1994), is a chemical attractant for adult nematodes as well as a nematocide that paralyses early larval stages, prevents dauer larvae exit, and inhibits egg hatching (Cinkornpumin et al., 2014). Most surprisingly, unlike other natural compounds so far described, ZTDO is a volatile nematocide that acts without direct contact. Because responses to ZTDO depend on the developmental stage in the P. pacificus life cycle, ZTDO represents a key semiochemical important for the coordination of the necromenic relationship between the nematodes and the beetles (Ruther et al., 2002; Leal, 2005; Chaisson and Hallem, 2012; Okumura et al., 2013).
Given the well-known ability of the nematode eggshell to resist chemical and biological assaults (Bird and McClure, 1976; Wharton, 1980), the ability of ZTDO to elicit novel embryonic lethal phenotypes is especially intriguing. The nematode eggshell is one of the most resistant biological structures and a major barrier against chemical and enzymatic nematocides. The nematode eggshell may consist of up to five layers, but the stereotypic nematode eggshell is a trilaminar structure consisting of an inner lipid layer, a middle chitinous layer, and an outer vitelline layer (Wharton, 1980; Rappleye et al., 1999). These layers assemble soon after fertilization of the oocyte by the sperm to prevent polyspermy as well as to protect the developing embryo from the environment. Nevertheless, the eggshell is still permeable to oxygen as well as water. Environmental factors can also penetrate the eggshell, sometimes in a species-specific manner. For example, the nematophagous fungi Paecilomyces lilacinus produces chitinases that degrade the outer two layers of the eggshell of the plant parasitic nematode Meloidogyne javanica, as well as proteases that destroy the inner lipid layer (Khan et al., 2004). Other filamentous fungi also use chitinases to attack the eggs of the parasitic nematode Ascaris lumbricoides during its free-living stage in the soil (Kunert, 1992). Even plant defence compounds, such as trans-2-hexenal, can significantly reduce the hatching rate of the pinewood nematode Bursaphelenchus xylophilus after a 12 h treatment (Cheng et al., 2016). Thus despite a remarkably resistant structure, the nematode eggshell represents both a regulator for host–parasite communication as well as a valid target for host-derived anthelmintics.
Although the chitinous layer provides rigidity, the lipid layer is widely perceived to act as a permeability barrier against most chemicals. Recent studies in the compost-dwelling Caenorhabditis elegans revealed a fourth layer between the embryo and the trilaminar eggshell that confers impermeability but is distinct from the lipid layer (Olson et al., 2012). Genetic analysis suggests that this permeability barrier consists of ascarosides with lipid side-chains. Although the eponymous ascarosides are found in the lipid layer of eggshells of Ascaridae, the conservation of this inner permeability barrier in ascarid species and other nematodes has not yet been examined. The permeability barrier in C. elegans can be compromised by a small compound, C22, that results in weakened eggshells sensitive to minor osmotic changes and light pressure. C22, however, acts specifically on the oocyte-to-embryo transition in C. elegans but not in other Caenorhabditis species, suggesting that the permeability barrier evolves quickly among nematodes.
In this study, we show that a minute amount of the sex pheromone ZTDO from the oriental beetle host is a potent embryocide that can permeabilize and perhaps also penetrate the P. pacificus eggshell. Exposure of early 2- to 8-cell embryos to the volatile form of ZTDO elicits a potent lethal effect on embryos that derails normal development and permanently prevents embryos from hatching. More curiously, most embryos continue to develop long after treatment has ended, with around half of the embryos developing muscles, gut, and pharynx-like structures well past the normal hatching time of 24 h. This embryonic lethality varied among global P. pacificus strains, with the strongest effect found in a Japanese strain isolated from the oriental beetle, and is correlated with ZTDO-induced eggshell permeability. However, eggshell permeability alone does not account for all of the ZTDO-induced lethality, which may suggest that zygotic factors also mediate ZTDO susceptibility.
MATERIALS AND METHODS
Nematode strains
All P. pacificus and C. elegans strains were maintained on 6 cm nematode growth media (NGM) plates seeded with OP50 and kept at 20°C as described by Hong et al. (2008). The following P. pacificus strains were used: PS312 (California wild-type, synonymous with RS2333), PS1843 (Port Angeles, WA, USA), Ppa-obi-1(tu404)I, csuls01[Ppa-obi-1p::gfp; Ppa-egl-20p::rfp; PS312 gDNA]X, RSB001 (La Reunion), RS5278 (Bolivia), RS5419 (La Reunion), RS5186 (Japan), RS5194 (Japan), and RLH163 (Mar Vista, CA, USA). Wild-type N2 C. elegans was used. Pristionchus pacificus populations include both hermaphrodites and ∼1% males with a life cycle of 4 days.
Lid-drop assay
The ZTDO assay was used to determine both the minimum lethal exposure duration of wild-type PS312 embryos and ZTDO susceptibility of other strains. To synchronize embryos, approximately 20 well-fed, young gravid hermaphrodites were isolated on 6 cm plates (T3308; Tritech, Los Angeles, CA, USA) with 100 µl of OP50 and then removed after an hour. For ZTDO treatment, 10 µl of 0.5% (Z)-7-tetradecen-2-ol (ZTDO; Bedoukian Research, Danbury, CT, USA) diluted in 100% ethanol (v/v) was added to the underside of the plate lids and sealed with Parafilm. Given the dimensions of the cylindrical plate (radius=2.5 cm, height=0.5 cm), the volume encompassing the embryos above, and thus not including, the agar is approximately 10 cm3. Thus the addition of 0.05 µl ZTDO results in at least 5.1 million-fold dilution in the headspace above the nematodes being tested. (E)-11-tetracenyl acetate (EDTA; Bedoukian Research) and methyl tetradecanoate (myristate; SigmaAldrich, St Louis, MO, USA) were diluted to 0.5% in ethanol and administered the same way as ZTDO. To stop the treatment, Parafilm and lids were removed after exposure and replaced with new lids. To determine hatching rates, the number of eggs was counted after synchronization (time 0) and unhatched embryos were counted at screening time (26 or 48 h). N (n)=number of experimental trials performed with control on different days (total sample size of nematodes). Trials with unusually high arrest rates in control embryos without odor treatment were omitted.
Microscopy
To determine the hatching rate, eggs and larvae were scored using a Leica S6E dissecting microscope (Wetzlar, Germany). For FM4-64 staining, embryos were viewed using differential interference contrast (DIC) on a Leica DM6000 upright microscope. Embryos were fixed in a 50:49 (egg salts:dH2O) solution on slides inside a small ring of Vaseline and covered with 22×22 mm coverslips on 75×25×1.0 mm slides. To determine permeability, embryos were fixed in a solution of 1:100 (FM4-64:egg buffer) from a 300 µmol l−1 FM4-64 stock solution (Molecular Probes, T3166, Eugene, OR, USA). Images were captured using the exposure time of 1 s and at an intensity level of two on the Leica Application Suite (LAS). Images were cropped using Adobe Photoshop CS6 (San Jose, CA, USA).
Anaerobic chamber
For the anaerobic experiments, a sealed jar and Oxoid AnaeroGen sachets (AN0035A, ThermoFisher Scientific, Waltham, MA, USA) were used. Oxygen levels were confirmed using Oxoid Anaerobic Indicator strips (BR0055, Thermo Fisher Scientific). An hour was added to anaerobic exposure times to allow oxygen levels to reach below 1%. Carbon dioxide levels reached between 9 and 13%, and no nitrogen was produced as a by-product from the sachets.
Statistical analysis
Statistical tests were analysed using GraphPad Prism (La Jolla, CA, USA) and Microsoft Excel.
RESULTS
ZTDO is a potent embryocide
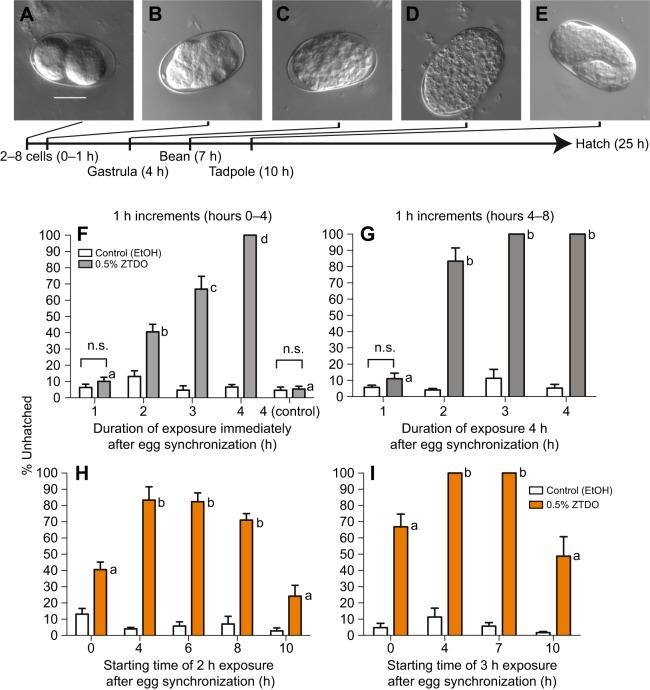
Our previous study showed that P. pacificus early-stage embryos exposed for 2 days to minute amounts of the Oriental beetle pheromone (z)-7-tetradecen-2-ol (ZTDO) severely retards embryonic development and almost all embryos remain at a pre-tadpole stage 2 days later, whereas most embryos exposed to the ethanol vehicle hatch approximately 1 day later (Cinkornpumin et al., 2014). To delineate the earliest embryonic stage most sensitive to ZTDO, we focused on its embryocidal effect on the first half of embryonic development using defined exposure durations, rather than continuous exposure. Because exposing embryos to less than 0.5% ZTDO for 2 days results in some embryos hatching, rather than varying the concentration of ZTDO we sought to determine the lethal time and susceptibility window necessary for 10 µl of volatile 0.5% ZTDO to prevent eggs from hatching after 24 h. In the lid-drop assay, staged embryos are exposed to a volatile source of 0.5% ZTDO under the lid of a culturing plate for a defined time, after which the lid is replaced with a new one to terminate the treatment (Fig. 1). This is approximately a 5.1×106-fold ZTDO dilution in the headspace above the assay arena. Unlike C. elegans, which tends to hold onto eggs in its uterus, wild-type P. pacificus PS312 young adult hermaphrodites typically hold on to only two eggs in the uterus. Eggs are usually laid at the 2-cell stage, although embryos can be expelled from the uterus as old as the 8-cell stage. Embryos synchronized to an hour range from two to 16 cells at 20°C (Fig. 2A–E) and P. pacificus embryogenesis requires 24 h at 20°C, compared with 18 h for C. elegans (Felix et al., 1999; Vangestel et al., 2008). The proportion of unhatched eggs, including unfertilized oocytes and spontaneously arrested embryos, are counted after 26 h ex utero. The additional 2 h is to accommodate the time needed to view the embryos. Roughly 95% of ethanol-treated control eggs hatch after 26 h (Fig. 2). ZTDO exposure on gravid hermaphrodites has a detectable but statistically insignificant effect on lowering the egg laying rate, but has no effect on the hatching rate of embryos exposed in utero (data not shown).
Fig. 1.
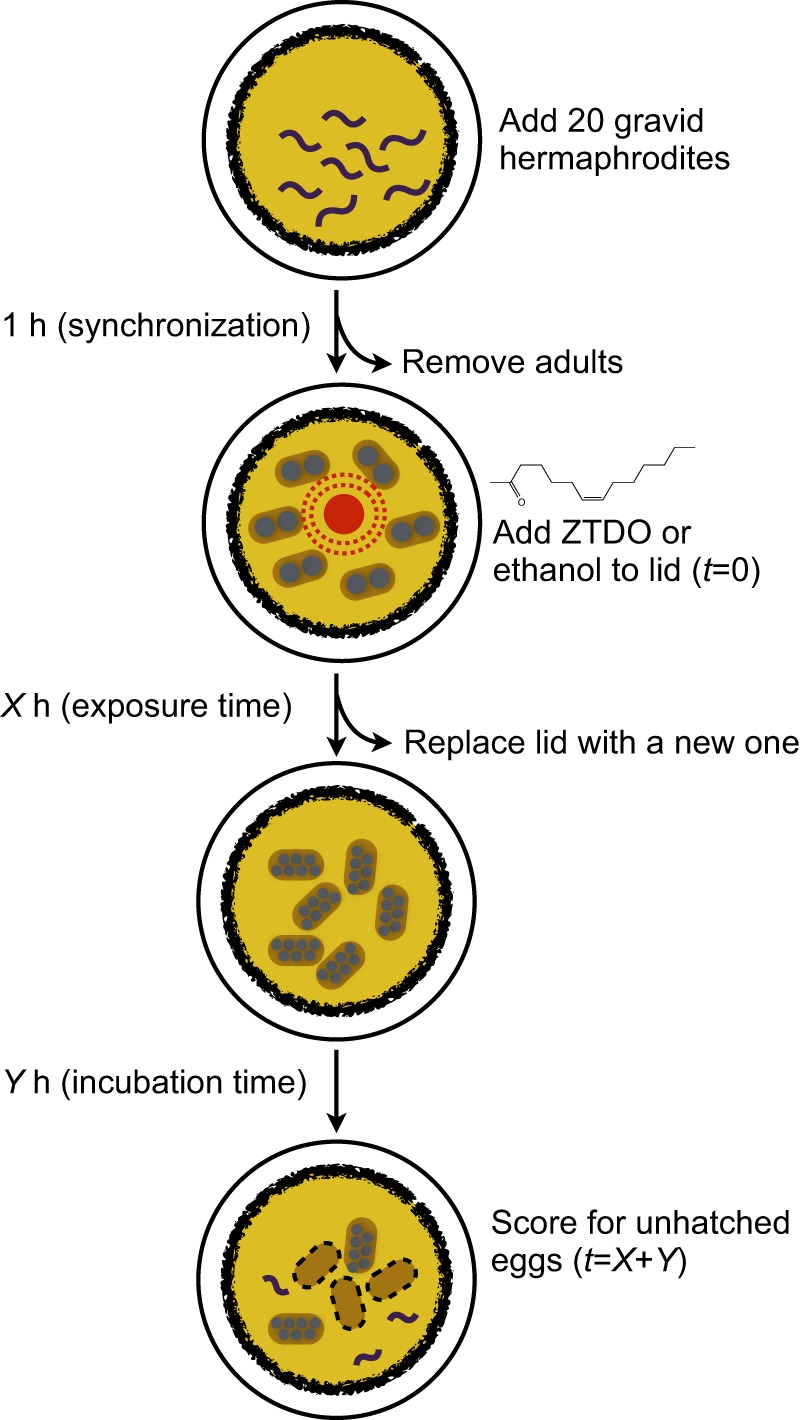
The lid-drop assay for ZTDO sensitivity. Synchronized 1-h-old ex utero embryos were exposed to 10 µl of either 0.5% ZTDO (z-7-tetradecen-2-ol) or control (ethanol) suspended from plate lids.
Fig. 2.
ZTDO sensitivity ex utero depends on exposure duration and embryonic stage. (A–E) Representative embryonic stages of untreated P. pacificus PS312 eggs ex utero at 20°C. Eggs are laid as early as the 2-cell stage and as late as the 8-cell stage. After an hour of synchronization, just prior to ZTDO exposure, the oldest embryo is at the 16-cell stage. Scale bar in A (20 µm) represents the same magnification for all images. Most ZTDO-exposed embryos arrest during gastrulation and the bean stage. Ten microliters of 0.5% ZTDO or 100% ethanol was suspended from each plate lid. (F–I) Mean hatching percentage. (F) Early embryos up to 4 h ex utero show increased percentage of developmental arrest with increasing exposure time to ZTDO. To determine the possible effects of residual ZTDO, untreated embryos were added to NGM plates previously exposed to ZTDO for 4 h (control). N (n) from left to right: 5 (147), 5 (132), 10 (420), 10 (432), 10 (432), 10 (406), 10 (476), 10 (454), 10 (486), 10 (416). (G) Older embryos between 4 and 8 h ex utero old show higher sensitivity to ZTDO. N (n) from left to right: 5 (296), 5 (309), 5 (305), 5 (383), 5 (262), 5 (308), 5 (355), 5 (287). (H) When exposed to ZTDO for only 2 h, 4- to 6-h-old embryos show higher rates of developmental arrest compared with just-laid and 8- to 10-h-old embryos. The 2-h exposure data are the same as in F and G and started at 0 and 4 h ex utero. N (n) from left to right: 10 (432), 10 (406), 5 (305), 5 (383), 5 (291), 5 (336), 5 (270), 5 (317), 5 (239), 5 (265). (I) When exposed to ZTDO for 3 h, 4- to 7-h-old embryos showed even higher rates of developmental arrest compared with just laid and 10-h-old embryos. The 3-h exposure data are the same as in H and I and started at 0 and 4 h ex utero. N (n): 10 (476), 10 (454), 5 (262), 5 (308), 5 (310), 5 (298), 5 (283), 5 (373). The difference between the ethanol control and ZTDO was significant (two-tailed unpaired t-test, P<0.05), except when indicated as not significant (n.s.). Each letter denotes a significant hatching difference between ZTDO exposure durations by one-way ANOVA with Tukey's correction for multiple comparisons. N, number of experimental trials performed with control on different days. n, total sample size of nematodes assayed. Error bars denote ±s.e.m.
We found that the earliest response to ZTDO occurs when synchronized embryos are exposed for at least 2 h, in which 40% of the embryos arrest. These embryos are between 120 and 180 min old and correspond to the start of gastrulation at the 28-cell stage, approximately 185 min after fertilization (Vangestel et al., 2008). Lethality reaches 100% when exposure is extended to 4 h (Fig. 2F). As a control, untreated embryos added to NGM OP50 plates previously exposed to ZTDO for 4 h do not show any responses to residual ZTDO. Thus the removal of the lid is sufficient to terminate the volatile ZTDO treatment. The ZTDO embryocidal effect is even stronger in older embryos that are exposed to the same dose of ZTDO between 4 and 8 h after synchronization, in which a 2 h exposure results in 80% embryonic lethality, and a 3 h exposure results in total lethality (Fig. 2G). These results show that the minimum lethal time for a 5.1 million-fold dilution of ZTDO is just 2 h rather than the 2 day assay used previously (Cinkornpumin et al., 2014), and that the later 4–8 h period ex utero is more sensitive than the earlier 0–4 h period.
To determine whether a shorter exposure time to achieve 100% lethal arrest is possible in other 2–3 h windows within the first 13 h of development ex utero, we exposed embryos to ZTDO for 2 h in 2 h time windows and found that lethality peaks at 80% (Fig. 2H). However, extending ZTDO exposure to 3 h between 4–7 h and 7–10 h ex utero results in the terminal arrest of all embryos, suggesting that the period between the gastrula and tadpole stage is much more sensitive to volatile ZTDO than the periods bracketing it (Fig. 2I). In C. elegans, the period leading to the tadpole stage is characterized by cell proliferation (Sulston et al., 1983), therefore it is likely that in P. pacificus ZTDO may interfere directly or indirectly with the process of cell division. In very rare circumstances, usually in association with bacterial contamination that results in thick lawns, ZTDO-exposed embryos show delayed hatching 48 h ex utero. Because all of the earliest embryos that were exposed to ZTDO for 4 h arrest, we performed subsequent characterizations using this standard regimen: 1–2 h of embryo synchronization, followed by 4 h of ZTDO exposure, and scored for hatching success after 26 h ex utero.
Natural variation in ZTDO sensitivity
Given that P. pacificus populations are found to be associated with several different beetle species globally (Herrmann et al., 2006a,b; Rodelsperger et al., 2014) and that chemotaxis attraction towards ZTDO differs in various P. pacificus strains (Hong et al., 2008; Koneru et al., 2016), we surmised that the embryocidal sensitivity to ZTDO may also vary across different strains. Using the lid-drop assay with synchronized embryos exposed to 0.5% ZTDO for 4 h, we observed that strong differences in embryocidal sensitivity correlated with geographic origins. Like the reference strain PS312 from California, the strains from the Pacific Rim (RLH163 California; PS1843 Washington; RS5194 and RS5186 Japan) have highly ZTDO-sensitive embryos (Fig. 3). In contrast, a strain from Bolivia (RS5278) and three strains from La Reunion Island in the Indian Ocean (RS5419, RS5413 and RSB001) all show 40% or higher of embryos hatching in the presence of ZTDO. C. elegans N2 is insensitive to ZTDO at the concentration tested, even after two days of exposure (Cinkornpumin et al., 2014). Interestingly, host origin is only weakly correlated with ZTDO sensitivity. The sensitive strain RLH163 was isolated from the masked chafer Cyclocephala pasadenae, while the RS5194 and RS5196 Japanese strains were isolated from the Oriental beetle Exomala orientalis. The four resistant strains from Bolivia and La Reunion were isolated from four different species of beetles (Rodelsperger et al., 2014). Because the oriental beetle pheromone is the only P. pacificus host compound commercially available, it is difficult to test whether the correlation between ZTDO sensitivity and geography, but not host species, is due to overlap in pheromone structure between Cyclocephala and Exomala in sensitive strains, or rapid host switching in resistant strains (McGaughran et al., 2013).
Fig. 3.
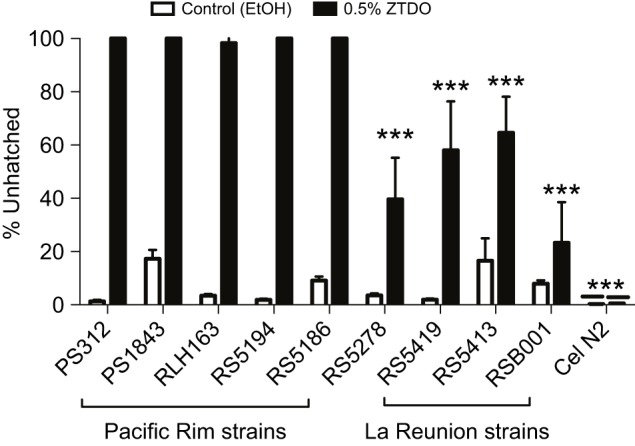
Natural variation in P. pacificus responses to the embryocide ZTDO. Early-stage embryos were exposed to 10 µl of 0.5% ZTDO or 100% ethanol suspended from plate lids for 4 h. Strains from the Pacific Rim – PS1843 (Washington), RLH163 (California), RS5194 (Japan), RS5186 (Japan) – resemble the reference strain PS312 (California). Strains from La Reunion (RS5419, RS5413, RSB001) and Bolivia (RS5278) are significantly less sensitive to ZTDO. Caenorhabditis elegans N2 do not respond to 0.5% ZTDO. The difference between the ethanol control and ZTDO was significant (two-tailed unpaired t-test, P<0.05), except for RSB001 and N2. Significant difference from PS312 was determined by one-way ANOVA followed by Dunnett's post hoc test (***P<0.0001). N (n): 10 (926), 10 (948), 15 (2894), 15 (3771), 15 (1899), 15 (2246), 15 (1678), 15 (1514), 15 (1482), 15 (2194), 15 (1965), 15 (2640), 15 (2735), 15 (2844), 15 (2077), 15 (2011), 15 (3497), 15 (3275), 15 (4335), 10 (3098). Error bars denote ±s.e.m.
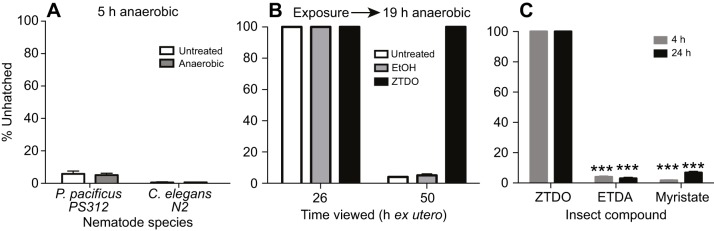
Anoxia does not phenocopy the effect of ZTDO
The development of the nematode embryo within the egg is aerobic and requires an external source of oxygen passing through the eggshell. Given the much larger molecular structure of ZTDO compared with those of water and oxygen, and the presumption that nematode eggshells are impervious to most small molecules (Carvalho et al., 2011; Chitwood and Chitwood, 1974), we wondered whether rather than penetrating the eggshell, ZTDO acts to arrest development by coating the eggshell and blocking essential gas exchange. To see whether oxygen deprivation would phenocopy the embryocidal effect of ZTDO, we incubated synchronized P. pacificus embryos in an anaerobic chamber for 5 h and then scored for hatching embryos at 24 h ex utero. Caenorhabditis elegans embryos were also deprived of oxygen for 5 h before resuming normal incubation on the plate and scored at 18 h ex utero (C. elegans has a shorter embryogenesis). However, the deprivation of oxygen for 5 h neither replicated the ZTDO embryo phenotype nor delayed hatching (Fig. 4A). Moreover, when we exposed synchronized P. pacificus embryos to ethanol or left them alone for 3 h, and then incubated them under anoxic condition for 19 h, most embryos hatched 24 h later, i.e. around 50 h ex utero. In contrast, no embryos hatched after 50 h when they were exposed to ZTDO prior to anaerobic treatment (Fig. 4B). Almost none of the ZTDO-exposed embryos hatched even 3 days after normal hatching time. These results suggest that like C. elegans embryos, P. pacificus embryos also undergo anoxia-induced arrest that delays development and hatching (Padilla and Ladage, 2014). However, unlike anoxia-induced arrest, the ZTDO-induced arrest is almost always irreversible in P. pacificus embryos at the minimum exposure regimen necessary to prevent hatching.
Fig. 4.
Anaerobic conditions and other insect compounds do not phenocopy the effects of ZTDO. (A) Synchronized P. pacificus and C. elegans embryos were deprived of oxygen at 20°C for 5 h, then allowed to recover with oxygen for 24 and 18 h, respectively. N (n): 3 (512), 3 (807), 3 (707), 3 (651). (B) Synchronized P. pacificus embryos were left alone (untreated) or exposed to ethanol or ZTDO for 3 h, followed by 19 h without oxygen. The anaerobic condition delayed egg hatching but only ZTDO pre-treatment arrested embryogenesis permanently. N (n): 4 (611), 5 (654), 5 (650). (C) Pristionchus pacificus PS312 was exposed to 10 µl of 0.5% of the insect compound suspended from plate lids for 4 or 24 h E-(11)-tetradecenyl acetate (ETDA). Significant differences in response to the compounds compared with ZTDO were determined by two-way ANOVA with Tukey's correction for multiple comparisons (***P<0.0001). N (n): 15 (1422), 15 (1690), 15 (1177), 15 (1090), 15 (2218), 15 (1496). Error bars denote ±s.e.m.
Finally, to investigate whether other insect-derived, long-chain hydrocarbon compounds attractive to P. pacificus can also act as embryocides, we performed the lid-drop assay using E-(11)-tetradecenyl acetate (ETDA), a moth sex pheromone from Spodoptera littoralis, and methyl myristate, an allomone from Musca flies (Hong and Sommer, 2006). We found that neither compound prevents P. pacificus embryos from hatching into healthy J2 larvae (Fig. 4C). Therefore, ZTDO is singular among volatile odors in acting like an embryocide.
Aberrant and incomplete embryonic development
Approximately a quarter (26%) of ZTDO-exposed early embryos stop development at the bean stage, while more than half arrest in later stages that exhibit partial cavity formation and muscle contraction. The ‘peanut’ stage, containing two unequal-sized lobes of cells, is a unique stage for ZTDO-treated embryos that seem to have initiated elongation but do not reach the tadpole stage (Fig. 5A). Interestingly, although muscle contraction does not start until after the tadpole stage in C. elegans, some ZTDO-treated peanut stage P. pacificus embryos exhibit movement. Sporadic twisting and whole-body bending movements can also be seen in the later 2- and 3-fold stages (Movie 1–3). More intriguingly, cavities appear in the peanut, 2-fold and 3-fold stages, with increasing proportion of these cavities occurring in these stages found at 48 h ex utero (Fig. 5B–E). Specifically, the proportion of peanut-stage embryos with cavity quadrupled between 26 and 48 h, while cavity formation doubled in 2- and 3-fold stage embryos during the same period. Cavities near the extremes of the aberrant embryos resemble partial pharynges (Fig. 5B,D), while cavities near the center of the embryos resemble partial intestines (Fig. 5C,E). One major difference in P. pacificus from C. elegans is the roughly 80-min delay in the first division of the E cell (endoderm blastomere) after the first division of the MS cell (mainly mesoderm blastomere), around 70 min after fertilization (Vangestel et al., 2008). This temporal separation between the MS and E cell divisions may help to explain the variation in ZTDO phenotypes – the MS cell divides shortly after starting the ZTDO treatment for some embryos, while the E cell division always occurs long after the start of the ZTDO treatment for all embryos. We surmised that in our assays, the stem cell of the endoderm lineage, the E cell, is exposed to ZTDO longer than the MS mesoderm precursor cell. The ability to contract muscles and coordinate cell movements to create cavities suggests at least partial developmental determination in these moribund embryos, though it is unclear whether certain cell lineages are more susceptible than others owing to cell identity or length of exposure.
Fig. 5.
Aberrant development in ZTDO-exposed P. pacificus embryos. Representative images of early-stage embryos exposed to 10 µl of 0.5% ZTDO suspended from plate lids for 4 h immediately following egg synchronization. The formation of a cavity-like structure for all embryonic stages increased with incubation time beyond the normal 26 h. (A) The ‘bean’ stage is the least developed of the zombryo stages, representing 26 and 32% of the total viewed at 26 and 48 h ex utero. (B) A ‘peanut’ stage embryo with an anterior cavity viewed at 48 h ex utero. (C) A ‘peanut’ stage embryo with a mid-body cavity viewed at 48 h ex utero. (D) A 2-fold stage embryo with an open anterior cavity viewed at 26 h ex utero. (E) A 3-fold stage embryo with a mid-body cavity viewed at 26 h ex utero. The asterisk denotes the cavity in each panel. Scale bar of 25 µm in B represents the same magnification for all images. N (n): 3 (115) for 26 h, 3 (93) for 48 h.
Although we did not follow the developmental trajectories of individual embryos, but rather sampled the exposed population for the two time periods in three separate experiments, we speculate that these developmentally advanced ‘zombryos’ attempt to continue cell differentiation and form the alimentary system as well as coordinated muscle contraction even when body elongation cannot be properly carried out and when embryogenesis is extended to twice the usual duration (see Movies 1–3). Curiously, zombryos viewed at 48 h ex utero show decreased proportion of 3-fold embryos compared with those viewed at 26 h. One explanation could be that the 3-fold embryos visible at 26 h contracted to resemble 2-fold embryos by 48 h. Unlike C. elegans, P. pacificus embryos transition to the pre-hatching J1 larvae inside the eggshell and molt just prior to hatching as J2 larvae (Lewis and Hong, 2014). However, the pre-hatching molt is unlikely to contribute to the hatching phenotype, as the embryos were exposed during early development before the gastrula stage. Interestingly, we found that some 2-fold stage zombryos exhibit Ppa-obi-1p::gfp cell expression characteristic of the pattern found in the epidermis of untreated pre-hatching J1 larvae (Cinkornpumin and Hong, 2011) (Fig. 6C). We interpret the expression of seam cell markers to mean partial differentiation of the epidermal lineage, despite retarded overall development. Thus for the small proportion of the ZTDO-treated embryos that become zombryos, partial development continues but not enough to trigger hatching or promote survival outside the eggshell. Even the more advanced 48-h-old 3-fold stage zombryos do not survive on NGM when we intervened to pop open the eggshell by compressing the zombryos between an agar pad and a coverslip. The variability and delay in the zombryo phenotype suggest that more than one cell type may be targeted by ZTDO. Because the punctuated and incomplete embryogenesis does not seem reversible or recoverable after several days, it is not clear when these zombryos actually cease living as embryos.
Fig. 6.
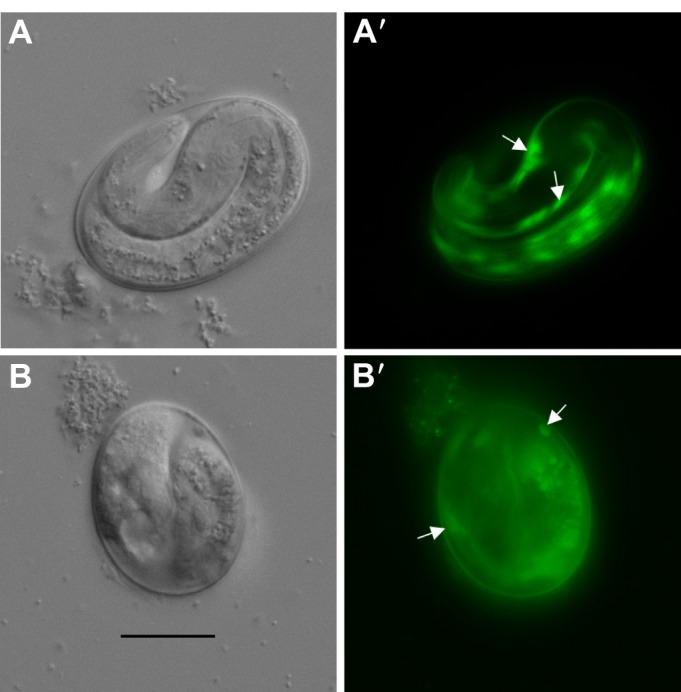
Expression of a J1 stage marker in arrested P. pacificus embryos. (A,A′) Seam cell expression Ppa-obi-1p::gfp (arrows) in untreated 24-h-old embryos. (B,B′) Similar seam cell expression (arrows) can be observed in ZTDO-induced arrested 24-h-old embryos. Scale bar in B (20 µm) represents the same magnification for all images.
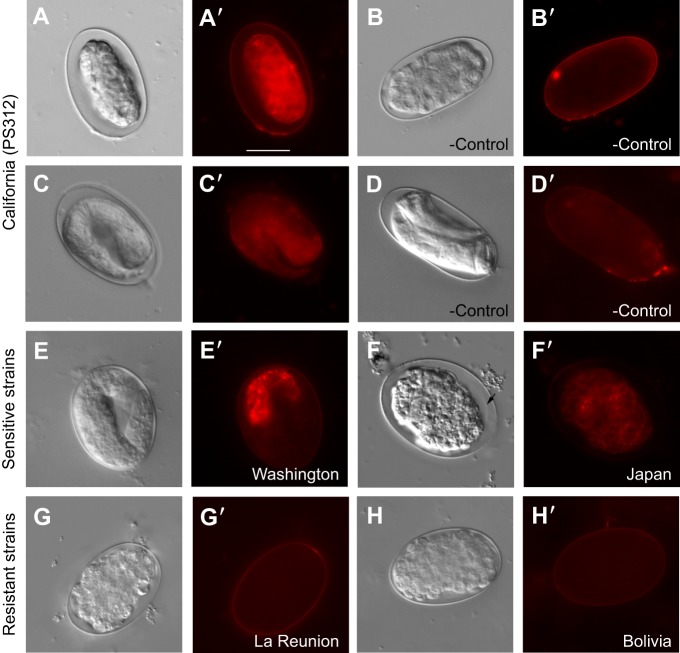
ZTDO increases eggshell permeability
Given that anoxia and other insect-derived pheromones do not reproduce the embryonic arrests similar to the ZTDO-exposure phenotype, we wondered if ZTDO enters through the eggshell to target embryonic tissues. We speculated that as we expose ZTDO to eggs after fertilization and ex utero, ZTDO is unlikely to interfere with eggshell assembly (Olson et al., 2012), but rather with eggshell function or integrity. Previous studies demonstrated that laser-permeabilized wild-type C. elegans embryos enable the fluorescent lipophilic dye FM4-64 to label the plasma membrane and endocytic structures (Rappleye et al., 1999). We found that FM4-64 strongly stains embryonic membranes of early P. pacificus embryos exposed to ZTDO for 4 h, but only weakly stains the eggshell in the ethanol-exposed control (Fig. 7A,B). This ZTDO-induced eggshell permeability appears to be irreversible, as FM4-64 also stains later 2-fold stage zombryos 20 h after ZTDO exposure ended, in contrast to the lack of staining in the control J1 pre-hatch larvae (Fig. 7C,D). ZTDO-treated embryos result in expanded peri-embryonic space, possibly because of shrinking of embryonic tissue. ZTDO-exposed embryos also shrink relative to the eggshell on the culturing NGM without mounting on agar, and exposure of embryos immersed in C. elegans Blastomere Culture Media (Shelton's Media) did not ameliorate the ZTDO effect (data not shown) (Skop et al., 2001), suggesting that a more permeable eggshell contributes only in part to the increased osmotic sensitivity.
Fig. 7.
FM4-64 membrane staining of P. pacificus embryos exposed to volatile ZTDO. Representative images of early embryos exposed to 10 µl of 0.5% ZTDO suspended from plate lids for 4 h. (A,A′) Wild-type PS312 exposed to ZTDO viewed immediately after treatment show stained embryonic tissue owing to a permeable eggshell. (B) Wild-type PS312 exposed to ethanol control viewed immediately after treatment show only weak tissue staining owing to an impermeable eggshell. (C,C′) Wild-type PS312 exposed to ZTDO viewed ∼20 h after treatment. (D,D′) Wild-type PS312 exposed to ethanol vehicle control viewed ∼20 h after treatment. (E,E′) Washington strain (PS1843) exposed to ZTDO viewed ∼20 h after treatment. (F,F′) Japan strain (RS5194) exposed to ZTDO viewed ∼20 h after treatment. Arrow indicates a possible embryonic plasma membrane. (G,G′) La Reunion strain (RSB001) exposed to ZTDO viewed immediately after treatment. (H,H′) Bolivia strain (RS5278) exposed to ZTDO viewed immediately after treatment. FM4-64 fluorescence images were taken at an intensity of 2 and exposure of 1 s. Scale bar in A′ (20 µm) represents the same magnification for all images.
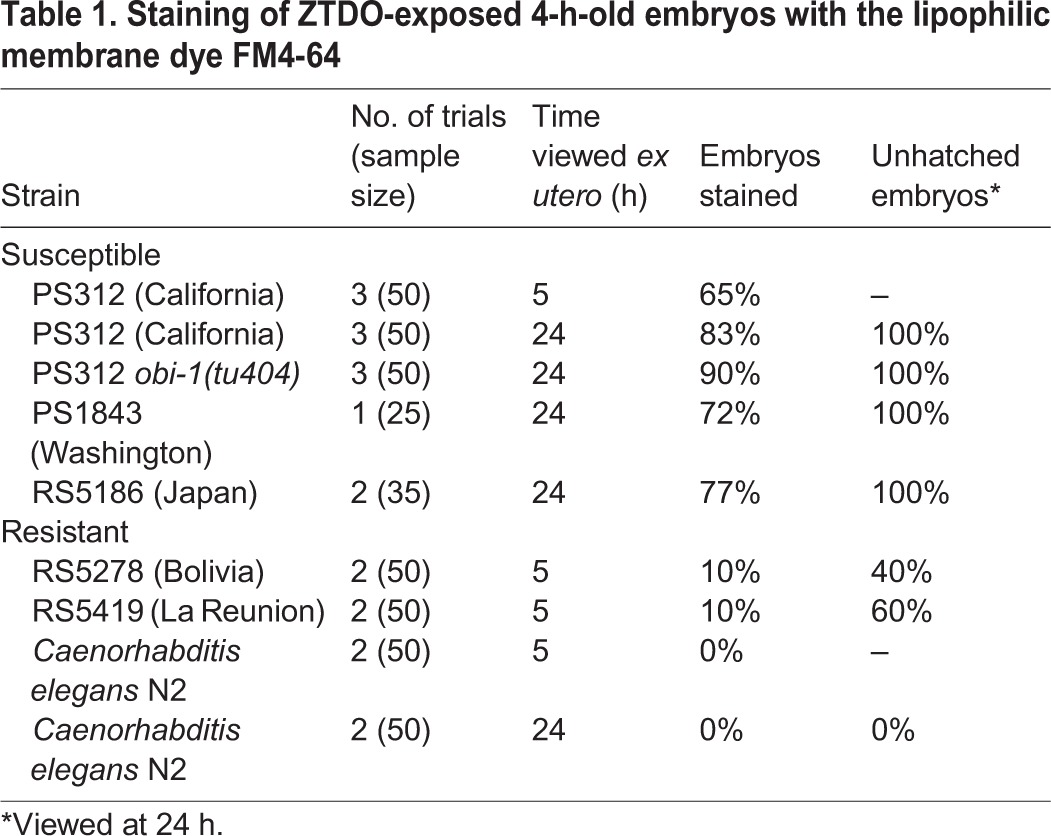
Next, we asked if the natural variation in ZTDO sensitivity is correlated with ZTDO-induced permeabilization of the eggshell. We found that like wild-type California PS312, late-stage embryos of sensitive strains from Washington (PS1843) and Japan (RS5194) remain permeable to FM4-64 (Fig. 7E′,F′). Although the cytoplasmic staining in the Japan strain is weaker than PS312, we discovered that the embryos show a more severe abnormal cellular morphology than PS312. Almost no treated Japanese embryos ever reach the 2- or 3-fold stages; a majority arrest in gastrulation and the bean stage, perhaps as a result of more extensive tissue damage. In contrast, partially ZTDO-resistant strains from La Reunion (RSB001) and Bolivia (RS5278) were largely impermeable to FM4-64, even when stained right after ZTDO exposure (Fig. 7G′,H′, cf. 7A′). Not all ZTDO-susceptible embryos, however, show cytoplasmic staining by FM4-64. Whereas 100% of PS312, Washington and Japan embryos do not hatch after a 4 h ZTDO treatment (Fig. 3), only 65–83% of the embryos show permeable eggshells (Table 1). Similarly, whereas ∼40% of Bolivia embryos do not hatch after ZTDO treatment, only 10% of the embryos show permeable eggshells. Only the P. pacificus La Reunion strain and C. elegans N2 show staining rates commensurate with ZTDO sensitivity at 10 and 0%, respectively. Also consistent with this trend, embryos of the ZTDO hypersensitive mutant Ppa-obi-1(tu404) (Cinkornpumin et al., 2014) are more likely to show FM4-64 staining than wild-type PS312. Because the molecular weight of ZTDO is lower than FM4-64 (210 versus 608), ZTDO may enter openings that would size-restrict FM4-64. Yet it is not clear whether ZTDO disrupts and penetrates the eggshell, or if disruption of the eggshell alone is sufficient to abolish embryogenesis. These results show that ZTDO can arrest development in part by increasing eggshell permeability and osmotic sensitivity, although ZTDO may also, in addition, damage embryonic tissue directly without detectable damage to the eggshell.
Table 1.
Staining of ZTDO-exposed 4-h-old embryos with the lipophilic membrane dye FM4-64
DISCUSSION
Unlike broad-spectrum nematocides such as ivermectin, aldicarb and monepantal that are administered in liquid or on solid media (Kampfe and Schutze, 1995; Goldman et al., 2007; Donnelly et al., 2013; Fru and Puoti, 2014; Wever et al., 2015), ZTDO is a synthetic version of the host Oriental beetle pheromone that exerts lethality as a volatile compound on P. pacificus embryos. We have uncovered a striking example of an odor that can arrest nematode embryos through the eggshell commonly perceived to be impermeable to large molecules. Sensitivity to ZTDO appears to be highest between the gastrula and tadpole stages, when 2 h of odor exposure can prevent ∼80% of embryos from hatching. A 3-h exposure during the 4–8 h period after synchronization is sufficient to achieve lethality, whereas a 4-h exposure during the 0–4 h period is necessary to arrest all embryos. ZTDO-exposed embryos arrest in various stages of incomplete embryogenesis that extend pre-hatching development from the usual 24 to 48 h at 20°C, while at the same time preventing the aberrant embryos from hatching indefinitely. Lethality caused by ZTDO exposure results in a spectrum of incomplete embryogenesis, with the most severe phenotype found in the Japanese strain that never progress to the active zombryo stage, to the less severe zombryo phenotype of elongated, active embryos with partial formation of pharyngeal-like or gut-like cavities in the wild-type PS312 embryos. Because the most susceptible P. pacificus strain comes from the Oriental beetle in Japan, in contrast to the La Reunion and Bolivia strains from other beetle species, the correlation of ZTDO susceptibility to beetle host in natural populations may play a role in maintaining host–parasite interactions.
Natural variation in ZTDO sensitivity is also correlated with ZTDO-induced permeabilization of the eggshell, although the percentage of embryonic staining is lower than the percentage of embryonic lethality. As ascarosides are highly diverse in different species of nematodes and ascaroside-derived ascaroside glycolipids are required for eggshell assembly (Olson et al., 2012), it is possible that the lipid-like ascaroside side-chain types and stoichiometry in the eggshell interact with the lipid moiety of ZTDO to contribute to the variations in ZTDO-induced permeability among different P. pacificus strains. To fully account for ZTDO's embryonic lethality, however, zygotic factors may also be involved. Early embryonic development up to the 50-cell stage in C. elegans and P. pacificus appears to be nearly identical, although the cell cycle is longer in P. pacificus (Vangestel et al., 2008; Schulze and Schierenberg, 2011). Earliest expression of zygotic transcription factors has been detected in the 4- to 6-cell stage in P. pacificus embryos (M. Maduro, personal communication) (Maduro et al., 2007), indicating that the maternal–zygotic transition occurs only one division later in P. pacificus compared with C. elegans. Two- to 8-cell embryos are exposed to ZTDO after an hour of egg-laying, when the oldest embryos are already 1 h into development at the start of exposure. Thus during the standard ZTDO exposure between 0 and 5 h ex utero, it is likely that zygotic gene products expressed at this time also play a role in mediating ZTDO sensitivity. In wild-type P. pacificus PS312, post-embryonic susceptibility to volatile ZTDO results in paralysed J2 and dauer larvae (Cinkornpumin et al., 2014). However, it is unlikely that ZTDO-induced larval paralysis and embryonic lethality are mediated by the same factor, because our results show that the earliest susceptible embryonic stage is prior to 4 h ex utero, before gastrulation and the formation of the differentiated cell types expressing OBI-1, a cholesteryl ester transfer protein homolog mediating ZTDO susceptibility in the J4 larvae.
Comparisons between the embryonic lethality caused by ZTDO and another embryocide reveal key differences in mode of action. In C. elegans, C22 is a potent and species-specific embryocide that disrupts the oocyte-to-embryo transition in the maternal environment (Weicksel et al., 2016). C22 acts only through the parental hermaphrodites and targets oocytes just prior to fertilization, but has no effect on any other developmental stages, such as embryos, larvae and adults. In contrast, ZTDO does not interfere with P. pacificus egg viability in utero, but ZTDO acts strongly on early embryos ex utero before the tadpole stage, as well as subsequent J2 and dauer larvae stages after hatching. C22-induced embryonic lethality requires up-regulation of the transcription factor LET-607 involved in protein trafficking, which in turn probably regulates the proper secretion of proteins needed for correct C. elegans eggshell assembly. Thus while C22 interferes with the permeability barrier during eggshell formation, ZTDO appears to permeabilize eggshells that are already formed. Both ZTDO and C22 increase eggshell permeability, which probably contributes to embryonic lethality owing to increased osmotic sensitivity. However, in the case of ZTDO, because the fraction of ZTDO-permeablized eggshell detected by FM4-64 staining is lower than the fraction of embryos susceptible to the lethal effects of ZTDO, hatching ability may be affected even when damage to the eggshell or embryonic tissue is not detectable by FM4-64 staining. Furthermore, C22-treated embryos seldom proceed beyond the 100-cell stage, whereas around half of the ZTDO-treated embryos reach the superficially elongated 2- and 3-fold stages and continue developing 24 h past the normal hatching time, albeit with severe developmental defects.
ZTDO is a naturally occurring beetle compound that could deter reproductive growth of its associated P. pacificus while the beetle host is alive, and may be a lever for attenuated antagonism to keep necromenic nematodes from becoming pathogenic as a part of an evolutionary detente (Thompson, 2005; Sudhaus, 2009). Because the physiological concentration of ZTDO that wild isolates of P. pacificus are likely to encounter is unknown, it is also possible that lower concentrations of ZTDO help to prolong rather than subvert embryonic development, as a way to coordinate with the death of the host beetle when bacterial food becomes imminent. For example, our result that natural variation in ZTDO susceptibility suggests that embryos of wild P. pacificus populations exposed to a pulse of ZTDO could perhaps delay hatching. In this respect, ZTDO-induced embryonic arrest may be analogous to host-mediated hatching behavior found in the plant parasitic nematode Heterodera glycines, whereby unidentified root exudate from fresh samples stimulates hatching (Tefft and Bone, 1985; Perry, 2002; Thapa et al., 2017). However, whereas host cues increased hatching rate but have no effect on pre-hatching development in H. glycines (Thapa et al., 2017), the early exposure and terminal embryogenesis in P. pacificus suggests that ZTDO affects hatching rate indirectly by disrupting pre-hatching development, rather than the mechanisms required for hatching per se. As rapid evolution of host pheromone-induced embryonic lethality can be an important mechanism for facilitating host switching, we speculate that the natural variation in susceptibility to ZTDO is due to factors that are fast evolving at the population level. To our knowledge, no prior study has shown that volatile insect pheromones can perturb nematode embryogenesis, and we hope this study establishes a necessary foundation to study the mode of action for ZTDO in the early embryo. In the future, identification of the direct targets of ZTDO in the eggshell and embryonic tissue will lead to important insight into how the strain-specific embryonic response is achieved. Genetic screens for suppressors of embryonic lethality will probably identify key factors mediating ZTDO recognition and pathway activation.
Acknowledgements
We thank B. Mojica and D. Kazerskiy for technical assistance, and Dr M. Maduro for sharing unpublished data.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: R.L.H., T.R.; Methodology: R.L.H., T.R.; Formal analysis: R.L.H.; Investigation: R.L.H., T.R.; Resources: R.L.H.; Data curation: R.L.H., T.R.; Writing - original draft: R.L.H., T.R.; Writing - review & editing: R.L.H., T.R.; Visualization: R.L.H.; Supervision: R.L.H.; Project administration: R.L.H.; Funding acquisition: R.L.H.
Funding
This work was supported by the National Institutes of Health (award 1SC3GM105579). Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://jeb.biologists.org/lookup/doi/10.1242/jeb.159665.supplemental
References
- Bird A. F. and McClure M. A. (1976). The tylenchid (Nematoda) egg shell: structure, composition and permeability. Parasitology 72, 19-28. 10.1017/S0031182000043158 [DOI] [Google Scholar]
- Byrne J. T., Maher N. J. and Jones P. W. (2001). Comparative responses of Globodera rostochiensis and G. pallida to hatching chemicals. J. Nematol. 33, 195-202. [PMC free article] [PubMed] [Google Scholar]
- Carvalho A., Olson S. K., Gutierrez E., Zhang K., Noble L. B., Zanin E., Desai A., Groisman A. and Oegema K. (2011). Acute drug treatment in the early C. elegans embryo. PLoS ONE 6, e24656 10.1371/journal.pone.0024656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaisson K. E. and Hallem E. A. (2012). Chemosensory behaviors of parasites. Trends Parasitol. 28, 427-436. 10.1016/j.pt.2012.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L., Xu S., Xu C., Lu H. and Zhang Z. (2016). Effects of trans-2-hexenal on reproduction, growth and behaviour and efficacy against the pinewood nematode, Bursaphelenchus xylophilus. Pest Manag. Sci. 73, 888-895. 10.1002/ps.4360 [DOI] [PubMed] [Google Scholar]
- Chitwood B. G. and Chitwood M. B. H. (1974). Introduction to Nematology. Baltimore, MD: University Park Press. [Google Scholar]
- Cinkornpumin J. K. and Hong R. L. (2011). RNAi mediated gene knockdown and transgenesis by microinjection in the necromenic Nematode Pristionchus pacificus. J. Vis. Exp., e3270 10.3791/3270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinkornpumin J. K., Wisidagama D. R., Rapoport V., Go J. L., Dieterich C., Wang X., Sommer R. J. and Hong R. L. (2014). A host beetle pheromone regulates development and behavior in the nematode Pristionchus pacificus. eLife 3, e03229 10.7554/eLife.03229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillman A. R., Chaston J. M., Adams B. J., Ciche T. A., Goodrich-Blair H., Stock S. P. and Sternberg P. W. (2012). An entomopathogenic nematode by any other name. PLoS Pathog. 8, e1002527 10.1371/journal.ppat.1002527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly J. L., Clark C. M., Leifer A. M., Pirri J. K., Haburcak M., Francis M. M., Samuel A. D. T. and Alkema M. J. (2013). Monoaminergic orchestration of motor programs in a complex C. elegans behavior. PLoS Biol. 11, e1001529 10.1371/journal.pbio.1001529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix M. A., Hill R. J., Schwarz H., Sternberg P. W., Sudhaus W. and Sommer R. J. (1999). Pristionchus pacificus, a nematode with only three juvenile stages, displays major heterochronic changes relative to Caenorhabditis elegans. Proc. R. Soc. Lond. B Biol. Sci. 266, 1617-1621. 10.1098/rspb.1999.0823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fru M. F. and Puoti A. (2014). Acquired resistance to monepantel in C. elegans: what about parasitic nematodes? Worm 3, e959416 10.4161/21624046.2014.959416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman A. S., Guisinger V. H., Aikins M., Amarillo M. L. E., Belizario V. Y., Garshong B., Gyapong J., Kabali C., Kamal H. A., Kanjilal S. et al. (2007). National mass drug administration costs for lymphatic filariasis elimination. PLoS Negl. Trop. Dis. 1, e67 10.1371/journal.pntd.0000067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann M., Mayer W. E. Sommer R. J. (2006a). Sex, bugs and Haldane's rule: the nematode genus Pristionchus in the United States. Front. Zool. 3 10.1186/1742-9994-3-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann M., Mayer W. E. and Sommer R. J. (2006b). Nematodes of the genus Pristionchus are closely associated with scarab beetles and the Colorado potato beetle in Western Europe. Zoology (Jena) 109, 96-108. 10.1016/j.zool.2006.03.001 [DOI] [PubMed] [Google Scholar]
- Herrmann M., Mayer W. E., Hong R. L., Kienle S., Minasaki R. and Sommer R. J. (2007). The nematode Pristionchus pacificus (Nematoda: Diplogastridae) is associated with the oriental beetle Exomala orientalis (Coleoptera: Scarabaeidae) in Japan. Zool. Sci. 24, 883-889. 10.2108/zsj.24.883 [DOI] [PubMed] [Google Scholar]
- Hong R. L. and Sommer R. J. (2006). Chemoattraction in Pristionchus nematodes and implications for insect recognition. Curr. Biol. 16, 2359-2365. 10.1016/j.cub.2006.10.031 [DOI] [PubMed] [Google Scholar]
- Hong R. L., Witte H. and Sommer R. J. (2008). Natural variation in Pristionchus pacificus insect pheromone attraction involves the protein kinase EGL-4. Proc. Natl. Acad. Sci. USA 105, 7779-7784. 10.1073/pnas.0708406105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim S. K. and Haydock P. (1999). Cadusafos inhibits hatching, invasion, and movement of the potato cyst nematode Globodera pallida. J. Nematol. 31, 201-206. [PMC free article] [PubMed] [Google Scholar]
- Kampfe L. and Schutze H. (1995). Reactions of Rhabditis oxycerca after long-term exposure to Aldicarb and Oxamyl. 3. Altered bionomic and physiological reactions. Nematologica 41, 449-467. 10.1163/003925995X00404 [DOI] [Google Scholar]
- Khan A., Williams K. L. and Nevalainen H. K. M. (2004). Effects of Paecilomyces lilacinus protease and chitinase on the eggshell structures and hatching of Meloidogyne javanica juveniles. Biol. Control 31, 346-352. 10.1016/j.biocontrol.2004.07.011 [DOI] [Google Scholar]
- Koneru S. L., Salinas H., Flores G. E. and Hong R. L. (2016). The bacterial community of entomophilic nematodes and host beetles. Mol. Ecol. 25, 2312-2324. 10.1111/mec.13614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunert J. (1992). On the mechanism of penetration of ovicidal fungi through egg-shells of parasitic nematodes. Decomposition of chitinous and ascaroside layers. Folia Parasitol. 39, 61-66. [PubMed] [Google Scholar]
- Leal W. S. (2005). Pheromone reception. Chem. Pheromones Other Semiochem. Ii 240, 1-36. [Google Scholar]
- Lewis V. M. and Hong R. L. (2014). Conserved behavioral and genetic mechanisms in the pre-hatching molt of the nematode Pristionchus pacificus. Evodevo 5, 31 10.1186/2041-9139-5-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maduro M. F., Broitman-Maduro G. and Mengarelli I. (2007). Maternal deployment of the embryonic SKN-1→ MED-1, 2 cell specification pathway in C. elegans. Dev. Biol. 301, 590-601. 10.1016/j.ydbio.2006.08.029 [DOI] [PubMed] [Google Scholar]
- Manegold A. and Kiontke K. (2001). The association of two Diplogasteroides species (Secernentea: Diplogastrina) and cockchafers (Melolontha spp., Scarabaeidae). Nematology 3, 603-606. 10.1163/156854101753389211 [DOI] [Google Scholar]
- McGaughran A., Morgan K. and Sommer R. J. (2013). Natural variation in chemosensation: lessons from an island nematode. Ecol. Evol. 3, 5209-5224. 10.1002/ece3.902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura E., Tanaka R. and Yoshiga T. (2013). Species-specific recognition of the carrier insect by dauer larvae of the nematode Caenorhabditis japonica. J. Exp. Biol. 216, 568-572. 10.1242/jeb.073593 [DOI] [PubMed] [Google Scholar]
- Olson S. K., Greenan G., Desai A., Müller-Reichert T. and Oegema K. (2012). Hierarchical assembly of the eggshell and permeability barrier in C. elegans. J. Cell Biol. 198, 731-748. 10.1083/jcb.201206008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla P. A. and Ladage M. L. (2014). Suspended animation, diapause and quiescence. Cell Cycle 11, 1672-1679. 10.4161/cc.19444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. N. (2002). Hatching. In The Biology of Nematodes (ed. Lee D.), pp. 147-169. New York: Taylor and Francis Books, Ltd. [Google Scholar]
- Rappleye C. A., Paredez A. R., Smith C. W., McDonald K. L. and Aroian R. V. (1999). The coronin-like protein POD-1 is required for anterior–posterior axis formation and cellular architecture in the nematode Caenorhabditis elegans. Genes Dev. 13, 2838-2851. 10.1101/gad.13.21.2838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodelsperger C., Neher R. A., Weller A. M., Eberhardt G., Witte H., Mayer W. E., Dieterich C. and Sommer R. J. (2014). Characterization of genetic diversity in the nematode Pristionchus pacificus from population-scale resequencing data. Genetics 196, 1153-1165. 10.1534/genetics.113.159855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruther J., Reinecke A., Tolasch T. and Hilker M. (2002). Phenol – Another cockchafer attractant shared by Melolontha hippocastani Fabr. and M. melolontha L. Z. Nat. C J. Biosci. 57, 910-913. 10.1515/znc-2002-9-1026 [DOI] [PubMed] [Google Scholar]
- Schulze J. and Schierenberg E. (2011). Evolution of embryonic development in nematodes. EvoDevo 2 10.1186/2041-9139-2-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skop A. R., Bergmann D., Mohler W. A. and White J. G. (2001). Completion of cytokinesis in C. elegans requires a brefeldin A-sensitive membrane accumulation at the cleavage furrow apex. Curr. Biol. 11, 735-746. 10.1016/S0960-9822(01)00231-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhaus W. (2009). Evolution of insect parasitism in rhabditid and diplogastrid nematodes. Adv. Arachnol. Dev. Biol. 12, 143-161. [Google Scholar]
- Sulston J. E., Schierenberg E., White J. G. and Thomson J. N. (1983). The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev. Biol. 100, 64-119. 10.1016/0012-1606(83)90201-4 [DOI] [PubMed] [Google Scholar]
- Tefft P. M. and Bone L. W. (1985). Plant-induced hatching of eggs of the soybean cyst nematode. J. Nematol. 17, 275-279. [PMC free article] [PubMed] [Google Scholar]
- Thapa S., Patel J. A., Reuter-Carlson U. and Schroeder N. E. (2017). Embryogenesis in the parasitic nematode Heterodera glycines is independent of host-derived hatching stimulation. BMC Dev. Biol. 17, 1-11. 10.1186/s12861-016-0144-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. N. (2005). The Geographical Mosaic of Coevolution. Chicago: The University of Chicago Press. [Google Scholar]
- Vangestel S., Houthoofd W., Bert W. and Borgonie G. (2008). The early embryonic development of the satellite organism Pristionchus pacificus: differences and similarities with Caenorhabditis elegans. Nematology 10, 301-312. 10.1163/156854108783900267 [DOI] [Google Scholar]
- Weicksel S. E., Mahadav A., Moyle M., Cipriani P. G., Kudron M., Pincus Z., Bahmanyar S., Abriola L., Merkel J., Gutwein M. et al. (2016). A novel small molecule that disrupts a key event during the oocyte-to-embryo transition in C. elegans. Development 143, 3540-3548. 10.1242/dev.140046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wever C. M., Farrington D. and Dent J. A. (2015). The validation of nematode-specific acetylcholine-gated chloride channels as potential anthelmintic drug targets. PLoS ONE 10, e0138804 10.1371/journal.pone.0138804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharton D. (1980). Nematode egg-shells. Parasitology 81, 447-463. 10.1017/S003118200005616X [DOI] [PubMed] [Google Scholar]
- Ye W., Torres-Barragan A. and Cardoza Y. (2010). Oscheius carolinensis n. sp. (Nematoda: Rhabditidae), a potential entomopathogenic nematode from vermicompost. Nematology 12, 121-135. 10.1163/156854109X458464 [DOI] [Google Scholar]
- Zhang A., Facundo H. T., Robbins P. S., Linn C. E., Hanula J. L., Villani M. G. and Roelofs W. L. (1994). Identification and synthesis of female sex pheromone of oriental beetle, Anomala orientalis (Coleoptera: Scarabaeidae). J. Chem. Ecol. 20, 2415-2427. 10.1007/BF02033210 [DOI] [PubMed] [Google Scholar]
- Zhang C., Liu J., Xu M., Sun J., Yang S., An X., Gao G., Lin M., Lai R., He Z. et al. (2008). Heterorhabditidoides chongmingensis gen. nov., sp. nov. (Rhabditida: Rhabditidae), a novel member of the entomopathogenic nematodes. J. Invertebr. Pathol. 98, 153-168. 10.1016/j.jip.2008.02.011 [DOI] [PubMed] [Google Scholar]