Abstract
INTRODUCTION
The relationship between the autonomic nervous system and restless legs syndrome (RLS) and periodic limb movements of sleep (PLMS) consists of varied and somewhat conflicting reports. In order to further elucidate these complexities, a retrospective analysis of polysomnography (PSG) records and clinical data was performed.
METHODS
Records from 233 adult subjects were randomly selected and organized into one of four groups ("non-RLS/PLMS" [n=61], "RLS" [n=60], "PLMS" [n=58], and "RLS/PLMS" [n=54]). Heart rate variability (HRV) analysis was based on 5-minute samples of 2-lead electrocardiogram data isolated from PSG recordings during wakefulness and NREM sleep, and included mean RR interval (labeled "NN") and standard deviation of the RR intervals (labeled "SDNN"), and HRV power, very low frequency (VLF), low frequency (LF), and high frequency (HF) spectral bands.
RESULTS
A significant reduction in the VLF band in the PLMS group as compared to the non-RLS/PLMS group (542±674 vs. 969±1025 ms2, p=0.038) was found in wakefulness. Statistically significant differences were seen in the PLMS group as compared to the non-RLS/PLMS group with a reduction in SDNN (p=0.001) and the HF (p=0.001) band, and an increase in HRV power (p=0.001), and the VLF (p=0.005) and LF (p=0.001) bands in NREM sleep.
CONCLUSIONS
The PLMS group exhibited reduced basal sympathetic activity in wakefulness, but basal sympathetic predominance during NREM sleep, distinguishing this group from the RLS and RLS/PLMS groups.
Keywords: Restless Legs Syndrome, Autonomic Nervous System, Heart Rate, Polysomnography, Sleep
INTRODUCTION
The relationships between the autonomic nervous system and restless legs syndrome (RLS) and periodic limb movements of sleep (PLMS) are not completely understood. The International Classification of Sleep Disorders, 3rd edition (ICSD-3) diagnostic criteria for RLS includes the urge to move the legs and/or the presence of uncomfortable sensations that occur primarily with rest/inactivity, are at least partially relieved with movement (as long as the movement persists), and have a circadian aspect in that the symptoms occur primarily in the evening or night. Periodic limb movements of sleep are noted during polysomnography (PSG) and are, defined by the ICSD-3 as episodes of repetitive, stereotypical leg movements during sleep, involving the rhythmic extension of the big toe and dorsiflexion of the ankle, occasionally accompanied by knee and hip flexion1.
Heart rate is modulated through effects of sympathetic and parasympathetic nervous systems, and analysis of changes in heart rate over time provides information about autonomic function. A reliable, non-invasive method to study these changes is heart rate variability (HRV), which has been utilized in patients with sleep disordered breathing and other sleep disturbances as a marker of autonomic dysfunction. In conditions associated with autonomic dysfunction, such as congestive heart failure, diabetes, end-stage renal disease, a decrease in HRV is typically found, with abnormal HRV being considered an independent risk factor for mortality2.
The literature linking RLS, PLMS, and autonomic disturbances is varied and somewhat conflicting. Small studies of subjects with RLS demonstrate reduced3,4, indicating a potential elevation of sympathetic activity, whereas others5 have failed to demonstrate HRV differences between subjects with RLS and healthy controls during NREM sleep. It is known that PLMS are preceded by cardiac acceleration, changes in the oscillatory activity of the cerebral cortex6, and surges in blood pressure7, however basal autonomic disturbance has not been demonstrated subjects with PLMS in NREM sleep (as measured through HRV)8. Other cardiovascular changes in sleep include increased HRV change during PLMS in subjects with RLS as compared to controls5 as well as increased blood pressure surges during PLMS in subjects with RLS as compared to controls7.
A more recent small study failed to demonstrate differences in cardiovascular responses (including HRV) between those patients with RLS and PLMS as compared to those with RLS but without PLMS4 in wakefulness8, but these 2 groups only had 6 patients each. Based on the limited literature on this topic, we hypothesize that patients with the combination of RLS and PLMS will demonstrate a basal HRV disturbance during wakefulness and NREM sleep as compared to the non-RLS/PLMS group, reflecting a more pronounced autonomic disturbance than that found in patients with either RLS or PLMS alone.
METHODS
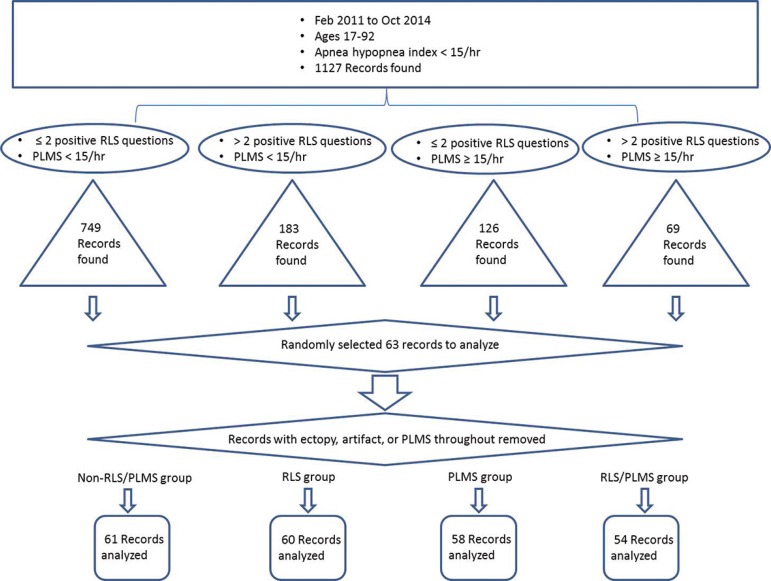
This study was approved by the institutional review board at Weill Cornell Medical College. Polysomnography records and clinical data from adult patients (ages 17-92) collected at the Weill Cornell Center for Sleep Medicine from February, 2011 to October, 2014 were retrospectively analyzed. All patients underwent PSG testing as part of the clinical evaluation of sleep disorders, including snoring, obstructive sleep apnea (OSA), insomnia, and/or hypersomnia. The initial exclusion criterion was an apnea hypopnea index (AHI) > 15/hr and/or missing or corrupt HRV data. Records were then classified into one of four groups depending on the clinical history and PSG findings: "non-RLS/PLMS" (essentially our control group; records with no RLS symptoms and PLMS < 15/hr were chosen), "RLS" (records with positive RLS symptoms and PLMS <15/hr were chosen), "PLMS" (records with no RLS symptoms and PLMS ≥ 15/hr were chosen), and "RLS/PLMS" (records with positive RLS symptoms and PLMS ≥ 15/hr were chosen) (Figure 1).
Figure 1.
Flow chart for PSG records chosen for analysis. RLS=restless legs syndrome; PLMS=periodic limb movements of sleep.
All subjects were provided a questionnaire to complete before the PSG, which includes questions regarding sleep schedules and symptoms, as well as questions regarding parasomnias, including RLS. The RLS-based questions were taken from the RLS Diagnostic Index9, which is, in turn, based the International Restless Legs Syndrome Study Group rating scale10,11. An answer of "yes" for more than 29 of the following RLS questions was considered a positive response: 1) kicking legs at night or during sleep; 2) leg movement due to discomfort or disagreeable sensations; 3) need to walk or rub legs to relieve discomfort; 4) symptoms worse when at rest; and/or 5) symptoms worse later in the day or at night.
The scoring of PLMS was performed by registered technologists, according to the American Academy of Sleep Medicine Scoring Manual guidelines12. A significant leg movement (LM) event was defined by a duration of 0.5-10 seconds, with an amplitude increase of 8µV in EMG voltage above resting EMG, with a minimum of four consecutive LM events, and a period length between LMs of 5-90 seconds12. The EMG criteria were as follows: sampling rate of 200 Hz, low frequency filter of 10 Hz, high frequency filter of 100 Hz, maximum electrode impedance of 5 KΩ, with 16-bit resolution.
For the non-RLS/PLMS group, 749 records were found that fulfilled the selection criteria; for the RLS group, 183 records were found that fulfilled the selection criteria; for the PLMS group, 126 records were found that fulfilled selection criteria; and finally, for the RLS/PLMS group, 69 records were found that fulfilled selection criteria. Of these, we randomly selected 63 per group, based on power analysis. We visually inspected ECG data from each record and if there was excessive artifact (muscle or otherwise), or excessive cardiac ectopy, that record was removed from analysis. For the NREM ECG data, we only used epochs that were free of PLMS. If we could not find PLM-free epochs, that record was discarded. For the non-RLS/PLMS group, 61 records were analyzed; for the RLS group, 60 records were analyzed; for the PLMS group, 58 records were analyzed; and finally, for the RLS/PLMS group, 54 records were analyzed. Demographic data and multiple facets of PSG data were compiled for both groups (see Table 1).
Table 1.
Demographic, and PSG characteristics of all four groups.
| Non-RLS/PLMS (n = 61) | RLS (n = 60) | P-value | Non-RLS/PLMS (n = 61) | PLMS (n = 58) | P-value | Non-RLS/PLMS (n = 61) | RLS/PLMS (n = 54) | P-value | |
|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 44.4 ± 17 | 47.2 ± 17 | 0.352 | 44.4 ± 17 | 55.6 ± 17* | <0.001 | 44.4 ± 17 | 57.3 ± 14* | <0.001 |
| Sex (%M) | 32/61 (52) | 24/60 (40) | N/A | 32/61 (52) | 31/58 (53) | N/A | 32/61 (52) | 22/54 (41) | N/A |
| BMI (kg/m2) | 26.9 ± 6 | 27.8 ± 6 | 0.411 | 26.9 ± 6 | 27.4 ± 6 | 0.659 | 26.9 ± 6 | 29.5 ± 7* | 0.026 |
| AHI (events/hr) | 4.5 ± 4 | 5.3 ± 4 | 0.272 | 4.5 ± 4 | 5.2 ± 4 | 0.361 | 4.5 ± 4 | 6.7± 4* | 0.005 |
| Sleep efficiency (%) | 76.1 ± 17 | 77.0 ± 12 | 0.728 | 76.1 ± 17 | 74.9 ± 14 | 0.683 | 76.1 ± 17 | 69.7 ± 17* | 0.025 |
| TST (min) | 353 ± 86 | 373 ± 73 | 0.217 | 353 ± 86 | 354 ± 91 | 0.928 | 353 ± 86 | 339 ± 110 | 0.431 |
| WASO (min) | 85 ± 68 | 91 ± 50 | 0.578 | 85 ± 68 | 98 ± 65 | 0.251 | 85 ± 68 | 116 ± 65* | 0.007 |
| PLMI (events/hr) | 2.4 ± 4 | 2.8 ± 4 | 0.888 | 2.4 ± 4 | 35.0 ± 23* | < 0.001 | 2.4 ± 4 | 40.3 ± 29* | < 0.001 |
BMI: Body mass index; AHI=apnea hypopnea index; TST: Total sleep time; WASO: Wake after sleep onset; PLMI: Periodic limb movement index.
Denotes a significant difference compared to non-RLS/PLMS group at p < 0.05.
An HRV analysis for each PSG meeting the selection criteria was then performed. The analysis was based on 5-minute PSG epochs of 2-lead ECG data isolated during continuous wakefulness (either at the start of the recording or in the middle of the night), and during NREM sleep, consistent with previous studies13,14. The data was then analyzed in the time- and frequency-domains using the SuperECG Package software (Mortara Instrument, Inc., Milwaukee, WI)15-18. Time-domain variables included mean RR interval (conventionally labeled "NN" to indicate "normal beats") and standard deviation of the RR intervals (conventionally labeled "SDNN")13. Frequency-domain variables included components of RR variability quantified by an autoregressive decomposition algorithm to compute spectral peak powers and their central frequencies, which are then classified into very low frequency (VLF; <0.04 Hz), low frequency (LF; 0.04-0.15 Hz), and high frequency (HF; 0.15-0.4 Hz) bands13.
Statistics
The HRV data was compiled and further analyzed using SPSS 21 software (IBM, Corp., Armonk, New York). An ANCOVA was performed to test for significant HRV differences between the non-RLS/PLMS, RLS, PLMS, and RLS/PLMS groups. Age was used as a co-variate because it differed significantly between groups as noted above. Of note, all variables were log transformed due to a non-normal distribution. The ANCOVA analysis was performed with the log transformed data to improve the ease of understanding the data; the means and standard deviations (SD) listed below are the actual values, not the log transformed values. A Holm's sequential Bonferroni procedure was used to control for type I error due to multiple testing19.
RESULTS
There were no significant differences in any demographic or PSG parameter between the non-RLS/PLMS and RLS groups. However, a significant difference in age was seen between the non-RLS/PLMS (44.4±17 years) and PLMS (55.6±17 years) groups, and between the non-RLS/PLMS and RLS/PLMS (57.3±14 years) groups. The RLS/PLMS group also revealed an elevated BMI and more disturbed sleep architecture, as evidenced by lower sleep efficiency and increased wake after sleep onset (WASO). As expected, there were also significant differences in PLM index between the non-RLS/PLMS and both RLS/PLMS and PLMS groups as shown on Table 1.
HRV in wakefulness
No significant differences were found in SDNN, HRV power, or any other HRV parameter during wakefulness between the non-RLS/PLMS and RLS, RLS/PLMs, and PLMS groups. There was, however, a significant reduction in the VLF band in the PLMS group as compared to the non-RLS/PLMS group (542±674 vs. 969±1025 ms2, p=0.038), as shown on Table 2.
Table 2.
HRV parameters of all four groups in wakefulness.
| Non- RLS/PLMS (n = 61) | RLS (n =60) | P-value | Non- RLS/PLMS (n = 61) | PLMS (n =58) | P-value | Non- RLS/PLMS (n = 61) | RLS/PUMS (n = 54) | P-value | |
|---|---|---|---|---|---|---|---|---|---|
| NN (ms) | 931 ± 125 | 921 ± 174 | 0.511 | 931 ± 125 | 937 ± 155 | 0.918 | 931 ± 125 | 905 ± 145 | 0.309 |
| SDNN (ms) | 55.7 ± 30.6 | 54.5 ± 36.5 | 0.647 | 55.7 ± 30.6 | 43.5 ± 24.2 | 0.095 | 55.7 ± 30.6 | 54.4 ± 41.7 | 0.731 |
| HRV Power (ms2) | 4011 ± 4365 | 4268 ± 7917 | 0.647 | 4011 ± 4365 | 2460 ± 3014 | 0.094 | 4011 ± 4365 | 4654 ± 9215 | 0.731 |
| VLF (ms2) | 969 ± 1025 | 761 ± 785 | 0.248 | 969 ± 1025 | 542 ± 674 | 0.038* | 969 ± 1025 | 907 ± 1602 | 0.519 |
| LF (ms2) | 1069 ± 1305 | 919 ± 1277 | 0.481 | 1069 ± 1305 | 600 ± 763 | 0.064 | 1069 ± 1305 | 820 ± 1094 | 0.348 |
| HF (ms2) | 1448 ± 1700 | 1714 ± 3610 | 0.788 | 1448 ± 1700 | 955 ± 1465 | 0.111 | 1448 ± 1700 | 1439 ± 2306 | 0.524 |
NN=R to R interval length; SDNN=standard deviation of R to R interval lengths; HRV=heart rate variability; VLF=very low frequency; LF=low frequency; HF=high frequency
Significant after controlling for multiple testing using the Holm’s sequential Bonferroni19
Please note: analysis was performed with the log transformed data, however to improve the ease of understanding the data the means and standard deviations listed below are the actual values, not the log transformed values
HRV in NREM sleep
There were no significant differences found in SDNN, HRV power, or the VLF, LF or HF bands during NREM sleep between the non-RLS/PLMS group and the RLS or RLS/PLMS groups. Statistically significant differences were seen in the PLMS group as compared to the non-RLS/PLMS group in regards to HRV parameters, with a reduction in SDNN (p=0.001) and the HF (p=0.001) band, and an increase in HRV power (p=0.001), and the VLF (p=0.005) and LF (p=0.001) bands, as shown on Table 3.
Table 3.
HRV parameters of all four groups in NREM sleep.
| Non- RLS/PLMS (n = 61) | RLS (n =60) | P-value | Non- RLS/PLMS (n = 61) | PLMS (n =58) | P-value | Non- RLS/PLMS (n = 61) | RLS/PUMS (n = 54) | P-value | |
|---|---|---|---|---|---|---|---|---|---|
| NN (ms) | 984 ± 148 | 957 ± 196 | 0.258 | 984 ± 148 | 976 ± 183 | 0.739 | 984 ± 148 | 967 ± 139 | 0.696 |
| SDNN (ms) | 45.7 ± 19.8 | 49.1 ± 33.3 | 0.548 | 45.7 ± 19.8 | 37.3 ± 33.6 | 0.001* | 45.7 ± 19.8 | 51.0 ±45.0 | 0.665 |
| HRV Power (ms2) | 2471 ± 2067 | 3482 ± 5255 | 0.548 | 2471 ± 2067 | 2489 ± 6161 | 0.001* | 2471 ± 2067 | 4572 ± 9948 | 0.665 |
| VLF (ms2) | 385 ± 299 | 503 ± 745 | 0.379 | 385 ± 299 | 472 ± 1177 | 0.005* | 385 ± 299 | 787 ±2356 | 0.770 |
| LF (ms2) | 689 ± 620 | 850 ± 1016 | 0.324 | 689 ± 620 | 749 ± 2263 | 0.001* | 689 ±620 | 1063 ± 2128 | 0.530 |
| HF (ms2) | 1132 ± 1142 | 1447 ± 2047 | 0.621 | 1132 ± 1142 | 1039 ± 2952 | 0.001* | 1132 ±1142 | 2044 ± 4862 | 0.604 |
NN=R to R interval length; SDNN=standard deviation of R to R interval lengths; HRV=heart rate variability; VLF=very low frequency; LF=low frequency; HF=high frequency
Significant after controlling for multiple testing using the Holm’s sequential Bonferroni19
Please note: analysis was performed with the log transformed data, however to improve the ease of understanding the data the means and standard deviations listed below are the actual values, not the log transformed values.
DISCUSSION
To the best of our knowledge, this is the first study examining basal HRV in a large number of subjects in both wake and NREM sleep across groups of subjects with RLS, PLMS, and the combination of RLS and PLMS, as compared to non-RLS/PLMS subjects during overnight polysomnography. Prior studies utilizing HRV analysis have focused on periods with leg movements in NREM sleep, given the fact that movement-related surges of sympathetic activity that are known to occur20,21; however, only a limited number of studies have investigated basal autonomic regulation in PLMS8,22. Our study revealed that the PLMS group had reduced basal sympathetic activity in wakefulness, but basal sympathetic predominance during NREM sleep, when compared to the non-RLS/PLMS group; these findings distinguished the PLMS group from both the RLS and the RLS/PLMS groups.
Altered HRV in the PLMS group in wakefulness
There was a significant reduction in wakefulness in the VLF band in the PLMS group, as compared to the non-RLS/PLMS group. Physiological interpretation of the VLF band still warrants further elucidation13, but may be related to sympathetic activity23. Additionally, in wakefulness, there was a reduction in the LF band in the PLMS group, which approached significance, and is a marker of sympathetic activity13,24. These findings indicate that there might be a reduction of sympathetic activity in wakefulness in the PLMS group, which is in direct contradiction with the prevailing thought of the long term effect of PLMS on the cardiovascular system25. The potential etiology of this finding is not clear, but may represent a "burn out" of the sympathetic nervous system, which would be similar to what has been noted in the locus coeruleus of subjects with post-traumatic stress disorder26,27.
Altered HRV in the PLMS group in NREM sleep
The relationship between the presence of PLMS and basal autonomic function in sleep is unsettled. On the one hand, some failed to produce convincing evidence indicating basal sympathetic predominance in subjects with PLMS5,8,28, others have reported that the presence of PLMS is related to basal sympathetic predominance and has a potentially negative impact on the cardiovascular system22. In our study, the altered HRV in the PLMS group during NREM sleep occurred across all measured parameters, and seems to point towards a basal sympathetic predominance in NREM sleep.
The SDNN and HRV total power parameters are considered to be global measures of variability14, and in this case there were changes in opposite directions. This suggests the presence of PLMS may have a global impact on the cardiac autonomic nervous system that is not fully understood yet, and/or that these two measurements are not sufficiently precise to detect a meaningful change. Of interest, similar global HRV change has been noted in young subjects with autonomic neuropathy associated with type 1 diabetes mellitus, leading to the authors concluding that young subjects with type 1 diabetes mellitus have autonomic nervous system behavior tending to randomness29; a similar behavior may be present in those with PLMS.
The HF band likely represents vagal modulation of sinus rhythm24. The fact that it was significantly reduced in the PLMS group compared to the non-RLS/PLMS group suggests that there is reduced parasympathetic influence in NREM sleep in these subjects. The LF band can be viewed either as a surrogate for sympathetic modulations, or may reflect both sympathetic and vagal activity13,24, or the baroreflex responsiveness to beat-to-beat variations in blood pressure30. This parameter was significantly elevated in the PLMS group as compared to the non-RLS/PLMS group in NREM sleep, suggesting that sympathetic influence was stronger in these subjects. Taken together, these two results imply that there exists a sympathetic predominance in the autonomic nervous system of those with PLMS in NREM sleep. Furthermore, there was also a significant elevation in the VLF band in NREM sleep, in the PLMS group compared to the non-RLS/PLMS group, suggesting that in NREM sleep, sympathetic activity may predominate in those with PLMS.
It should be noted that electrocardiographic data time-locked to the occurrence of PLMS were not analyzed in our study, and thus we cannot conclude that there would be further sympathetic surges during PLMS as described in prior studies5,31-35. It has been hypothesized that the repetitive abnormal HR rises connected with PLMS might have a long-term negative effect on the cardiovascular system. Despite this, other studies have reported that there is a disconnect of PLMS from arousals36 and thus leg movements are thought to be either a consequence or a co-occurrence, rather than the cause, of sympathetic activation in PLMS21. However, as discussed by Wu et al, basal sympathetic predominance during the intermovement interval in subjects with PLMS may be present22; this is in agreement with our findings.
Normal HRV in the RLS and RLS/PLMS groups
Similar to the case with PLMS, the literature regarding basal HRV is disparate for RLS. Whereas large epidemiological surveys have found a significant comorbidity between RLS and cardiovascular disease37-41, a prior study failed to demonstrate differences in HRV between subjects with RLS and healthy non-RLS/PLMSs during NREM sleep5. Another study revealed no difference in HRV measured during wakefulness in six subjects with RLS/PLMS as compared to six non-RLS/PLMSs4. Given our large sample size, our study strengthens these two latter results, and suggests that there does not seem to be a basal autonomic disturbance in wake in subjects with either RLS or PLMS, and more importantly, in those with the combined RLS/PLMS condition.
It has been posited that a reduction in HRV across age42 may occur. Both the PLMS group and the RLS/PLMS combination group in our study were significantly older than the non-RLS/PLMS group, so age could have played some role in reducing HRV in the PLMS group; but this would not explain why the PLMS group and the RLS/PLMS group were not similarly affected. However, the RLS/PLMS group was also significantly older and the HRV in this group was not significantly different to the non-RLS/PLMS group in both wakefulness and sleep. Moreover, we adjusted for age in our analysis, so any effect in this regard should have been minimized.
Limitations
Our study suffers from several limitations, the main issue being its retrospective design, which may have affected the classification of the groups. In particular, the non-RLS/PLMS group, despite acting as our control group, was not necessarily without any sleep abnormality. Rather, these were subjects simply free of RLS, PLMS, and OSA, and actually may have been suffering with other conditions such as insomnia, hypersomnia, or other parasomnias, which admittedly may have had effects on HRV. Despite this issue, the retrospective design allowed us to analyze a considerable amount of subjects per group; additionally, our data was comprised of a diverse population of subjects seen in Manhattan, New York, at our comprehensive sleep disorder center.
Similarly, those with RLS may have been falsely deemed so, as this designation was based on information from the questionnaire and chart review. However, all diagnostic algorithms tend to focus on presenting symptoms43, and in the RLS epidemiology, symptoms, and treatment (REST) primary care study, sleep-related symptoms, daytime sleepiness, and discomfort in the legs (pain, twitching and jerks, uncomfortable feelings) accounted for the most troublesome symptoms44. If a patient presents with insomnia/sleep problems and an urge to move, or complains of unpleasant sensations in the legs, it is recommended that follow up questions be asked; these are based on the RLS-Diagnostic Index, a validated diagnostic algorithm9. The most important questions concern the urge to move the legs and the worsening of symptoms at rest9,43. If a patient answers yes to three or more of these questions43, there is a high likelihood of RLS being present. Thus, our screening for RLS, while compiled in a retrospective manner, is suitable for grouping purposes.
Another important limitation to mention is our exclusion criteria; there are multiple factors, both known and unknown, that can affect HRV analysis. Among them includes the use of anti-hypertensive medications45, the use of anti-depressant46, and the presence of psychiatric diseases47. Additionally, there is data suggesting gender-related differences in cardiac autonomic tone that could have potentially impacted HRV analysis in that there has been found a predominance of parasympathetic over sympathetic tone in women, and vice versa in men48,49. Finally, obesity is a well-known risk factor of low HRV and overstimulation of the sympathetic nervous system50. These factors may have played a role in our study, but given the relatively large number of diverse subjects analyzed, we feel the confounding effects were minimized. Large, prospective studies analyzing HRV in subjects with RLS and PLMS are needed.
Regarding the HRV analysis itself, we attempted to locate 5-minute epochs free of PLMs, but in the case of HRV measured during wakefulness, the presence of PLM in wakefulness and/or RLS may have affected these measurements. The EKG segments during wakefulness that we chose occurred at any point during the PSG record, even in the middle of the night, and HRV could have been affected by this.
Finally, the PLMS group and the RLS/PLMS group were both significantly older than the non-RLS/PLMS group; this should not have been an issue, however, as age was controlled for in analysis. Additionally, while there were significant differences between the non-RLS/PLMS group and the RLS/PLMS groups in sleep efficiency and WASO, these findings were not totally unexpected. The RLS/PLMS group had more sleep disruption, which is what one would except given the presence of both RLS and PLMS. The RLS/PLMS group also had a significantly higher BMI and AHI compared to the non-RLS/PLMS group. These differences, however, should not have played a major role in HRV analysis, as in both groups the BMI was in the overweight range (25-29.9 kg/m2), and the AHI was very close to the cutoff (≥ 5/hr) for only mild OSA. As evidence of these differences not being a major issue, the non-RLS/PLMS and RLS/PLMS groups had similar HRV values.
CONCLUSIONS
This current report demonstrates somewhat disparate results in that there is reduced basal sympathetic activity in wakefulness in subjects with PLMS, but basal sympathetic predominance during NREM sleep in the same subjects. It argues against a role for basal HRV disturbance in subjects with RLS and the combination of RLS/PLMS during wakefulness and NREM sleep. Although in clinical practice it is tempting to group subjects with RLS and PLMS in the same category, it may be that these conditions are fundamentally different entities. The findings of our current study add to the literature important information regarding HRV on a large sample of subjects with RLS, PLMS and RLS/PLMS combination during wakefulness and sleep and highlight the need for further studies to fully elucidate the effects of these conditions on the autonomic nervous system, and in relation to cardiovascular control.
REFERENCES
- 1.American Academy of Sleep Medicine . International Classification of Sleep Disorders. 3rd ed. Darien: American Academy of Sleep Medicine; 2014. International classification of sleep disorders. [Google Scholar]
- 2.Kleiger RE, Stein PK, Bigger JT., Jr Heart rate variability: measurement and clinical utility. Ann Noninvasive Electrocardiol. 2005;10(1):88–101. doi: 10.1111/j.1542-474X.2005.10101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cikrikcioglu MA, Hursitoglu M, Erkal H, Kinas BE, Sztajzel J, Cakirca M, et al. Oxidative stress and autonomic nervous system functions in restless legs syndrome. Eur J Clin Invest. 2011;41(7):734–742. doi: 10.1111/j.1365-2362.2010.02461.x. [DOI] [PubMed] [Google Scholar]
- 4.Izzi F, Placidi F, Romigi A, Lauretti B, Marfia GA, Mercuri NB, et al. Is autonomic nervous system involved in restless legs syndrome during wakefulness. Sleep Med. 2014;15(11):1392–1397. doi: 10.1016/j.sleep.2014.06.022. [DOI] [PubMed] [Google Scholar]
- 5.Manconi M, Ferri R, Zucconi M, Clemens S, Rundo F, Oldani A, et al. Effects of acute dopamine-agonist treatment in restless legs syndrome on heart rate variability during sleep. Sleep Med. 2011;12(1):47–55. doi: 10.1016/j.sleep.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 6.Iriarte J, Urrestarazu E, Alegre M, Valencia M, Artieda J. Oscillatory cortical changes during periodic limb movements. Sleep. 2004;27(8):1493–1498. doi: 10.1093/sleep/27.8.1493. [DOI] [PubMed] [Google Scholar]
- 7.Pennestri MH, Montplaisir J, Fradette L, Lavigne G, Colombo R, Lanfranchi PA. Blood pressure changes associated with periodic leg movements during sleep in healthy subjects. Sleep Med. 2013;14(6):555–561. doi: 10.1016/j.sleep.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Palma JA, Alegre M, Valencia M, Artieda J, Iriarte J, Urrestarazu E. Basal cardiac autonomic tone is normal in patients with periodic leg movements during sleep. J Neural Transm (Vienna) 2014;121(4):385–390. doi: 10.1007/s00702-013-1116-8. [DOI] [PubMed] [Google Scholar]
- 9.Benes H, Kohnen R. Validation of an algorithm for the diagnosis of Restless Legs Syndrome: The Restless Legs Syndrome-Diagnostic Index (RLS-DI) Sleep Med. 2009;10(5):515–523. doi: 10.1016/j.sleep.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Walters AS. Toward a better definition of the restless legs syndrome. The International Restless Legs Syndrome Study Group. Mov Disord. 1995;10(5):634–642. doi: 10.1002/mds.870100517. [DOI] [PubMed] [Google Scholar]
- 11.Walters AS, LeBrocq C, Dhar A, Hening W, Rosen R, Allen RP, et al. Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Med. 2003;4(2):121–132. doi: 10.1016/s1389-9457(02)00258-7. [DOI] [PubMed] [Google Scholar]
- 12.Berry RB, Brooks R, Gamaldo BR, Harding CE, Lloyd SM, Marcus RM, et al. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications, Version 2.2. Darien: American Academy of Sleep Medicine; 2015. [Google Scholar]
- 13.Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology Eur Heart J. 1996;17(3):354–381. [PubMed] [Google Scholar]
- 14.Postuma RB, Lanfranchi PA, Blais H, Gagnon JF, Montplaisir JY. Cardiac autonomic dysfunction in idiopathic REM sleep behavior disorder. Mov Disord. 2010;25(14):2304–2310. doi: 10.1002/mds.23347. [DOI] [PubMed] [Google Scholar]
- 15.Penn State College of Medicine. Department of Public Health Sciences . Super ECG Software Standard Operational Procedures for Air Pollution and Cardiac Risk (APACR) Study. Hershey: 2008. [Google Scholar]
- 16.Liao D, Shaffer ML, Rodriguez-Colon S, He F, Li X, Wolbrette DL, et al. Acute adverse effects of fine particulate air pollution on ventricular repolarization. Environ Health Perspect. 2010;118(7):1010–1015. doi: 10.1289/ehp.0901648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodríguez-Colón SM, Li X, Shaffer ML, He F, Bixler EO, Vgontzas AN, et al. Insulin resistance and circadian rhythm of cardiac autonomic modulation. Cardiovasc Diabetol. 2010;9:85–85. doi: 10.1186/1475-2840-9-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He F, Shaffer ML, Li X, Rodriguez-Colon S, Wolbrette DL, Williams R, et al. Individual-level PM2.5 exposure and the time course of impaired heart rate variability: the APACR Study. J Expo Sci Environ Epidemiol. 2011;21(1):65–73. doi: 10.1038/jes.2010.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holm S. A Simple Sequentially Rejective Multiple Test Procedure. Sour Scand J Stat. 1979;6(2):65–70. [Google Scholar]
- 20.Ferrillo F, Beelke M, Canovaro P, Watanabe T, Aricò D, Rizzo P, et al. Changes in cerebral and autonomic activity heralding periodic limb movements in sleep. Sleep Med. 2004;5(4):407–412. doi: 10.1016/j.sleep.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 21.Guggisberg AG, Hess CW, Mathis J. The significance of the sympathetic nervous system in the pathophysiology of periodic leg movements in sleep. Sleep. 2007;30(6):755–766. doi: 10.1093/sleep/30.6.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu MN, Lai CL, Liu CK, Yen CW, Liou LM, Hsieh CF, et al. Basal sympathetic predominance in periodic limb movements in sleep with obstructive sleep apnea. J Sleep Res. 2015;24(6):722–729. doi: 10.1111/jsr.12314. [DOI] [PubMed] [Google Scholar]
- 23.Ergun U, Demirci M, Nurlu G, Komürcü F. Power spectral analysis of heart rate variability: normal values of subjects over 60 years old. Int J Neurosci. 2008;118(8):1165–1173. doi: 10.1080/00207450701820845. [DOI] [PubMed] [Google Scholar]
- 24.Eckberg DL. Physiological basis for human autonomic rhythms. Ann Med. 2000;32(5):341–349. doi: 10.3109/07853890008995937. [DOI] [PubMed] [Google Scholar]
- 25.Cuellar NG. The effects of periodic limb movements in sleep (PLMS) on cardiovascular disease. Heart Lung. 2013;42(5):353–360. doi: 10.1016/j.hrtlng.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 26.Anisman H, Pizzino A, Sklar LS. Coping with stress, norepinephrine depletion and escape performance. Brain Res. 1980;191(2):583–588. doi: 10.1016/0006-8993(80)91311-6. [DOI] [PubMed] [Google Scholar]
- 27.Husain AM, Miller PP, Carwile ST. Rem sleep behavior disorder: potential relationship to post-traumatic stress disorder. See comment in PubMed Commons below. J Clin Neurophysiol. 2001;18(2):148–157. doi: 10.1097/00004691-200103000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Walters AS, Rye DB. Review of the relationship of restless legs syndrome and periodic limb movements in sleep to hypertension, heart disease, and stroke. Sleep. 2009;32(5):589–597. doi: 10.1093/sleep/32.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Souza NM, Giacon TR, Pacagnelli FL, Barbosa MP, Valenti VE, Vanderlei LC. Dynamics of heart rate variability analysed through nonlinear and linear dynamics is already impaired in young type 1 diabetic subjects. Cardiol Young. 2016;26(7):1383–1390. doi: 10.1017/S104795111500270X. [DOI] [PubMed] [Google Scholar]
- 30.Sleight P, La Rovere MT, Mortara A, Pinna G, Maestri R, Leuzzi S, et al. Physiology and pathophysiology of heart rate and blood pressure variability in humans: is power spectral analysis largely an index of baroreflex gain? Clin Sci (Lond) 1995;88(1):103–109. doi: 10.1042/cs0880103. [DOI] [PubMed] [Google Scholar]
- 31.Winkelman JW. The evoked heart rate response to periodic leg movements of sleep. Sleep. 1999;22(5):575–580. doi: 10.1093/sleep/22.5.575. [DOI] [PubMed] [Google Scholar]
- 32.Sforza E, Juony C, Ibanez V. Time-dependent variation in cerebral and autonomic activity during periodic leg movements in sleep: implications for arousal mechanisms. Clin Neurophysiol. 2002;113(6):883–891. doi: 10.1016/s1388-2457(02)00066-4. [DOI] [PubMed] [Google Scholar]
- 33.Sforza E, Pichot V, Barthelemy JC, Haba-Rubio J, Roche F. Cardiovascular variability during periodic leg movements: a spectral analysis approach. Clin Neurophysiol. 2005;116(5):1096–1104. doi: 10.1016/j.clinph.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 34.Pennestri MH, Montplaisir J, Colombo R, Lavigne G, Lanfranchi PA. Nocturnal blood pressure changes in patients with restless legs syndrome. Neurology. 2007;68(15):1213–1218. doi: 10.1212/01.wnl.0000259036.89411.52. [DOI] [PubMed] [Google Scholar]
- 35.Siddiqui F, Strus J, Ming X, Lee IA, Chokroverty S, Walters AS. Rise of blood pressure with periodic limb movements in sleep and wakefulness. Clin Neurophysiol. 2007;118(9):1923–1930. doi: 10.1016/j.clinph.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 36.Manconi M, Ferri R, Zucconi M, Bassetti CL, Fulda S, Aricò D, et al. Dissociation of periodic leg movements from arousals in restless legs syndrome. Ann Neurol. 2012;71(6):834–844. doi: 10.1002/ana.23565. [DOI] [PubMed] [Google Scholar]
- 37.Ulfberg J, Nyström B, Carter N, Edling C. Prevalence of restless legs syndrome among men aged 18 to 64 years: an association with somatic disease and neuropsychiatric symptoms. Mov Disord. 2001;16(6):1159–1163. doi: 10.1002/mds.1209. [DOI] [PubMed] [Google Scholar]
- 38.Ohayon MM, Roth T. Prevalence of restless legs syndrome and periodic limb movement disorder in the general population. J Psychosom Res. 2002;53(1):547–554. doi: 10.1016/s0022-3999(02)00443-9. [DOI] [PubMed] [Google Scholar]
- 39.Phillips B, Hening W, Britz P, Mannino D. Prevalence and correlates of restless legs syndrome: results from the 2005 National Sleep Foundation Poll. Chest. 2006;129(1):76–80. doi: 10.1378/chest.129.1.76. [DOI] [PubMed] [Google Scholar]
- 40.Winkelman JW, Finn L, Young T. Prevalence and correlates of restless legs syndrome symptoms in the Wisconsin Sleep Cohort. Sleep Med. 2006;7(7):545–552. doi: 10.1016/j.sleep.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 41.Winkelman JW, Shahar E, Sharief I, Gottlieb DJ. Association of restless legs syndrome and cardiovascular disease in the Sleep Heart Health Study. Neurology. 2008;70(1):35–42. doi: 10.1212/01.wnl.0000287072.93277.c9. [DOI] [PubMed] [Google Scholar]
- 42.Greiser KH, Kluttig A, Schumann B, Swenne CA, Kors JA, Kuss O, et al. Cardiovascular diseases, risk factors and short-term heart rate variability in an elderly general population: the CARLA study 2002-2006. Eur J Epidemiol. 2009;24(3):123–142. doi: 10.1007/s10654-009-9317-z. [DOI] [PubMed] [Google Scholar]
- 43.Garcia-Borreguero D, Stillman P, Benes H, Buschmann H, Chaudhuri KR, Gonzalez Rodríguez VM, et al. Algorithms for the diagnosis and treatment of restless legs syndrome in primary care. BMC Neurol. 2011;11:28–28. doi: 10.1186/1471-2377-11-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hening W, Walters AS, Allen RP, Montplaisir J, Myers A, Ferini-Strambi L. Impact, diagnosis and treatment of restless legs syndrome (RLS) in a primary care population: the REST (RLS epidemiology, symptoms, and treatment) primary care study. Sleep Med. 2004;5(3):237–246. doi: 10.1016/j.sleep.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 45.Kiselev AR, Gridnev VI, Prokhorov MD, Karavaev AS, Posnenkova OM, Ponomarenko VI, et al. Effects of antihypertensive treatment on cardiovascular autonomic control: a prospective study. Anadolu Kardiyol Derg. 2014;14(8):701–710. doi: 10.5152/akd.2014.5107. [DOI] [PubMed] [Google Scholar]
- 46.Prasko J, Latalova K, Diveky T, Grambal A, Kamaradova D, Velartova H, et al. Panic disorder, autonomic nervous system and dissociation - changes during therapy. Neuro Endocrinol Lett. 2011;32(5):641–651. [PubMed] [Google Scholar]
- 47.Alvares GA, Quintana DS, Hickie IB, Guastella AJ. Autonomic nervous system dysfunction in psychiatric disorders and the impact of psychotropic medications: a systematic review and meta-analysis. J Psychiatry Neurosci. 2016;41(2):89–104. doi: 10.1503/jpn.140217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rossy LA, Thayer JF. Fitness and gender-related differences in heart period variability. Psychosom Med. 1998;60(6):773–781. doi: 10.1097/00006842-199811000-00022. [DOI] [PubMed] [Google Scholar]
- 49.Evans JM, Ziegler MG, Patwardhan AR, Ott JB, Kim CS, Leonelli FM, et al. Gender differences in autonomic cardiovascular regulation: spectral, hormonal, and hemodynamic indexes. J Appl Physiol (1985) 2001;91(6):2611–2618. doi: 10.1152/jappl.2001.91.6.2611. [DOI] [PubMed] [Google Scholar]
- 50.Tentolouris N, Liatis S, Katsilambros N. Sympathetic system activity in obesity and metabolic syndrome. Ann N Y Acad Sci. 2006;1083:129–152. doi: 10.1196/annals.1367.010. [DOI] [PubMed] [Google Scholar]