Abstract
The RNA-binding iron regulatory proteins IRP1 and IRP2 are inactivated by either Fe-S cluster insertion or protein degradation mediated by the E3 ligase component FBXL5. However, the mechanisms for coordination between Fe-S cluster assembly, FBXL5, and IRP1/IRP2 activity are poorly defined. A new study reveals that FBXL5 plays a critical role in limiting IRP1 and IRP2 overaccumulation when cytosolic Fe-S cluster assembly is impaired in order to maintain optimal iron levels for cell viability.
Introduction
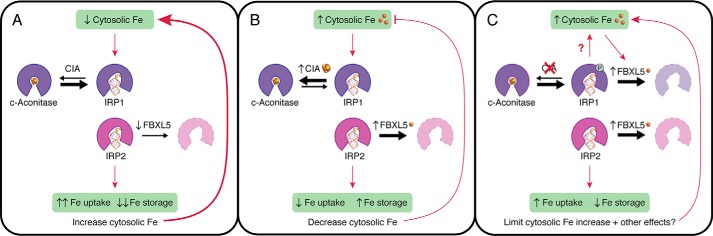
Control of intracellular iron levels in animal cells is regulated at the post-transcriptional level by iron regulatory protein 1 (IRP1)2 and its paralog IRP2 (1). When iron is depleted, both IRP1 and IRP2 bind to mRNAs that encode proteins involved in iron homeostasis, either stabilizing or translationally blocking the target mRNA, with the net result of increasing cytosolic iron levels by stimulating iron uptake and usage and mobilizing iron stores (Fig. 1A). When iron levels are sufficient, IRP1 and IRP2 are deactivated by two distinct pathways: IRP1 is converted to the enzyme aconitase by insertion of a [4Fe-4S] cluster that precludes RNA binding (2, 3), whereas IRP2, which does not bind an Fe-S cluster, is targeted for degradation by the iron-stabilized E3 ligase component FBXL5 (Fig. 1B) (4, 5). Deletion of FBXL5 or both IRPs is embryonic lethal in mammalian models (1), highlighting the importance of these iron regulation systems to viability. Previous studies have suggested coordination between the Fe-S cluster insertion and protein degradation pathways (6); however, the specific mechanisms regulating this cross-talk were unclear.
Figure 1.
Coordination between CIA, FBXL5, IRP1 and IRP2 in control of iron metabolism. A, when cytosolic iron (Fe) levels are low, Fe-S cluster insertion into IRP1 by the CIA pathway is decreased, driving an increase in the RNA-binding apo-form. Concurrently, FBXL5 is degraded in the absence of iron, leading to increased steady-state levels of its ubiquitination substrate IRP2. The net result is increased RNA binding by both IRP1 and IRP2, resulting in increased iron uptake and decreased iron storage. B, with sufficient cytosolic iron, the CIA pathway inserts an Fe-S cluster in IRP1, preventing RNA binding. Iron binding also stabilizes FBXL5, leading to increased degradation of IRP2. Reduced IRP1/2 RNA-binding activity decreases iron uptake while increasing iron storage, with the net result of lowering cytosolic iron. C, CIA silencing disrupts Fe-S insertion into IRP1 and increases IRP1 phosphorylation, presumably leading to increased cytosolic iron pools that stabilize FBXL5. The expanded FBXL5 pool functions to degrade both apo-IRP1 and IRP2 to curb RNA binding, limiting the increase in cytosolic iron and possibly other downstream effects of CIA dysfunction.
The insertion of an Fe-S cluster in IRP1 requires the cytosolic iron-sulfur assembly (CIA) pathway, which specifically functions in maturation of cytosolic and nuclear Fe-S proteins (7). In the early stages of the CIA pathway, a transient [4Fe-4S] cluster is assembled on the scaffold proteins NUBP1 and NUBP2. In the later stages, the assembled cofactor is delivered to Fe-S acceptor proteins via specific targeting complexes. A previous study revealed that FAM96A (CIA2A) is specifically required for Fe-S cluster trafficking to IRP1, based on RNAi gene silencing studies in which IRP1 protein levels and cytosolic aconitase activity decreased with FAM96A depletion (6). Unexpectedly, FAM96A silencing also decreased IRP2 protein levels, even though IRP2 is not an Fe-S protein. Because FBXL5 promotes ubiquitination and degradation of IRP2 and apo-IRP1 in an iron-dependent manner (4, 5), this study uncovered a potentially intriguing, unexplained connection between the CIA machinery, FBXL5 activity, and iron regulation.
The current report by Eisenstein and co-workers (8) sought to illuminate this regulatory circuit by asking an important question: How does FBXL5 control IRP1 and IRP2 activity when the CIA system is disrupted? To address this issue, FBXL5 and CIA factors were silenced alone or in combination in HEK cells, and the downstream effects were evaluated. Cell viability was significantly decreased when FBXL5 knockdown was combined with knockdown of either early (NUBP2) or late acting (FAM96A) CIA components or with expression of an RNA-binding IRP13C>3S mutant that is unable to bind an Fe-S cluster. Based on these results, the authors postulated that when the IRP1 Fe-S switch is not functional (via CIA disruption or IRP1 mutation), cell viability is dependent on induction of FBXL5 activity to limit apo-IRP1 and IRP2 overaccumulation. To support this hypothesis, they demonstrated that CIA silencing leads to reduced protein levels and RNA binding by both IRP1 and IRP2, whereas combining CIA dysfunction with FBXL5 knockdown largely reverses these effects. Phosphorylation of Ser-138 in IRP1, which is known to destabilize the Fe-S cluster and stimulate IRP1 degradation, also increases with CIA silencing (1). Furthermore, FBXL5 levels rise with CIA silencing, suggesting that FBXL5 induction promotes degradation of both IRP1 and IRP2 in the absence of the CIA machinery (8).
To better understand the mechanism for FBXL5 control of IRP1/IRP2 levels, Eisenstein and co-workers (8) also investigated IRP1 ubiquitination. Earlier studies demonstrated that IRP2 is targeted for ubiquitination by FBXL5 in an iron-dependent manner (4, 5), but the impact of CIA dysfunction on IRP1 ubiquitination was unknown. If the apo-IRP1 pool is expanded via CIA knockdown, is more IRP1 available for FBXL5-dependent ubiquitination? Eisenstein and co-workers (8) confirm this hypothesis, supporting the proposition that apo-IRP1 rather than Fe-S IRP1 is the preferred substrate for FBXL5 targeting (4, 5). As FBXL5 activity impacted IRP1/IRP2 levels, the situation was also examined in reverse, measuring the effect of increased IRP1/IRP2 expression on FBXL5 levels. In this case, overexpression of IRP1 or IRP2 leads to elevated levels of FBXL5 that further increases with added iron, revealing a feedback mechanism for controlling excessive IRP1 or IRP2 RNA-binding activity (8). Overall, these results demonstrated that upon apo-IRP1 and IRP2 accumulation, iron-dependent induction of FBXL5 is critical for degrading excess IRPs and limiting overaccumulation of the RNA-binding forms.
In summary, this study provides strong evidence that the CIA Fe-S insertion and FBXL5 protein degradation pathways are coordinated to maintain tight control over IRP1/IRP2 RNA-binding activity. Tipping this balance by inhibiting CIA function leads to iron-dependent induction of FBXL5 to limit both IRP1 and IRP2 RNA-binding activity (Fig. 1C). Elimination of both regulation pathways compromises cell viability, presumably due to IRP overaccumulation. But why exactly is apo-IRP1/IRP2 overaccumulation toxic to cells? One logical conclusion is that the increased RNA-binding activity leads to iron toxicity due to excessive iron uptake and mobilization. However, this possibility seems unlikely as addition of iron to the growth media partially rescued the synthetic growth defect of CIA/FBXL5 silencing (8). Future studies are required to fully understand the toxic effects of IRP overaccumulation. In addition, the increased phosphorylation of IRP1 with CIA silencing reveals another layer of regulation that has yet to be fully explored. Finally, this study focused on only two CIA factors, including a late-acting CIA component that specifically delivers Fe-S cluster to IRP1. How does silencing other CIA components that target different Fe-S cluster proteins impact IRP1/IRP2 and FBXL5 activity? Presumably, the coordination between FBXL5 and CIA activity is tied to changes in the bioavailability and/or speciation of cytosolic iron pools, but these changes have yet to be directly measured due to the difficulty in developing accurate probes for bioavailable iron. This study has thus opened up new avenues of study to explore, while providing a deeper understanding of the checks and balances in place to control the intracellular iron bank.
This work was supported by National Institutes of Health Grant GM118164. The author declares that she has no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- IRP1
- iron regulatory protein 1
- CIA
- cytosolic iron-sulfur assembly.
References
- 1. Anderson C. P., Shen M., Eisenstein R. S., and Leibold E. A. (2012) Mammalian iron metabolism and its control by iron regulatory proteins. Biochim. Biophys. Acta 1823, 1468–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Haile D. J., Rouault T. A., Tang C. K., Chin J., Harford J. B., and Klausner R. D. (1992) Reciprocal control of RNA-binding and aconitase activity in the regulation of the iron-responsive element binding protein: role of the iron-sulfur cluster. Proc. Natl. Acad. Sci. U.S.A. 89, 7536–7540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Walden W. E., Selezneva A. I., Dupuy J., Volbeda A., Fontecilla-Camps J. C., Theil E. C., and Volz K. (2006) Structure of dual function iron regulatory protein 1 complexed with ferritin IRE-RNA. Science 314, 1903–1908 [DOI] [PubMed] [Google Scholar]
- 4. Salahudeen A. A., Thompson J. W., Ruiz J. C., Ma H. W., Kinch L. N., Li Q., Grishin N. V., and Bruick R. K. (2009) An E3 ligase possessing an iron-responsive hemerythrin domain is a regulator of iron homeostasis. Science 326, 722–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vashisht A. A., Zumbrennen K. B., Huang X., Powers D. N., Durazo A., Sun D., Bhaskaran N., Persson A., Uhlen M., Sangfelt O., Spruck C., Leibold E. A., and Wohlschlegel J. A. (2009) Control of iron homeostasis by an iron-regulated ubiquitin ligase. Science 326, 718–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stehling O., Mascarenhas J., Vashisht A. A., Sheftel A. D., Niggemeyer B., Rösser R., Pierik A. J., Wohlschlegel J. A., and Lill R. (2013) Human CIA2A-FAM96A and CIA2B-FAM96B integrate iron homeostasis and maturation of different subsets of cytosolic-nuclear iron-sulfur proteins. Cell Metab. 18, 187–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lill R., Dutkiewicz R., Freibert S. A., Heidenreich T., Mascarenhas J., Netz D. J., Paul V. D., Pierik A. J., Richter N., Stümpfig M., Srinivasan V., Stehling O., and Mühlenhoff U. (2015) The role of mitochondria and the CIA machinery in the maturation of cytosolic and nuclear iron-sulfur proteins. Eur. J. Cell Biol. 94, 280–291 [DOI] [PubMed] [Google Scholar]
- 8. Johnson N. B., Deck K. M., Nizzi C. P., and Eisenstein R. S. (2017) A synergistic role of IRP1 and FBXL5 proteins in coordinating iron metabolism during cell proliferation. J. Biol. Chem. 292, 15976–15989 [DOI] [PMC free article] [PubMed] [Google Scholar]