Abstract
The transmembrane signaling protein Notch, which is crucial for embryonic cell fate decisions, has 36 extracellular EGF domains that are glycosylated in variable and complex ways. A new study shows that O-fucose and O-glucose stabilize the repeats but that extension of glucose by xylose weakens stability, explained by the binding of the glycan to a protein groove. This work shows how different types of glycosylation can distinctly influence protein stability and structure.
Introduction
The emergence of metazoa, in which vital functions are allocated to specialized cells and organs, required the development of cellular communication systems capable of defining cellular individuality. Remarkably, the enormous morphological complexity that we see in the animal kingdom is believed to be the result of evolutionary adaptations in <20 signal transduction pathways, and only seven of these control cell-cell interactions during embryonic development (1). The pathways relevant in development commonly exert their functions through the regulation of transcription of target genes. Considering the wide diversity and strongly context-dependent signaling patterns controlled by these receptors, it becomes immediately clear that the direction and tuning of cell fate signaling must be controlled at many different levels. Takeuchi et al. (2) provide new insights into how one key signaling component, Notch, is regulated by its glycosylation status.
The Notch signaling pathway is one of these archetypical pathways involved in cell fate decisions. Cell surface-localized Notch binds to the membrane-bound DSL proteins Delta or Jagged/Serrate on other cells. Receptor-ligand engagement generates force and unfolding of a regulatory region, which becomes cleaved in two consecutive steps to separate the transcriptionally active Notch intracellular domain, which traffics to the nucleus to impact transcriptional programs. In complex with the protein ligand, the Notch extracellular domain is endocytosed by the signaling cell (3). Notch regulation occurs on many levels, starting at the level of Notch protein biosynthesis through post-translational modification by a group of endoplasmic reticulum (ER)2-localized glycosyltransferases. These enzymes glycosylate the 36 tandem epidermal growth factor (EGF)-like repeats that form the prominent structural module in the N-terminal ligand-binding portion of Notch. Based on mass spectrometric, enzymatic, and structural data, four types of protein O-linked glycans are known to occur on EGF repeats (Fig. 1A).
Figure 1.
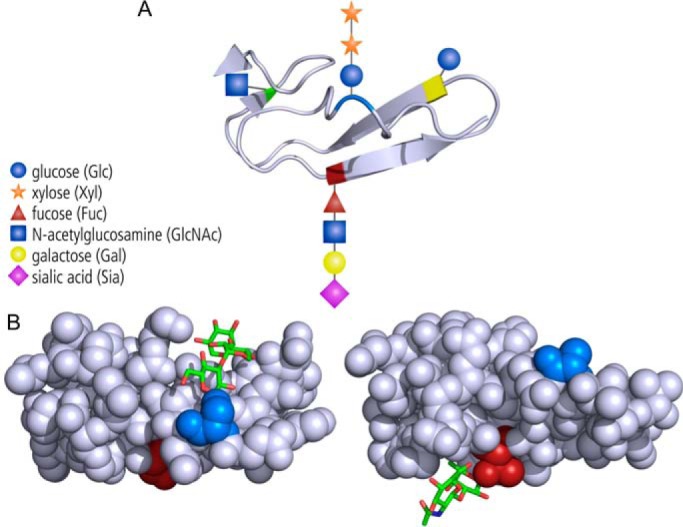
Glycans occupy different sides of EGF repeats. A, the four types of O-glycans that can be found on distinct consensus sequences of EGF repeats, but not all occurring on every repeat. Blue, serine modified by O-glucose that can be extended by two xyloses. Red, serine or threonine with an extended O-fucose-linked glycan. Yellow and green, serine or threonine modified with O-glucose and O-N-acetylglucosamine (O-GlcNAc) residues. B, the serine (blue) linked O-glucose trisaccharide (Xyl-Xyl-Glc) occupies a cleft of the EGF repeat (left, from Protein Data Bank code 5VYG (2)), whereas the O-linked fucose (GlcNAc-Fuc) linked to a threonine (red) on the opposite side of the domain seems to be more exposed (right, Protein Data Bank code 4D0E (10)).
The Haltiwanger group (4) has been a pioneer in defining the presence of O-fucose and O-glucose on Notch proteins. The respective glycosylating enzymes, POFUT1 and POGLUT1, recognize specific consensus sequences C2X3(S/T)C3 and C1XSX(P/A)C2 on EGF repeats. The soluble enzymes differ structurally from other ER-localized glycosyltransferases, which are typically multitopic membrane proteins. Moreover, the EGF repeat-specific enzymes act on folded domains, whereas the classical ER glycosyltransferases act on the nascent protein chains. Based on their previous observation that a modification system exists in the ER with which the folding quality of thrombospondin repeat-containing proteins is monitored (5), Takeuchi and colleagues (2) wanted to study whether the POFUT1/POGLUT1 system exerts a similar noncanonical ER quality control function for EGF repeat-containing proteins.
To pursue this question, the authors first examined whether Notch surface transport depends on POFUT1 and/or POGLUT1 activity. To this end, they used the well-established Notch1-positive cell model HEK297T and deleted the POFUT1 and POGLUT1 genes individually and in combination. Notch1 surface expression was monitored by FACS analysis. Notch expression was reduced by a factor of 2 in single knockout cells and showed further reduction in double knockouts. Importantly, the reduced surface expression was accompanied by intracellular accumulation of full-length Notch1, demonstrating that the absence of modifications catalyzed by POFUT1 and POGLUT1 restrains Notch1 from transport to the Golgi and thus from access to the protease furin, which cleaves and activates it. When the same mutant cell lines were used to evaluate the secretion of a soluble Notch1 fragment composed of the 36 EGF repeats, knocking out either enzyme prevented secretion. Together, these findings suggest that EGF modifications catalyzed by POFUT1 and POGLUT1 promote Notch ER-Golgi transport both independently and additively.
The significance of this connection between proper glycosylation and Notch maturation might be mirrored by a recently described clinical model of hereditary muscular dystrophy. Patients harbor a missense mutation in POGLUT1 that strongly impairs its enzymatic activity. In muscle satellite cells, a pool of cells essential for muscle regeneration and homeostasis, Notch signaling is severely diminished, which causes a drastic reduction in cell numbers, cumulating in a lack of regeneration capacity in these patients (6).
Having determined that POFUT1 and POGLUT1 are required for Notch protein biosynthesis, the authors wanted to understand how these glycosylations impact the EGF repeats. To answer this question, they used an in vitro assay system that monitored denaturation kinetics under the influence of the reducing agent DTT, thus quantifying protein stability. Recombinant glycosyltransferases were used to successively introduce the desired modifications to a recombinant model EGF repeat containing both O-glucose and O-fucose sites. In addition to the two initial modifications, the effect of extension of O-glucose by two xylosyltransferases (Fig. 1A) was investigated. The contributions of different modifications to protein stability were determined individually and in combination. The results clearly showed that the two protein modifications stabilize EGF repeats in an additive manner. Interestingly, the addition of the first xylose to O-glucose canceled out the stabilizing effect of glucose, whereas addition of a second xylose restored stability. The same opposing effects of glucose and xylose were seen in genetic experiments in flies: Whereas transfer of O-glucose increased Notch signaling (7), the positive effect was weakened after formation of the disaccharide Glc-Xyl (8). Repeating the unfolding experiments with an EGF repeat of Notch revealed similar fine-tuning of stability by glycosylation. Considering that this fragment is involved in controlling Notch-Delta interactions, this result provides novel insight into how sugars participate in steering Notch functions. Finally, the authors solved the crystal structure of an EGF repeat decorated with the full O-glucose trisaccharide Xyl-Xyl-Glc. In a way not known for other O-glycan modifications, the trisaccharide was found to interact strongly with the amino acids of the EGF repeat, folding completely back into a surface groove (Fig. 1B). This detailed structural insight establishes a new basis to explain the protein-modulatory functions of glycan modifications: Different glycans are found on different sides of the EGF repeats (Fig. 1, A and B) and modify Notch signaling in different ways. The O-fucose interacts directly with Notch ligands (9), whereas O-glucose on the opposite side of the domain might have a more direct function on stability of the EGF repeat itself.
The 36 EGF repeats of Notch contain, to a variable extent, consensus sites for the four different types of glycosylation (Fig. 1A), which are, depending on the expression of different glycosyltransferases, changeably modified. This results in a mutable system of enormous complexity. We are slowly beginning to understand the effects of different modifications on both the molecular and systemic level, but there is still a long way to go before we will fully comprehend the multifaceted glycosylation of Notch.
This work was supported by Deutsche Forschungsgemeinschaft Grant BA4091/5-1 and Cluster of Excellence REBIRTH (From Regenerative Biology to Reconstructive Therapy, EXC 62/2). The authors declare that they have no conflicts of interest with the contents of this article.
- ER
- endoplasmic reticulum.
References
- 1. Pires-daSilva A., and Sommer R. J. (2003) The evolution of signaling pathways in animal development. Nat. Rev. Genet. 4, 39–49 [DOI] [PubMed] [Google Scholar]
- 2. Takeuchi H., Yu H., Hao H., Takeuchi M., Ito A., Li H., and Haltiwanger R. S. (2017) O-Glycosylation modulates the stability of epidermal growth factor-like repeats and thereby regulates Notch trafficking. J. Biol. Chem. 292, 15964–15973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kovall R. A., Gebelein B., Sprinzak D., and Kopan R. (2017) The canonical Notch signaling pathway: Structural and biochemical insights into shape, sugar, and force. Dev. Cell 41, 228–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moloney D. J., Shair L. H., Lu F. M., Xia J., Locke R., Matta K. L., and Haltiwanger R. S. (2000) Mammalian Notch1 is modified with two unusual forms of O-linked glycosylation found on epidermal growth factor-like modules. J. Biol. Chem. 275, 9604–9611 [DOI] [PubMed] [Google Scholar]
- 5. Vasudevan D., Takeuchi H., Johar S. S., Majerus E., and Haltiwanger R. S. (2015) Peters plus syndrome mutations disrupt a noncanonical ER quality-control mechanism. Curr. Biol. 25, 286–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Servián-Morilla E., Takeuchi H., Lee T. V., Clarimon J., Mavillard F., Area-Gómez E., Rivas E., Nieto-González J. L., Rivero M. C., Cabrera-Serrano M., Gómez-Sánchez L., Martínez-López J. A., Estrada B., Márquez C., Morgado Y., Suárez-Calvet X., Pita G., Bigot A., Gallardo E., Fernández-Chacón R., Hirano M., Haltiwanger R. S., Jafar-Nejad H., and Paradas C. (2016) A POGLUT1 mutation causes a muscular dystrophy with reduced Notch signaling and satellite cell loss. EMBO Mol. Med. 8, 1289–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Acar M., Jafar-Nejad H., Takeuchi H., Rajan A., Ibrani D., Rana N. A., Pan H., Haltiwanger R. S., and Bellen H. J. (2008) Rumi is a CAP10 domain glycosyltransferase that modifies Notch and is required for Notch signaling. Cell 132, 247–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee T. V., Sethi M. K., Leonardi J., Rana N. A., Buettner F. F., Haltiwanger R. S., Bakker H., and Jafar-Nejad H. (2013) Negative regulation of notch signaling by xylose. PLoS. Genet. 9, e1003547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Luca V. C., Jude K. M., Pierce N. W., Nachury M. V., Fischer S., and Garcia K. C. (2015) Structural biology. Structural basis for Notch1 engagement of Delta-like 4. Science 347, 847–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Taylor P., Takeuchi H., Sheppard D., Chillakuri C., Lea S. M., Haltiwanger R. S., and Handford P. A. (2014) Fringe-mediated extension of O-linked fucose in the ligand-binding region of Notch1 increases binding to mammalian Notch ligands. Proc. Natl. Acad. Sci. U.S.A. 111, 7290–7295 [DOI] [PMC free article] [PubMed] [Google Scholar]


