ABSTRACT
The restimulation of an immune memory response by in vitro culture of blood cells with a specific antigen has been used as a way to gauge immunity to vaccines for decades. In this commentary we discuss a less appreciated application to support vaccine process development. We report that human whole blood from pre-primed subjects can generate a profound adjuvant-modulated, antigen-specific response to several different vaccine formulations. The response is able to differentiate subtle changes in the quality of an immune memory response to vaccine formulations and can be used to select optimal conditions relating to a particular manufacture process step. While questions relating to closeness to in vivo vaccination remain, the approach is another big step nearer to the more relevant human response. It has special importance for new adjuvant development, complementing other preclinical in vivo and in vitro approaches to considerably de-risk progression of novel vaccines before and throughout early clinical development. Broader implications of the approach are discussed.
KEYWORDS: Adjuvant, Human whole blood, Immune response, Memory T cells, Vaccine
Introduction
An ability to restimulate and monitor a vaccine-specific immune response in vitro can provide valuable insight to how a vaccine might perform in vivo. The information may be particularly relevant to the longer-term memory T cell population that can be stimulated and monitored after several days of in vitro culture with antigen and which is the most likely target for boosting by vaccines. The cultured response is due to a heterogeneous population found in peripheral circulation, broadly referred to as central memory T cells (Tcm).1,2 The Tcm population plays an important role in reactive memory and differs from effector or effector memory cells (Tem) that perform an effector function immediately upon contact with the cognate antigen. The Tcm possess surface molecules that direct them to secondary lymphoid tissue to receive antigenic re-stimulation to become effector T cells. The Tcm are long-lasting, respond upon stimulation by secreting key cytokines (such as IFNγ), and in this way are thought to be instrumental in enabling other cells to engage the pathogen and mount a protective immune response.3 The generation and maintenance of the longer-term memory cells is therefore central to vaccinology and for the rational design of vaccines.4
During preclinical development, the stimulation of human cells has been shown to be a suitable strategy to predict vaccine performance. For example, previous studies have cultured the peripheral blood mononuclear cells (PBMCs) from individual subjects in a specialized culture (referred to as MIMIC®) that can enact a ‘trial-in-a-tube’, with the immunogenicity readout used as a surrogate for a potential clinical response.5
Another in vitro application is for the immunomonitoring of clinical trials, where the immune response, typically in peripheral blood samples, is analyzed exhaustively to determine immune response profiles, predictive of function or efficacy. Such testing is especially important for ‘first-in-human’ trials (phase I) and adaptive trial designs.
An equally important, but possibly less appreciated, role is when a vaccine candidate progresses from preclinical development, to bioprocess development and the manufacture of a product to support Phase I through to Phase III clinical trials. Here the vaccine candidate will be formulated, scaled-up and manufactured using a quality by design (QbD) methodology.6 During the specialized formulation development cycle, the physiochemical properties of several different vaccine formulations are configured in the context of quality attributes considered to be critical to safety and efficacy. The critical quality attributes (CQAs) must include an immunogenicity or potency readout, which is arguably, the most critical attribute driving formulation and process development. Typically, the immunogenicity of formulations is tested in animal models. However, this approach faces several drawbacks, the most notable being whether the response in an animal truly translates to human. It is also questionable whether an animal model has sufficient sensitivity to screen precision formulations.
As a way to complement and leverage animal immunogenicity and other preclinical data such as MIMIC, as well as to eventually bridge to early clinical trial data, we have looked to include an in vitro culture that uses diluted fresh human whole blood (hWB). Unlike other PBMC-based technologies, the hWB approach is considerably easier to perform and better suited to a bioprocess development facility where many formulations and processes need to be evaluated quickly using a more streamlined readout.
The application discussed herein is specific to a vaccine candidate intended to boost or modulate pre-existing immunity. A good example is the tuberculosis (TB) vaccine candidate H4-IC31 that will boost the response primed by a prior Bacillus Calmette-Guerin (BCG) vaccination.7 However, there are many other examples, especially for booster vaccines targeted to adolescence, adult and elderly populations, where there is intent to modulate pre-existing immunity. Some examples include vaccines against the Varicella Zoster (Shingles), Pertussis, Diphtheria and Tetanus. In this commentary, specific features of the hWB approach for vaccine formulation development are highlighted and discussed.
An adjuvant-modulated antigen-specific response
When the hWB approach was in early development, it was clear that many groups had performed the shorter 6–24 hr culture period to monitor both non-specific innate immune signals and specific effector cell responses. Indeed, several commercial companies now use hWB cultures as a diagnostic (e.g. the QuantiFERON technology), or to support specific questions for the pharmaceutical industry (e.g., the ProStorm Cytokine Release Assay by ProImmune). Although the short-term hWB culture has a clear role in vaccine formulation development, it is not discussed any further here.
Another more important challenge, at least for vaccine development, is the ability of a candidate vaccine to elicit a longer-term antigen-specific immune memory response. Several groups have used diluted hWB over an extended 6–7 day culture period to monitor this activity.8,9 A key goal in bioprocess development is to combine an antigen with an adjuvant in an optimal formulation as a ‘single-vial’ presentation. It was apparent that few, if any, groups had considered the antigen-adjuvant combination in the longer-term hWB culture. Early in development of the ‘single-vial’ formulation, an unexpected finding was the ability to stimulate a profound adjuvant-modulated, antigen-specific response.10,11 To our best knowledge, no other group had reported this phenomenon before. For a vaccine developer, an ability to imitate or at least simulate, an anticipated clinical response in human cells has several translational benefits. Not least, it is a relatively simple way to evaluate a functionally relevant response to a complete formulation (antigen and adjuvant) in human cells. A general approach for screening formulations is outlined briefly below.
Screening human volunteers
To screen specific vaccine formulations, the first step is to identify subjects who have an antigen-specific memory response in peripheral circulation. These subjects tend to generate a variable but consistent memory response, assumed to be driven by Tcm, over several months, possibly years.12
To select which subject to use for a formulation screen, the diluted fresh hWB from 17 randomly recruited volunteers were stimulated with several different vaccine candidate antigens mixed with or without a control TLR4-Agonist adjuvant (TLR4-A). Cultures were established in 10 wells of a 96 U-well microtiter plate and the interferon gamma (IFNγ) released in pooled supernatant was monitored 6–7 d later. A summary of the responses for all subjects is shown in Table 1. The results demonstrate that an adjuvant-modulated antigen-specific response to different antigens and in a large proportion of subjects can be readily detected. In many cases, the presence of the adjuvant was a prerequisite to detecting an antigen-specific response. It was also clear that some antigens stimulated a better response than others, possibly relating to higher precursor frequencies of memory cell populations in the peripheral blood. The TA (TB antigen H4) stimulated a particularly good response in the presence of the adjuvant. As expected, the live attenuated virus (LAV) vaccine on its own stimulated a substantial response; however; the response was also augmented in the presence of TLR4-A. Taken together, the data provides valuable knowledge on the overall quality of the memory population being stimulated in the presence of an adjuvant with different antigens.
Table 1.
Magnitude of the IFNγ response by different subjects to different antigens in the presence or absence of a TLR4-Agonist adjuvant (TLR4-A).
| Subject | FA | FA+ TLR4-A | TA | TA+ TLR4-A | LAV | LAV+ TLR4-A | PA | PA+ TLR4-A | TLR4-A |
|---|---|---|---|---|---|---|---|---|---|
| S01 | — | — | — | + | + | ++ | — | — | — |
| S02 | — | — | — | — | — | — | — | — | — |
| S03 | — | — | — | + | — | + | — | — | — |
| S05* | — | — | — | + | — | + | — | — | — |
| S06 | — | — | — | — | — | — | — | — | — |
| S07 | — | — | — | — | — | + | — | — | — |
| S08 | — | — | — | + | + | + | — | — | — |
| S09 | — | — | — | — | — | + | — | — | — |
| S10 | — | ++ | + | ++ | ++ | +++ | — | + | — |
| S11 | + | ++ | — | — | ++++ | ++++ | — | + | — |
| S12 | + | ++ | — | ++ | ++++ | ++++ | — | ++ | + |
| S13 | — | ++ | — | — | ++++ | ++++ | — | — | — |
| S14 | — | — | — | — | + | + | — | — | — |
| S15 | — | — | — | — | — | — | — | — | — |
| S16 | — | — | — | + | — | — | — | + | — |
| S17 | — | + | + | ++++ | + | + | — | — | — |
| S18 | — | + | ++ | +++ | — | + | — | — | — |
FA = Flu antigen, TA = Tuberculosis antigen - H4, LAV = Live attenuated virus, PA = Pertussis Antigen, TLR4-A = TLR4-Agonist adjuvant
The hWB cultures were stimulated with 1ug/ml of antigen (LAV was used at 1 × 105 PFU/ml) and the IFNγ released in supernatants was determined by standard ELISA 6 d later.
The IFNγ (pg/ml) measured in supernatant is indicated as:
<120 -
120–500 +
500–1000 ++
1000–1500 +++
>1500 ++++
Subject was selected for an H4-IC31 formulation screen in Figure 1.
Screening vaccine formulations
Having identified subjects with a responsive memory population, one subject was selected to use in a specific vaccine formulation screen. For the example shown in Fig. 1, subject S05 was chosen to evaluate formulations of TA, the TB antigen H4, but in this case, adjuvanted with IC31®, a TLR9 Agonist adjuvant.13 The formulations screened were produced by 3 different manufacture process conditions (MPC 1–3) (Fig. 1). The diluted fresh hWB was dispensed as before into a 96 U-well microtiter plate, but using a total of 30 wells/formulation. For this multi-well methodology, wells are not seen as replicates but rather as discrete individual cultures.14 A statistical difference between the responses by each formulation can be determined across all individual wells by the Mann Whitney U test. Thus, the assay unveiled differences in the manufacturing process, identifying MPC 3 as suboptimal. The use of multiple wells allows for the response to be assessed in terms of its overall quality. The variability of the response across different wells highlights an anticipated complexity in the phenotype of the memory response in human cells.15
Figure 1.
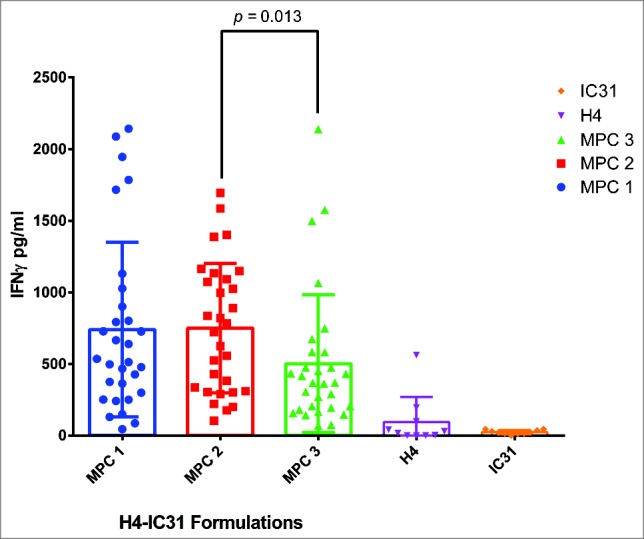
A comparison of 3 vaccine manufacture process conditions. The diluted fresh hWB from subject S05 was cultured with 3 different H4-IC31 formulations (MPC 1–3) using 30 wells/formulation in a standard 96 U-well microtiter plate. All supernatants were harvested after 10 d culture as previously reported.10 The H4 (0.1 μg/ml) and IC31 (4.0 nmol KLK) alone controls used 10 wells each. The graph shows a scatter plot +/− SD of the IFNγ released by individual wells to highlight differences in the overall quality of the response to each formulation. The MPC 3 formulation yielded significantly lower levels of IFNγ than the MPC 2 formulation (p = 0.013) (Mann Whitney U test).
Quality of antigenic restimulation
To date, banked PBMCs isolated from peripheral blood are the basis of most cell based assays. However, the immune system is a complex combination of innate and adaptive pathways that can discern signals external or internal to cells to initiate an appropriate response against infections. Also a broad range of cells types that possess differing receptors (such as TLRs) and soluble mediators is used. Therefore, frozen PBMCs that can offer an important degree of consistency, may not necessarily provide a complete picture of the physiological response, especially relevant to new adjuvants.
Herein, we show that the fresh hWB can stimulate a profound adjuvant-modulated antigen-specific response in the longer-term culture.10 It is not clear why the adjuvant has such a profound effect on the in vitro antigen-specific response. The most likely explanation is that in addition to conventional PBMCs, several other cell types such as neutrophils and soluble factors are present in hWB and these may be required to generate optimal innate signals.16,17 An adjuvant may cooperate with such cells to modulate the antigen-specific memory response that emerges several days later. Cultures also use autologous plasma and the presence of autologous antibody may be needed to generate additional optimal antigen-specific signals.18 Finally, while we are confident that the effects in hWB are antigen-specific, we cannot rule out the possibility for bystander synergistic effects. If confirmed, such effects may be far reaching.19
Although the response from a selected subject is relatively consistent it can vary considerably. These responses can be normalized by comparing directly to a reference formulation monitored concurrently, both within and between subjects. An expected variability in the response between subjects will be important to understand.
The data may have implications for ways to optimally restimulate cultures in vitro. Vaccine induced T cell responses are typically monitored by restimulating PBMCs with soluble antigens and/or peptides. However, soluble antigen and peptide are known to be poorly immunogenic. Apart from ingested food antigens that are well tolerated, it is difficult to conceive a situation, in vivo where soluble protein and/or peptide alone can stimulate a longer-term memory T cell response in the absence of some kind of ‘danger signal’. It is for this reason that vaccine antigens are formulated as particulates or combined with suitable adjuvants to imitate infection.
The hWB data suggest that like vaccines, soluble antigen and/or peptide may need to be presented in the context of an innate immunomodulating event to generate a more relevant and optimal response in vitro. It is also likely that responses generated in the absence of adjuvant (or innate signals) may be underestimated and appear with more varied phenotypes, analogous to an orchestral performance with no conductor.
One potential gap in understanding innate signals and adjuvantation, is the potential role for Tem. These cells elicit an effector function immediately upon contact with antigen and may play a role, similar to an adjuvant, in alerting danger and in modulating the longer-term antigen-specific response. Studies have shown that Tem and Tcm correlate, but the correlation is lost over time.2,20 More studies are required to clarify the role of effector cells either alone or in synergy with an adjuvant to stimulate a robust protective memory population.
While an IFNγ readout has been used throughout, detailed studies are currently underway to understand proteomic and phenotypic (mass cytometry) properties associated with the modulated vaccine response in hWB culture. Biomarkers or signatures of an adjuvant-specific memory response should hopefully link to clinical data and would be invaluable to monitor for the manufacture of a quality product.
Concluding remarks
It is accepted that the in vitro hWB culture may be some way from the actual events required to recall an immune response in vivo. Activation of specific cells at the injection site, drainage to the lymph node and subsequent activities cannot be readily replicated in vitro. Nevertheless, an ability to at least simulate an adjuvant-modulated response is a big step forward in allowing new adjuvanted vaccine formulations to be monitored closely in an autologous culture in the presence of all components of blood. The approach offers a new functionally relevant readout closely aligned to MIMIC and the principals of QbD, permitting, at least in early clinical phases, some linkage to functionality and CQAs. The approach is also aligned closely with a commitment to implement a 3R's strategy (replacement, reduction and refinement of the use of animal testing), fitting neatly between preclinical and clinical evaluations.
In terms of ease of use, the diluted fresh human blood can be collected from a local health center and applied to pre-prepared plates in a matter of minutes. With minimal hands-on time, the hWB culture becomes readily amenable to automation. Thus, both the number of antigens and subjects screened can be substantially amplified. The ability to reveal antigen-specific responsive cells easily in this way may have several potential applications beyond screening formulations, possibly touching on the potential for personalized medicine.
At least by Phase III, a streamlined in vitro potency assay, appropriate to a controlled (GMP) environment, needs to be developed. Ideally the potency test would leverage all studies, both clinical and pre-clinical. The readouts from hWB and MIMIC and any other in vitro approach should strongly support development to such an assay. Ultimately in vitro studies on human cells will help determine relevant critical quality attributes (CQAs) that can be applied throughout manufacture under QbD principals. Such approaches may also considerably de-risk progression before and throughout clinical development.
Disclosure of potential conflicts of interest
All authors are employees of Sanofi Pasteur. Sanofi Pasteur was involved in the design and conduct of the review and the decision to publish. Stephen Todryk has no conflict of interest.
Acknowledgments
We would like to thank all volunteers who donated blood to the study and to Karen Chiarelli and staff in the Health Center who coordinated sample collection and communication with the laboratory. Thanks also to Emily Xiao and team from the Formulation and Stability Platform who provided manufacture process samples as well as to Michael Cohen who helped review the document.
Funding
Sanofi Pasteur.
References
- [1].Godkin AJ, Thomas HC, Openshaw PJ. Evolution of epitope-specific memory CD4+ T cells after clearance of hepatitis C virus. J Immunol 2002; 169(4):2210-4; PMID:12165552; https://doi.org/ 10.4049/jimmunol.169.4.2210 [DOI] [PubMed] [Google Scholar]
- [2].Todryk SM, Pathan AA, Keating S, Porter DW, Berthoud T, Thompson F, Klenerman P, Hill AV. The relationship between human effector and memory T cells measured by ex vivo and cultured ELISPOT following recent distal priming. Immunology 2009; 128(1):83-91; PMID:19689738; https://doi.org/ 10.1111/j.1365-2567.2009.03073.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Sullusto F, Lanzavecchia A, Araki K, Ahmed R. From vaccines to memory and back. Immunity 2010; 33(4):451-63; PMID:21029957; https://doi.org/ 10.1016/j.immuni.2010.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ahmed R, Grey D. Immunological memory and protective immunity: Understanding their rection. Science 1996; 272(5258):54-60; PMID:8600537; https://doi.org/ 10.1126/science.272.5258.54 [DOI] [PubMed] [Google Scholar]
- [5].Higbee RG, Byers AM, Dhir V, Drake D, Fahlenkamp HG, Gangur J, Kachurin A, Kachurina O, Leistritz D, Ma Y, et al.. An immunologic model for rapid vaccine assessment - a clinical trial in a test tube. Altern Lab Anim 2009; 37(Supp 1):19-27; PMID:19807200 [DOI] [PubMed] [Google Scholar]
- [6].Junker B, Kosinski M, Geer D, Mahajan R, Chartrain M, Meyer B, Dephillips P, Wang Y, Henrickson F, Ezis K, et al.. Design-for-six-sigma for development of a bioprocess quality-by-design framework. PDA J Pharm Sci Technol 2011; 65(3):254-86; PMID:22293236; https://doi.org/ 10.5731/pdajpst.2011.00739 [DOI] [PubMed] [Google Scholar]
- [7].Geldenhuys H, Mearns H, Miles DJ, Tameris M, Hockey D, Shi Z, Bennet S, Andserson P, Kromann I, Hoff ST, et al.. The tuberculosis vaccine H4:IC31 is safe and induces a persistent polyfunctional CD4 T cell response in South African males: A randomized controlled trial. Vaccine 2015; 33(30):3592-9; PMID:26048780; https://doi.org/ 10.1016/j.vaccine.2015.05.036 [DOI] [PubMed] [Google Scholar]
- [8].Sutherland JS, Lalor MK, Black GF, Ambrose LR, Loxton AG, Cheqou NN, Kassa D, Mihret A, Howe R, Mayanja-Kizza H, et al.. Analysis of host responses to mycobacterium tuberculosis anitgens in a multi-site study of subjects with different TB and HIV infection states in sub-Saharan Africa. PLoS One 2013; 8(9):e74080; PMID:24040170; https://doi.org/ 10.1371/journal.pone.0074080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Dockrell HM, Black GF, Weir RE, Fine PE. Whole blood assays for interfon-gamma: Practicalities and potential for use as diagnostic tests in the field. Lepr Rev 2000; 71(Suppl):S60-2; PMID:11201889 [DOI] [PubMed] [Google Scholar]
- [10].Aboutorabian S, Hakimi J, Boudet E, Montano S, Dookie A, Roque C, Ausar SE, Rahman N, Brookes RH. A high ratio of IC31 (R) adjuvant to antigen is necessary for H4 TB vaccine immunomodulation. Hum Vaccin Immunother 2015; 11(6):1449-55; PMID:25997147; https://doi.org/ 10.1080/21645515.2015.1023970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Brookes RH, Hakimi J, Ha Y, Aboutorabian S, Ausar SE, Hasija M, Smith SG, Todryk SM, Dockrell HM, Rahman N. Screening vaccine formulations for biological activity using fresh human whole blood. Hum Vaccin Immunother 2014; 10(4):1129-35; PMID:24401565; https://doi.org/ 10.4161/hv.27657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Shen-Orr SS, Furman D, Kidd BA, Hadad F, Lovelace P, Juang YW, Rosenberg-Hasson Y, Mackey S, Grisar FA, Pickman Y, et al.. Defective signaling in the JAK-STAT pathway tracks with chronic inflammation and cardiovascular risk in aging humans. Cell Syst 2016; 3(4):374-84.e4; PMID:27746093; https://doi.org/ 10.1016/j.cels.2016.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Schellack C, Prinz K, Egyed A, Fritz JH, Wittmann B, Ginzler M, Swatosch G, Zauner W, Kast C, Akira S, et al.. IC31, a novel adjuvant signaling via TLR9, induces potent cellular and humoral immune response. Vaccine 2006; 24(26):5461-72; PMID:16678312; https://doi.org/ 10.1016/j.vaccine.2006.03.071 [DOI] [PubMed] [Google Scholar]
- [14].Hakimi J, Aboutorabian S, To F, Ausar SE, Rahman N, Brookes RH. Screening vaccine formulations in fresh whole human blood. Methods Mol Biol 2017; 1494:295-304; PMID:27718203; https://doi.org/ 10.1007/978-1-4939-6445-1_22 [DOI] [PubMed] [Google Scholar]
- [15].Su LF, Han A, McGuire HM, Furman D, Newell EW, Davis MM. The promised land of human immunology. Cold Spring Harb Symp Quant Biol 2013; 78:203-13; PMID:24638855; https://doi.org/ 10.1101/sqb.2013.78.022905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Montovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol 2011; 11(8):519-31; PMID:21785456; https://doi.org/ 10.1038/nri3024 [DOI] [PubMed] [Google Scholar]
- [17].Vono M, Lin A, Norby-Teglund A, Koup R, Liang F, Lore K. Neutrophils acquire antigen presentation capacity to memory CD4+ T cells in vitro and ex vivo. Blood 2017; 129(14):1991-2001; PMID:28143882; https://doi.org/ 10.1182/blood-2016-10-744441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].O'Gorman WE, Huang H, Wei YL, Davis KL, Leipold MD, Bendall SC, Kidd BA, Dekker CL, Maecker HT, Chien YH, et al.. The split virus influenza vaccine rapidly activates immune cells through Fcy receptors. Vaccine 2014; 32(45):5989-97; PMID:25203448; https://doi.org/ 10.1016/j.vaccine.2014.07.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Saadatian-Elahi M, Aaby P, Shann F, Netea MG, Levy O, Louis J, Picot V, Greenberg M, Warren W. Heterologous vaccine effects. Vaccine 2016; 34(34):3923-30; PMID:27312214; https://doi.org/ 10.1016/j.vaccine.2016.06.020 [DOI] [PubMed] [Google Scholar]
- [20].Todryk SM, Walther M, Bejon P, Hutchings C, Thompson FM, Urban BC, Porter DW, Hill AV. Multiple functions of human T cells generated by experimental malaria challenge. Eur J Immunol 2009; 39(11):3042-51; PMID:19658096; https://doi.org/ 10.1002/eji.200939434 [DOI] [PubMed] [Google Scholar]


