Abstract
Mitochondria play a primary role in the pathophysiology of Parkinson's disease (PD), and small molecules that counteract the initial stages of disease may offer therapeutic benefit. In this regard, we have examined whether the off-target effects of the Food and Drug Administration (FDA)–approved anti-helminth drug nitazoxanide (NTZ) on mitochondrial respiration could possess any therapeutic potential for PD. Results indicate that MPP+-induced loss in oxygen consumption rate (OCR) and ATP production by mitochondria were ameliorated by NTZ in real time by virtue of its mild uncoupling effect. Pretreatment of cells with NTZ mitigated MPP+-induced loss in mitochondrial OCR and reactive oxygen species (ROS). Similarly, addition of NTZ to cells pretreated with MPP+ could reverse block in mitochondrial OCR and reactive oxygen species induced by MPP+ in real time. The observed effects of NTZ were found to be transient and reversible as removal of NTZ from incubation medium restored the mitochondrial respiration to that of controls. Apoptosis induced by MPP+ was ameliorated by NTZ in a dose-dependent manner. In vivo results demonstrated that oral administration of NTZ (50 mg/kg) in an acute MPTP mouse model of PD conferred significant protection against the loss of tyrosine hydroxylase (TH)-positive neurons of substantia nigra. Based on the above observations we believe that repurposing of NTZ for PD may offer therapeutic benefit.
Keywords: bioenergetics, mitochondria, neurodegeneration, oxidative stress, Parkinson's disease, MPTP, nitazoxanide, uncoupler
Introduction
Parkinson's disease (PD)3 is one of the most common neurodegenerative disorders that is characterized primarily by the loss of dopaminergic neurons in the substantia nigra pars compacta (SNPc) leading to dopamine deficit in the striatum. Mitochondria were found to play an indispensable role in the onset and progression of PD (1–3). In fact, PD mimetics, such as MPP+ and rotenone, happen to be the direct inhibitors at the complex-I of mitochondria (1–3). Also, some of the PD-associated genes, such as parkin and pink-1 have been shown to directly affect the mitochondrial integrity by regulating its quality control, and mutations in these genes were found to be associated with familial PD (4–6). Indeed, complex-I activities were reported to be significantly reduced in postmortem samples of SNPc and platelets of PD patients (7, 8). Further, the catalytic subunits of complex-I derived from PD patients were found to be oxidatively damaged (8). Moreover, the pathogenesis of PD appears to be converging on common pathways, such as mitochondrial dysfunction followed by oxidative stress and protein aggregation, which ultimately leads to apoptosis (9, 10). Therapeutic strategies employing antioxidants and iron chelators have been utilized in PD animal models with limited success (11–13). Our earlier results demonstrated the neuroprotective effects of the mitochondria-targeted antioxidant MitoQ in cellular and animal models of PD (14). In this regard, targeting the very initial steps of disease pathology employing small molecules capable of enhancing mitochondrial respiration and mitigating reactive oxygen species (ROS) appears to be the promising strategy in preventing the downstream biochemical abnormalities. A recent study identified the mitochondrial respiration–enhancing effects of some of the FDA-approved drugs, including the anti-helminth drug nitazoxanide (NTZ) (15). Moreover, NTZ happens to be a highly bioavailable drug through oral route and has a better safety profile with a half-life of nearly 3.5 h and can reach the CNS (16, 17). In the present study, we have investigated the efficacy of NTZ in dopaminergic cell lines against MPP+-induced alterations in the mitochondrial bioenergetics as well as in an acute MPTP mouse model of PD in both preventive and therapeutic models. Our findings demonstrate that the mild uncoupling effects of NTZ could mitigate MPP+-induced deleterious effects in both cellular and animal models of PD.
Results
NTZ prevents MPP+-induced block in mitochondrial respiration and restores ATP production by its mild uncoupling effect
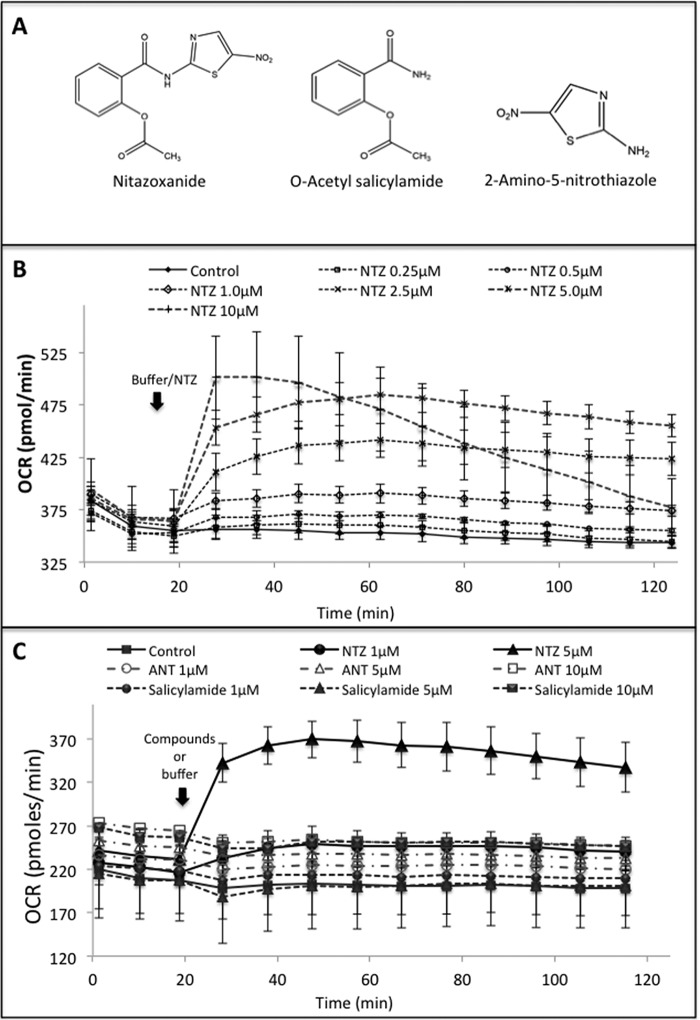
Based on some of the recent reports on the ability of NTZ in enhancing mitochondrial oxygen consumption rate (OCR), we examined whether this property of NTZ could ameliorate MPP+-induced dysfunction in mitochondrial respiration. To examine this, we added either buffer or different concentrations of NTZ (0.25–10 μm) directly to the human dopaminergic cells seeded in 24-well plate through one of the ports of the Seahorse flux analyzer and real-time OCRs were measured. Results indicate that NTZ dose-dependently increased cellular OCR as soon as it was added to the cells (Fig. 1B). A substantial increase in OCR was evident starting from 1 μm NTZ wherein an increase in the OCR from 220 to 250 pmol/min was evident, whereas at 5 μm concentration there was a steep and significant increase from 220 to 369 pmol/min, and the values remained nearly constant until 2 h. At 10 μm NTZ, although there was a steep increase in OCR during the initial 20 min, it gradually decreased until 2 h. Because NTZ consists of two basic chemical scaffolds/moieties, i.e. 2-amino-5-nitrothiazole (ANT) and O-acetyl-salicylamide (OAS), we next examined whether the effect of NTZ is because of any of these scaffolds. Addition of either ANT (1–10 μm) or OAS (1–10 μm) did not induce any increase in cellular OCR unlike NTZ (Fig. 1C).
Figure 1.
Effect of NTZ and its chemical moieties on mitochondrial OCR. A, chemical structures of nitazoxanide, O-acetyl salicylamide, and 2-amino-5-nitrothiazole. B, dose-dependent effect of NTZ on mitochondrial OCR: SK-N-SH cells in 24-well plates were treated with different concentrations of NTZ (0.25–10 μm) and cellular OCR was measured using Seahorse extracellular flux analyzer. C, effect of NTZ (1 and 5 μm) and its chemical moieties, salicylamide (1, 5, and 10 μm) and 2-amino-5-nitrothiazole (ANT) (1, 5, and 10 μm), on cellular OCR was measured using Seahorse extracellular flux analyzer. Control cells were treated with equal volumes of buffer in place of compounds. Data are representative of the mean ± S.D. of samples analyzed in triplicates (n = 3) from two independent experiments.
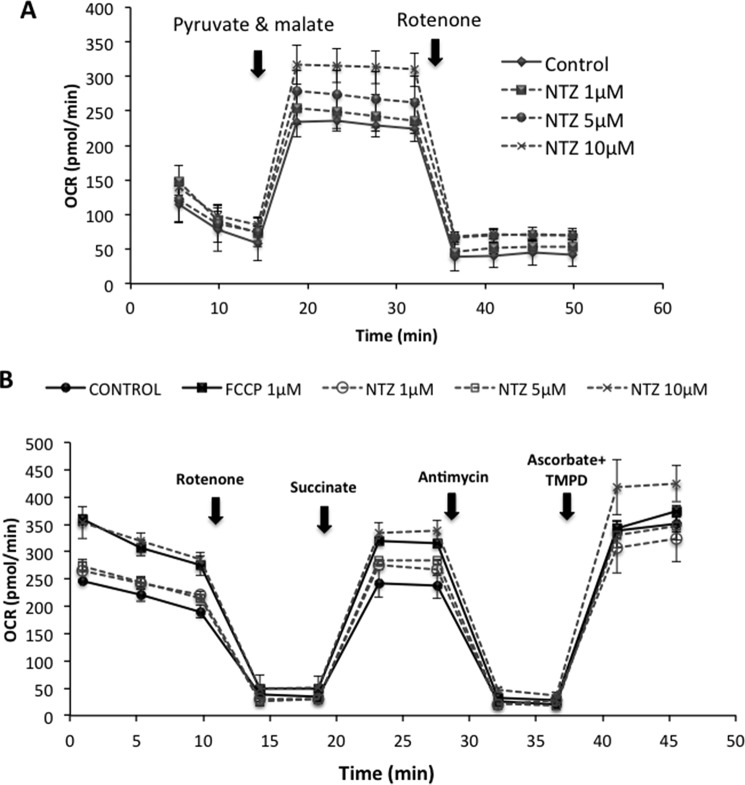
Next, to examine whether the increase in OCR by NTZ is because of mitochondrial respiration or not, we have added assay medium that is devoid of pyruvate and malate to dopaminergic cells in a 24-well Seahorse assay plate in the presence or absence of NTZ (1–10 μm) and found no increase in the OCR. However, addition of pyruvate and malate instantaneously increased OCR in control and NTZ-treated cells (Fig. 2A). There was a dose-dependent increase in OCR in cells incubated with increasing concentrations of NTZ (Fig. 2A). Addition of rotenone (1.5 μm) almost completely inhibited the OCR in control as well as NTZ-treated cells to similar extents, indicating that NTZ-induced OCR is derived from mitochondrial respiration (Fig. 2A). To examine whether NTZ affects mitochondrial respiration at specific mitochondrial complexes, we have performed electron flow assay using permeabilized human dopaminergic cells in the presence and absence of different concentrations of NTZ (1–10 μm) (Fig. 2B). Results indicate that there was a dose-dependent increase in OCR by NTZ in the pyruvate and malate (complex-I)–, succinate (complex-II)–, as well as ascorbate and TMPD (complex-IV)–induced respiration (Fig. 2B).
Figure 2.
Effect of NTZ on overall mitochondrial OCR and at individual respiratory complexes. A and B, human SK-N-SH neuroblastoma cells were incubated with different concentrations of NTZ (1, 5, and 10 μm) on (A) pyruvate and malate-driven respiration and (B) OCR at individual respiratory complexes and was monitored by electron flow assay in permeabilized cells as mentioned in “Experimental Procedures.” Data are representative of the mean ± S.D. of samples analyzed in triplicates (n = 3) from three independent experiments.
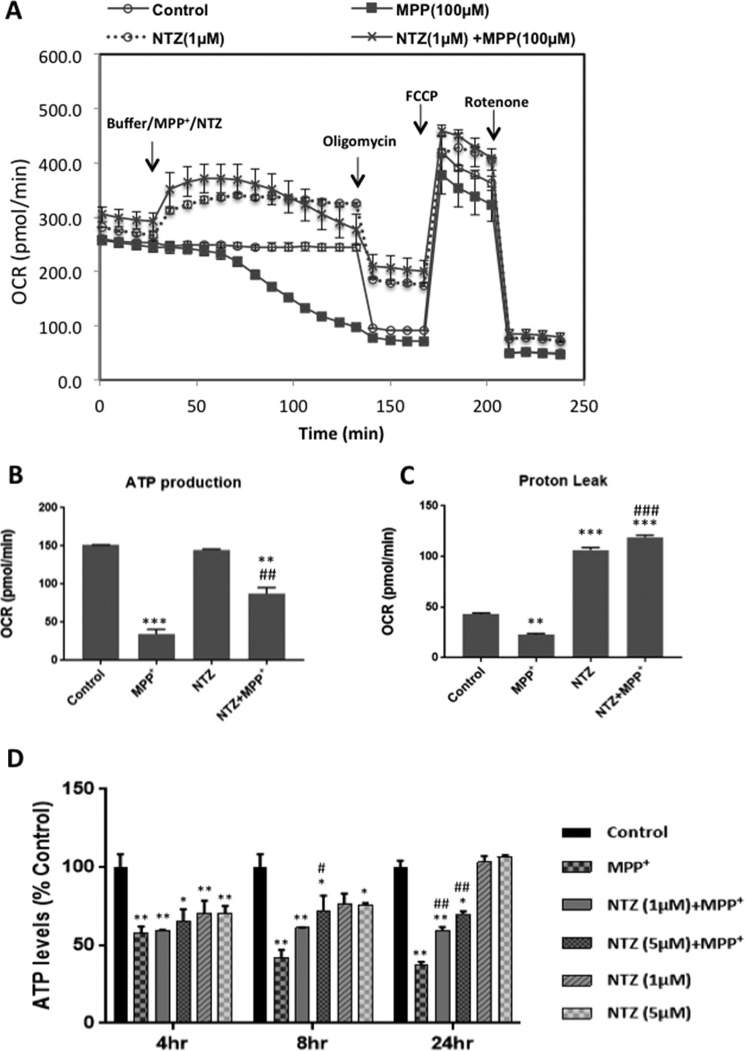
To examine whether NTZ mitigates MPP+-induced block in the mitochondrial respiration, dopaminergic cells were seeded in 24-well assay plates and Mito Stress assay was performed with MPP+ in the presence or absence of NTZ, whereas control cells were treated with assay medium in place of MPP+ or NTZ under similar experimental conditions. Addition of MPP+ (100 μm) gradually decreased the mitochondrial respiration as indicated by the decrease in OCR and virtually no respiration was evident at 2 h following addition of MPP+. At this stage addition of oligomycin had negligible effect on the OCR, indicating that ATP generation was almost completely ceased in cells treated with MPP+ (Fig. 3A). However, when MPP+ was added along with NTZ (1 μm), there was a significant increase in the respiration (OCR) as compared with MPP+ treatment alone or controls which persisted until 2 h (Fig. 3A). Addition of oligomycin to MPP+ + NTZ–treated cells exhibited a decline in OCR, indicating that cells were capable of generating ATP (Fig. 3, A and B). Under similar conditions, NTZ alone enhanced mitochondrial respiration and NTZ-treated cells could generate ATP nearly to the same extent as control cells (Fig. 3, A and B). However, at higher concentrations of NTZ (>5 μm) cells exhibited a dose-dependent decrease in ATP production, possibly because of more uncoupling (data not shown). Nevertheless, there was a significant increase in proton leak in both NTZ alone and NTZ + MPP+–treated conditions (Fig. 3, A and C). Addition of FCCP to cells resulted in a steep increase in OCR under all the treatment conditions, indicating the presence of intact functional mitochondria (Fig. 3A).
Figure 3.
NTZ mitigates MPP+-induced mitochondrial dysfunction. A, SK-N-SH cells were treated with MPP+ in the presence and absence of NTZ (1 μm) and Mito Stress assay was performed as described in “Experimental Procedures.” B and C, bar diagrams representing (B) ATP production and (C) protein leak from the data obtained from (A). D, SK-N-SH cells were incubated with NTZ (1 μm and 5 μm) for 60 min before exposure to MPP+ for 4, 8, and 24 h and cellular ATP content was analyzed by luciferase-based kit as mentioned in the “Experimental Procedures” section. Data shown are the representative of mean ± S.E. from triplicate measurements per each condition (n = 3) for (A) from three independent experiments. B–D, values indicated are the mean ± S.E. of triplicate measurements per each condition from two different experiments (n = 6). ***, p < 0.001; **, p < 0.01; *, p < 0.05 as compared with controls. ###, p < 0.001; ##, p < 0.01; #, p < 0.05 as compared with MPP+ group as analyzed by two-way ANOVA with Tukey's multiple comparisons test.
To revalidate the obtained results and to examine the long-term effects of NTZ on MPP+-induced block in ATP production, human dopaminergic cells were treated with MPP+ in the presence and absence of NTZ (1 and 5 μm) for 4, 8, and 24 h and cellular ATP was estimated by luciferase-based assay method (Fig. 3D). Results demonstrate that treatment with MPP+ for 4, 8, and 24 h inhibited cellular ATP levels to nearly 40, 56, and 60%, respectively, as compared with controls. At the 8 h time period, NTZ could significantly improve cellular ATP levels as compared with MPP+ treatment alone, but not at the 4 h time point. However, following 24 h incubation, NTZ significantly protected from MPP+-induced loss in cellular ATP levels (Fig. 3D). NTZ per se decreased ATP levels as compared with controls at 4 and 8 h time periods, possibly because of its uncoupling effect. However, ATP levels were found to be nearly the same as controls at 24 h time period, indicating that the uncoupling effect of NTZ could be a transient event (Fig. 3D).
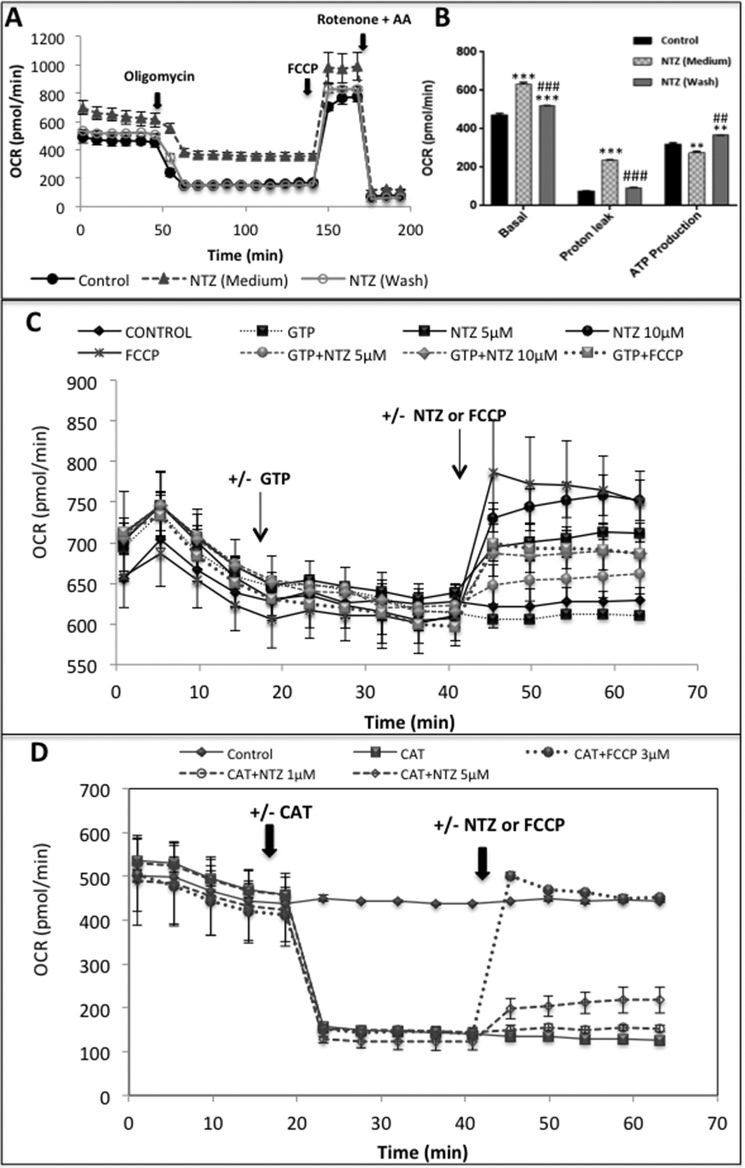
The observed effects of NTZ were found to be reversible as cells treated with NTZ (1 μm in assay medium) for 1 h exhibited a significant increase in basal respiration and proton leak with a concomitant decrease in ATP production (Fig. 4, A and B). However, replacement of NTZ-containing medium with fresh assay medium restored the basal respiration, proton leak, and ATP levels significantly (Fig. 4, A and B). The observed uncoupling effects of NTZ may not be operative through uncoupling proteins (UCPs) or adenine nucleotide translocase as NTZ could induce OCR in the presence of GTP (UCP inhibitor) and carboxyatractyloside, a specific inhibitor of adenine nucleotide transferase (Fig. 4, C and D).
Figure 4.
NTZ-induced uncoupling effects are reversible and do not involve UCPs or adenine nucleotide translocase. A, SK-N-SH cells were treated with NTZ (1 μm) for 1 h in a 24-well Seahorse assay plate. In few wells the NTZ containing medium was replaced with fresh assay medium after 1 h incubation and Mito Stress assay was performed as described in “Experimental Procedures.” B, bar diagrams representing basal respiration, proton leak, and ATP production from the data obtained from (A). C, cells treated with the UCP inhibitor GTP (4 mm) for 20 min, and NTZ (5 μm) or FCCP (3 μm) was added and OCR was recorded further for 25 min. Control cells were treated with equal volumes of buffer under similar conditions. D, cells were treated with the adenine nucleotide translocase inhibitor CAT (3 μg/ml) for 20 min, and later NTZ (1 and 5 μm) or FCCP (3 μm) was added and OCR was recorded further for 25 min. Control cells were treated with equal volumes of buffer under similar conditions. A, C, and D, data are the representative of mean ± S.E. of triplicate measurements per each condition (n = 3) from two independent experiments. B, values indicated are the mean ± S.E. of triplicate measurements per each condition from two independent experiments (n = 6). ***, p < 0.001; **, p < 0.01 as compared with controls. ###, p < 0.001; ##, p < 0.01 as compared with NTZ (medium) group as analyzed by two-way ANOVA with Tukey's multiple comparisons test.
NTZ restores respiration in partially damaged mitochondria by MPP+
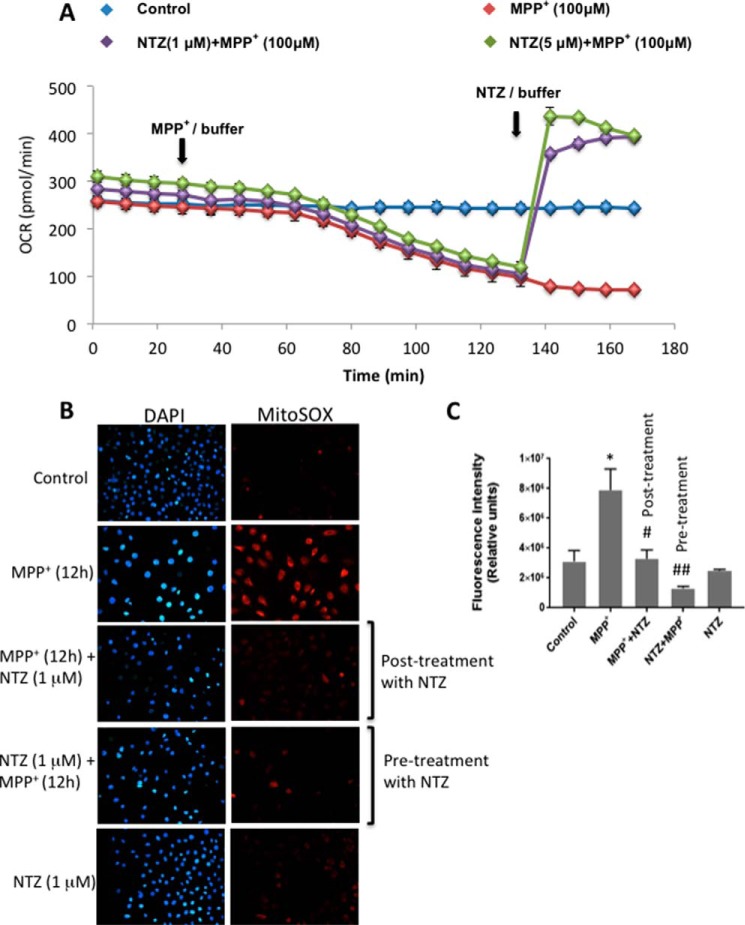
As the above results suggest that NTZ mitigates MPP+-induced block in mitochondrial respiration, we next examined whether NTZ has the potential to restore respiration in mitochondria that were preincubated with MPP+ in which the respiration is completely ceased. To examine this, human neuroblastoma cells were incubated with MPP+ (100 μm) for 2 h because at this time point there was no or negligible OCR as compared with control cells (Fig. 5A). Interestingly, addition of NTZ (1 and 5 μm) to MPP+-treated cells immediately reversed the block in mitochondrial respiration at both the concentrations examined, and the values were found to be substantially higher than control cells (Fig. 5A).
Figure 5.
Reversal of MPP+-induced block in mitochondrial respiration by NTZ. A, following 20 min of recording basal OCR in SK-N-SH cells, MPP+ was added through port A and OCR was recorded further for 2 h. Later, NTZ (1 and 5 μm) was added through port B and OCR was measured. Buffer was added in place of MPP+ in controls, whereas, buffer was added in place of NTZ for MPP+-treated samples. Values indicated are the mean ± S.E. of triplicate measurements (n = 3) and the experiment was repeated two times. B, SK-N-SH cells were either pretreated with NTZ (1 μm) for 1 h before incubation with MPP+ (1 mm) for 12 h or posttreated for 1 h following 12 h incubation with MPP+ (1 mm). At the end of incubation MitoSOX staining was performed as described in “Experimental Procedures” and fluorescence images were captured from five different fields of view. C, the average intensity units from five different fields of view from two independent experiments (n = 8–10) were represented as mean ± S.D. *, p < 0.05 as compared with controls. ##, p < 0.01; #, p < 0.05 as compared with MPP+ group by two-way ANOVA with Sidak's multiple comparisons test.
Similarly, dopaminergic cells treated with MPP+ (1 mm) for 12 h exhibited a 2.5-fold increase in mitochondrial ROS as measured by MitoSOX staining (Fig. 5, B and C). Either pretreatment of cells for 1 h with NTZ followed by 12 h incubation with MPP+ or posttreatment of NTZ for 1 h to cells that were preincubated with MPP+ for 12 h significantly reduced MitoSOX staining to nearly the control values (Fig. 5, B and C).
NTZ ameliorates MPP+-induced loss of mitochondrial membrane potential, ROS, and apoptosis
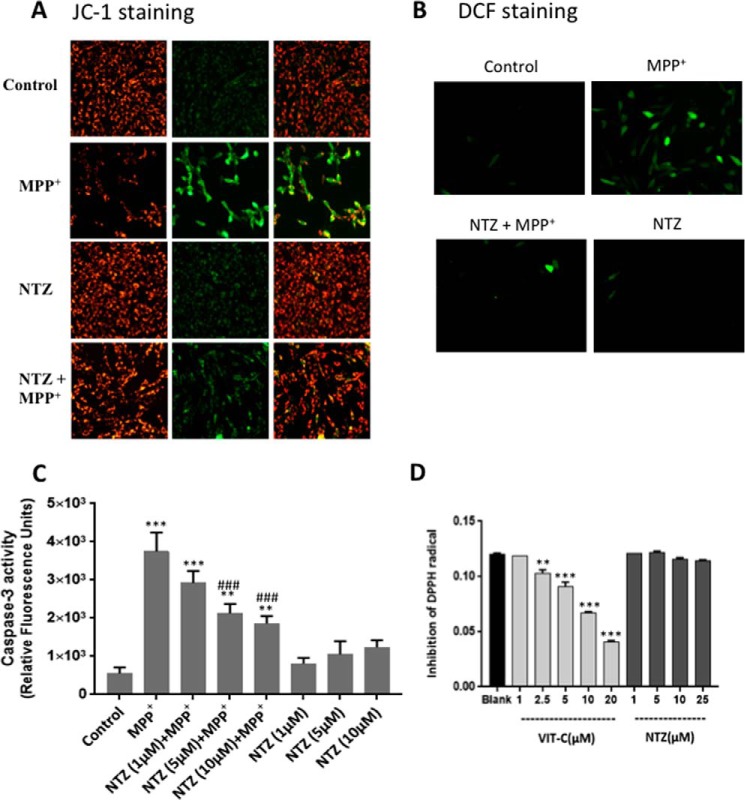
Addition of MPP+ (500 μm) to N27 cells (rat midbrain-derived dopaminergic cell line) for 24 h resulted in a substantial increase in depolarized mitochondria as indicated by an increase in the net green fluorescence (JC-1 staining) as well as cellular ROS (DCF staining) (Fig. 6, A and B). However, pretreatment of cells with NTZ (1 μm) prevented MPP+-induced loss in mitochondrial membrane potential as well as oxidative stress (Fig. 6, A and B). Also, treatment of N27 cells with MPP+ (0.5 mm) for 24 h resulted in a 6.8-fold increase in apoptosis as indicated by caspase-3 activity (Fig. 6C). However, pretreatment of cells with 1, 5, or 10 μm NTZ prior to the addition of MPP+ (500 μm) resulted in a significant decrease in the caspase-3 activation from 6.8-fold to 5.3-, 3.8-, and 3.3-fold, respectively. Under similar experimental conditions, NTZ per se at these concentrations did not induce any significant apoptosis in cells (Fig. 6C).
Figure 6.
NTZ suppressed MPP+-induced loss of mitochondrial membrane potential, ROS generation, and cellular apoptosis. Rat mesencephalic N27 cells were treated with NTZ (1 μm) for 1 h before exposure to MPP+ (500 μm) for 24 h. A, mitochondrial membrane potential was analyzed by JC-1 staining. B, ROS production was determined by H2DCF-DA as described in “Experimental Procedures.” C, caspase-3 activity in cells treated with MPP+ in the presence and absence of NTZ (1, 5, and 10 μm). D, DPPH radical scavenging activity of NTZ and vitamin C was employed as a positive control. A and B, data are the representative of three independent experiments. C and D, values are the mean ± S.E. of triplicate measurements from two independent experiments (n = 6). ***, p < 0.001; **, p < 0.01 as compared with controls. ###, p < 0.001 as compared with MPP+ group. For (C) two-way ANOVA with Sidak's test and for (D) one-way ANOVA with Dunnett's test was performed.
To know whether the observed effects of NTZ could be because of any of its antioxidant activity, in vitro DPPH radical scavenging activity of NTZ (1–25 μm) was performed and vitamin C was used as a positive control. As shown in Fig. 6D, NTZ did not exhibit any antioxidant activity (DPPH radical scavenging activity) up to 25 μm concentration. However, under similar conditions, vitamin C dose-dependently inhibited DPPH radical production (Fig. 6D).
NTZ prevents MPTP-induced loss of TH-positive neurons in vivo
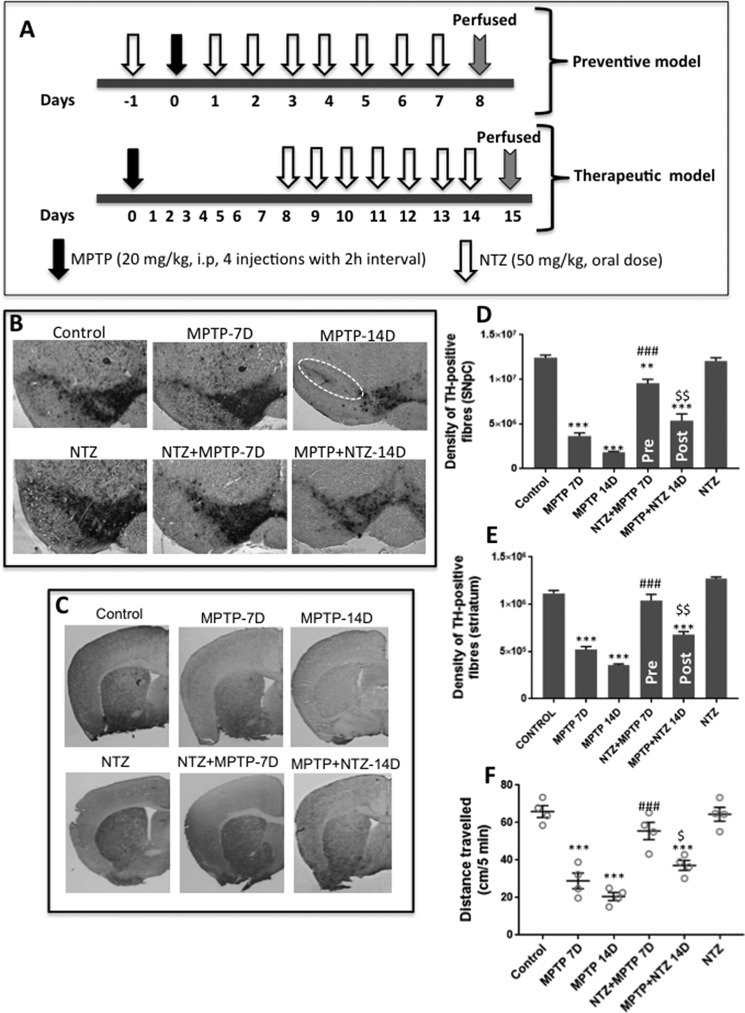
To evaluate the in vivo effects in a preventive model, NTZ (50 mg/kg body weight) was administered orally for 7 days following treatment with MPTP (20 mg/kg, four injections at 2-h intervals) in an acute mouse model of PD (Fig. 7, A–C). For therapeutic model, NTZ (50 mg/kg body weight) was administered orally starting from 8th day post MPTP treatment (20 mg/kg, four injections at 2-h intervals) and continued the same until 14th day. Results demonstrate that although there was an intense staining of TH-positive neurons in control animals, staining of the same was significantly reduced on days 7 and 14 following MPTP treatment (Fig. 7B). However, a significant recovery in TH staining was evident in NTZ + MPTP–treated group as compared with MPTP treatment alone. Under similar experimental conditions, administration of NTZ alone did not induce any significant loss in TH staining (Fig. 7, B–F). In the therapeutic model, administration of NTZ post MPTP treatment could not significantly restore the TH staining of SNPc or striatum to that of control values or 7th day post MPTP treatment. However, as compared with day 14 MPTP treatment, the TH staining in the SNPc and striatum was significantly restored in MPTP + NTZ group. The above data clearly suggest that NTZ could not reverse the loss of TH-positive neurons but has the ability in delaying the death of existing dopamine neurons of SNPc (Fig. 7, B–E). The locomotor activity of the animals as measured by open field test reflected the above findings (Fig. 7F). MPTP-treated animals exhibited a time-dependent loss in the locomotor activity as compared with controls. Pretreatment with NTZ (NTZ+MPTP 7D group) significantly alleviated MPTP-induced (MPTP 7D group) loss in motor activity. However, in the therapeutic model, administration of NTZ 7 days post MPTP treatment for 7 consecutive days significantly restored motor activity of mice as compared with 14 days post MPTP treatment (MPTP 14D group), but the values were not significant as compared with 7 days post MPTP treatment (MPTP 7D group). Mice treated with NTZ alone exhibited motor activity similar to that of control group (Fig. 7F).
Figure 7.
NTZ mitigates MPTP-induced loss of TH neurons and locomotor activity in vivo. Effects of NTZ on MPTP-induced loss of dopamine neurons as measured by TH-positive staining in the SNPc and striatum of mouse brain in an acute mouse model of PD. A, schematic representation of in vivo experiment depicting the therapeutic and preventive models employed. B and C, TH-positive staining and TH-positive fiber density in the SNPc and striatum of mice. D and E, densitometric analysis of TH-positive staining from SNPc and striatum from three different mice (n = 3). F, locomotor activity of animals as measured by open field test at the end of experiment (n = 4). D and E, data are the mean ± S.E. (n = 3). F, values indicated are the mean ± S.E. from four animals (n = 4). ***, p < 0.001; **, p < 0.01 as compared with controls. ###, p < 0.001 as compared with MPTP 7D group. $$, p < 0.01; $, p < 0.05 as compared with MPTP 14D group as analyzed by two-way ANOVA with Tukey's multiple comparisons test.
Discussion
Mitochondrial dysfunction was found to be associated with several neurodegenerative diseases, and defects in various mitochondrial respiratory chain complexes have been detected in Alzheimer's, Parkinson's, and Huntington's diseases (18, 19). Several lines of evidence implicate PD as a free radical disease involving mitochondrial dysfunction leading to the production of free radicals and energy failure (10, 20, 21). Increased oxidative damage and aggregation of the protein α-synuclein (α-syn) were found to be characteristic hallmarks of PD (22).
In fact, various attempts have been made to understand the pathways leading to the aggregation of α-syn, and inhibitors of α-syn aggregation, active and passive immunization against α-syn, are under focus (23). As oxidative stress resulting from mitochondrial damage happens to play a pivotal role in the pathophysiology of PD, numerous attempts have been made over the decades to find a cure employing antioxidants and iron chelators, AAV2-neurturin, creatine, and pioglitazone, but they were found to have no significant effect on disease progression (24–27). In fact, we reported earlier that the mitochondria-targeted antioxidant MitoQ, as well as some of the lipophilic iron chelators, confer significant protection in cellular or animal models of PD (13, 14). However, several of these observations could not reach clinics because they are either toxic (26) or by the time the disease is evident, it was found to be too late to rescue the remaining dopamine neurons (28, 29). Currently, isradipine, caffeine, nicotine, inosine, glial cell-line derived neurotrophic factor, and α-syn immunotherapy are being focused on or under clinical trials for the treatment of PD (27).
Recently, more attention has been paid to understanding the role of mitochondrial uncoupling proteins (UCPs) as their activity was found to have a profound influence on mitochondrial biogenesis, calcium flux, free radical production, and neuronal function. In fact, UCP-4A was found to play a protective role in PD models (30–32). Based on the above observations, attempts have been made to examine the protective role of pharmacological small molecule uncouplers in neurodegenerative disease processes. Earlier reports indicate that uncouplers, such as FCCP, 2,4-dinitrophenol (DNP), were found to be toxic and this was attributed to their plausible off-target effects and narrow therapeutic index (33, 34). However, the liver-targeted form of DNP viz. DNP methyl ether was found to protect against fatty liver and hyperglycemia because of its wide therapeutic index (35). Also, low concentrations of DNP were found to protect from cerebral ischemia as well as amyloid-β peptide–induced toxicity by scavenging mitochondrial free radicals (36, 37). Because of the increasing importance of uncouplers in therapeutic research, new molecules are being synthesized that are less toxic (34, 35).
In line with the above observations, repurposing of FDA-approved drugs appears to be a safer option for PD patients in terms of both safety as well as cost effectiveness, provided they can confer significant protection. Recently, the respiration-enhancing properties of FDA-approved drugs have been assessed and nitazoxanide was among them (15). It was interesting to find that NTZ happens to be one of the safest and most bioavailable drugs that can reach the CNS. Oral doses of 4 g in healthy adults did not induce any significant adverse effects, and in animals the LD50 was found to be more than 10 g/kg (oral) (16, 17). Although NTZ was designed to inhibit pyruvate-ferredoxin oxidoreductase (PFOR) under anaerobic conditions, it was also found to enhance mitochondrial respiration in mammalian cells in recent studies (15, 38). Prompted by these initial observations, we have examined the effect of NTZ and some of its core functional groups, such as ANT and OAS, on cellular OCR and noticed that NTZ dose-dependently enhanced OCR as measured by Seahorse flux analyzer in real time, but not the individual scaffolds (Fig. 1). NTZ-induced OCR is not specific to individual respiratory complexes, but appears to exhibit this effect on the overall mitochondrial respiration (Fig. 2). Also, NTZ-induced OCR was associated with an increase in the proton leak with a concomitant decrease in ATP production and these effects were found to be reversible (Figs. 3D and 4, A and B). The observed uncoupling effects of NTZ do not appear to be mediated through either UCPs or adenine nucleotide translocase as treatment of cells with GTP or carboxyatractyloside (CAT) could not prevent NTZ-induced OCR (Fig. 4, C and D). Treatment of cells with the parkinsonian mimetic MPP+ resulted in a gradual decrease in the mitochondrial respiration and by the end of 2 h there was hardly any noticeable OCR in MPP+-treated cells. However, the mitochondria were in a highly coupled state at this stage as addition of FCCP induced a rapid increase in OCR in MPP+-treated cells. It has been well established that higher levels of ROS accumulate in mitochondria following perturbation of electron transport chain in a tightly coupled mitochondria (33). However, by allowing protons into the mitochondrial matrix uncouplers play a pivotal role in neutralizing mitochondrial ROS and essentially we found the same in cells treated with NTZ and MPP+. NTZ per se increased OCR because of its mild uncoupling effect with a negligible reduction in ATP levels at 1 μm concentration; however, it could significantly relieve the block in OCR and increase ATP production in MPP+-treated cells (Fig. 3). Also, treatment of cells with NTZ significantly reversed MPP+-induced loss in cellular ATP levels by 24 h (Fig. 3D). Nevertheless, no significant cell death was observed even at the highest concentrations of NTZ employed for up to 24 h (data not shown). NTZ instantaneously enhanced the mitochondrial OCR in cells that were pretreated with MPP+ for 2 h, wherein, there was a complete block in respiration implying that NTZ could restore respiration in partially damaged mitochondria (Fig. 5A). In fact, the mitochondrial ROS induced by MPP+ over a period of time could be nullified soon after the addition of NTZ (Fig. 5, B and C), indicating that it has good therapeutic potential. As symptoms of PD are apparent following a significant loss of dopamine neurons of SNPc, compounds that can energize the existing cellular mitochondria and quench ROS might hold greater therapeutic potential in terms of delaying the disease progression. Also, NTZ prevented the loss of mitochondrial membrane potential, accumulation of ROS, as well as apoptosis induced by MPP+ over a 24-h time period (Fig. 6, A–C) possibly because of its mild uncoupling effect as it did not possess any antioxidant activity per se (Fig. 6D). Oral administration of NTZ conferred significant protection in an acute MPTP mouse model of PD against the loss of TH-positive neurons of SNPc and locomotor activity in both preventive and therapeutic models (Fig. 7, A–F).
A survey of the literature at this juncture indicates that uncouplers of mitochondrial respiration not only mitigate mitochondrial free radicals but, during this process, also activate cellular autophagy (39, 40). These properties appear to be the most sought after ones for any anti-parkinsonism drug as they might clear the buildup of abnormal protein aggregates and improve the cellular respiration. In line with the above observations, it has been reported recently that NTZ enhances autophagy (41), and we also observed similar results (data not shown). Although a recent study indicated that preformed α-synuclein aggregates could not be cleared merely by inducing autophagy, it was surmised that enhanced autophagic flux could prevent accumulation of protein aggregates in cells (42). Hence, the mild uncoupling effects of NTZ in conjunction with its autophagy-enhancing property might confer potential therapeutic benefit to counter PD.
Overall, the present study demonstrates the ability of NTZ in mitigating MPP+-induced biochemical abnormalities under both in vitro and in vivo conditions by preventing the buildup of toxic mitochondrial ROS. NTZ also improved mitochondrial OCR and nullified ROS in cells that were preexposed to MPP+, indicating that it may have disease-delaying effects. Because NTZ is an FDA-approved drug with a very good safety profile and has the ability to reach the CNS, it appears to be a promising candidate for the treatment of PD. However, the chronic effects of NTZ, if any, needs to be carefully examined to take the present findings further.
Experimental procedures
Materials
Nitazoxanide; MPP+ iodide; MPTP hydrochloride; oligomycin; FCCP; rotenone; antimycin A (AA); pyruvate; malate; succinate; ascorbate; TMPD; ATP; and the dyes, JC-1, carboxyatractyloside (CAT), GTP, and carboxyl H2-DCF-DA, were purchased from Sigma. 2-Amino-5-nitrothiazole (ANT) was from HiMedia (India). O-acetyl-salicylamide was a generous gift from Dr. Chinaraju Bhimapaka from CSIR–Indian Institute of Chemical Technology (India). Luciferase-based assay kit for ATP measurement was from Sigma.
Cell culture
The human SK-N-SH and rat N27 dopaminergic cells were grown in Dulbecco's modified Eagle's medium and RPMI 1640 (containing 10% fetal bovine serum, 2 mmol/liter l-glutamine, 100 units/ml penicillin, and 100 units/ml streptomycin), respectively, at 37 °C in a humidified atmosphere of 5% CO2 and 95% air. For cell culture experiments, after attaining 70–80% confluence and before 12 h of treatments, the cell culture media were replaced with 2% serum-containing media. Unless otherwise mentioned, cells were pretreated with NTZ 60 min before exposure to MPP+ for indicated time intervals.
Mito Stress assay
Mitochondrial bioenergetics employing whole cells was measured in a Seahorse XF24 extracellular flux analyzer. SK-N-SH cells (30,000/well) were plated in 24-well Seahorse assay plate a day before the experiment. Before taking the readings, media were replaced with assay medium and cells in 24-well assay plate were incubated in a non-CO2 incubator for 1 h as per the manufacturer's protocol (Seahorse Bioscience). The four ports of the plate were filled with either NTZ (1–5 μm, final concentration) or MPP+ (100 μm, final concentration), oligomycin (1 μm, final concentration), FCCP (1 μm, final concentration), rotenone + antimycin A (2 μm each, final concentration), respectively. Oxygen consumption rate (OCR) was recorded after the addition of contents from each port for a period of 20 min to 2 h as per the design of experiment. At the end of experiment, cells were lysed using 0.1 NaOH and the protein content was measured by Bradford assay (Sigma). The recordings were normalized to the total cellular protein content in each well.
In other experiments, basal OCR was monitored for 20 min and then NTZ or its individual chemical moieties, such as O-acetyl salicylamide (OAS) and 2-amino-5-nitrothiazole (ANT), were added directly through port A, whereas control cells were treated with equal volumes of buffer and OCR was monitored further for 2 h.
To examine the ability of NTZ in reversing MPP+-induced block in mitochondrial respiration, cells in 24-well Seahorse plate were treated directly with MPP+ (100 μm final concentration) through port A, whereas control cells received equal volumes of buffer in place of MPP+. Following 2 h of treatment with continuous monitoring of OCR, NTZ (1 and 5 μm final concentration) was added to MPP+ treated cells. In parallel, control and MPP+ treatments alone were added with equal volumes of buffer and OCR was monitored further for 50 min.
To examine the reversible nature of NTZ-mediated effects, cells were pretreated with NTZ (1 μm) for 1 h. Before placing the plate into the flux analyzer, medium containing NTZ was replaced with fresh assay medium in some wells and the plate was incubated in a non-CO2 incubator for additional 1 h and Mito Stress assay was performed as described above.
ANT and UCP inhibition assay
SK-N-SH cells (30,000/well) were plated and grown in 24-well Seahorse analyzer plate for 24 h. Just before the initiation of assay, cells were washed twice with 1× Mitochondrial Assay Solution (MAS) buffer and permeabilized with 1.0 nm XF PMP reagent (Seahorse Bioscience) and simultaneously cells were supplemented with pyruvate (10 mm), malate (1 mm), and ADP (4 mm). OCR was recorded for 20 min before the addition of GTP (4 mm) or CAT (3 μg/ml) through port A, whereas control cells were treated with equal volumes of buffer through port A. OCR was recorded further for 20 min and cells were treated with either NTZ or FCCP through port B (control cells were treated with equal volumes of buffer) and the changes in OCR were recorded for an additional 25 min before terminating the assay.
Electron flow assay
SK-N-SH cells (30,000/well) were plated in 24-well Seahorse assay plate. Before initiating the experiment, the complete media was replaced with Mitochondrial Assay Solution (1×: 70 mm sucrose, 220 mm mannitol, 10 mm KH2PO4, 5 mm MgCl2, 2 mm HEPES, 1.0 mm EGTA, and 0.2% (w/v) fatty acid–free BSA, pH 7.4 at 37 °C) and XF PMP reagent as recommended by the manufacturer (Seahorse Bioscience). Briefly, pyruvate (10 mm) and malate (1 mm) were used as substrates along with ADP (4 mm). Final concentrations of the compounds that were injected through respective ports into each well of 24-well plate are as follows: (a) rotenone (2 μm), (b) succinate (10 mm), (c) antimycin A (2 μm), (d) ascorbate (10 mm) + TMPD (0.5 mm). Initially, pyruvate- and malate-induced OCR was measured for 10 min, rotenone was added through port A and the drop in OCR was recorded for 10 min. Later, succinate was added through port B and OCR was recorded for 10 min for succinate-induced OCR. Similarly, antimycin A was added through port C and the drop in OCR was recorded for 10 min. Lastly, ascorbate and TMPD were added through port D to measure the complex-IV (cytochrome C oxidase)–induced OCR.
DPPH-antioxidant assay
The antioxidant activity of NTZ was analyzed using DPPH (2, 2-diphenyl-1-picrylhydrazyl) assay as described earlier (43). Briefly, different concentrations of compounds in 2 μl were added to the reaction mixture containing methanolic solution of DPPH (100 μm, 98 μl) in 96-well plate. The plate was incubated in dark for 30 min and absorbance was measured at 520 nm using a microplate reader (EnSpire, PerkinElmer).
JC-1 staining
The membrane-permeable dye JC-1 was used to assess the changes in mitochondrial membrane potential in N27 cells as described earlier (14). Briefly, following the termination of incubation, cells were washed twice with Dulbecco's phosphate-buffered saline (DPBS) and treated with JC-1 at a final concentration of 5 μg/ml in fresh serum-free culture medium and then incubated at 37 °C for 30 min. Later, cells were gently washed with DPBS and fixed with 4% paraformaldehyde and the fluorescence images were captured using Olympus fluorescence microscope under FITC and rhodamine filter settings.
Caspase-3 activity assay
The increase in fluorescence because of the release of 7-amino-4-trifluoromethylcoumarin (AFC) from the tetra peptide substrate, acetyl-DEVD-AFC was considered as a measure of caspase-3 activity and was performed as described previously using N27 cells (13). Increase in fluorescence because of the release of AFC (excitation 400 nm and emission 505 nm) was monitored every 5 min for up to 1 h at 37 °C in a microplate reader (EnSpire, PerkinElmer). The obtained fluorescence values were normalized to the total protein content.
ATP measurement by luminescence
Cellular ATP levels were measured using ATP bioluminescent somatic cell assay kit according to the manufacturer's protocol (Sigma). Briefly, following the termination of incubation, cells in 6-well plates were collected by trypsinization and 50 μl of cell suspension was added to 150 μl of 1.5× ATP releasing reagent (Sigma). The samples were mixed thoroughly and the emitted luminescence was measured immediately in a microplate reader (EnSpire, PerkinElmer).
ROS detection
Intracellular ROS generation was monitored using the fluorogenic probe 6-carboxy-2′,7′-dichlorofluorescein diacetate (carboxy H2DCF-DA) (Sigma) as described previously (13). The mitochondrial superoxide was measured using MitoSOX dye as per the manufacturer's protocol (Invitrogen). Briefly, following the termination of incubation, medium was aspirated and N27 cells were washed twice with DPBS. Fresh serum-free culture medium containing 10 μm carboxy H2DCF-DA or MitoSOX was added and the cells were incubated further for 30 min at 37 °C in a humidified cell culture incubator. Later, the medium was aspirated, cells were washed three times with ice cold DPBS and fixed using 4% paraformaldehyde. The presence of ROS in cells was measured by the increase in the intensity of DCF fluorescence and MitoSOX red fluorescence under FITC and rhodamine filter settings, respectively, using an Olympus fluorescence microscope from at least five different fields of view.
In vivo experiments
Male C57BL-6J mice (6–8 weeks), weighing 22–25 g were divided into six groups of six animals in each group: (a) control, (b) MPTP (7 days), (c) MPTP (14 days), (d) NTZ, (e) NTZ + MPTP (pretreatment with NTZ, preventive model), and (f) MPTP + NTZ (posttreatment with NTZ, therapeutic model). Acute dosage of MPTP was given to mice (20 mg/kg, four injections at 2-h intervals) intraperitoneally as described earlier (44). For preventive model, a day before MPTP injection, NTZ was administered orally (50 mg/kg) and continued daily for 7 days post MPTP treatment. To study the therapeutic effects, NTZ (50 mg/kg) was administered on the 8th day following MPTP treatment and the dosage was continued up to the 14th day. Following the termination of experiment, animals in each group were perfused (whole body perfusion) using 4% paraformaldehyde in phosphate buffered saline. The fixed and frozen midbrain region was sectioned (25 μm) using a cryotome (Leica). The viability of dopamine neurons of SNPc and the TH fiber density in striatum was analyzed by immunohistochemistry using anti-tyrosine hydroxylase antibody (Sigma) followed by alkaline phosphatase–labeled secondary antibody (Sigma) for SNPc and biotinylated HRP-labeled secondary antibody followed by staining with NovaRED substrate for striatum (Vector Laboratories). TH-positive neurons were visualized using an inverted microscope (Olympus) and density of TH staining was quantified employing ImageJ software. All the experimental protocols used were approved by Institutional Animal Ethical Committee (IAEC) and were in accordance with the NIH guidelines for the care and use of laboratory animals.
To examine the effects of NTZ on locomotor activity of mice, an open field test (OFT) was performed after the end of the experiment. Briefly, the mouse was placed at the center of the arena and allowed to explore the apparatus freely for 5 min with the experimenter out of the animal's sight. A white square arena (50 × 50 × 45 cm) was used for the test. The total distance traveled by each animal was analyzed using video-tracking software (SMART, Panlab, Barcelona, Spain).
Statistical analysis
Unless otherwise stated, all the experiments were performed at least two times and each condition was analyzed in triplicates (n = 6). Results were expressed as mean ± S.D. or mean ± S.E. as indicated. Statistical significance was determined by either one-way (single variable) or two-way (for multiple variables) analysis of variance (ANOVA) using GraphPad Prism software version 7.03.
Author contributions
N. A. performed the majority of the in vitro experiments. S. N. P. performed in vivo experimentation. R. L. V. performed in vitro experiments. H. G. R. performed in vivo experiments. S. T. performed in vivo experiments. S. V. K. conceptualized the idea, interpreted the results, and wrote the manuscript.
Acknowledgment
We greatly acknowledge the help of Dr. Sumana Chenna in statistical analysis.
This work was supported by CSIR, India XII FYP projects MiND (BSC-0115) and SMiLE (CSC-0111) and by the Department of Biotechnology, India Research project. This work was also supported by research fellowships from the University Grant Commission, Government of India (to S. T.) and from CSIR, India (to S. N. P. and R. L. V.). The authors declare that they have no conflicts of interest with the contents of this article.
- PD
- Parkinson's disease
- NTZ
- nitazoxanide
- ROS
- reactive oxygen species
- H2DCF-DA
- dichlorofluorescein diacetate
- OCR
- oxygen consumption rate
- DNP
- 2,4-dinitrophenol
- MPP+
- 1-methyl-4-phenylpyridinium ion
- MPTP
- 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- ANT
- 2-amino-5-nitrothiazole
- OAS
- O-acetyl salicylamide
- CAT
- carboxyatractyloside
- SNPc
- substantia nigra pars compacta
- TH
- tyrosine hydroxylase
- UCP
- uncoupling protein
- DPPH
- 2,2-diphenyl-1-picrylhydrazyl
- TMPD
- N,N,N′,N′-tetramethyl-p-phenylenediamine
- DPBS
- Dulbecco's phosphate-buffered saline.
References
- 1. Ramsay R. R., Salach J. I., Dadgar J., and Singer T. P. (1986) Inhibition of mitochondrial NADH dehydrogenase by pyridine derivatives and its possible relation to experimental and idiopathic parkinsonism. Biochem. Biophys. Res. Commun. 135, 269–275 [DOI] [PubMed] [Google Scholar]
- 2. Trevor A. J., Singer T. P., Ramsay R. R., and Castagnoli N. Jr. (1987) Processing of MPTP by monoamine oxidases: implications for molecular toxicology. J. Neural Transm. Suppl. 23, 73–89 [DOI] [PubMed] [Google Scholar]
- 3. Mizuno Y., Saitoh T., and Sone N. (1987) Inhibition of mitochondrial NADH-ubiquinone oxidoreductase activity by 1-methyl-4-phenylpyridinium ion. Biochem. Biophys. Res. Commun. 143, 294–299 [DOI] [PubMed] [Google Scholar]
- 4. Lutz A. K., Exner N., Fett M. E., Schlehe J. S., Kloos K., Lämmermann K., Brunner B., Kurz-Drexler A., Vogel F., Reichert A.S., Bouman L., Vogt-Weisenhorn D., Wurst W., Tatzelt J., Haass C., and Winklhofer K.F. (2009). Loss of parkin or PINK1 function increases Drp1-dependent mitochondrial fragmentation. J. Biol. Chem. 284, 22938–22951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pickrell A. M., and Youle R. J. (2015) The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson's disease. Neuron 85, 257–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Morais V. A., Haddad D., Craessaerts K., De Bock P. J., Swerts J., Vilain S., Aerts L., Overbergh L., Grünewald A., Seibler P., Klein C., Gevaert K., Verstreken P., and De Strooper B. (2014). PINK1 loss-of-function mutations affect mitochondrial complex I activity via NdufA10 ubiquinone uncoupling. Science 344, 203–207 [DOI] [PubMed] [Google Scholar]
- 7. Ryan B. J., Hoek S., Fon E. A., and Wade-Martins R. (2015) Mitochondrial dysfunction and mitophagy in Parkinson's: from familial to sporadic disease. Trends Biochem. Sci. 40, 200–210 [DOI] [PubMed] [Google Scholar]
- 8. Burté F., Carelli V., Chinnery P. F., and Yu-Wai-Man P. (2015) Disturbed mitochondrial dynamics and neurodegenerative disorders. Nat. Rev. Neurol. 11, 11–24 [DOI] [PubMed] [Google Scholar]
- 9. Gandhi S., and Wood N. W. (2005) Molecular pathogenesis of Parkinson's disease. Hum. Mol. Genet. 14, Spec. No. 2, 2749–2755 [DOI] [PubMed] [Google Scholar]
- 10. Bose A., and Beal M. F. (2016) Mitochondrial dysfunction in Parkinson's disease. J. Neurochem. 139, 216–231 [DOI] [PubMed] [Google Scholar]
- 11. Kieburtz K. (2006) Issues in neuroprotection clinical trials in Parkinson's disease. Neurology 66, S50–S57 [DOI] [PubMed] [Google Scholar]
- 12. Kaur D., Yantiri F., Rajagopalan S., Kumar J., Mo J. Q., Boonplueang R., Viswanath V., Jacobs R., Yang L., Beal M. F., DiMonte D., Volitaskis I., Ellerby L., Cherny R. A., Bush A. I., and Andersen J. K. (2003) Genetic or pharmacological iron chelation prevents MPTP-induced neurotoxicity in vivo: A novel therapy for Parkinson's disease. Neuron 37, 899–909 [DOI] [PubMed] [Google Scholar]
- 13. Kalivendi S. V., Kotamraju S., Cunningham S., Shang T., Hillard C. J., and Kalyanaraman B. (2003) 1-Methyl-4-phenylpyridinium (MPP+)-induced apoptosis and mitochondrial oxidant generation: Role of transferrin-receptor-dependent iron and hydrogen peroxide. Biochem. J. 371, 151–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ghosh A., Chandran K., Kalivendi S. V., Joseph J., Antholine W. E., Hillard C. J., Kanthasamy A., Kanthasamy A., and Kalyanaraman B. (2010) Neuroprotection by a mitochondria-targeted drug in a Parkinson's disease model. Free Radic. Biol. Med. 49, 1674–1684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sahdeo S., Tomilov A., Komachi K., Iwahashi C., Datta S., Hughes O., Hagerman P., and Cortopassi G. (2014) High-throughput screening of FDA-approved drugs using oxygen biosensor plates reveals secondary mitofunctional effects. Mitochondrion 17, 116–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. White C. A. (2004) Nitazoxanide: A new broad spectrum antiparasitic agent. Expert Rev. Anti. Infect. Ther. 2, 43–49 [DOI] [PubMed] [Google Scholar]
- 17. Rossignol J. F. (2014) Nitazoxanide: A first-in-class broad-spectrum antiviral agent. Antiviral Res. 110, 94–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Carvalho C., Correia S. C., Cardoso S., Plácido A. I., Candeias E., Duarte A. I., and Moreira P. I. (2015) The role of mitochondrial disturbances in Alzheimer, Parkinson and Huntington diseases. Expert Rev. Neurother. 15, 867–884 [DOI] [PubMed] [Google Scholar]
- 19. Orth M., and Schapira A. H. (2001) Mitochondria and degenerative disorders. Am. J. Med. Genet. 106, 27–36 [DOI] [PubMed] [Google Scholar]
- 20. Requejo-Aguilar R., and Bolaños J. P. (2016) Mitochondrial control of cell bioenergetics in Parkinson's disease. Free Radic. Biol. Med. 100, 123–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schapira A. H., and Gegg M. (2011) Mitochondrial contribution to Parkinson's disease pathogenesis. Parkinsons Dis. 2011, 159160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Norris E. H., and Giasson B. I. (2005) Role of oxidative damage in protein aggregation associated with Parkinson's disease and related disorders. Antioxid. Redox Signal. 7, 672–684 [DOI] [PubMed] [Google Scholar]
- 23. Török N., Majláth Z., Szalárdy L., and Vécsei L. (2016) Investigational α-synuclein aggregation inhibitors: Hope for Parkinson's disease. Expert Opin. Investig. Drugs. 25, 1281–1294 [DOI] [PubMed] [Google Scholar]
- 24. Gassen M., and Youdim M. B. (1999) Free radical scavengers: Chemical concepts and clinical relevance. J. Neural Transm. Suppl. 56, 193–210 [DOI] [PubMed] [Google Scholar]
- 25. Jellinger K. A. (1999) The role of iron in neurodegeneration: Prospects for pharmacotherapy of Parkinson's disease. Drugs Aging 14, 115–140 [DOI] [PubMed] [Google Scholar]
- 26. Cole G. M. (2003) Ironic fate: Can a banned drug control metal heavies in neurodegenerative diseases? Neuron 37, 889–890 [DOI] [PubMed] [Google Scholar]
- 27. Kalia L. V., Kalia S. K., and Lang A. E. (2015) Disease-modifying strategies for Parkinson's disease. Movement Disorders 30, 1442–1450 [DOI] [PubMed] [Google Scholar]
- 28. Smith R. A., and Murphy M. P. (2010) Animal and human studies with the mitochondria-targeted antioxidant MitoQ. Ann. N.Y. Acad. Sci. 1201, 96–103 [DOI] [PubMed] [Google Scholar]
- 29. Snow B. J., Rolfe F. L., Lockhart M. M., Frampton C. M., O'Sullivan J. D., Fung V., Smith R. A., Murphy M. P., and Taylor K. M. (2010) A double-blind, placebo-controlled study to assess the mitochondria-targeted antioxidant MitoQ as a disease-modifying therapy in Parkinson's disease. Movement Disorders 25, 1670–1674 [DOI] [PubMed] [Google Scholar]
- 30. Andrews Z. B., Diano S., and Horvath T. L. (2005) Mitochondrial uncoupling proteins in the CNS: In support of function and survival. Nat. Rev. Neurosci. 6, 829–840 [DOI] [PubMed] [Google Scholar]
- 31. Lindholm D., Eriksson O., and Korhonen L. (2004) Mitochondrial proteins in neuronal degeneration. Biochem. Biophys. Res. Commun. 321, 753–758 [DOI] [PubMed] [Google Scholar]
- 32. Wu K., Liu J., Zhuang N., and Wang T. (2014) UCP4A protects against mitochondrial dysfunction and degeneration in pink1/parkin models of Parkinson's disease. FASEB J. 28, 5111–5121 [DOI] [PubMed] [Google Scholar]
- 33. Kenwood B. M., Weaver J. L., Bajwa A., Poon I. K., Byrne F. L., Murrow B. A., Calderone J. A., Huang L., Divakaruni A. S., Tomsig J. L., Okabe K., Lo R. H., Cameron Coleman G., Columbus L., Yan Z., et al. (2013) Identification of a novel mitochondrial uncoupler that does not depolarize the plasma membrane. Mol. Metab. 3, 114–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lou P. H., Hansen B. S., Olsen P. H., Tullin S., Murphy M. P., and Brand M. D. (2007) Mitochondrial uncouplers with an extraordinary dynamic range. Biochem. J. 407, 129–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Perry R. J., Kim T., Zhang X. M., Lee H. Y., Pesta D., Popov V. B., Zhang D., Rahimi Y., Jurczak M. J., Cline G. W., Spiegel D. A., and Shulman G. I. (2013) Reversal of hypertriglyceridemia, fatty liver disease, and insulin resistance by a liver-targeted mitochondrial uncoupler. Cell Metab. 18, 740–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Korde A. S., Pettigrew L. C., Craddock S. D., and Maragos W. F. (2005) The mitochondrial uncoupler 2,4-dinitrophenol attenuates tissue damage and improves mitochondrial homeostasis following transient focal cerebral ischemia. J. Neurochem. 94, 1676–1684 [DOI] [PubMed] [Google Scholar]
- 37. De Felice F. G., and Ferreira S. T. (2006) Novel neuroprotective, neuritogenic and anti-amyloidogenic properties of 2,4-dinitrophenol: The gentle face of Janus. IUBMB Life 58, 185–191 [DOI] [PubMed] [Google Scholar]
- 38. Senkowski W., Zhang X., Olofsson M. H., Isacson R., Höglund U., Gustafsson M., Nygren P., Linder S., Larsson R., and Fryknäs M. (2015) Three-dimensional cell culture-based screening identifies the anthelmintic drug nitazoxanide as a candidate for treatment of colorectal cancer. Mol. Cancer Ther. 14, 1504–1516 [DOI] [PubMed] [Google Scholar]
- 39. Di Santo N., and Ehrisman J. (2014) A functional perspective of nitazoxanide as a potential anticancer drug. Mutat. Res. 768, 16–21 [DOI] [PubMed] [Google Scholar]
- 40. Liu D., Zhang Y., Gharavi R., Park H. R., Lee J., Siddiqui S., Telljohann R., Nassar M. R., Cutler R. G., Becker K. G., and Mattson M. P. (2015) The mitochondrial uncoupler DNP triggers brain cell mTOR signaling network reprogramming and CREB pathway upregulation. J. Neurochem. 134, 677–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lam K. K., Zheng X., Forestieri R., Balgi A. D., Nodwell M., Vollett S., Anderson H.J., Andersen R.J., Av-Gay Y., and Roberge M. (2012) Nitazoxanide stimulates autophagy and inhibits mTORC1 signaling and intracellular proliferation of Mycobacterium tuberculosis. PLoS Pathog. 8, e1002691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tanik S. A., Schultheiss C. E., Volpicelli-Daley L. A., Brunden K. R., and Lee V. M. (2013) Lewy body-like α-synuclein aggregates resist degradation and impair macroautophagy. J. Biol. Chem. 288, 15194–15210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sharma O. P., and Bhat T. K. (2009) DPPH antioxidant assay revisited. Food Chemistry 113, 1202–1205 [Google Scholar]
- 44. Kalivendi S. V., Yedlapudi D., Hillard C. J., and Kalyanaraman B. (2010) Oxidants induce alternative splicing of α-synuclein: Implications for Parkinson's disease. Free Radic. Biol. Med. 48, 377–383 [DOI] [PMC free article] [PubMed] [Google Scholar]