ABSTRACT
Cdk5 deregulation is highly neurotoxic in Alzheimer's disease (AD). We identified Mcl-1 as a direct Cdk5 substrate using an innovative chemical screen in mouse brain lysates. Our data demonstrate that Mcl-1 levels determine the threshold for cellular damage in response to neurotoxic insults. Mcl-1 is a disease-specific target of Cdk5, which associates with Cdk5 under basal conditions, but is not regulated by it. Neurotoxic insults hyperactivate Cdk5 causing Mcl-1 phosphorylation at T92. This phosphorylation event triggers Mcl-1 ubiquitylation, which directly correlates with mitochondrial dysfunction. Consequently, ectopic expression of phosphorylation-dead T92A-Mcl-1 fully prevents mitochondrial damage and subsequent cell death triggered by neurotoxic treatments in neuronal cells and primary cortical neurons. Notably, enhancing Mcl-1 levels offers comparable neuroprotection to that observed upon Cdk5 depletion, suggesting that Mcl-1 degradation by direct phosphorylation is a key mechanism by which Cdk5 promotes neurotoxicity in AD. The clinical significance of the Mcl-1-Cdk5 axis was investigated in human AD clinical specimens, revealing an inverse correlation between Mcl-1 levels and disease severity. These results emphasize the potential of Mcl-1 upregulation as an attractive therapeutic strategy for delaying or preventing neurodegeneration in AD.
KEY WORDS: Cdk5, Mcl-1, Alzheimer's disease, Neurodegeneration, Chemical genetic, Neuroprotection
Highlighted Article: Cdk5-mediated degradation of Mcl-1 by direct phosphorylation promotes neurotoxicity. Mcl-1 upregulation, or Cdk5 inhibition, is potentially an attractive therapeutic strategy for preventing neurodegeneration in AD.
INTRODUCTION
Alzheimer's disease (AD) is a progressive neurodegenerative disease, which currently affects an estimated 5.3 million Americans. This number is expected to increase to 16 million by 2050, with an estimated increase of ∼1 million new cases per year. Currently, the only available drugs for AD are N-methyl D-aspartate receptor antagonists and acetylcholinesterase inhibitors, which target symptoms, but do not prevent neurodegeneration (Sun et al., 2012). In this study, we focused on cyclin-dependent kinase 5 (Cdk5), which is deregulated in AD and contributes to all three hallmarks: formation of β-amyloid (Aβ) plaques and neurofibrillary tangles (NFT), and extensive neurodegeneration (Shukla et al., 2012).
Cdk5 is an atypical cyclin-dependent kinase (Cdk) family member, which has evolved to be functionally divergent from its family (Dhavan and Tsai, 2001; Shah and Lahiri, 2014, 2017; Shah and Rossie, 2017). Cdk5 requires a binding partner for activation; however, it is not activated by classical cyclins such as cyclin A, cyclin D and cyclin E, which activate other family members. Instead, it possesses its own specific protein partners, p35 (also known as Cdk5r1), p39 (also known as Cdk5r2) and cyclin I (CCNI), which are highly expressed in the central nervous system, particularly in postmitotic neurons (Tsai et al., 1994; Tang et al., 1995; Brinkkoetter et al., 2010). Cyclin I has not been shown to activate any other Cdks, suggesting that it might be a unique Cdk5 activator.
Likewise, Cdk5 also interacts with its own set of negative regulators. It is not inhibited by p27Kip1 (also known as Cdkn1b), p21Cip1 (also known as Cdkn1a) or p57Kip2 (also known as Cdkn1c), which inhibit other Cdk family members. Instead, Cdk5 activity is inhibited by binding to glutathione-S-transferase P (GSTP1), cyclin D1 (CCND1) and cyclin E (CCNE) (Sun et al., 2011; Modi et al., 2012; Odajima et al., 2011). Additionally, Cdk5 is not phosphorylated at T14, and phosphorylation at Y15 by c-Abl, Eph receptor A4 (EphA4) or Fyn was shown to activate it, instead of inhibition observed for its family members (Zukerberg et al., 2000; Sasaki, et al., 2002; Fu et al., 2007). Notably, one study reported that phosphorylation at Y15 has no impact on Cdk5 activity (Kobayashi et al., 2014).
Although all Cdk family members are crucial orchestrators of the cell cycle, Cdk5 does not participate in the normal cell cycle. However, in postmitotic neurons, deregulation of Cdk5 activity aberrantly activates various components of the cell cycle, resulting in neuronal death (Chang et al., 2012). Cdk5 is highly expressed and active in postmitotic neurons, where other Cdks are minimally expressed. During embryogenesis, Cdk5 is essential for brain development and regulates various neuronal pathways, including neuronal migration, neurite outgrowth, axon guidance and synapse formation (Ohshima et al., 1996). Postbirth, Cdk5 activity is crucial for higher cognitive functions such as synaptic plasticity, learning and memory formation, long term behavioral changes and drug addiction (Heller et al., 2016; Hernandez et al., 2016). Reduced activity and hyperactivity of Cdk5 are equally neurotoxic, and give rise to various neurodevelopmental and neurological disorders. For example, reduction or loss of Cdk5 activity is associated with mental retardation, schizophrenia, epilepsy and attention-deficit/hyperactivity disorder (ADHD) (Venturin et al., 2004; Engmann et al., 2011; Patel et al., 2004; Drerup et al., 2010). By contrast, hyperactivation of Cdk5 occurs in many neurodegenerative diseases, including AD, Parkinson's disease (PD), ischemic stroke and amyotrophic lateral sclerosis (ALS), and is highly destructive (Hisanaga and Endo, 2010; Meyer et al., 2014).
In AD, a variety of neurotoxic signals such as excitotoxicity, Aβ, ischemia and oxidative stress increase intracellular Ca2+ levels in neurons, causing the activation of calpain, which cleaves p35 into p25 and p10. p25 has a 5- to 10-fold longer half-life than p35 and lacks the membrane anchoring signal. This results in the constitutive activation and mislocalization of the Cdk5-p25 complex, which results in the phosphorylation of a variety of nonphysiological targets, culminating in the formation of neurotoxic NFT and β-amyloid, and neuronal death (Sun et al., 2009; Chang et al., 2010). Cdk5 regulates neurotoxic β-amyloid formation both at transcriptional and post-translational levels. Cdk5 increases β-secretase transcription via Stat3, leading to increased β-amyloid formation (Wen et al., 2008). Cdk5 increases β-secretase activity by directly phosphorylating it at T252 (Song et al., 2015). We have shown that phosphorylation of FOXO3a (also known as FOXO3) at S43, S173, S294 and S325 by deregulated Cdk5 also contributes to neurotoxic Aβ formation (Shi et al., 2016). A crucial role of Cdk5 in exacerbating AD pathogenesis was recently shown in a mouse model, in which specific inhibition of aberrant Cdk5 activity using a peptide inhibitor attenuated NFT formation and restored synaptic and cognitive functions (Shukla et al., 2017).
In this study, we uncovered a novel mechanism by which deregulated Cdk5 harnesses induced myeloid cell leukemia sequence 1 (Mcl-1) and promotes neuronal death. Using an innovative chemical genetic screen, we identified Mcl-1 as a direct substrate of Cdk5 kinase in mouse brain lysates. Mcl-1 is a prosurvivor member of the B-cell lymphoma 2 (Bcl-2) family, which is essential for neuronal development (Arbour et al., 2008). Germline knockout of Mcl-1 causes peri-implantation lethality on embryonic day 3.5 (Rinkenberger et al., 2000). During cortical neurogenesis, Mcl-1 is highly expressed in neural precursors and newly committed neurons in the cortical plate. Not surprisingly, loss of Mcl-1 in neuronal progenitors results in extensive apoptosis. Mcl-1 is also highly expressed in postmitotic neurons and is associated with neuronal protection following prolonged seizures (Mori et al., 2004). Loss of Mcl-1 sensitizes neurons to DNA damage-induced death (Arbour et al., 2008). In this study, we investigated the mechanism of Mcl-1 regulation upon neurotoxic conditions and its consequences in a mouse hippocampal cell line (HT22 cells), mouse primary neurons and AD human clinical specimens.
RESULTS
Mcl-1 is a direct substrate of Cdk5
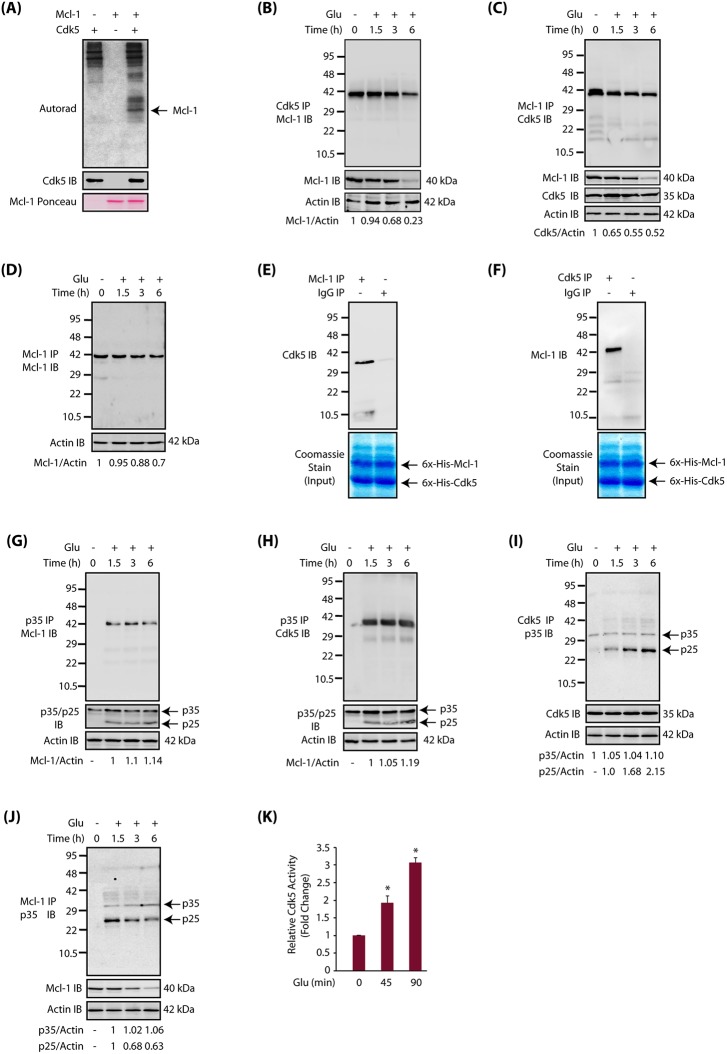
The chemical-genetic approach utilizes an engineered kinase that possesses a ‘hole’ in the active site, which in the presence of a radioactive orthogonal ATP analog [e.g. N6-(phenethyl) ATP], specifically transfers the radioactive tag (32P) to its substrates. These ATP analogs are not accepted by wild-type kinases, permitting unbiased identification of direct substrates of the engineered kinase in a global environment (Shah et al., 1997; Shah and Shokat, 2003; Shah and Vincent, 2005; Kim and Shah, 2007; Johnson et al., 2011, 2012; Wang et al., 2017a,b). Using these design criteria, we previously generated an analog-sensitive mutant of Cdk5 (named Cdk5-as1) that efficiently accepted N-6-phenethyl-ATP (PE-ATP) as the orthogonal ATP analog. Using Cdk5-as1 and [32P] PE-ATP, we previously identified several direct Cdk5 substrates including GM130 (also known as Golga2), peroxiredoxin 1, peroxiredoxin 2, lamin A, lamin B, Cdc25a, Cdc25b, Cdc25c and Foxo3 (Sun et al., 2008a,b; Chang et al., 2011, 2012; Shi et al., 2016). In this study, we focused on Cdk5-mediated regulation of Mcl-1 signaling. As proteomic screens can often lead to false positives, we tested whether Cdk5 directly phosphorylates Mcl-1 using an in vitro kinase assay. We found that Cdk5 directly phosphorylates Mcl-1, confirming the result obtained from the chemical genetic screen (Fig. 1A).
Fig. 1.
Mcl-1 is a disease-specific target of Cdk5. (A) Mcl-1 is a direct substrate of Cdk5. Cdk5-p25 complex was subjected to a kinase assay with either [32P]ATP alone (lane 1), or with 6x-His-Mcl-1 and [32P]ATP (lane 3) for 15 min. Lane 2 shows Mcl-1 incubated with [32P]ATP. (B) Cdk5 and Mcl-1 association under normal and neurotoxic conditions in HT22 cells. Cdk5 was immunoprecipitated from either control or glutamate-treated HT22 cells (for 0–6 h), and the association of Cdk5 and Mcl-1 was analyzed. Total Mcl-1 and actin levels were probed in whole-cell lysates. (C) Cdk5 and Mcl-1 association under normal and neurotoxic conditions in HT22 cells. Mcl-1 was immunoprecipitated from either control or glutamate-treated HT22 cells (for 0–6 h), and Mcl-1 and Cdk5 association was analyzed. Total Mcl-1, Cdk5 and actin levels were probed in whole-cell lysates. (D) Mcl-1 levels decrease in Mcl-1 immune complexes. Mcl-1 was immunoprecipitated from control and glutamate-treated HT22 cells (for 0–6 h), and its levels analyzed. Actin was used as a control. (E) Cdk5 binds Mcl-1 directly. Mcl-1 and Cdk5 association was analyzed using recombinant Cdk5 and Mcl-1 in an in vitro pull-down assay. Mcl-1 on beads was incubated with 6×-His-Cdk5 and binding analyzed. The lower panel shows the input (6×-His-Cdk5 and 6×-His-Mcl-1). (F) Cdk5 and Mcl-1 bind directly. Mcl-1 and Cdk5 association was analyzed using recombinant Cdk5 and Mcl-1 in an in vitro pull-down assay. The lower panel shows the input (6×-His-Cdk5 and 6×-His-Mcl-1). (G) Glutamate stimulates the association of p35/p25 with Mcl-1. p35/p25 were immunoprecipitated from either control or glutamate-treated HT22 cells (for 0–6 h), and the association of p35/p25 with Mcl-1 analyzed. The lower panel shows the relative levels of p35 and p25 in whole-cell lysate from untreated and glutamate-treated cells. Actin was used as a loading control. (H) Cdk5 associates with p35/p25 upon glutamate stimulation. p35/p25 were immunoprecipitated from either control or glutamate-treated HT22 cells (for 0–6 h), and the association of p35/p25 with Cdk5 analyzed. (I) Cdk5 associates with p25 upon glutamate stimulation. (J) Glutamate stimulates association of p25 and Mcl-1. Mcl-1 was immunoprecipitated from either control or glutamate-treated HT22 cells (for 0–6 h), and the association of Mcl-1 and p35/p25 was analyzed. Results in A-J are from at least three independent experiments. (K) Cdk5 activity increases upon glutamate treatment in HT22 cells. The Cdk5 kinase assay was performed as described in the Materials and Methods. *P<0.05, compared with untreated HT22 cells.
Mcl-1 is a disease-specific target of Cdk5
To uncover the significance of Mcl-1 phosphorylation by Cdk5, we determined whether they associate with each other in HT22 cells under normal and/or neurotoxic conditions. We chose glutamate as the neurotoxic signal. Glutamate excitotoxicity is a major contributor to AD pathogenesis (Hosie et al., 2012). HT22 cells were treated with glutamate for 1.5, 3 and 6 h, and Cdk5 immune complexes were isolated and Mcl-1 levels analyzed. Surprisingly, Mcl-1 and Cdk5 were associated in control cells, and the level of association remained almost the same until 1.5 h of glutamate treatment. However, as time progressed, Mcl-1 association with Cdk5 decreased and only ∼20% of Mcl-1 remained associated after 6 h (Fig. 1B). This result suggested that either glutamate treatment results in the dissociation of Cdk5 and Mcl-1 or that the levels of Mcl-1 decline with time. We have previously observed that Cdk5 levels remain constant during glutamate treatment in HT22 cells. Therefore, Mcl-1 levels were analyzed in glutamate-treated HT22 cells, revealing a time-dependent decrease in Mcl-1 levels (Fig. 1B). We conducted the reciprocal experiment, in which Mcl-1 was isolated from control and glutamate-treated cells (for 1.5, 3 and 6 h), which also confirmed that Cdk5 and Mcl-1 associate under basal conditions. Similarly, there was a time-dependent decrease in Cdk5 and Mcl-1 association due to the decrease in Mcl-1 levels (Fig. 1C). We also isolated the Mcl-1 immune complex from control and glutamate-treated cells, which revealed decreased levels of Mcl-1 as time progressed (Fig. 1D). This result suggests that the observed decrease in Cdk5 levels in Mcl-1 immune complexes is not due to decreased binding of Cdk5, but instead results from overall reduced Mcl-1 levels.
To examine whether the interaction between Cdk5 and Mcl-1 was direct, we conducted an in vitro pull-down assay using recombinant Cdk5 and Mcl-1. Initially, Mcl-1 immune complexes were isolated. They were then incubated with recombinant Cdk5, which pulled down Cdk5 (Fig. 1E). Similarly, we isolated a Cdk5 immune complex and incubated it with recombinant Mcl-1, which pulled down Mcl-1 (Fig. 1F). These in vitro binding assays confirmed that Cdk5 and Mcl-1 bind each other directly.
This finding prompted us to investigate whether Mcl-1 binds to the Cdk5 activator p35 or p25 in HT22 cells. p35/p25 immune complexes were isolated from untreated and glutamate-treated HT22 cells, and their potential binding to Mcl-1 analyzed. Glutamate treatment triggered the formation of p25 as expected (Fig. 1G). Contrary to Cdk5 immune complexes, which showed Mcl-1 binding in both untreated and treated cells, Mcl-1 was only present in glutamate-treated cells, suggesting that Mcl-1 presumably associates with p35 and/or p25 via Cdk5 (Fig. 1G). Therefore, we next investigated Cdk5 levels in p35/p25 immune complexes, which revealed that Cdk5 associates with p35/p25 only in glutamate-treated cells (Fig. 1H). Taken together, these findings indicate that glutamate treatment triggers Cdk5 binding to p35/p25, which brings Mcl-1 to p35/p25 immune complexes in glutamate-treated cells (Fig. 1G).
Because Mcl-1 associates with Cdk5 under basal conditions, we next investigated whether p35 or p25 was present in this complex in the absence of any neurotoxic signal. Cdk5 immune complexes were isolated from control and glutamate-treated cells, and p35/p25 levels analyzed. As shown in Fig. 1I, a negligible amount of p35 associates with Cdk5 in control cells. However, glutamate-treatment increased the formation of p25, which associated with Cdk5 in a time-dependent manner (Fig. 1I). More importantly, the absence of any p35 or p25 in the Cdk5 immune complex in control cells indicated that Mcl-1 associates with monomeric inactive Cdk5 in the absence of any neurotoxic signal.
This finding also indicated that glutamate treatment should increase the binding of p25 to Cdk5 in the Cdk5-Mcl-1 complex. Therefore, we isolated Mcl-1 immune complexes from control and glutamate-treated HT22 cells, and investigated the levels of p35 and p25. As expected, no p35 or p25 was present in the Mcl-1 immune complex in control cells; however, glutamate treatment in HT22 cells resulted in increased presence of p25 in the Mcl-1 immune complex (Fig. 1J). Since p25 binding increases Cdk5 activity, we also measured Cdk5 activity in control and glutamate-treated cells using an in vitro kinase assay. As shown in Fig. 1K, Cdk5 activity increases ∼2-fold after 45 min of glutamate treatment. Taken together, these findings suggest that monomeric inactive Cdk5 binds to Mcl-1 under basal conditions, but does not phosphorylate it. Glutamate treatment activates Cdk5 leading to Mcl-1 phosphorylation, suggesting that Mcl-1 is a disease-specific target of Cdk5.
Cdk5 regulates the subcellular localization of Mcl-1 in HT22 cells
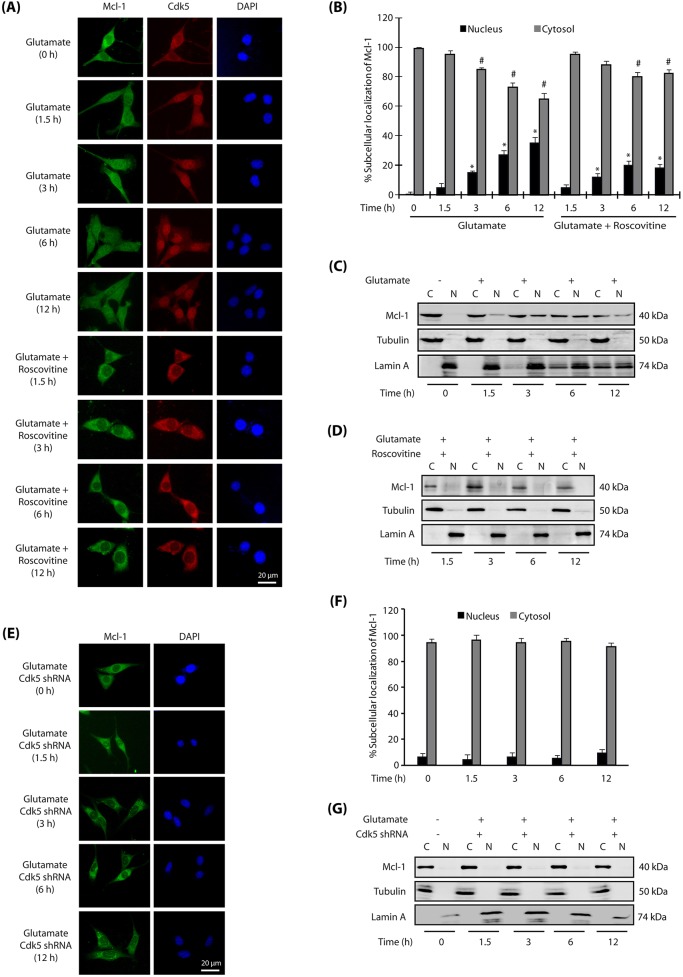
Association of Cdk5 and Mcl-1 upon glutamate treatment prompted us to investigate their subcellular localization in HT22 cells. Both Cdk5 and Mcl-1 were predominantly cytosolic in HT22 cells (Fig. 2A). Glutamate treatment resulted in increased nuclear translocation of both Cdk5 and Mcl-1 in a time-dependent manner (Fig. 2A,B). After 6–12 h of glutamate treatment, ∼35% cells displayed nuclear Mcl-1, whereas >80% revealed nuclear Cdk5. Notably, when Cdk5 was inhibited using a small molecule inhibitor roscovitine, both Mcl-1 and Cdk5 remained cytoplasmic, suggesting that Cdk5 not only shows a similar subcellular localization to that of Mcl-1, but is also responsible for nuclear translocation of Mcl-1 upon glutamate stimulation in HT22 cells (Fig. 2A,B).
Fig. 2.
Cdk5 promotes nuclear localization of Mcl-1 upon glutamate stimulation. (A) Glutamate stimulates nuclear translocation of Cdk5 and Mcl-1. HT22 cells were treated with glutamate for 0–12 h with or without roscovitine, and then immunostained as described in the Materials and Methods. Representative images are shown. (B) Percentage of cells showing nuclear translocation of Mcl-1. At least 100 cells from 10 random frames were counted. Data are mean±s.e.m. from three independent experiments. *P<0.05 vs nucleus fraction control; #P<0.05 vs cytoplasmic fraction control, analyzed by two-way analysis of variance. (C,D) Subcellular fractionation of Mcl-1 in glutamate-treated HT22 cells in the absence or presence of roscovitine, as described in the Materials and Methods, with α-tubulin as the cytoplasmic marker and lamin A as the nuclear marker. N, nuclear fraction; C, cytoplasmic fraction. (E) Cdk5 knockdown inhibits nuclear translocation of Mcl-1. HT22 cells were treated with Cdk5 shRNA for 30 h, followed by glutamate treatment for 0–12 h. (F) The percentage of cells showing nuclear translocation of Mcl-1. Data are mean±s.e.m. from three independent experiments. (G) Cdk5 shRNA-infected HT22 cells were treated with glutamate (0–12 h) and fractionated as described in the Materials and Methods, with α-tubulin as the cytoplasmic marker and lamin A as the nuclear marker. Scale bars: 20 μm.
To further validate these results, we performed cytosolic and nuclear fractionation of control and HT22 cells treated with glutamate for different time periods. Similar to the results obtained using immunofluorescence, subcellular fractionation also showed a time-dependent increase in the translocation of Mcl-1 to the nucleus, which increased with increased glutamate exposure time (Fig. 2C). Cdk5 inhibition using roscovitine fully abrogated this translocation, confirming that Cdk5 activity is responsible for the migration of Mcl-1 to the nucleus (Fig. 2D).
Depletion of Cdk5 inhibits nuclear translocation of Mcl-1
Roscovitine is a highly potent inhibitor of Cdk5; however, it is not monospecific and inhibits Cdk1 and Cdk2 with almost equal potency (Meijer et al., 1997). Therefore, we knocked down Cdk5 and analyzed Mcl-1 subcellular localization upon glutamate treatment. Ablation of Cdk5 fully prevented the nuclear translocation of Mcl-1 upon glutamate treatment (analyzed up to 12 h), thereby supporting the results obtained using roscovitine (Fig. 2E,F). Finally, we also generated nuclear and cytosolic fractions of control, glutamate-treated and Cdk5 shRNA-treated cells, which too revealed complete inhibition of Mcl-1 nuclear translocation in Cdk5-depleted cells (Fig. 2G). Collectively, these results demonstrate that Cdk5 controls the migration of Mcl-1 to the nucleus upon deregulation. Importantly, cellular fractionation experiments showed increased levels of lamin A in the cytosolic fraction of glutamate-treated cells (Fig. 2C), but significantly lower levels in Cdk5-inhibited (Fig. 2D) or Cdk5-depleted (Fig. 2G) cells. This finding is consistent with our previous study showing rapid dispersion of the nuclear envelope in glutamate-treated HT22 cells and primary neurons, triggered by Cdk5-mediated phosphorylation of lamin A and lamin B1 (Chang et al., 2011).
Cdk5 phosphorylates Mcl-1 at T92
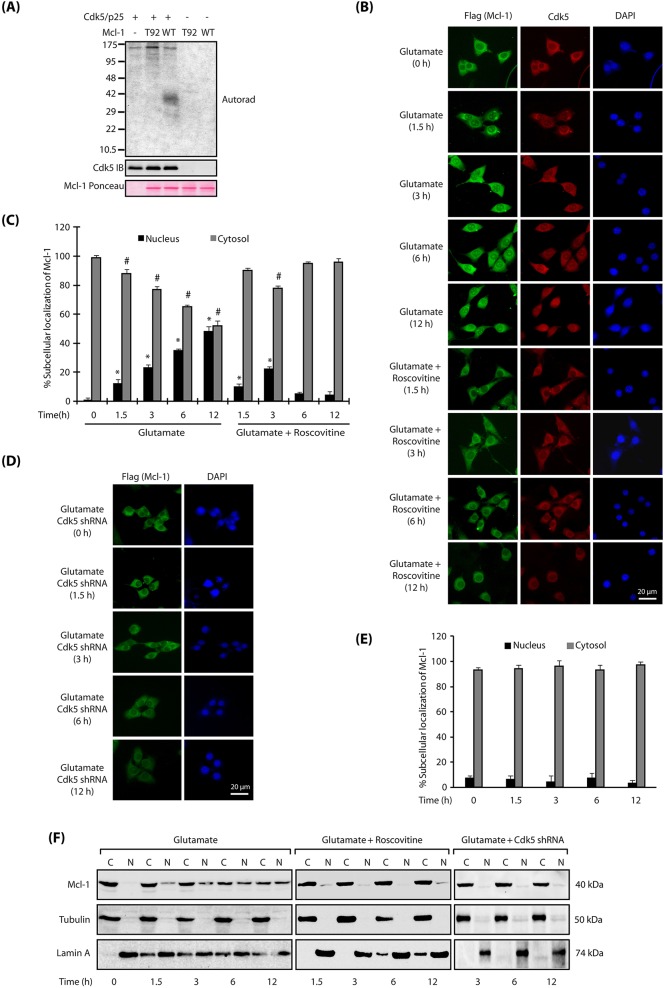
To uncover the mechanism by which Cdk5 regulates Mcl-1, we examined Cdk5-mediated phosphorylation sites on Mcl-1. Cdk5 prefers to phosphorylate SP/TP sites which are flanked by basic residues. This preference suggested T92 as a potential Cdk5 site on Mcl-1. Therefore, we generated the corresponding phospho-dead mutant (T92A-Mcl-1) and subjected it to an in vitro kinase assay using Cdk5-p25. Wild-type Mcl-1 was efficiently phosphorylated, but T92A-Mcl-1 showed no phosphorylation, confirming that T92 is the only Cdk5 phosphorylation site on Mcl-1 (Fig. 3A).
Fig. 3.
Cdk5 directly phosphorylates Mcl-1 at T92. (A) The Cdk5-p25 complex (Cdk5/p25) phosphorylates Mcl-1 at T92. Recombinant 6×-His-tagged wild type and Mcl-1 mutant were subjected to a kinase assay with Cdk5-p25. (B) Glutamate stimulates nuclear translocation of Cdk5 and Mcl-1 in Mcl-1-HT22 cells. Mcl-1-HT22 cells were treated with glutamate for 0–12 h with or without roscovitine, and then immunostained with anti-Flag and Cdk5 antibody. Representative images are shown. (C) The percentage of cells showing nuclear translocation of Mcl-1. Data are mean±s.e.m. from three independent experiments. *P<0.05 vs nucleus fraction control; #P<0.05 vs cytoplasmic fraction control, analyzed by two-way analysis of variance. (D) Cdk5 knockdown inhibits nuclear translocation of Mcl-1. Cdk5 shRNA-infected Mcl-1-HT22 cells were treated with glutamate (0–12 h). Representative images are shown. (E) The percentage of cells showing nuclear translocation of Mcl-1. Data are mean±s.e.m. from three independent experiments. (F) Subcellular fractionation of Mcl-1 in glutamate-treated Mcl-1-HT22 cells in the absence or presence of roscovitine or Cdk5 shRNA, with α-tubulin as the cytoplasmic marker and lamin A as the nuclear marker. Scale bars: 20 μm.
Cdk5 deregulation triggers Mcl-1 nuclear translocation independent of its phosphorylation at T92
We next investigated whether phosphorylation of Mcl-1 at T92 is responsible for nuclear translocation of Mcl-1 upon glutamate treatment. Therefore, we generated stable HT22 cells expressing either Flag-tagged wild-type Mcl-1 or Flag-tagged T92A-Mcl-1, and analyzed their subcellular localization with or without glutamate exposure for 1.5, 3, 6 and 12 h. As expected, wild-type Mcl-1-expressing HT22 cells revealed nuclear translocation of Mcl-1 upon glutamate treatment, which increased to ∼50% after 12 h of treatment (Fig. 3B,C). Inhibition of Cdk5 activity using roscovitine, or depletion of Cdk5 using corresponding shRNA, largely prevented nuclear translocation of Mcl-1 (Fig. 3B–E). Finally, these findings were corroborated by analyzing the nuclear and cytosolic fractions of wild-type Mcl-1-HT22 cells, which were either control, glutamate-treated, glutamate and roscovitine-treated, or glutamate and Cdk5 shRNA-treated for varying periods. As shown in Fig. 3F, glutamate treatment increased the nuclear population of Mcl-1, which was fully prevented by Cdk5 inhibition or depletion. These results thus confirm that deregulated Cdk5 triggers nuclear translocation of wild-type Mcl-1 in neuronal cells.
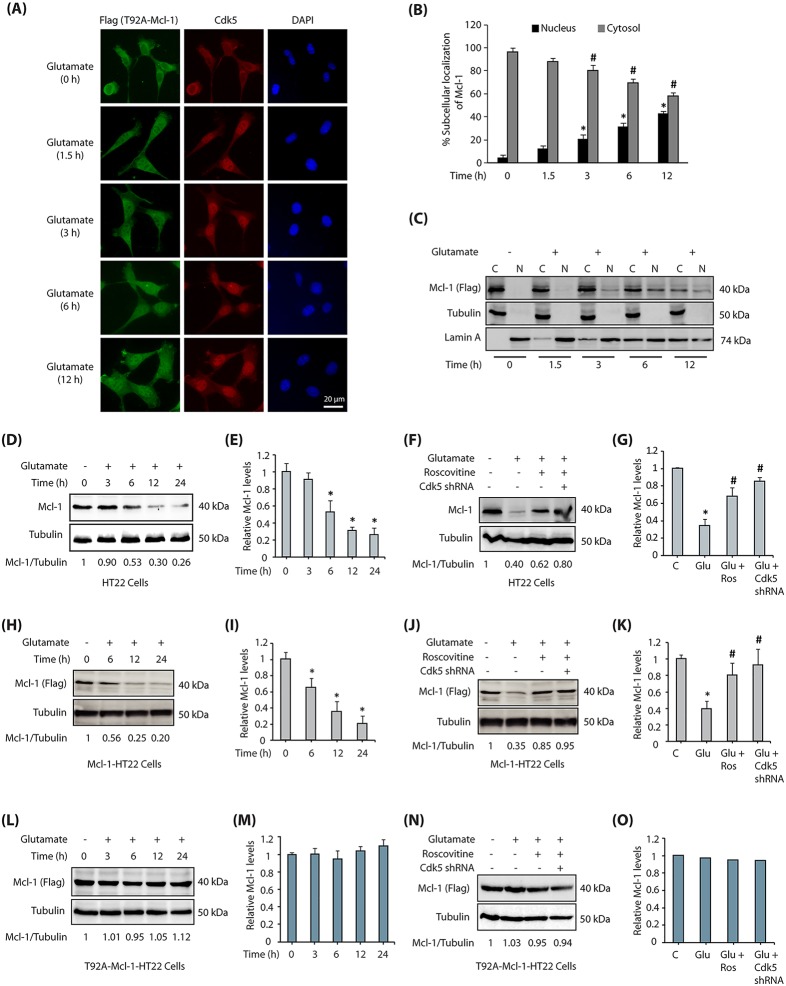
Interestingly, when T92A-Mcl-1-expressing HT22 cells were exposed to glutamate, mutant Mcl-1 still migrated to the nucleus, suggesting that phosphorylation of T92 is not responsible for its nuclear translocation (Fig. 4A,B). These findings were confirmed in cytosolic and nuclear fractions of glutamate-exposed T92A-Mcl-1 HT22 cells, which revealed both cytosolic and nuclear residence of T92A-Mcl-1 in glutamate-exposed cells (Fig. 4C).
Fig. 4.
Mcl-1 translocation to the nucleus is phosphorylation independent. (A) Mcl-1 phospho-dead mutant translocates to the nucleus upon glutamate stimulation. T92A-Mcl-1-HT22 cells were treated with glutamate for 0–12 h, and then immunostained with anti-Flag antibody. Representative images are shown. Scale bar: 20 μm. (B) The percentage of cells showing nuclear translocation of Mcl-1. Data are mean±s.e.m. from three independent experiments. *P<0.05 vs nucleus fraction control; #P<0.05 vs cytoplasmic fraction control, analyzed by two-way analysis of variance. (C) Subcellular fractionation of T92A-Mcl-1 in glutamate-treated T92A-Mcl-1-HT22 cells, with α-tubulin as the cytoplasmic marker and lamin A as the nuclear marker. T92A-Mcl-1 was visualized using anti-Flag antibody. (D) Mcl-1 levels decrease upon glutamate treatment. HT22 cells were treated with glutamate for 0–24 h and the total levels of Mcl-1 analyzed. (E) Average relative ratios of Mcl-1 band intensities to alpha-tubulin band intensities upon glutamate treatment, as obtained from three independent experiments. *P<0.05, compared with untreated HT22 cells. (F) Cdk5 inhibition or ablation prevents the decrease in Mcl-1 levels in glutamate-treated HT22 cells. HT22 cells were treated with glutamate for 12 h, in the presence and absence of roscovitine or Cdk5 shRNA and then the total levels of Mcl-1 were analyzed. (G) Mcl-1 protein levels in HT22 cells in response to glutamate treatment with or without roscovitine and Cdk5 shRNA. Data are mean±s.e.m. from three independent experiments. *P<0.05, compared with untreated HT22 cells; #P<0.05, compared with glutamate-treated cells. (H) Wild-type Flag-Mcl-1 levels decrease upon glutamate treatment. Flag-Mcl-1-HT22 cells were treated similarly to in D and total levels of Flag-Mcl-1 analyzed using Flag antibody. (I) Average relative ratios of Flag (Mcl-1) band intensities to α-tubulin band intensities upon glutamate treatment, as obtained from three independent experiments. *P<0.05, compared with untreated Mcl-1-HT22 cells. (J) Cdk5 inhibition or ablation prevents the decrease in wild-type Flag-Mcl-1 levels in glutamate-treated HT22 cells. Flag-Mcl-1-HT22 cells were treated similarly to in F and Mcl-1 levels analyzed using Flag antibody. (K) Wild-type Flag-Mcl-1 protein levels in HT22 cells in response to glutamate treatment with or without roscovitine and Cdk5 shRNA. Data are mean±s.e.m. of three independent experiments. *P<0.05, compared with untreated HT22 cells. #P<0.05, compared with glutamate-treated cells. (L) T92A-Mcl-1 levels do not vary significantly with glutamate treatment. Flag-tagged T92A-Mcl-1-HT22 cells were treated similarly to in D and total levels of Mcl-1 analyzed using Flag antibody. (M) Average relative ratios of Flag (Mcl-1) band intensities to α-tubulin band intensities upon glutamate treatment, as obtained from three independent experiments. (N) T92A-Mcl-1 levels do not vary significantly in the absence or presence of Cdk5 inhibitor or Cdk5 shRNA in glutamate-treated cells. T92A-Mcl-1-HT22 cells were treated similarly to in F and Mcl-1 levels analyzed. (O) Flag-T92A-Mcl-1 protein levels in HT22 cells in response to glutamate treatment with or without roscovitine and Cdk5 shRNA. Data are mean±s.e.m. from three independent experiments.
Taken together, these results demonstrate that although deregulated Cdk5 is responsible for the nuclear translocation of Mcl-1, it is not caused by T92 phosphorylation. Our data show that Cdk5 deregulation also causes dispersion of the nuclear envelope, as is evident by the presence of nuclear lamina in the cytosolic fractions in Fig. 2C, Fig. 3F and Fig. 4C (Chang et al., 2011). Therefore, we predict that disruption of the nuclear lamina mislocalizes Mcl-1 to the nucleus. When Cdk5 is inhibited or depleted, the nuclear lamina remains intact (Fig. 2D,G and Fig. 3F), and maintains the cytoplasmic residence of Mcl-1 despite glutamate treatment.
Cdk5-mediated phosphorylation of Mcl-1 triggers its degradation by ubiquitylation
An increase in Mcl-1 levels is often linked with cell survival (Mori et al., 2004). Therefore, we investigated the consequences of Mcl-1 phosphorylation in HT22 cells under neurotoxic conditions. Mcl-1 levels rapidly decreased upon glutamate treatment in a time-dependent manner (Fig. 4D). As shown in Fig. 4E, we observed a >70% decrease in Mcl-1 levels in HT22 cells exposed to glutamate for 24 h in three independent experiments. Importantly, Cdk5 inhibition or Cdk5 depletion largely prevented this decrease in Mcl-1 levels under neurotoxic conditions, suggesting that Cdk5 is responsible for the observed reduction in Mcl-1 levels (Fig. 4F). Fig. 4G depicts the impact of Cdk5 inhibition on Mcl-1 levels in glutamate-treated cells from three independent experiments (Fig. 4G).
To investigate a potential role of T92 phosphorylation in this event, both wild-type Flag-tagged Mcl-1 and Flag-tagged T92A-Mcl-1 HT22 cells were treated with glutamate for increasing time periods. As expected, Flag-Mcl-1 was degraded in wild-type Mcl-1 HT22 cells (Fig. 4H). Fig. 4I shows the average impact of glutamate treatment on Mcl-1 levels from three independent experiments. Importantly, Mcl-1 degradation was prevented if Cdk5 activity was inhibited or depleted (Fig. 4J,K). By contrast, T92A-Mcl-1 HT22 cells showed no change in Mcl-1 levels upon glutamate exposure (Fig. 4L,M), and were unaffected by Cdk5 inhibition or depletion (Fig. 4N,O). Taken together, these results confirm that Cdk5-mediated phosphorylation of Mcl-1 is responsible for the decrease in Mcl-1 levels.
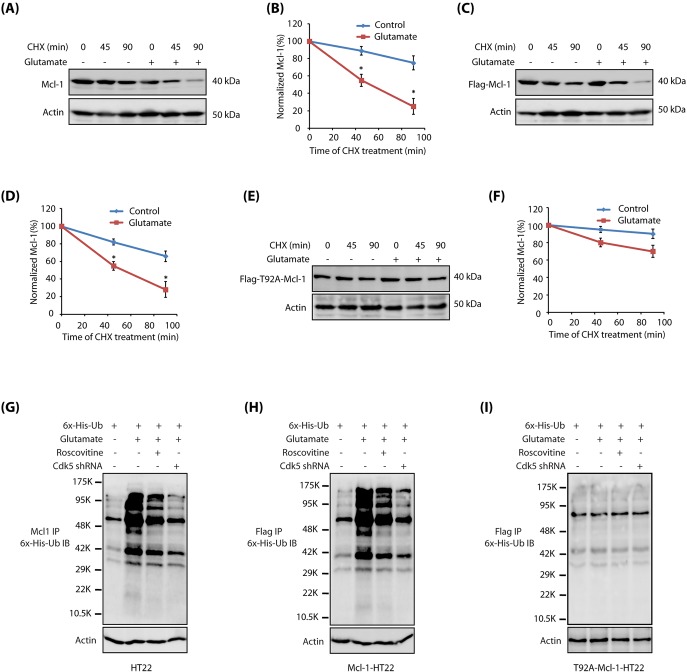
Mcl-1 has a short half-life of <1 hour (Adams and Cooper, 2007). Therefore, we investigated whether glutamate exposure resulted in the degradation of Mcl-1. HT22 cells were treated with cycloheximide to inhibit protein synthesis, and their degradation kinetics analyzed with and without glutamate treatment. Exposure to glutamate resulted in reduced Mcl-1 levels, suggesting that the Cdk5-mediated decrease in Mcl-1 levels upon glutamate stimulation is caused by increased degradation (Fig. 5A,B). To investigate the impact of Mcl-1 phosphorylation on its stability, we also analyzed its protein degradation profile in Flag-Mcl-1-HT22 and Flag-T92A-Mcl-1-HT22 cells upon glutamate treatment. These cells were exposed to cycloheximide, then treated with glutamate for varying periods, and the levels of ectopically expressed wild-type and mutant Mcl-1 were examined using Flag antibody. Although wild-type Mcl-1 was rapidly degraded upon glutamate treatment (Fig. 5C,D), T92A-Mcl-1 was significantly resistant to glutamate toxicity (Fig. 5E,F). Taken together, these results indicate that Cdk5-mediated phosphorylation of Mcl-1 triggers its degradation.
Fig. 5.
Cdk5-mediated phosphorylation of Mcl-1 at T92 triggers Mcl-1 degradation by ubiquitylation. (A) Glutamate stimulation promotes Mcl-1 degradation. HT22 cells were treated with 10 µg/ml of the protein synthesis inhibitor cycloheximide for 30 min, followed by control or 5 mM glutamate treatment. Cells were harvested at various time points (0, 45 and 90 min) and Mcl-1 protein levels determined. CHX, cycloheximide. (B) Graphical representation of Mcl-1 degradation rate. The results of densitometric scanning are shown with Mcl-1 signal normalized to α-tubulin signal. Data are mean±s.e.m. from three independent experiments. (C) Flag-Mcl-1-HT22 cells were treated as described above and total levels of Mcl-1 analyzed using Flag antibody. (D) Graphical representation of Flag (Mcl-1) degradation rate. The results of densitometric scanning are shown with Flag (Mcl-1) signal normalized to α-tubulin signal. Data are mean±s.e.m. from three independent experiments. (E) T92A-Mcl-1 is resistant to glutamate-induced degradation. T92A-Mcl-1-HT22 cells were treated as described above and T92A-Mcl-1 levels analyzed using Flag antibody. (F) Graphical representation of Mcl-1 degradation rate. The results of densitometric scanning are shown with Flag (Mcl-1) signal normalized to α-tubulin signal. Data are mean±s.e.m. from three independent experiments. (G) Mcl-1 degradation is mediated by ubiquitylation. 6×-His-ubiquitin was expressed in HT22 cells, followed by glutamate treatment for 12 h in the presence and absence of roscovitine or Cdk5 shRNA. Mcl-1 was immunoprecipitated and potential ubiquitylation analyzed using 6×-His antibody. Representative data are shown from at least three independent experiments. (H) Wild-type Mcl-1 is ubiquitylated in response to glutamate treatment. Flag-Mcl-1-HT22 cells were treated as described above and ubiquitylated proteins were isolated and analyzed. Representative data are shown from at least three independent experiments. (I) T92A-Mcl-1 is resistant to ubiquitylation upon glutamate treatment. T92A-Mcl-1-HT22 cells were treated similarly and ubiquitylated proteins were isolated and analyzed. Representative data are shown from at least three independent experiments.
We next examined whether Mcl-1 degradation is mediated by ubiquitylation. We expressed 6×-His-ubiquitin in HT22 cells, which were exposed to glutamate for 12 h. Mcl-1 was then immunoprecipitated and potential ubiquitylation analyzed. As shown in Fig. 5G, glutamate treatment resulted in robust ubiquitylation of Mcl-1, which was significantly inhibited upon Cdk5 inhibition or depletion. Similar results were obtained in Flag-Mcl-1-HT22 cells upon glutamate treatment, confirming that Cdk5 promotes Mcl-1 degradation by ubiquitylation in glutamate-exposed HT22 cells (Fig. 5H). By contrast, T92A-Mcl-1 cells showed little ubiquitylation of mutant Mcl-1 (analyzed using Flag antibody) upon glutamate exposure, thereby suggesting that Cdk5-mediated phosphorylation of Mcl-1 at T92 contributes to Mcl-1 degradation (Fig. 5I).
Mcl-1 levels set the threshold for neuronal vulnerability
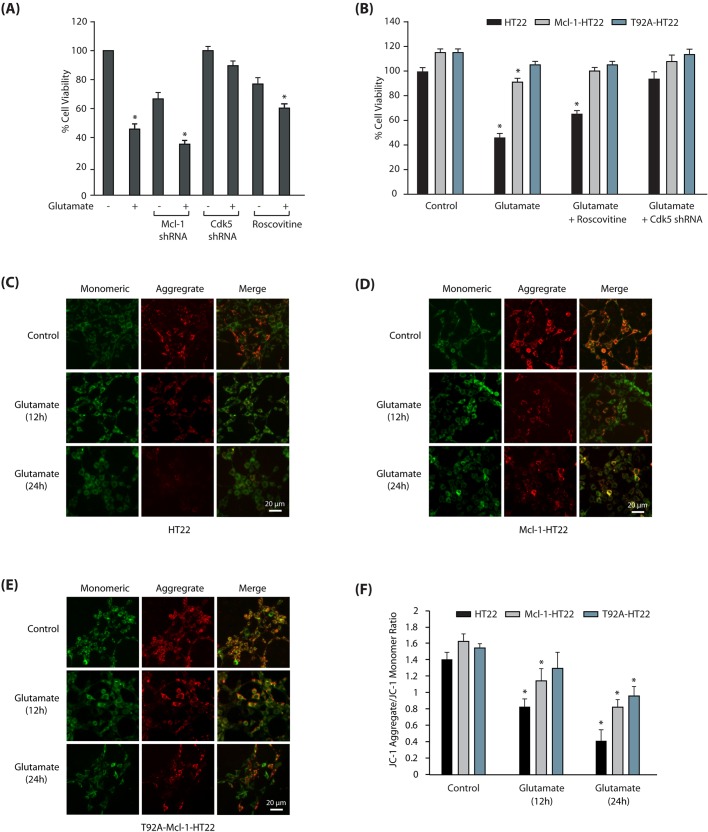
As Mcl-1 is an anti-apoptotic protein, we hypothesized that Cdk5-mediated degradation of Mcl-1 may be one of the mechanisms by which Cdk5 promotes neurotoxicity. We observed ∼50% loss of cell viability after 24 h of glutamate treatment in HT22 cells. Mcl-1 knockdown in these cells resulted in a modest decrease in cell viability (∼60%) (Fig. 6A). This result was not surprising, as Mcl-1 had already been depleted by >70% following glutamate treatment (Fig. 4D,E); therefore, its knockdown did not reveal any significant impact on cell viability. By contrast, in normal HT22 cells, Mcl-1 knockdown resulted in ∼30% loss of cell viability, suggesting that Mcl-1 is important for cell survival under normal and neurotoxic conditions in neuronal cells. Cdk5 inhibition using roscovitine was partially neuroprotective, which is presumably due to its inhibition of Cdk1 and Cdk2, both of which are required for the normal cell cycle. Cdk5 depletion was highly neuroprotective in glutamate-treated neuronal cells, confirming a central role of deregulated Cdk5 in promoting neurotoxic cascades.
Fig. 6.
Mcl-1 degradation promotes mitochondrial dysfunction and neurotoxicity. (A) Mcl-1 ablation is neurotoxic in HT22 cells. Scrambled-shRNA infected, Cdk5-shRNA- infected, Mcl-1-shRNA-infected or roscovitine-exposed HT22 cells were treated with glutamate for 24 h. Cell viability was examined using an MTT assay. *P<0.05, compared with untreated HT22 cells. (B) Ectopic expression of T92A-Mcl-1 rescues cells from glutamate toxicity. HT22, Mcl-1-HT22 and T92A-Mcl-1-HT22 cells were treated with glutamate in the absence or presence of roscovitine and Cdk5 shRNA. Cell viability was examined using an MTT assay. *P<0.05, compared with respective control cells. (C) Glutamate stimulation induces mitochondrial depolarization in HT22 cells. Mitochondrial depolarization was analyzed using JC-1 dye. (D) Glutamate stimulation induces mitochondrial depolarization in Mcl-1-HT22 cells. (E) T92A-Mcl-1-HT22 cells are more resistant to mitochondrial depolarization than control HT22 cells. (F) Ratio of JC-1 aggregates versus monomers in HT22, Mcl-1-HT22 and T92A- Mcl-1-HT22 cells. Data are mean±s.e.m. from three independent experiments. *P<0.05, compared with untreated cells. Scale bars: 20 μm.
The correlation between Mcl-1 degradation and increased cell death prompted us to overexpress Mcl-1 in HT22 cells as a potential therapeutic approach to rescue cells exposed to neurotoxic conditions. Overexpression of Mcl-1 was indeed highly neuroprotective and >80% of the cells survived, compared to 50% of regular HT22 cells. Phosphorylation-dead T92A-Mcl-1 expression rescued >90% of the cells from neurotoxicity, which underscores the significance of Mcl-1 upregulation as an approach for AD therapy (Fig. 6B).
Phosphorylation-resistant Mcl-1 protects cells from mitochondrial dysfunction
Mcl-1 serves as a critical regulatory point in mitochondrial-induced apoptosis by regulating the permeability of cytochrome c (Thomas et al., 2010). Release of cytochrome c triggers the formation of an apoptosome complex, thereby initiating a caspase cascade leading to neuronal apoptotic death. Therefore, we next investigated whether protection of Mcl-1 from Cdk5-mediated degradation rescues mitochondrial dysfunction triggered by glutamate stimulation in neuronal cells. Potential mitochondrial depolarization was analyzed using JC-1 dye in HT22, Mcl-1-HT22 and T92A-Mcl-1-HT22 cells exposed to glutamate for 12 h and 24 h. JC-1 is a cationic dye, used as an indicator of mitochondrial potential. When mitochondria are hyperpolarized, JC-1 accumulates at high concentration in the matrix, leading to its aggregation, resulting in red to orange emission. When mitochondria are depolarized, JC-1 is present predominantly as a monomer that yields green fluorescence. Importantly, the ratio of red to green fluorescence depends only on the membrane potential and is independent of mitochondrial size, shape, and density. Use of dual-color fluorescence detection thus allows researchers to perform ratiometric semiquantitative assessment of mitochondrial polarization states (Perry et al., 2011).
As shown in Fig. 6C, glutamate stimulation for 12 h and 24 h initiated robust mitochondrial depolarization in HT22 cells at 12 h, which significantly increased at 24 h. Although wild-type Mcl-1-expressing HT22 cells were relatively more resistant to mitochondrial dysfunction at 12 h and 24 h, T92A-Mcl-1-HT22 exhibited higher resilience to glutamate-induced neurotoxicity (Fig. 6D,E respectively). Fig. 6F displays the ratio of JC-1 aggregates versus monomers in HT22, Mcl-1-HT22 and T92A-Mcl-1-HT22 cells.
Deregulated Cdk5 triggers nuclear translocation of Mcl-1 in primary neurons upon excitotoxicity
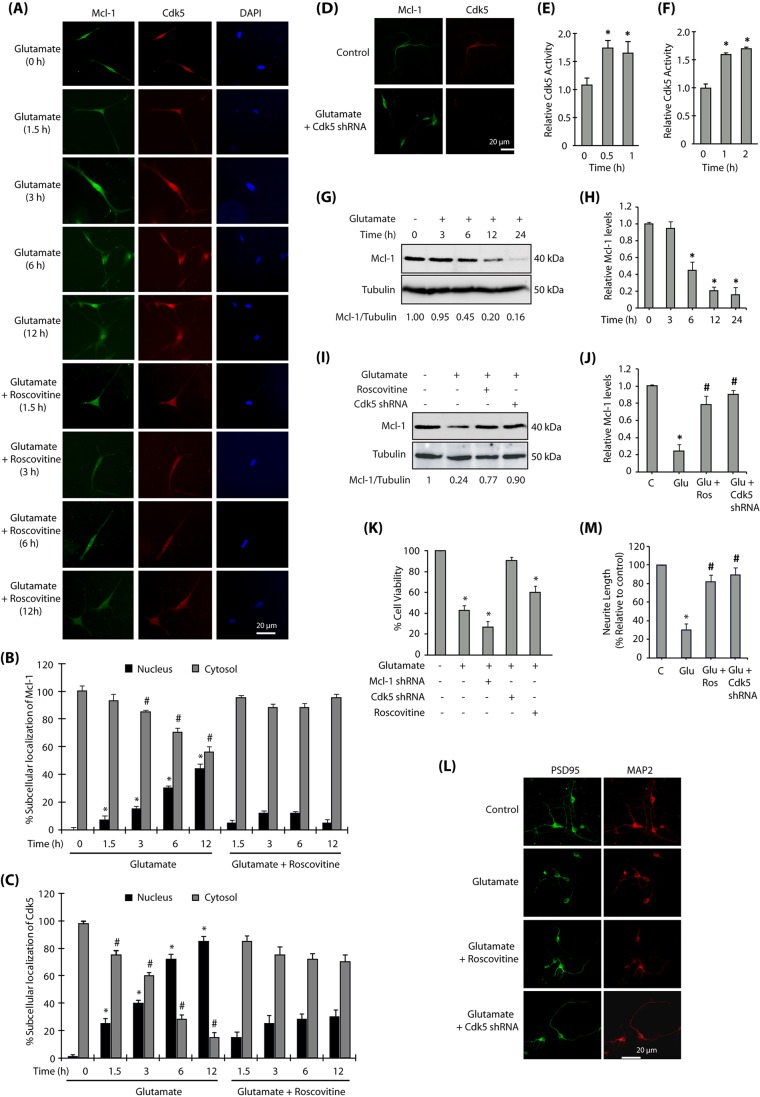
To examine a potential role of Cdk5 in regulating Mcl-1 levels and its consequences under more pathologically relevant conditions, we exposed mature primary neurons to 100 µM glutamate to induce excitotoxicity (Hosie et al., 2012). We initially analyzed whether glutamate stimulation affects the subcellular localization of Mcl-1 and if this event was regulated by Cdk5. Similar to the results obtained in HT22 cells, glutamate stimulation of primary cortical neurons exhibited a time-dependent increase in nuclear translocation of Mcl-1, which was essentially prevented when Cdk5 was inhibited using roscovitine or knocked down using Cdk5 shRNA (Fig. 7A–D). These results confirm that Cdk5 deregulation upon excitotoxicity triggers nuclear translocation of Mcl-1. In parallel, we also examined Cdk5 activity in primary cortical neurons, which confirmed Cdk5 activation upon exposure to glutamate (Fig. 7E). To further confirm that Cdk5 deregulation was caused by p25, we transduced TAT-p25, which too revealed similar levels of Cdk5 activation in primary neurons (Fig. 7F). The fusion of the TAT sequence with proteins enables protein transduction into cells when directly added to the culture (Becker-Hapak et al., 2001). We have previously shown that TAT-p25 transduction efficiently activates Cdk5 in cells (Sun et al., 2008a). TAT-fusion red fluorescent protein (TAT-RFP) was used as a control.
Fig. 7.
Mcl-1 signaling in primary neurons. (A) Cdk5 promotes nuclear translocation of Mcl-1 in primary neurons. Primary cortical neurons were treated with glutamate (100 µM) for 12 h in the presence or absence of roscovitine (10 µM), and then immunostained. (B) The percentage of cells showing nuclear translocation of Mcl-1. Data are mean±s.e.m. from three independent experiments. *P<0.05 vs nucleus fraction control; #P<0.05 vs cytoplasmic fraction control, analyzed by two-way analysis of variance. (C) The percentage of cells showing nuclear translocation of Cdk5. Data are mean±s.e.m. from three independent experiments. *P<0.05 vs nucleus fraction control; #P<0.05 vs cytoplasmic fraction control analyzed by two-way analysis of variance. (D) Cdk5 depletion inhibits nuclear translocation of Mcl-1. Primary cortical neurons were treated with glutamate (100 µM) for 12 h in the presence or absence of Cdk5 shRNA, and then immunostained. (E) Cdk5 kinase activity increases upon glutamate treatment in primary cortical neurons. Primary cortical neurons were treated with glutamate for 0.5 h and 1 h, and Cdk5 kinase activity was analyzed as described in the Materials and Methods. Data are mean±s.e.m. from three independent experiments. *P<0.05, compared with untreated primary neurons. (F) TAT-p25 transduction increases Cdk5 activity in primary cortical neurons. 200 nM TAT-p25 was transduced in primary neurons and Cdk5 kinase activity measured as described in the Materials and Methods. Data are mean±s.e.m. from three independent experiments. *P<0.05, compared with untreated primary neurons. (G) Mcl-1 is degraded by glutamate treatment. Primary cortical neurons were treated with glutamate for 0–24 h and the total levels of Mcl-1 analyzed. (H) Average relative ratios of Mcl-1 band intensities to α-tubulin band intensities upon glutamate treatment, as obtained from three independent experiments. *P<0.05, compared with untreated primary neurons. (I) Cdk5 depletion or inhibition prevents the decrease in Mcl-1 levels in glutamate-treated cells. Mcl-1 levels were analyzed following glutamate (12 h), roscovitine and Cdk5 shRNA treatments. (J) Mcl-1 protein levels in primary cortical neurons in response to glutamate treatment with or without roscovitine and Cdk5 shRNA. Data are mean±s.e.m. from three independent experiments. *P<0.05, compared with untreated primary neurons; #P<0.05, compared with glutamate-treated cells. (K) Inhibition and ablation of Cdk5 inhibits neurotoxicity while ablation of Mcl-1 promotes neurotoxicity in primary cortical neurons. Cdk5-shRNA- or Mcl-1-shRNA-infected primary neurons (30 h) were treated with glutamate (24 h). Cell viability was tested by an MTT assay. *P<0.05, compared with untreated neurons. (L) Cdk5 inhibition or ablation prevents synaptic neurotoxicity in primary neurons. Primary cortical neurons pre-treated with roscovitine or Cdk5 shRNA were treated with glutamate for 12 h, and then immunostained for PSD95 and MAP2. Representative images are shown. (M) Quantification of relative neurite length in cortical neurons exposed to various treatments. *P<0.05, compared with untreated primary neurons; #P<0.05, compared with glutamate-treated neurons. ImageJ 1.46r with add-in NeuriteQuant was used for morphological analysis. *P<0.05, compared with untreated neurons; #P<0.05, compared with glutamate-treated cells. Scale bars: 20 μm.
Cdk5 is responsible for Mcl-1 degradation under excitotoxic conditions in primary neurons
We next analyzed whether excitotoxicity affects the levels of Mcl-1 and whether this process is Cdk5 dependent. Mature primary cortical neurons were treated with glutamate for varying periods and Mcl-1 levels analyzed. As shown in Fig. 7G, excitotoxicity triggered a rapid loss in Mcl-1 levels (>50% in 6 h), similar to that observed in HT22 cells (Fig. 4D). Fig. 7H displays the alterations in Mcl-1 levels in response to excitotoxicity from three independent experiments.
A potential role of Cdk5 in degrading Mcl-1 upon excitotoxicity was examined using roscovitine and Cdk5 shRNA, both of which prevented Mcl-1 degradation under neurotoxic conditions (Fig. 7I,J), confirming that deregulated Cdk5 is responsible for degrading Mcl-1 in primary neurons. To investigate the significance of Mcl-1 levels in Cdk5-induced neurotoxicity, we examined cell viability in mature primary neurons treated with 100 µM glutamate. Excitotoxicity resulted in ∼60% loss of neuronal viability over 24 h (Fig. 7K). More importantly, Mcl-1 knockdown further sensitized the neurons to excitotoxic conditions resulting in >70% loss of cell viability. This finding is highly significant, as glutamate treatment already reduces Mcl-1 levels to ∼20% of its original level (Fig. 7G,H), and additional reduction in Mcl-1 levels via knockdown further sensitizes these neurons, suggesting that Mcl-1 degradation is an important contributor to Cdk5-induced neurotoxicity. Cdk5 depletion fully prevented the loss of neuronal viability induced by excitotoxicity, thus highlighting a central role of Cdk5 in this process.
Finally, we analyzed the role of Cdk5 in inducing synaptic toxicity under excitotoxic conditions by investigating the distribution of PSD95, a postsynaptic protein (also known as DLG4), in primary cortical neurons. Untreated cortical neurons immunostained with PSD95 (green) and MAP2 (a neuronal protein, red) antibodies displayed long neurites, which shrank severely upon glutamate treatment with a concomitant decrease in PSD95 levels (Fig. 7L,M). Inhibition or ablation of Cdk5 prevented PSD95 downregulation, highlighting a role of deregulated Cdk5 in synaptic toxicity.
Mcl-1 levels show inverse correlation with disease severity in AD pathogenesis
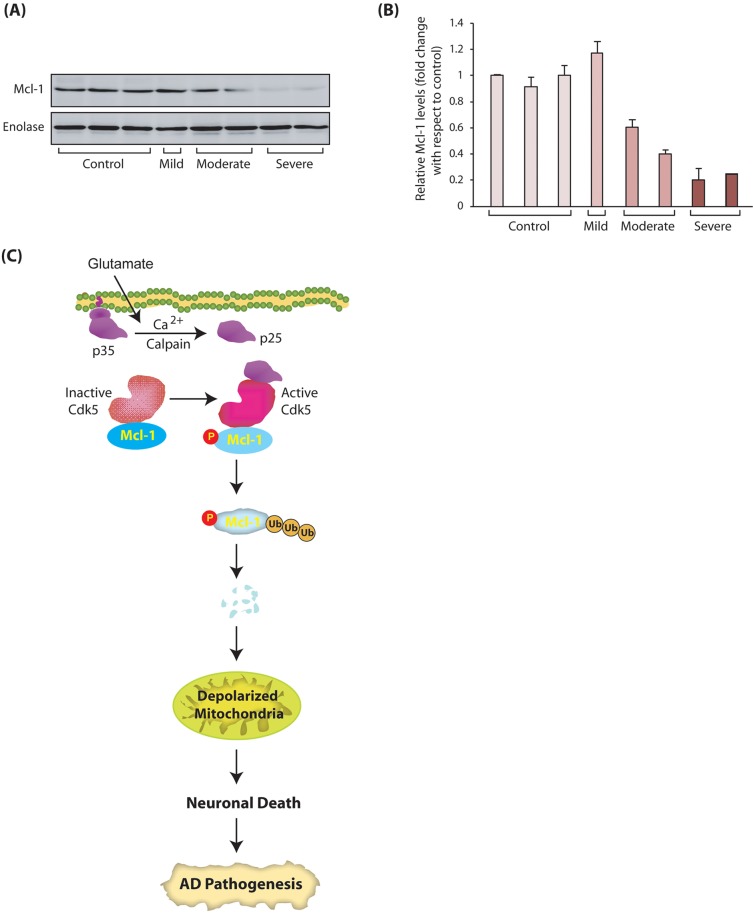
To investigate the clinical significance of our findings, we examined Mcl-1 levels in human AD and age-matched control brain tissues. We obtained the tissues from prefrontal cortex area 9 (Brodmann's area 9), a prefrontal association region located in the superior frontal gyrus of the brain, which is severely affected in AD (Braak and Braak, 1991). These neurons are extremely vulnerable in AD, and their loss strongly associates with the severity of the disease, with >90% loss occurring in the end stages of AD (Bussière et al., 2003). Mcl-1 levels were analyzed in tissues obtained from patients with mild (n=1), moderate (n=2) and severe (n=2) AD, along with age-matched controls (n=3). Although a limited number of specimens were analyzed in this study, among these clinical tissues, Mcl-1 levels exhibited a strong correlation with disease severity and decreased substantially as the disease progressed, with minimal Mcl-1 levels observed in the patients with severe AD (Fig. 8A). Fig. 8B shows the mean Mcl-1 levels in age-matched controls and AD patients from three independent experiments (Fig. 8B). These results supported our finding that Mcl-1 degradation is one of the key mechanisms in the progression of AD pathology.
Fig. 8.
Mcl-1 levels show inverse correlation with disease severity in AD clinical specimens. (A) Mcl-1 levels in human AD and age-matched control brain tissues. Mcl-1 levels were analyzed in tissues obtained from patients with mild (n=1), moderate (n=2) and severe (n=2) AD, along with age-matched controls (n=3). (B) Average Mcl-1 levels in age-matched controls and AD patients from three independent experiments. (C) Schematic of our model showing the link between Cdk5-mediated phosphorylation of Mcl-1 and neuronal death. Monomeric Cdk5 associates with Mcl-1. Glutamate stimulation triggers the formation of p25 formation, which binds the Cdk5-Mcl-1 complex, activating Cdk5. Deregulated Cdk5 phosphorylates Mcl-1 at T92, triggering its degradation by ubiquitylation. Mcl-1 degradation causes mitochondrial dysfunction and subsequent neuronal death.
DISCUSSION
Three spliced variants of Mcl-1 are known to exist in human tissues: full length or the long isoform (Mcl-1L), with an antiapoptotic function, the short isoform (Mcl-1S), with a proapoptotic function, and Mcl-1ES, also with a proapoptotic function (Kozopas et al., 1993; Bae et al., 2000; Kim et al., 2009). Previous studies have established that only the long isoform of Mcl-1 is expressed in the brain and is associated with neuronal survival (Mori et al., 2004). Although a crucial oncogenic role of Mcl-1 in cancer has been well established, only a handful of studies have analyzed its contribution in neurological disorders. A recent study revealed that β-amyloid injection into the lateral ventricles of mice results in downregulation of Mcl-1 due to a decrease in the levels of myocardin-related transcription factor-A (MRTF-A, also known as Mkl1), a transcriptional activator of Mcl-1 (Zhang et al., 2016). By contrast, another recent study reported that injection of β-amyloid in mice upregulates Mcl-1 mRNA leading to neuroprotection (Tao et al., 2017). Similarly, preconditioning of neurons with a sublethal dose of 5-aminoimidazole-4-carboxamide riboside (AICAR), activates AMP-activated protein kinase (AMPK), which in turn upregulates the transcription of Mcl-1, providing neuroprotection (Anilkumar et al., 2013). Taken together, these findings highlight a neuroprotective role of Mcl-1; however, it remains unclear whether Mcl-1 is upregulated or downregulated in response to neurotoxic insults in AD models. Similarly, it is not known whether Mcl-1 levels are regulated post-translationally in AD models. Mcl-1 levels have also not been analyzed in AD patient specimens and their correlation with disease progression remains unknown.
In this study, we have unraveled the mechanism by which Cdk5 deregulation directly leads to the degradation of Mcl-1 resulting in neurotoxicity in a model of AD (Fig. 8C). Our data demonstrate that Mcl-1 levels determine the threshold for cellular damage in response to neurotoxic insults. Interestingly, under basal conditions, inactive Cdk5 remains associated with Mcl-1, although it does not affect its levels. We also investigated potential binding of p35 and p25 to Mcl-1 independent of Cdk5 in an in vitro binding assay using purified recombinant Mcl-1, p35 and p25. Recombinant p25 and p35 on beads were independently incubated with 6×-His-Mcl-1. p35 and p25 immune complexes showed no binding with Mcl-1, confirming that p35 or p25 do not bind Mcl-1 directly (data not shown). However, glutamate stimulation triggers the association of p25 with the Cdk5-Mcl-1 complex, leading to Mcl-1 phosphorylation at T92 and rapid degradation via ubiquitylation. Degradation of Mcl-1 directly correlates with mitochondrial dysfunction and subsequent neuronal death (Fig. 8C).
Importantly, a recent study has shown that serum and KCl deprivation triggers apoptosis in cerebral granule neurons via phosphorylation of Mcl-1 at S140 and T144 by JNK1. Phosphorylation of Mcl-1 at T144 primes it for phosphorylation by GSK3β which phosphorylates it further at S159, triggering its degradation (Magiera et al., 2013). Consequently, the phospho-dead S140A, T144A double mutant of Mcl-1 is degradation-resistant and rescues cells from apoptosis.
In contrast to the results from Magiera et al. (2013), our results indicated a crucial role of Cdk5 in mediating neurotoxicity in a Mcl-1-dependent manner. We observed that ectopic expression of phospho-resistant T92A-Mcl-1 is fully capable of rescuing neurons from cell death by preventing mitochondrial dysfunction upon neurotoxic insults, which was comparable to the neuroprotection observed upon Cdk5 depletion. This finding suggests that Mcl-1 degradation is one of the key mechanisms by which Cdk5 promotes neurodegeneration. Furthermore, the clinical significance of our finding was confirmed in specimens from AD patients, in which a strong inverse correlation was observed between Mcl-1 levels and disease severity compared to age-matched controls. These findings strongly emphasize the potential of Mcl-1 upregulation as an attractive therapeutic strategy for the treatment of AD and perhaps other neurological disorders. This is in sharp contrast with cancer, for which degradation of Mcl-1 is highly desirable for eradicating diseased cells. Therefore, Mcl-1 upregulation in the brain needs to be highly tissue-specific, and could delay, and possibly prevent, neurodegeneration.
In conclusion, Cdk5 deregulation is known to trigger many neurotoxic cascades in AD pathogenesis, rendering Cdk5 a highly attractive therapeutic target for the treatment of AD. This study identified a new mechanism by which Cdk5 deregulation leads to rapid degradation of a pro-survival protein Mcl-1, a process that is extremely neurotoxic. Together, these results strongly emphasize the potential of Mcl-1 upregulation or Cdk5 inhibition as attractive therapeutic strategies for delaying and perhaps preventing neurodegeneration in AD patients.
MATERIALS AND METHODS
Glutamate, 3-(4,5-dimethyldiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and poly-l-lysine were obtained from Sigma-Aldrich. Roscovitine was purchased from LC Laboratories. Antibodies against Cdk5 (C-8), Cdk5 (DC-17), p35 (C-19), actin (C-2), α-tubulin (TU-02), lamin A (H-102), MAP2 (H300), PSD95 (7E3) and enolase (N-14) were purchased from Santa Cruz Biotechnology (Chang et al., 2011, 2012). Validated antibodies against Mcl-1 were purchased from One World Lab. All antibodies were used at 1:1000 dilution and are listed in Table S1. Validation of the Mcl-1 and Cdk5 antibodies is shown in Fig. S1.
Cell culture
HT22 cells were a kind gift from David Schubert (Salk Institute, La Jolla, CA). HEK293T cells were purchased from ATCC. HT22 cells, HEK293T and Phoenix cells were cultured as previously reported (Sun et al., 2011). All cells were tested for potential contamination.
Isolation of primary cortical neurons
The primary cortical neurons were isolated from E17 CD1 mice embryos as reported previously (Chang et al., 2012). All experiments were performed after 12 days in culture. Briefly, cortices were isolated and treated with trypsin (0.2 mg/ml) at 37°C for 30 min, followed by trituration in minimum essential media (MEM) supplemented with DNase (130 U/ml) and 10% FBS. They were then passed through a cell strainer (40 µM mesh). After centrifugation at 200 g for 4 min, the isolated cortical neurons were seeded on poly-L-lysine-coated coverslips in a 24-well plate at a density of 5×104 cells, or in a six-well plate at a density of 1×106 cells, in MEM supplemented with 5% FBS, 5% horse serum, 0.5 mM glutamine, 2.6 g/l glucose, 2.2 g/l NaHCO3 and 1 mM pyruvate. The medium was changed after 12 h and half the amount of medium was replaced every 5 days.
Expression plasmids and constructs
Flag-tagged human Mcl-1 was cloned into TAT-HA and VIP3 vectors at BamHI and EcoRI sites. Flag-tagged Mcl-1 mutant was generated using overlapping PCR.
Expression and purification of Cdk5, p25 and Mcl-1
6×-His-Cdk5 and 6×-His-p25 were generated and purified as reported previously (Chang et al., 2012). 6×-His tagged wild-type and mutant Mcl-1 were expressed in E. coli and purified according to our published procedures (Sun et al., 2008a,b).
Transfection and retroviral infection
For generating stable cell lines, Flag-tagged wild-type and mutant Mcl-1 plasmid (Addgene plasmid #25371, deposited by Roger Davis) (Morel et al., 2009) were transiently transfected using calcium phosphate into Phoenix cells. The retroviruses were harvested and used to infect HT22 cells as reported previously (Shah and Shokat, 2002).
In vitro kinase assays
For in vitro labeling, 2 µg of 6×-His-tagged wild-type or mutant Mcl-1 were incubated with Cdk5-p25 and 0.5 mCi of [γ-32P]ATP in a kinase buffer. Reactions were terminated by adding SDS sample buffer, after which the proteins were separated by SDS-PAGE, transferred to PVDF membrane and exposed for autoradiography. For determining Cdk5 activity in control and glutamate-treated cells, Cdk5 immune complexes were isolated, and subjected to an in vitro kinase assay using [32P]ATP and 5 µg Cdk5 substrate peptide (KHHKSPKHR) in a final volume of 30 µl kinase buffer (50 mM Tris-HCl, pH 8.00, 20 mM MgCl2). After 30 min, the reactions were terminated by spotting 25 µl of the reaction mixture on p81 phosphocellulose disks (Whatman) and immersing in 100 ml 10% acetic acid for 25 min, followed by three washes in 0.5% phosphoric acid (3–5 min each) and finally rinsing with acetone. Radioactivity was measured in a liquid scintillation counter (Packard Instrument Company, CA).
Mcl-1 shRNA
Cdk5 shRNA (mouse) was generated in our previous study (Chang et al., 2011). Mcl-1 shRNA was cloned into pLKO.1 vector (Addgene plasmid #10878, deposited by David Root) (Moffat et al., 2006). Mcl-1 shRNA (mouse) primer sequences were as follows: forward primer: 5′-CCGGGGGACTGGCTAGTTAAACAAACTCGAGTTTGTTTAACTAGCCAGTCCCTTTTTG-3′; reverse primer: 5′-AATTCAAAAAGGGACTGGCTAGTTAAACAAACTCGAGTTTGTTTAACTAGCCAGTCCC-3′. Scrambled shRNA, Cdk5 and Mcl-1 shRNA lentiviruses were generated and used for infecting HT22 cells for 30 h. Mcl-1 and Cdk5 shRNA validation are shown in Fig. S1A,B.
Western blotting
Cells were lysed in modified RIPA buffer, supplemented with protease inhibitors. Equal amounts of cell extracts were used for western blotting.
MTT assay
Cells were seeded in 96-well plates at 5×103 cells per 100 µl per well and cultured for 24 h. The MTT assay was conducted as reported previously (Sun et al., 2008a). At the end of treatment, MTT (0.5 mg/ml) was added and incubated for an additional 30 min. The media were aspirated and the cells on wells dissolved in DMSO (100 µl). The absorbance value at 590 nm was measured using a microplate reader (Tecan Spectrafluor Plus, Männedorf). Experiments were repeated three times in triplicate wells to ensure reproducibility.
Immunoprecipitation of Cdk5 and Mcl-1 from HT22 cells
HT22 cells were treated with 5 mM glutamate for 1.5, 3 or 6 h. Cells were then harvested and lysed in RIPA buffer. After centrifugation, whole-cell lysate was mixed with protein A Sepharose beads (Sigma-Aldrich) and antibody for either Cdk5 or Mcl-1. After incubation overnight, immunocomplexes were washed and then subjected to western blotting using either Cdk5 or Mcl-1 antibodies.
Cdk5 and Mcl-1 in vitro binding assay
To investigate whether Mcl-1 directly binds Cdk5 in an in vitro assay, recombinant 6×-His-Mcl-1 was incubated with either Mcl-1 antibody or IgG control in the presence of protein A Sepharose beads for 4 h at 4°C. Both immune complexes were washed and incubated with 6×-His-Cdk5 for an additional 4 h. The beads were washed, loaded on SDS-PAGE gels, transferred to PVDF membrane and probed using Cdk5 antibody. For reciprocal immunoprecipitation, recombinant 6×-His-Cdk5 was incubated with either Cdk5 antibody or IgG control. Both immune complexes were washed and incubated with 6×-His-Mcl-1. Subsequently, the beads were washed, loaded on SDS-PAGE, transferred onto PVDF membrane and probed using Mcl-1 antibody.
Subcellular fractionation
Subcellular fractionation of HT22 cells was conducted as described previously (Chang et al., 2011). Briefly, cells were lysed in subcellular fractionation buffer (250 mM sucrose, 20 mM HEPES, pH 7.4, 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1 mM PMSF, 1 mM DTT) and then passed through a 25 G needle (10×). The cell lysate was further incubated on ice for 20 min. The nuclear pellet was obtained after centrifugation at 900 g for 5 min. The supernatant was centrifuged at 6000 g for 10 min. The cytosolic fraction was obtained after the removal of supernatant. Both nuclear and cytosolic fractions were mixed with SDS loading dye. Proteins were separated using SDS-PAGE and transferred to PVDF membrane. Tubulin was used as the cytoplasmic marker and lamin A as the nuclear marker.
Immunofluorescence
HT22 cells were grown on poly-L-lysine-coated coverslips for 24 h before subjecting to various treatments. Roscovitine (10 μM) was added 0.5 h before glutamate treatment and Cdk5 shRNA lentivirus was added 30 h prior to glutamate treatment. Cells were fixed with 4% formaldehyde in PBS for 20 min at room temperature, and then washed three times with PBS. The cells were blocked in 1% FBS, 2% BSA and 0.1% triton X-100 in PBS for 1 h at 25°C. Cells were labeled with antibodies (Cdk5, Mcl-1, MAP2, PSD95 or Flag) for 2 h in PBS, followed by incubation with fluorescein isothiocyanate-conjugated goat anti-mouse-IgG secondary antibody (1:1000 dilution, cat. no. 115-095-003, Jackson Immunoresearch), fluorescein isothiocyanate-conjugated donkey anti-rabbit-IgG secondary antibody (1:1000 dilution, cat. no. 711-095-152, Jackson Immunoresearch), Texas Red-conjugated goat anti-rabbit-IgG secondary antibody (1:500 dilution, T6391, Invitrogen) or Texas Red-conjugated goat anti-mouse-IgG secondary antibody (1:500 dilution, cat. no. T6390, Invitrogen). Cells were visualized using an Eclipse E600 microscope (Nikon Instruments). To quantify cytoplasmic and nuclear localization, the average fluorescence intensity in the nucleus was compared with the average fluorescence intensity of an area of equal size in the cytosol. This value was plotted with respect to different treatment times. The ratio between the two was used to define whether the localization of the protein in the cell is cytoplasmic or nuclear. If the ratio was more than 1, the protein was considered to be positive for nuclear localization.
Protein stability assay
HT22 cells were grown to 60–70% confluency and treated with 10 μg/ml protein synthesis inhibitor cycloheximide (30 min pretreatment) with or without glutamate treatment. Cells were harvested at various time points and protein levels analyzed.
Ubiquitylation assay
HT22 cells were transfected with 6×-His-ubiquitin followed by glutamate treatment for 12 h in the presence and absence of roscovitine or Cdk5 shRNA. After 6 h of glutamate treatment, MG132 (Sigma-Aldrich) was added to a final concentration of 10 μM for an additional 6 h to stabilize ubiquitylated proteins. After 12 h of glutamate treatment, cells were lysed and Mcl-1 was immunoprecipitated using anti-Mcl-1 antibody. The proteins were then separated by SDS-PAGE and potential ubiquitylation was analyzed using anti-6×-His antibody. Each experiment was performed at least three times independently.
Mitochondria polarization measurement
Mitochondrial depolarization was measured as reported previously (Sun et al., 2008b). Briefly, HT22, Mcl-1-HT22 and T92A-Mcl-1-HT22 cells were treated with glutamate for 12 h or 24 h. Subsequently, 0.8 µg/µl JC-1 was added from a 40 µg/µl stock solution in DMSO and incubated for 10 min. JC-1 was removed and PBS was added for imaging. Representative images were taken from each sample. The percentage of cells with depolarized mitochondria was counted as the average of 100 cells from at least five random fields.
Statistical analysis
Data are expressed as mean±s.e.m. and were statistically evaluated with one-way ANOVA followed by the Bonferroni post hoc test using GraphPad Prism 5.04 software (GraphPad Software). P<0.05 was considered statistically significant.
Acknowledgements
We thank Dr David Schubert for HT22 cells and New York Brain Bank for providing clinical specimens.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: K.S.; Methodology: K.N.; Validation: K.N.; Formal analysis: K.N., K.S.; Investigation: K.N., K.S.; Data curation: K.N.; Writing - original draft: K.S.; Writing - review & editing: K.N., K.S.; Visualization: K.S.; Supervision: K.S.; Project administration: K.S.; Funding acquisition: K.S.
Funding
This work was supported by the National Institute on Aging (R21-AG 47447 to K.S. and P50 AG008702 to the New York Brain Bank, New York). Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://jcs.biologists.org/lookup/doi/10.1242/jcs.205666.supplemental
References
- Adams K. W. and Cooper G. M. (2007). Rapid turnover of mcl-1 couples translation to cell survival and apoptosis. J. Biol. Chem. 282, 6192-6200. 10.1074/jbc.M610643200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anilkumar U., Weisová P., Düssmann H., Concannon C. G., König H.-G. and Prehn J. H. M. (2013). AMP-activated protein kinase (AMPK)-induced preconditioning in primary cortical neurons involves activation of MCL-1. J. Neurochem. 124, 721-734. 10.1111/jnc.12108 [DOI] [PubMed] [Google Scholar]
- Arbour N., Vanderluit J. L., Le Grand J. N., Jahani-Asl A., Ruzhynsky V. A., Cheung E. C. C., Kelly M. A., MacKenzie A. E., Park D. S., Opferman J. T. et al. (2008). Mcl-1 is a key regulator of apoptosis during CNS development and after DNA damage. J. Neurosci. 28, 6068-6078. 10.1523/JNEUROSCI.4940-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae J., Leo C. P., Hsu S. Y. and Hsueh A. J. W. (2000). MCL-1S, a splicing variant of the antiapoptotic BCL-2 family member MCL-1, encodes a proapoptotic protein possessing only the BH3 domain. J. Biol. Chem. 275, 25255-25261. 10.1074/jbc.M909826199 [DOI] [PubMed] [Google Scholar]
- Becker-Hapak M., McAllister S. S. and Dowdy S. F. (2001). TAT-mediated protein transduction into mammalian cells. Methods 24, 247-256. 10.1006/meth.2001.1186 [DOI] [PubMed] [Google Scholar]
- Braak H. and Braak E. (1991). Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 82, 239-259. 10.1007/BF00308809 [DOI] [PubMed] [Google Scholar]
- Brinkkoetter P. T., Pippin J. W. and Shankland S. J. (2010). Cyclin I-Cdk5 governs survival in post-mitotic cells. Cell Cycle 9, 1729-1731. 10.4161/cc.9.9.11471 [DOI] [PubMed] [Google Scholar]
- Bussière T., Giannakopoulos P., Bouras C., Perl D. P., Morrison J. H. and Hof P. R. (2003). Progressive degeneration of nonphosphorylated neurofilament protein-enriched pyramidal neurons predicts cognitive impairment in Alzheimer's disease: stereologic analysis of prefrontal cortex area 9. J. Comp. Neurol. 463, 281-302. 10.1002/cne.10760 [DOI] [PubMed] [Google Scholar]
- Chang K. H., Pablo Y., Lee H., Lee H., Smith M. and Shah K. (2010). Cdk5 is a major regulator of p38 cascade: relevance to neurotoxicity in Alzheimer's disease. J. Neurochem. 113, 1221-1229. 10.1111/j.1471-4159.2010.06687.x [DOI] [PubMed] [Google Scholar]
- Chang K.-H., Multani P. S., Sun K.-H., Vincent F., de Pablo Y., Ghosh S., Gupta R., Lee H.-P., Lee H. G., Smith M. A. et al. (2011). Nuclear envelope dispersion triggered by deregulated Cdk5 precedes neuronal death. Mol. Biol. Cell 22, 1452-1462. 10.1091/mbc.E10-07-0654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K.-H., Vincent F. and Shah K. (2012). Deregulated Cdk5 triggers aberrant activation of cell cycle kinases and phosphatases inducing neuronal death. J. Cell Sci. 125, 5124-5137. 10.1242/jcs.108183 [DOI] [PubMed] [Google Scholar]
- Dhavan R. and Tsai L.-H. (2001). A decade of CDK5. Nat. Rev. Mol. Cell Biol. 2, 749-759. 10.1038/35096019 [DOI] [PubMed] [Google Scholar]
- Drerup J. M., Hayashi K., Cui H., Mettlach G. L., Long M. A., Marvin M., Sun X., Goldberg M. S., Lutter M. and Bibb J. A. (2010). Attention-deficit/hyperactivity phenotype in mice lacking the cyclin-dependent kinase 5 cofactor p35. Biol. Psychiatry 68, 1163-1171. 10.1016/j.biopsych.2010.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engmann O., Hortobágyi T., Pidsley R., Troakes C., Bernstein H.-G., Kreutz M. R., Mill J., Nikolic M. and Giese K. P. (2011). Schizophrenia is associated with dysregulation of a Cdk5 activator that regulates synaptic protein expression and cognition. Brain 134, 2408-2421. 10.1093/brain/awr155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu W. Y., Chen Y., Sahin M., Zhao X. S., Shi L., Bikoff J. B., Lai K. O., Yung W. H., Fu A. K., Greenberg M. E. et al. (2007). Cdk5 regulates EphA4-mediated dendritic spine retraction through an ephexin1-dependent mechanism. Nat. Neurosci. 10, 67-76. 10.1038/nn1811 [DOI] [PubMed] [Google Scholar]
- Heller E. A., Hamilton P. J., Burek D. D., Lombroso S. I., Peña C. J., Neve R. L. and Nestler E. J. (2016). Targeted epigenetic remodeling of the Cdk5 gene in nucleus accumbens regulates cocaine- and stress-evoked behavior. J. Neurosci. 36, 4690-4697. 10.1523/JNEUROSCI.0013-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez A., Tan C., Mettlach G., Pozo K., Plattner F. and Bibb J. A. (2016). Cdk5 modulates long-term synaptic plasticity and motor learning in dorsolateral striatum. Sci. Rep. 6, 29812 10.1038/srep29812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisanaga S.-i. and Endo R. (2010). Regulation and role of cyclin-dependent kinase activity in neuronal survival and death. J. Neurochem. 115, 1309-1321. 10.1111/j.1471-4159.2010.07050.x [DOI] [PubMed] [Google Scholar]
- Hosie K. A., King A. E., Blizzard C. A., Vickers J. C. and Dickson T. C. (2012). Chronic excitotoxin-induced axon degeneration in a compartmented neuronal culture model. ASN Neuro. 4, e00076 10.1042/AN20110031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E. O., Chang K.-H., de Pablo Y., Ghosh S., Mehta R., Badve S. and Shah K. (2011). PHLDA1 is a critical negative regulator and effector of AURKA kinase in breast cancer. J. Cell Sci. 124, 2711-2722. 10.1242/jcs.084970 [DOI] [PubMed] [Google Scholar]
- Johnson E. O., Chang K.-H., Ghosh S., Venkatesh C., Giger K., Low P. S. and Shah K. (2012). LIMK2 is a crucial regulator and effector of Aurora-A-kinase-mediated malignancy. J. Cell Sci. 125, 1204-1216. 10.1242/jcs.092304 [DOI] [PubMed] [Google Scholar]
- Kim S. and Shah K. (2007). Dissecting yeast Hog1 MAP kinase pathway using a chemical genetic approach. FEBS Lett. 581, 1209-1216. 10.1016/j.febslet.2007.02.032 [DOI] [PubMed] [Google Scholar]
- Kim J.-H., Sim S.-H., Ha H.-J., Ko J.-J., Lee K. and Bae J. (2009). MCL-1ES, a novel variant of MCL-1, associates with MCL-1L and induces mitochondrial cell death. FEBS Lett. 583, 2758-2764. 10.1016/j.febslet.2009.08.006 [DOI] [PubMed] [Google Scholar]
- Kobayashi H., Saito T., Sato K., Furusawa K., Hosokawa T., Tsutsumi K., Asada A., Kamada S., Ohshima T. and Hisanaga S.-i. (2014). Phosphorylation of cyclin-dependent kinase 5 (Cdk5) at Tyr-15 is inhibited by Cdk5 activators and does not contribute to the activation of Cdk5. J. Biol. Chem. 289, 19627-19636. 10.1074/jbc.M113.501148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozopas K. M., Yang T., Buchan H. L., Zhou P. and Craig R. W. (1993). MCL1, a gene expressed in programmed myeloid cell differentiation, has sequence similarity to BCL2. Proc. Natl. Acad. Sci. USA 90, 3516-3520. 10.1073/pnas.90.8.3516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magiera M. M., Mora S., Mojsa B., Robbins I., Lassot I. and Desagher S. (2013). Trim17-mediated ubiquitination and degradation of Mcl-1 initiate apoptosis in neurons. Cell Death Differ. 20, 281-292. 10.1038/cdd.2012.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer L., Borgne A., Mulner O., Chong J. P. J., Blow J. J., Inagaki N., Inagaki M., Delcros J.-G. and Moulinoux J.-P. (1997). Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur. J. Biochem. 243, 527-536. 10.1111/j.1432-1033.1997.t01-2-00527.x [DOI] [PubMed] [Google Scholar]
- Meyer D. A., Torres-Altoro M. I., Tan Z., Tozzi A., Di Filippo M., DiNapoli V., Plattner F., Kansy J. W., Benkovic S. A., Huber J. D. et al. (2014). Ischemic stroke injury is mediated by aberrant Cdk5. J. Neurosci. 34, 8259-8267. 10.1523/JNEUROSCI.4368-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modi P. K., Komaravelli N., Singh N. and Sharma P. (2012). Interplay between MEK-ERK signaling, cyclin D1, and cyclin-dependent kinase 5 regulates cell cycle reentry and apoptosis of neurons. Mol. Biol. Cell 23, 3722-3730. 10.1091/mbc.E12-02-0125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffat J., Grueneberg D. A., Yang X., Kim S. Y., Kloepfer A. M., Hinkle G., Piqani B., Eisenhaure T. M., Luo B., Grenier J. K., et al. (2006). A lentiviral RNAi library for human and mouse genes applied to an arrayed viral high-content screen . Cell 124, 1283-1298. 10.1016/j.cell.2006.01.040 [DOI] [PubMed] [Google Scholar]
- Morel C., Carlson S. M., White F. M. and Davis R. J. (2009). Mcl-1 integrates the opposing actions of signaling pathways that mediate survival and apoptosis. Mol. Cell. Biol. 29, 3845-3852. 10.1128/MCB.00279-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M., Burgess D. L., Gefrides L. A., Foreman P. J., Opferman J. T., Korsmeyer S. J., Cavalheiro E. A., Naffah-Mazzacoratti M. G. and Noebels J. L. (2004). Expression of apoptosis inhibitor protein Mcl1 linked to neuroprotection in CNS neurons. Cell Death Differ. 11, 1223-1233. 10.1038/sj.cdd.4401483 [DOI] [PubMed] [Google Scholar]
- Odajima J., Wills Z. P., Ndassa Y. M., Terunuma M., Kretschmannova K., Deeb T. Z., Geng Y., Gawrzak S., Quadros I. M., Newman J. et al. (2011). Cyclin E constrains Cdk5 activity to regulate synaptic plasticity and memory formation. Dev. Cell 21, 655-668. 10.1016/j.devcel.2011.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohshima T., Ward J. M., Huh C. G., Longenecker G., Veeranna, Pant H. C., Brady R. O., Martin L. J. and Kulkarni A. B. (1996). Targeted disruption of the cyclin-dependent kinase 5 gene results in abnormal corticogenesis, neuronal pathology and perinatal death. Proc. Natl. Acad. Sci. USA 93, 11173-11178. 10.1073/pnas.93.20.11173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel L. S., Wenzel H. J. and Schwartzkroin P. A. (2004). Physiological and morphological characterization of dentate granule cells in the p35 knock-out mouse hippocampus: evidence for an epileptic circuit. J. Neurosci. 24, 9005-9014. 10.1523/JNEUROSCI.2943-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry S. W., Norman J. P., Barbieri J., Brown E. B. and Gelbard H. A. (2011). Mitochondrial membrane potential probes and the proton gradient: a practical usage guide. BioTechniques 50, 98-115. 10.2144/000113610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinkenberger J. L., Horning S., Klocke B., Roth K. and Korsmeyer S. J. (2000). Mcl-1 deficiency results in peri-implantation embryonic lethality. Genes Dev. 14, 23-27. 10.1101/gad.14.1.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y., Cheng C., Uchida Y., Nakajima O., Ohshima T., Yagi T., Taniguchi M., Nakayama T., Kishida R., Kudo Y. et al. (2002). Fyn and Cdk5 mediate semaphorin-3A signaling, which is involved in regulation of dendrite orientation in cerebral cortex. Neuron 35, 907-920. 10.1016/S0896-6273(02)00857-7 [DOI] [PubMed] [Google Scholar]
- Shah K. and Lahiri D. K. (2014). Cdk5 activity in the brain - multiple paths of regulation. J. Cell Sci. 127, 2391-2400. 10.1242/jcs.147553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah K. and Lahiri D. K. (2017). A tale of the good and bad: remodeling of the microtubule network in the brain by Cdk5. Mol. Neurobiol. 54, 2255-2268. 10.1007/s12035-016-9792-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah K. and Rossie S. (2017). Tale of the good and bad Cdk5: remodeling of the actin cytoskeleton in the brain. Mol. Neurobiol. (In press) 10.1007/s12035-017-0525-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah K. and Shokat K. M. (2002). A chemical genetic screen for direct v-Src substrates reveals ordered assembly of a retrograde signaling pathway. Chem. Biol. 9, 35-47. 10.1016/S1074-5521(02)00086-8 [DOI] [PubMed] [Google Scholar]
- Shah K. and Shokat K. M. (2003). A chemical genetic approach for the identification of direct substrates of protein kinases. Methods Mol. Biol. 233, 253-271. 10.1385/1-59259-397-6:253 [DOI] [PubMed] [Google Scholar]
- Shah K. and Vincent F. (2005). Divergent roles of c-Src in controlling platelet-derived growth factor-dependent signaling in fibroblasts. Mol. Biol. Cell 16, 5418-5432. 10.1091/mbc.E05-03-0263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah K., Liu Y., Deirmengian C. and Shokat K. M. (1997). Engineering unnatural nucleotide specificity for Rous sarcoma virus tyrosine kinase to uniquely label its direct substrates. Proc. Natl. Acad. Sci. USA 94, 3565-3570. 10.1073/pnas.94.8.3565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C., Viccaro K., Lee H.-g. and Shah K. (2016). Cdk5-FOXO3a axis: initially neuroprotective, eventually neurodegenerative in Alzheimer's disease models. J. Cell Sci. 129, 1815-1830. 10.1242/jcs.185009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla V., Skuntz S. and Pant H. C. (2012). Deregulated Cdk5 activity is involved in inducing Alzheimer's disease. Arch. Med. Res. 43, 655-662. 10.1016/j.arcmed.2012.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla V., Seo J., Binukumar B. K., Amin N. D., Reddy P., Grant P., Kuntz S., Kesavapany S., Steiner J., Mishra S. K. et al. (2017). TFP5, a peptide inhibitor of aberrant and hyperactive Cdk5/p25, attenuates pathological phenotypes and restores synaptic function in CK-p25Tg mice. J. Alzheimers Dis. 56, 335-349. 10.3233/JAD-160916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W. J., Son M. Y., Lee H. W., Seo H., Kim J. H. and Chung S. H. (2015). Enhancement of BACE1 activity by p25/Cdk5-mediated phosphorylation in Alzheimer's disease. PLoS ONE 10, e0136950 10.1371/journal.pone.0136950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun K.-H., de Pablo Y., Vincent F., Johnson E. O., Chavers A. K. and Shah K. (2008a). Novel genetic tools reveal Cdk5's major role in Golgi fragmentation in Alzheimer's disease. Mol. Biol. Cell 19, 3052-3069. 10.1091/mbc.E07-11-1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun K.-H., De Pablo Y., Vincent F. and Shah K. (2008b). Deregulated Cdk5 promotes oxidative stress and mitochondrial dysfunction. J. Neurochem. 107, 265-278. 10.1111/j.1471-4159.2008.05616.x [DOI] [PubMed] [Google Scholar]
- Sun K.-H., Lee H.-g., Smith M. A. and Shah K. (2009). Direct and indirect roles of Cdk5 as an upstream regulator in the JNK cascade: relevance to neurotoxic insults in Alzheimer's disease. Mol. Biol. Cell 20, 4611-4619. 10.1091/mbc.E09-05-0433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun K.-H., Chang K. H., Clawson S., Ghosh S., Mirzaei H., Regnier F. and Shah K. (2011). Glutathione S-transferase P1 is a critical regulator of Cdk5 kinase activity. J. Neurochem. 118, 902-914. 10.1111/j.1471-4159.2011.07343.x [DOI] [PubMed] [Google Scholar]
- Sun X., Jin L. and Ling P. (2012). Review of drugs for Alzheimer's disease. Drug Discov. Ther. 6, 285-290. 10.5582/ddt.2012.v6.6.285 [DOI] [PubMed] [Google Scholar]
- Tang D., Yeung J., Lee K.-Y., Matsushita M., Matsui H., Tomizawa K., Hatase O. and Wang J. H. (1995). An isoform of the neuronal cyclin-dependent kinase 5 (Cdk5) activator. J. Biol. Chem. 270, 26897-26903. 10.1074/jbc.270.45.26897 [DOI] [PubMed] [Google Scholar]
- Tao C. C., Hsu W. L., Ma Y. L., Cheng S. J. and Lee E. H. Y. (2017). Epigenetic regulation of HDAC1 SUMOylation as an endogenous neuroprotection against Aβ toxicity in a mouse model of Alzheimer's disease. Cell Death Differ. 24, 597-614. 10.1038/cdd.2016.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas L. W., Lam C. and Edwards S. W. (2010). Mcl-1; the molecular regulation of protein function. FEBS Lett. 584, 2981-2989. 10.1016/j.febslet.2010.05.061 [DOI] [PubMed] [Google Scholar]
- Tsai L.-H., Delalle I., Caviness V. S. Jr, Chae T. and Harlow E. (1994). p35 is a neural-specific regulatory subunit of cyclin-dependent kinase 5. Nature 371, 419-423. 10.1038/371419a0 [DOI] [PubMed] [Google Scholar]
- Venturin M., Guarnieri P., Natacci F., Stabile M., Tenconi R., Clementi M., Hernandez C., Thompson P., Upadhyaya M., Larizza L. et al. (2004). Mental retardation and cardiovascular malformations in NF1 microdeleted patients point to candidate genes in 17q11.2. J. Med. Genet. 41, 35-41. 10.1136/jmg.2003.014761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Nikhil K., Viccaro K., Chang L., White J. and Shah K. (2017a). Phosphorylation-dependent regulation of ALDH1A1 by aurora kinase a: insights on their synergistic relationship in pancreatic cancer. BMC Biol. 15, 1-10. 10.1186/s12915-016-0335-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Nikhil K., Viccaro K., Lei C., Jacobsen M., Sandusky G. and Shah K. (2017b). Aurora A-Twist1 axis promotes highly aggressive phenotypes in pancreatic carcinoma. J. Cell Sci. 130, 1078-1093. 10.1242/jcs.196790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Y., Yu W. H., Maloney B., Bailey J., Ma J., Marié I., Maurin T., Wang L., Figueroa H., Herman M. et al. (2008). Transcriptional regulation of beta-secretase by p25/cdk5 leads to enhanced amyloidogenic processing. Neuron 57, 680-690. 10.1016/j.neuron.2008.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Pan H.-y., Hu X.-m., Cao X.-l., Wang J., Min Z.-l., Xu S.-q., Xiao W., Yuan Q., Li N. et al. (2016). The role of myocardin-related transcription factor-A in Aβ25-35 induced neuron apoptosis and synapse injury. Brain Res. 1648, 27-34. 10.1016/j.brainres.2016.07.003 [DOI] [PubMed] [Google Scholar]
- Zukerberg L. R., Patrick G. N., Nikolic M., Humbert S., Wu C. L., Lanier L. M., Gertler F. B., Vidal M., Van Etten R. A. and Tsai L.-H. (2000). Cables links Cdk5 and c-Abl and facilitates Cdk5 tyrosine phosphorylation, kinase upregulation, and neurite outgrowth. Neuron 26, 633-646. 10.1016/S0896-6273(00)81200-3 [DOI] [PubMed] [Google Scholar]