Abstract
Hypertrophic cardiomyopathy (HCM) is the most common inherited cardiac disease in humans and results in significant morbidity and mortality. Research over the past 25 years has contributed enormous insight into this inherited disease particularly in the areas of genetics, molecular mechanisms, and pathophysiology. Our understanding continues to be limited by the heterogeneity of clinical presentations with various genetic mutations associated with HCM. Transgenic mouse models have been utilized especially studying the genotypic and phenotypic interactions. However, mice possess intrinsic cardiac and hemodynamic differences compared to humans and have limitations preventing their direct translation. Other animal models of HCM have been studied or generated in part to overcome these limitations. HCM in cats shows strikingly similar molecular, histopathological, and genetic similarities to human HCM, and offers an important translational opportunity for the study of this disease. Recently, inherited left ventricular hypertrophy in rhesus macaques was identified and collaborative investigations have been conducted to begin to develop a non-human primate HCM model. These naturally-occurring large-animal models may aid in advancing our understanding of HCM and developing novel therapeutic approaches to this disease. This review will highlight the features of HCM in humans and the relevant available and developing animal models of this condition.
Keywords: familial hypertrophic cardiomyopathy, left ventricular hypertrophy, cardiomyopathy, animal models, genetics
Introduction
Hypertrophic cardiomyopathy (HCM) has been recognized for more than fifty years, with first pathological description published in 1958, followed by the first clinical report in the early 1960s [1]. Since then, clinical, biochemical, pathologic, and genetic studies have made enormous progress to better understand this inherited disease. However, this information has not yet been translated into practical information necessary for the prevention or treatment of HCM. This is in part because of the unexpected heterogeneity and complexity in phenotypic presentations with underlying genetic causes of HCM. To date, over 1,500 mutations in at least 20 genes encoding the myofilaments of the sarcomere or Z-disc have been identified to be causative or associated with HCM. This polygenetic feature of HCM makes a homogenous study population challenging to identify given the large numbers required for successful human clinical trials [2]. Controlled prospective studies are necessary to identify pre-clinical signs of disease in human HCM patients, but such studies are complicated, expensive, and require lengthy periods of longitudinal follow-up. Mouse models of HCM overcome some of these challenges to advance our understanding of HCM. However, they have other limitations that prevent direct translation to human medicine because of intrinsic differences in their cardiac physiology, response to cardiac stress, hemodynamics, and the inherent resistance of rodent hearts to arrhythmia due in part to the small size and rapid kinetics of their hearts. The need for more direct translational models, particularly large-animal models, of HCM is clearly identified.
HCM is also known to be the most common inherited cardiac disease in the domestic cats [3]. Large numbers of biochemical, genetic, and clinical studies have been conducted evaluating feline HCM over the last few decades. These studies revealed that feline HCM follows incredibly similar clinical and pathological presentations as human HCM, and shows promise to serve as an animal model of human HCM. At least two cat breeds share genetic similarity to human HCM cases where mutations in myosin binding protein C (MYBPC) have been reported as a major contributor to development of HCM [4,5]. However, a majority of feline HCM cases are of yet unknown genetic etiology and other undiscovered mutations are likely involved in developing feline HCM. Recently a unique large animal model of HCM is observed in the rhesus macaque, and pedigree investigation has revealed that this condition is also familial and likely heritable [6,7]. Once clinical, molecular, pathologic, and genetic studies are completed, this may serve as an excellent non-human primate model of HCM and further our ability to perform meaningful research into various aspects of HCM including novel therapeutics.
In this review, current knowledge and understanding of pathophysiological, clinical, diagnostic, and genetic aspects of human HCM are reviewed. Furthermore, translational and comparative models of naturally occurring or transgenic HCM in other species such rodents, cats, and non-human primates are summarized and discussed to identify the potentials for propelling further translational investigations of HCM.
General Pathophysiology of Hypertrophic Cardiomyopathy
Hypertrophied myocytes with disorganized sarcomeric alignment are hallmark histopathological changes in human HCM (Figure 1) [8]. The hypertrophied myocytes may possess unusually shaped nuclei and chaotic myofibril alignment [8]. They also frequently have abnormalities in the coronary arteriolar walls [9], elongation or malformation of mitral valve leaflets [10], and presence of myocardial fibrosis, leading to further functional abnormalities of the heart [11].
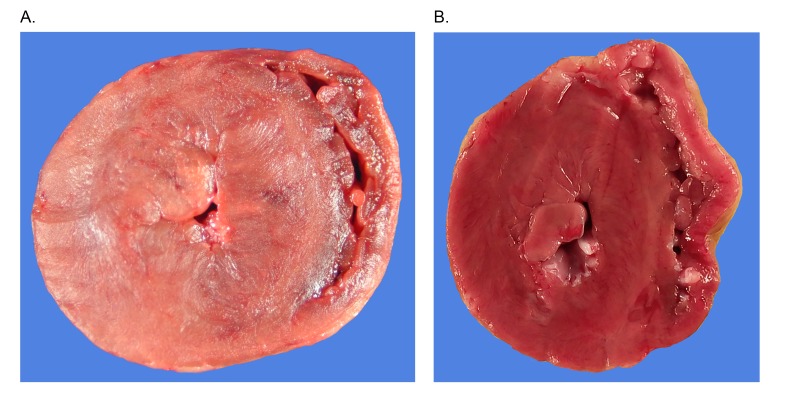
Figure 1.
Gross necropsy images of (A) cat and (B) rhesus macaque with severe left ventricular hypertrophy. Hearts were transversely transected midway between the apex and base of the hearts (Gross necropsy image of rhesus macaque courtesy of R. Reader).
Primary components of the functional disturbances observed with HCM are reduced stroke volume with diastolic dysfunction, reduced chamber size, and decreased compliance of the left ventricle (LV) [12]. Stroke volume can be even further reduced by the presence of left ventricular outflow obstruction (LVOTO). LVOTO most commonly develops secondary to systolic anterior motion (SAM) of the mitral valve generating mechanical interference along the pathway of the LV outflow tract. This causes increased pressure in the LV lumen and elevated flow velocity through the LV outflow tract. Elevated LV outflow tract velocity can further worsen SAM and lead to the development of mitral regurgitation (Figure 2). LVOTO can also occur at the mid-cavity or apex of the LV lumen resulting from symmetric or asymmetric hypertrophy of LV anterior and/or posterior walls [13]. Therefore, the degree of reduced stroke volume due to HCM may vary depending on the degrees of diastolic dysfunction, myocyte hypertrophy, LVOTO, mitral regurgitation and cardiac systolic dysfunction. Additionally, it is important to note that LVOTO may be dynamic and often dependent on physiological state, making its presence more common and more severe with exercise or catecholamine stimulation [14,15].
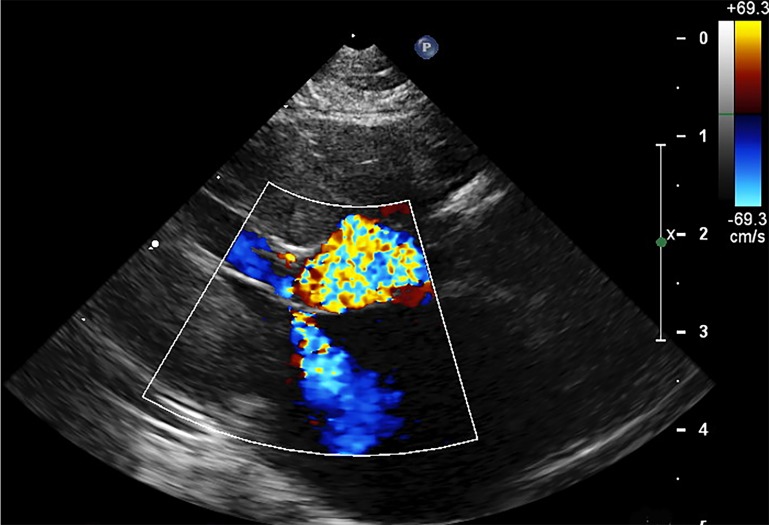
Figure 2.
2-Dimensional right parasternal left ventricular outflow tract view in systole with color-Doppler showing left ventricular outflow tract obstruction secondary to systolic anterior motion of the mitral valve in a cat with HCM. Mitral valve regurgitation is also evident.
Hypertrophic Cardiomyopathy in Humans
A previous population analysis reported in 1995 estimated the prevalence of human HCM to be at least 1 in 500 adults [2,16]. HCM is identified across all ethnic groups as well as in both sexes [17]. The overall prevalence of HCM is lower in African-American patients compared to white patients, but sudden death due to HCM is more frequent in African-Americans in the United States [18,19]. Male patients are overrepresented to female patients, while female patients tend to be diagnosed with HCM at older ages with symptoms of heart failure (HF) [18-20]. However, the overall prevalence of HCM may be underestimated since many HCM individuals do not develop any obvious clinical symptoms during their life spans [21]. Asymptomatic HCM is frequently diagnosed during family screening for HCM or diagnostic examination for other cardiovascular diseases on electrocardiography (ECG), echocardiography, or cardiovascular magnetic resonance images (CMR) [2].
Clinical Manifestations in Humans
HCM is associated with a broad spectrum of clinical manifestations from no symptoms with normal lifestyle and life span to serious clinical complications [21,22]. The common clinical symptoms related to HCM include sudden death (SD), heart failure (HF), and atrial fibrillation (AF). Age is a key factor that impacts the likelihood of developing the clinical symptoms, where SD events are most common in young patients, while HF and AF are more common in middle-aged to elderly patients [23]. In fact, HCM remains the most common cause of athletic field deaths in high school and college age students in the United States [24]. These SD events may occur without warning during physical activity, and are thought to be primarily due to development of ventricular tachycardia and fibrillation [22].
The clinical signs of HF in HCM patients include difficulty breathing, which is often associated with the presence of diastolic dysfunction, LVOTO, mitral regurgitation, and systolic dysfunction [21,22]. About one-third of HCM patients with HF do not have significant LVOTO at rest, but develop LVOTO during exercise or under stress [25]. Exercise induced LVOTO identifies patients possessing higher risk of HF development, and thus therapeutic intervention before developing HF symptoms may become indicated.
Approximately 25 percent of HCM patients develop atrial fibrillation (AF) during their clinical courses and thus AF is most commonly identified clinical symptom of HCM [26]. Development of AF due to HCM is associated with increasing age, greater left atrial size, and impaired left atrial ejection fraction [27]. Manifestation of AF itself may not result in serious clinical conditions, although it is often associated with the presence of LVOTO and progressive HF [28]. In addition, AF due to HCM is a risk factor for developing thromboembolic disease (e.g., stroke). Interestingly, no significant relationship in human HCM has been reported between AF and SD [26].
Genetics of HCM in Humans
HCM in humans is an inherited disease with an autosomal dominant pattern and incomplete penetrance [29]. Since the first sarcomeric gene mutation associated with HCM was identified approximately 25 years ago, more than 1,500 mutations across at least 20 genes encoding myofilament proteins have been identified and linked to human HCM [2,29]. Approximately 70 percent of human HCM cases are primarily associated with mutations in two major genes: beta-myosin heavy chain (MYH) and myosin-binding protein C (MYBPC) [28]. These proteins are the major components of the thick filament of sarcomeres providing structural support and regulating muscle contractions. Notably, HCM patients possessing a mutation in one of these two genes are observed to have increased severity of HCM compared to HCM patients with mutations in other genes (Table 1) [30]. Despite these advancements in understanding the genetic etiology of HCM in many cases, this genetic data has not altered the treatments or provided enough information to prognosticate or prevent HCM in a clinical setting. This is mainly due to the vast devastating genetic heterogeneity of this condition, where various mutations are identified within the MYH and MYBPC genes [29]. For example, some sarcomeric mutations in these major genes don’t cause significant left ventricular hypertrophy (LVH), but rather lead patients to develop other clinical manifestations of HCM including diastolic dysfunction, AF, and SD [31]. Other studies have shown that different mutations in these genes may result in different morphology of LVH [48]. In addition, about 3 to 5 percent of HCM affected humans have more than one HCM-associated mutation and the clinical symptoms in patients with multiple mutations are generally more severe and confer greater risk of SD [32].
Table 1. Identified genetic mutations in association with HCM in various species.
| Species | Genes | References |
| Human | ACTC1 (Actin, Alpha, Cardiac Muscle 1) | [114-116] |
| ACTN2 (Actin Alpha 2) | [117,118] | |
| CALR3 (Calreticulin 3) | [119] | |
| CSRP3 (Cysteine and Glycine Rich Protein 3) | [37,38] | |
| JPH2 (Junctophilin 2) | [120,121] | |
| MYBPC3 (Myosin Binding Protein C, Cardiac) | [122-130] | |
| MYH7 (Myosin Heavy Chain 7) | [131-139] | |
| MYL2 (Myosin Light Chain 2) | [140-142] | |
| MYL3 (Myosin Light Chain 3) | [140,143,144] | |
| MYOM1 (Myomesin-1) | [41] | |
| MYOZ2 (Myozenin 2) | [40,145] | |
| NEXN (Nexilin F-actin Binding Protein) | [146] | |
| PLN (Phospholamban) | [147,148] | |
| PRKAG2 (Protein Kinase AMP-Activated Catalytic Subunit α2) | [149-152] | |
| TCAP (Titin-Cap) | [39,153] | |
| TNNI3 (Troponin I3, Cardiac Type) | [34,115,154,155] | |
| TNNT2 (Troponin T2, Cardiac Type) | [35,100,156-158] | |
| TPM1 (Tropomyosin 1) | [156,159,160] | |
| TTN (Titin) | [36,161] | |
| VCL (Vinculin) | [162] | |
| Cat | MYBPC3 (Myosin Binding Protein C, Cardiac) | [4,5,163] |
| Pig | Not yet defined | |
| Dog | Not yet defined | |
| Rhesus macaque | Not yet defined | Under investigation |
Genes encoding cardiac troponins (TNNI3, TNNT2, TNNC1) are found to play a key role in developing HCM in humans (Table 1). The cardiac troponin is composed of three subunits including cardiac troponin C (cTnC), cardiac troponin I (cTnI), and cardiac troponin T (cTnT). Among these genes, TNNI3 is specific to cTnI and a single protein isoform has been identified, while TNNT2 is specific to cTnT and twelve different isoforms have been described [33]. Previous studies noted that several missense mutations in TNNI3 and TNNT2 were found to be associated with developing varied clinical manifestations of HCM in humans. For example, TNNI3 gene mutation (R145G missense mutation) is shown to be associated with developing significant LVH [34], while TNNT2 gene mutation (179N missense mutation) was reported to be associated with higher incidence of SD and minimal LVH [35].
Genes encoding proteins within the Z-disc and M-band are also known to have mutations associated with human HCM. These genetic variants were identified within the genes encoding titin (TTN), muscle LIM protein (CSRP), myozenin 2 (MYOZ2) and telethonin (TCAP) [36-40]. These mutations in Z-disc result in alterations of protein-protein interactions leading to excessive response to mechanical load and ultimately result in LVH. Similarly, a mutation in myomesin as an M-band protein has been identified in human HCM patients [41].
Although many mutations have been reported for HCM in humans, the association of specific mutations with the clinical characteristics of HCM is still controversial because of genotypic and phenotypic heterogeneities. These mutations cannot be routinely used to prognosticate human HCM as large cohorts of a single mutation are infrequent and thus comparing clinical outcomes by mutation is incredibly challenging [42]. Additionally, the presence of modifier genes that can modulate the phenotype of HCM, renders genotype-based prognostication even more challenging. For example, a few genetic variants of the angiotensin II type 2 receptor gene were described to be associated with the severity of LVH in human HCM patients with sarcomeric gene mutations [43]. Another study evaluated the calmodulin (CALM3) gene and reported a mutation that modifies HCM expression by altering an intracellular calcium sensitization in human HCM [44].
Diagnosis of HCM in Humans
Electrocardiographic (ECG) abnormalities are frequently seen, occurring in approximately 95 percent of human HCM in one study [45]. Typical ECG findings in humans with HCM include increased precordial voltages and ST segment and T wave abnormalities with LVH, prominent deep and narrow Q-waves with asymmetric LVH, and p-wave abnormalities (P-mitrale) with left atrial enlargement [46]. These ECG abnormalities are not specific to HCM, and further evaluations are necessary to rule out other diseases resulting in the similar ECG abnormalities (e.g., myocardial infarction).
Imaging plays a key role in diagnosing HCM. The two imaging modalities frequently used to diagnose HCM in humans include echocardiography and cardiovascular magnetic resonance images (CMR). HCM is typically diagnosed by two-dimensional (2D) echocardiography with LV wall thickness (more than 15 mm in humans) in diastole, after excluding other possible causes of LVH, such as aortic stenosis, systemic hypertension, infiltrative disease, storage disorders, and athlete’s heart (3) [22]. 2D echocardiography also identifies the pattern and location of LVH. The common locations for LVH described on 2D echocardiography include basal anterior septum, anterior free wall, and the posterior septum at the mid-LV level [47]. Two-dimensional echocardiography can be used to assess the outflow tract and valves for regurgitations or SAM under various conditions such as exercise, Valsalva maneuver, and isoproterenol administration. Using continuous-wave, pulsed-wave, and color-Doppler techniques, echocardiography can determine the LV outflow gradients to determine the presence of LVOTO before and after these physiologic manipulations. CMR can provide additional information about cardiac structure. CMR may identify the LVH in segments not visualized well with echocardiography and further characterize structural abnormalities of the mitral valve or papillary muscles. CMR combined with late gadolinium enhancement can also be used to quantify myocardial fibrosis which is reported to be a predictor of SD [48].
Cardiac biomarkers have been frequently utilized to assess the degree and severity of human HCM. Several studies showed that elevated serum cTnT level was associated with degree of LVH and diastolic dysfunction, increased risk of developing SD, HF, AF, and thromboembolic disease (e.g., stroke) in HCM patients [49]. cTnI is also found to be associated with the degree of LVH and diastolic dysfunction due to HCM [50]. In addition, the recently developed highly sensitive troponin (hs-Tn) assays have significantly higher sensitivity and specificity for diagnosing HCM and elevated hs-Tn level is associated with a greater risk of developing complications such as SD and HF [50]. B-type natriuretic peptide (BNP) concentration has also been used for diagnosing and prognosticating HF due to HCM, and the degree of LVH is also known to be independently associated with BNP level [51]. BNP level is significantly elevated in human HCM patients with HF. BNP level is shown to trend higher as the severity of HF increases [52]. However, there is significant overlap of serum BNP concentrations in HCM patients with HF and without HF [52]. Therefore, diagnosing the presence and degree of HF in patients with HCM based just solely on the BNP levels may have limited utility and must always be interpreted in concert with other diagnostic tests.
There are many laboratories offering screening to genotype patients for known mutations associated with HCM. However, using single mutations to predict clinical characteristics, prognosticate, or choose an appropriate treatment option is unreliable for individual patients due to the significant heterogeneity of HCM. Currently, the most useful application for genetic testing is for family screenings, particularly when individuals have relatives previously diagnosed with HCM [28].
Hypertrophic Cardiomyopathy in Animal Models
Molecular and biochemical effects of the individual mutations associated with HCM have been extensively investigated in vitro where the function of altered proteins can be directly evaluated. Proving the mutation results in clinical manifestation also requires studies in vivo with extensive pedigree analysis of HCM affected families or with studies using an animal model. Developing an animal HCM model provides considerable data in relatively short periods of time, especially regarding genotype and phenotype interactions as well as disease progression. With this purpose in mind, multiple animal models of HCM were developed over time [53].
Hypertrophic Cardiomyopathy in Mouse Models
Mouse models have been utilized commonly to study human cardiac disease, because 99 percent of human genes have direct murine orthologues and mice have high reproductive efficiency and short life spans [54]. In 1996, the first mouse model of HCM with a MYH7 mutation was created and they provided a great deal of information regarding the natural history of MYH7 associated HCM [55]. In 1999, another lineage of mice with HCM and an alpha-MYH mutation was created [56]. The heterozygous mice with this mutation had the typical features of HCM including LVH, but homozygous animals died shortly after birth. Since then, mouse models with nearly every known human gene mutation have been created.
For mutations in the TNNT2 and TNNI3 genes, transgenic mouse models were created and morphological and functional changes with these mutations were investigated. For example, I79N missense mutation in the TNNT2 was reported to be associated with higher risk of SD without any significant LVH, [35] while the transgenic mice with this mutation developed higher contractility and diastolic dysfunction of the heart, potentially explaining the mortality especially during exercise [57]. Transgenic mice with TNNI3 mutation also developed LVH due to compensatory mechanisms showing that this mutation caused higher energy to create a contraction force and reduced diastolic function as a result of prolonged calcium ion and force transitions [36].
Using transgenic mouse models allows us to investigate genetic and phenotypic interactions in relatively a short period of time [58,59]. However, mice have significantly different contractile function from humans, mainly due to their small size. In addition, the murine heart is composed mainly of alpha-myosin, while the human heart consists mainly of beta-myosin [53,54]. Due to these differences, the mouse models typically do not represent all aspects of human HCM making the direct translation of novel therapies from mouse to human incredibly challenging [58]. Therefore, information obtained from transgenic mouse models must be interpreted carefully, and need to be extended into larger animal models and eventually into humans to fully understand the genetic and phenotypic interactions of HCM.
Hypertrophic Cardiomyopathy in Feline Models
HCM is also the most common inherited cardiac disease in the domestic cat with a recent estimate as high as 1 in 7 (14.7 percent of a general population of 780 cats screened once by echocardiogram) [60]. Approximately 57 percent of feline idiopathic cardiomyopathies are hypertrophic [61,62]. In 1970, a study evaluated the postmortem characteristics of feline cardiac disease that leads to congestive HF and identified feline HCM as analogous to the human form of this disease [63]. Since then, abundant research characterizing the pathologic, genetic, biochemical, and echocardiographic features of this condition in cats has been published. Histopathology of feline HCM is similar to that of human HCM, characterized by disorganized arrangement of myocytes and abnormal intramural coronary arterial walls (Figure 1) [64]. As in human HCM, clinical manifestations of feline HCM vary from asymptomatic to serious complications such as SD, HF, and thromboembolic diseases. There are, however, some marked differences in the clinical manifestations of HCM between humans and cats. In human HCM, clinical symptoms have a broad spectrum, and often occur as an asymptomatic form. The most common disease manifestation of human HCM is SD in young adults and HF in middle-aged to elderly patients [16,17]. In contrast, feline HCM is most commonly associated with congestive heart failure (CHF) characterized pulmonary edema and/or pleural effusion [65]. HF. In one clinical study of cats with respiratory distress, more than 50 percent of those diagnosed with CHF had HCM [66]. This high prevalence of CHF in cats with HCM might be associated with the significant phenotypic and genotypic differences between humans and cats. Another possibility is that feline HCM is more prevalent in apparently healthy patients and we simply do not see these healthy cats in veterinary hospitals due to a lack of standardized screening protocols. Supporting this hypothesis is a study that screened apparently healthy cats by echocardiography and ECG, which found HCM in 14.7 percent of the population. This data more closely resembles the high number of asymptomatic human HCM cases [61,62].
Despite the discrepancy of clinical manifestations, the morphological differences of human and feline HCM are minimal [67,68]. This fact supports the proposal that HCM is basically the same disease in humans and cats [67]. Feline HCM is diagnosed echocardiographically in cats with left ventricular diastolic wall thickness that exceeds 6 mm (Figure 3) [69]. LVH can be asymmetric and it is often seen in the LV anterior wall and papillary muscles. Left atrial chamber size is often enlarged, but it is not an intrinsic feature of HCM [70]. LVOTO secondary to SAM is frequently identified with feline HCM as in human HCM (Figure 2) [62]. Systolic function (e.g., ejection fraction) of the left ventricle is usually normal or hyperdynamic, although progressive reduction in systolic function is noted in some HCM cats presumably secondary to myocardial fibrosis and/or ischemia [71].
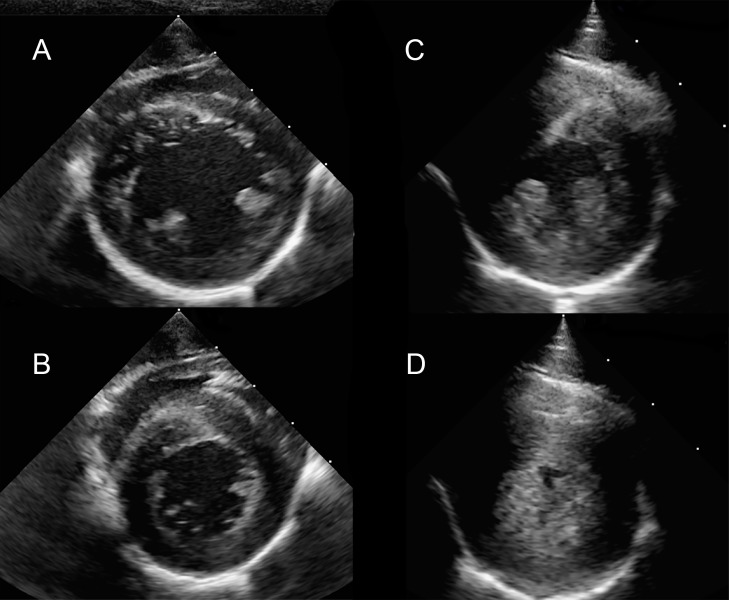
Figure 3.
Right parasternal short axis imaging at the level of the papillary muscle in a normal rhesus macaque (A and B) and a rhesus macaque with severe left ventricular hypertrophy consistent with HCM (C and D). The images are presented as systole (B and D) and diastole (A and C).
Approximately 30 percent of human HCM cases are associated with LVOTO at rest and the presence of LVOTO at rest is associated with poorer outcome and higher risk of SD [72]. It is possible that LVOTO may be responsible for elevating ventricular and atrial filling pressures, reducing coronary perfusion, and therefore inducing development of subsequent ischemia and myocardial fibrosis. In cats with HCM, the prevalence of LVOTO is as high as 67 percent [69]. This is considerably higher than observed in human HCM patients [25]. Notably, when humans with HCM are assessed immediately after exercise, the proportion of the HCM cases that demonstrate LVOTO exceeds 60 percent [15,25]. Therefore, the high prevalence of SAM in cats might reflect sympathetic activation associated with a hospital visit because SAM and LVOTO are clearly dependent on physiological state. Interestingly, a few retrospective studies reported that feline HCM with LVOTO is associated with lower mortality than those without LVOTO [65,69]. The effect of LVOTO on morbidity and mortality in cats is thus unclear. The better outcomes with LVOTO in feline HCM may be simply a reflection that these cats are evaluated earlier in the course of disease due to their frequent development of a significant heart murmur.
As in humans, feline HCM is also an inherited disease with autosomal dominant pattern and incomplete penetrance. In 2005, the first genetic mutation associated with feline HCM was identified in the MYBPC3 gene of Maine Coon cats [4]. Shortly after, a second mutation in the same gene but at a difference locus was also identified in Ragdoll cats [5]. Among these mutations, the A31P missense mutation in MYBPC3 gene is the most studied and present in approximately 30 to 40 percent of Maine Coon cats [30]. Young male cats are overrepresented for the development of HCM while female Maine Coon cats tend to develop HCM later in their life with milder clinical signs than male respectively [73].
Maine Coon cats with a heterozygous A31P mutation status often do not develop disease or manifest reduced clinical severity of HCM, demonstrating low and incomplete penetrance (6 percent). Conversely, most homozygous cats develop classical echocardiographic changes of HCM earlier in their life with high penetrance [19,68,74-76]. However, recent study showed Maine Coon cats with the A31P MYBPC3 heterozygous mutation may have significantly altered cardiac function even if they do not develop clear morphological changes characteristic of HCM. In one study, Maine Coon cats heterozygous for the A31P mutation in MYBPC3 gene without obvious clinical signs had impaired diastolic function diagnosed based on 2D and tissue Doppler images [76]. Similar findings are noted in Ragdoll cats with the R820W missense mutation in MYBPC3 [77]. Overall homozygous mutations in MYBPC3 are associated with higher chance of developing physiological and functional abnormalities due to HCM, while heterozygous cats for either mutation are associated with a lower chance of developing HCM related changes [74,75]. To date, true longitudinal studies are rare in feline HCM and thus more research is needed to firmly determine disease penetrance and expressivity.
Findings of these major mutations in Maine Coon and Ragdoll cats were led by the identification of similar mutations in human HCM patients and feline candidate gene sequencing [78]. It is likely that additional genes will be identified in cats with HCM, and these may be distinct from the genes or mutations already known in human HCM patients. Thus far, the identified genetic mutations associated with HCM in cats are breed specific and represent only a small fraction of the reported feline HCM cases. The improved annotation of the feline genome has revolutionized the ability to utilize high throughput technologies in the search for causative HCM variants in cats. With the recently published estimates of 14.7 percent of mixed breed cats harboring subclinical HCM, genetic discovery is likely to increase dramatically in the coming years [60].
Plasma cTnI and cTnT concentrations are significantly higher in cats with HCM compared to healthy cats, and even higher with CHF than without CHF due to HCM [79,80]. A recent study however failed to show correlation between changes in troponin concentration and the severity of feline HCM [80]. Natriuretic peptides including NTproBNP and NTproANP have also been investigated in feline HCM, and found to be significantly elevated in HCM affected cats [81]. Notably, elevation of NTproBNP is also observed in cats with asymptomatic HCM and thus might be useful as a screening tool to identity high-risk patients [82]. However, as in cTnI and cTnT, only weak correlations between NTproBNP concentrations and the severity of LVH were noted. This contrasts with human HCM where plasma NTproBNP as well as cTnI and cTnI concentrations are strongly correlated with the severity of disease [82].
Hypertrophic Cardiomyopathy in Non-human Primate Models
Until recently, few case reports of SD in association with LVH were documented in several non-human primates such as captive chimpanzees (Pan troglodytes), captive western lowland gorillas (Gorilla gorilla gorilla), and owl monkeys (aotus spp.) [83,84]. Naturally-occurring LVH with a high rate of SD has been identified and recently reported in captive rhesus macaques. They present clinical and pathologic manifestations resembling HCM in humans [6,85]. Pedigree analysis in this cohort suggested that LVH in rhesus macaques is an inherited disease as in human and feline HCM, and is dominated by founder effects [7,85]. The LVH in rhesus macaques is often symmetric demonstrating both LV posterior and anterior wall thickening with occasionally hypertrophied papillary muscle (Figure 1) [6,85]. These changes were accompanied by a significant reduction of the LV chamber size. Interestingly, unlike human and feline HCM, the histologic lesions of LVH in rhesus macaques lack the classical myocyte disarray, significant myocardial fibrosis, and intramural coronary arterial wall abnormalities. These histological discrepancies may imply that there are pathologic and genetic differences between HCM in humans and rhesus macaques. Nonetheless, disorganized myocyte architecture can be seen in regions with normal to mildly thickened myocardium in human HCM calling into question the specificity and importance of this lesion [86]. This pattern challenges the ideas that the degree of LVH is related to the degree of disorganized myocyte architecture, which is thought to be associated with the degree of clinical manifestation. In fact, as in human patients with HCM, SD remains the primary clinical manifestation of LVH in rhesus macaques, and this similarity suggests that investigating LVH in rhesus macaques may be worthwhile in determining the associations among the histological change, clinical manifestations, and genetic influences.
The only clinical presentation described thus far with LVH in rhesus macaques is SD, with the diagnosis of LVH made by postmortem examination. Echocardiographic screening of family members also demonstrated a high prevalence of LVH, diastolic dysfunction and mitral regurgitation in rhesus macaques genetically related to LVH-affected individuals that died suddenly (Figure 3). cTnI was also measured in LVH-affected and healthy rhesus macaques, but no significant difference was noted between these groups [6]. No significant difference in the overall incidence of LVH between males and female rhesus macaques was noted, while the male rhesus macaques with LVH had a higher rate of SD than female rhesus macaques with LVH [20,85]. These findings may be influenced by social and behavioral differences between male and female rhesus macaques. The male macaques had physiological activity somewhat similar to young athletes who more commonly develop SD due to HCM. In rhesus macaques with LVH, no direct mechanisms of SD has been determined although it has been speculated to be due to ventricular arrhythmias as in human patients with HCM [47]. Investigation into the possible causative mutation and phenotypic entities of LVH in rhesus macaques is underway. This new animal model of HCM is exciting and may represent the most direct translational approach to the study of human HCM. Continued phenotypic and genotypic investigations are essential to establish this HCM model in non-human primates.
Hypertrophic Cardiomyopathy in Other Species (rabbits, fish, pigs, dogs)
Rabbits have advantages for studying human cardiovascular disease due to their cardiovascular system closely resembling that of humans, when compared to murine models [87]. This is especially true regarding calcium ion handling during contraction cycle and an altered calcium ion flux during heart failure [88]. Rabbit myosin composition more closely resembles the human heart than murine heart, which is comprised of mainly alpha myosin. Transgenic rabbit models with mutations in beta-MYH or cTnI were previously created and produced severe defects including LVH and a high incidence of premature death [89,90].
Zebrafish are another important animal model for HCM. These fish are an especially important model for studying cardiac development, due to the external development of the heart, enabling the assessment of cardiac gene function during embryologic formation. Also, zebrafish embryos with HCM-associated gene modifications can continue to grow for several days since they initially do not rely on the cardiovascular system for oxygen delivery. Finally, despite the simpler structure of the zebrafish heart compared to mammals, the essential genes responsible for heart development are largely conserved [91]. One research group developed a zebrafish model of a TNNT2 human mutation to assess its impact during heart development. They discovered that the TNNT2 mutation in zebrafish led to a similar phenotype with matching myocardial hypertrophy pathways. Also, they observed that the embryonic hearts developed cardiomyocyte hyperplasia. This led them to conclude that sarcomeric mutations can impact the cardiomyocytes much earlier than previously understood and that despite similar transcriptional responses they possess clear differences when compared to adult hearts [92].
Naturally occurring heritable HCM was also reported in pigs, and many of the pathologic and echocardiographic characteristics of HCM in pigs closely resemble those in humans with HCM [93-95]. Other structural and biochemical features in pigs are similar to those in other species, including increased amounts of collagen matrix, abnormalities in intramural coronary arteries, and decreased calcium ATPase activity within the LV [96-98]. Although the HCM-affected pig is a potential model for studying the pathogenesis of HCM-associated disease and assisting in design of therapeutic interventions, genetic and heritability investigations are not fully characterized and further phenotypic and genotypic studies are required.
HCM is a rare disease in dogs, but there are limited reports characterizing naturally occurring HCM in dogs with similar histopathologic and clinical abnormalities to feline and human HCM [68]. In the small number of reports on canine HCM some breed predilection was proposed which included Rottweilers, Dalmatians, and various other breeds [68,99]. The infrequency of this condition in dogs renders it impossible to draw meaningful conclusions at this time.
Beyond the Genetic Mutations
Many mutations are found to be associated with HCM in humans, but the molecular pathways resulting in the broad spectrum of HCM phenotypes remain unclear. Understanding these mechanisms are the cornerstone of developing novel HCM therapies that aim to modify the natural course of this disease. Several hypotheses explaining the development of the HCM phenotype are supported to varying degrees in the literature and underscore the complex pathophysiology of this condition. The initially proposed hypothesis was that mutations results in a decreased myocardial contractility and subsequent LVH due to compensatory mechanisms [100]. However, some studies identified gain-of-function mutations with increased myocardial contractility, suggesting decreased myocardial contractility is an incomplete explanation of phenotypic HCM [101,102].
Altered myocardial calcium ion sensitivity is another mechanism proposed for the various phenotypes of HCM. Genetic mutations in myofilament proteins increase the sensitivity of calcium ions, alter calcium ion handling, and change the calcium reuptake at the sarcoplasmic reticulum [103]. This may result in development of HCM-associated cardiac arrhythmias which can be reduced or eliminated through the administration of calcium channel blocking drugs [104]. This effect was noted even in the absence of LVH, which indicates that arrhythmias with HCM are not entirely secondary to structural abnormalities.
Diastolic dysfunction and cardiac arrhythmias observed with HCM are commonly attributed to myocardial fibrosis, left ventricular hypertrophy, and myocyte disarray [105,106]. The molecular mechanisms leading to the development of myocardial fibrosis in HCM is incompletely understood and largely attributed to ongoing myocardial apoptosis, microvascular ischemia, or fibroblast proliferation linked to sarcomeric mutations [9,107]. In human HCM patients, severity of myocardial fibrosis has been identified as an independent risk factor for adverse outcomes [108]. Significant myocardial fibrosis however is not a major feature of naturally occurring LVH of other species, such as rhesus macaques with SD [85]. Further investigations are thus necessary to better understand the role of myocardial fibrosis and its relationship to clinical symptoms of HCM in humans and other animals.
Several studies have reported that myocardial dysfunction sometime seen in HCM patients was due to a combination of inefficient ATP utilization at the sarcomere concurrent with increased energy demand [109]. One study showed that myocardial contraction cycles were faster in mutated sarcomeres necessitating a greater energy demand due to their inefficient energy utilization [110]. This effect might partially explain diastolic dysfunction and ventricular arrhythmias in HCM patients [111]. Recently, a novel small molecule, MYK-461, was tested in mouse models of HCM and found to reduce contractility by decreasing ATPase activity of the cardiac myosin heavy chain, and thus lower myocardial energy demands through more efficient utilization of ATP [112]. This effect is also demonstrated in feline models with HCM [113]. MYK-461 was further shown to suppress the development of LVH, myocyte disarray and fibrosis in genetic mouse models of HCM [112]. The results of this novel therapy support the role of inefficient ATP utilization in HCM. It also indicates that hyperdynamic contraction may be a key component of HCM pathophysiology, and that novel inhibitors of myocardial contraction, such as MYK-461, may represent promising therapeutic options for HCM in humans and other species.
Conclusions and Outlook
HCM is a devastating heritable condition in humans affecting 1 in 500 and frequently leading to SD in young athletes and HF in older individuals. Past research has revealed a variety of genotypic and phenotypic information regarding HCM, which has been extensively tested and described through the use of animal models. Analysis of mouse HCM models indicate phenotypic and genotypic interactions that suggest specific mutations result in specific disease phenotypes. However, there are significant limitations to directly translate these findings into human HCM due to significant cardiac and hemodynamic differences between the two species. Naturally occurring large animal models of HCM are necessary to bridge this divide. Naturally occurring HCM in cats is morphologically and histologically similar to human HCM. Moreover, similar mutations in association with HCM in some breeds of cats have been also identified in human HCM patients. These findings indicate that feline HCM research may result in translational advancements and better define the complex genotype-phenotype relationships that limit clinical advancements in human HCM therapy. Recent investigation and development of a non-human primate model of naturally occurring LVH in rhesus macaques may also eventually shed light on the complex molecular pathogenesis of this condition and help to advance the diagnostic and therapeutic approaches to HCM. The constant evolution of new technologies, such as the availability of low-cost next generation sequencing and push towards precision medicine may also guide our understanding of genotype-phenotype relationships in this complex disease. The description of new animal models and development of novel therapeutics rooted in the molecular pathogenesis of this disease offer promise for the future diagnosis and management of HCM across many species.
Glossary
- AF
atrial fibrillation
- BNP
brain natriuretic peptide
- CMR
cardiovascular magnetic resonance image
- ECG
electrocardiography
- HCM
hypertrophic cardiomyopathy
- HF
heart failure
- LVH
left ventricular hypertrophy
- LV
left ventricle
- LVOTO
left ventricular outflow tract obstruction
- MYBPC
Myosin-binding protein C
- MYH
myosin heavy chain
- SAM
systolic anterior motion
- SD
sudden death
- cTnC
cardiac troponin C
- cTnI
cardiac troponin I
- cTnT
cardiac troponin T
- 2D
two-dimensional
Author Contributions
Yu Ueda and Joshua A. Stern jointly contributed to the research and writing of this review article.
References
- Teare D. Asymmetrical hypertrophy of the heart in young adults. Br Heart J. 1958;20(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingles J, Burns C, Barratt A, Semsarian C. Application of Genetic Testing in Hypertrophic Cardiomyopathy for Preclinical Disease Detection. Circ Cardiovasc Genet. 2015;8(6):852–9. [DOI] [PubMed] [Google Scholar]
- Ferasin L, Sturgess CP, Cannon MJ, Caney SM, Gruffydd-Jones TJ, Wotton PR. Feline idiopathic cardiomyopathy: a retrospective study of 106 cats (1994-2001). J Feline Med Surg. 2003;5(3):151–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meurs KM, Sanchez X, David RM, Bowles NE, Towbin JA, Reiser PJ, et al. A cardiac myosin binding protein C mutation in the Maine Coon cat with familial hypertrophic cardiomyopathy. Hum Mol Genet. 2005;14(23):3587–93. [DOI] [PubMed] [Google Scholar]
- Meurs KM, Norgard MM, Ederer MM, Hendrix KP, Kittleson MD. A substitution mutation in the myosin binding protein C gene in ragdoll hypertrophic cardiomyopathy. Genomics. 2007;90(2):261–4. [DOI] [PubMed] [Google Scholar]
- Haertel AJ, Stern JA, Reader JR, Spinner A, Roberts JA, Christe KL. Antemortem Screening for Left Ventricular Hypertrophy in Rhesus Macaques (Macaca mulatta). Comp Med. 2016;66(4):333–42. [PMC free article] [PubMed] [Google Scholar]
- Kanthaswamy S, Reader R, Tarara R, Oslund K, Allen M, Ng J, et al. Large scale pedigree analysis leads to evidence for founder effects of Hypertrophic Cardiomyopathy in Rhesus Macaques (Macaca mulatta). J Med Primatol. 2014;43(4):288–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maron BJ, Anan TJ, Roberts WC. Quantitative analysis of the distribution of cardiac muscle cell disorganization in the left ventricular wall of patients with hypertrophic cardiomyopathy. Circulation. 1981;63(4):882–94. [DOI] [PubMed] [Google Scholar]
- Maron BJ, Wolfson JK, Epstein SE, Roberts WC. Intramural (“small vessel”) coronary artery disease in hypertrophic cardiomyopathy. J Am Coll Cardiol. 1986;8(3):545–57. [DOI] [PubMed] [Google Scholar]
- Klues HG, Maron BJ, Dollar AL, Roberts WC. Diversity of structural mitral valve alterations in hypertrophic cardiomyopathy. Circulation. 1992;85(5):1651–60. [DOI] [PubMed] [Google Scholar]
- Factor SM, Butany J, Sole MJ, Wigle ED, Williams WC, Rojkind M. Pathologic fibrosis and matrix connective tissue in the subaortic myocardium of patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 1991;17(6):1343–51. [DOI] [PubMed] [Google Scholar]
- Wigle ED, Rakowski H, Kimball BP, Williams WG. Hypertrophic cardiomyopathy. Clinical spectrum and treatment. Circulation. 1995;92(7):1680–92. [DOI] [PubMed] [Google Scholar]
- Sherrid MV. Dynamic Left Ventricular Outflow Obstruction in Hypertrophic Cardiomyopathy Revisited: Significance, Pathogenesis, and Treatment. Cardiol Rev. 1998;6(3):135–45. [DOI] [PubMed] [Google Scholar]
- Mingo S, Benedicto A, Jimenez MC, Perez MA, Montero M. Dynamic left ventricular outflow tract obstruction secondary to catecholamine excess in a normal ventricle. Int J Cardiol. 2006;112(3):393–6. [DOI] [PubMed] [Google Scholar]
- Shah JS, Esteban MT, Thaman R, Sharma R, Mist B, Pantazis A, et al. Prevalence of exercise-induced left ventricular outflow tract obstruction in symptomatic patients with non-obstructive hypertrophic cardiomyopathy. Heart. 2008;94(10):1288–94. [DOI] [PubMed] [Google Scholar]
- Maron BJ, Gardin JM, Flack JM, Gidding SS, Kurosaki TT, Bild DE. Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Echocardiographic analysis of 4111 subjects in the CARDIA Study. Coronary Artery Risk Development in (Young) Adults. Circulation. 1995;92(4):785–9. [DOI] [PubMed] [Google Scholar]
- Maron BJ. Hypertrophic cardiomyopathy: an important global disease. Am J Med. 2004;116(1):63–5. [DOI] [PubMed] [Google Scholar]
- Maron BJ, Carney KP, Lever HM, Lewis JF, Barac I, Casey SA, et al. Relationship of race to sudden cardiac death in competitive athletes with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2003;41(6):974–80. [DOI] [PubMed] [Google Scholar]
- Movahed MR, Strootman D, Bates S, Sattur S. Prevalence of suspected hypertrophic cardiomyopathy or left ventricular hypertrophy based on race and gender in teenagers using screening echocardiography. Cardiovasc Ultrasound. 2010;8:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivotto I, Maron MS, Adabag AS, Casey SA, Vargiu D, Link MS, et al. Gender-related differences in the clinical presentation and outcome of hypertrophic cardiomyopathy. J Am Coll Cardiol. 2005;46(3):480–7. [DOI] [PubMed] [Google Scholar]
- Maron BJ, Casey SA, Hauser RG, Aeppli DM. Clinical course of hypertrophic cardiomyopathy with survival to advanced age. J Am Coll Cardiol. 2003;42(5):882–8. [DOI] [PubMed] [Google Scholar]
- Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, et al. 2011 ACCF/AHA Guideline for the Diagnosis and Treatment of Hypertrophic Cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the American Association for Thoracic Surgery, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2011;58(25):e212–60. [DOI] [PubMed] [Google Scholar]
- Maron BJ, Rowin EJ, Casey SA, Haas TS, Chan RH, Udelson JE, et al. Risk stratification and outcome of patients with hypertrophic cardiomyopathy >=60 years of age. Circulation. 2013;127(5):585–93. [DOI] [PubMed] [Google Scholar]
- Maron BJ. Sudden death in young athletes. N Engl J Med. 2003;349(11):1064–75. [DOI] [PubMed] [Google Scholar]
- Maron MS, Olivotto I, Zenovich AG, Link MS, Pandian NG, Kuvin JT, et al. Hypertrophic cardiomyopathy is predominantly a disease of left ventricular outflow tract obstruction. Circulation. 2006;114(21):2232–9. [DOI] [PubMed] [Google Scholar]
- Olivotto I, Cecchi F, Casey SA, Dolara A, Traverse JH, Maron BJ. Impact of atrial fibrillation on the clinical course of hypertrophic cardiomyopathy. Circulation. 2001;104(21):2517–24. [DOI] [PubMed] [Google Scholar]
- Maron BJ, Haas TS, Maron MS, Lesser JR, Browning JA, Chan RH, et al. Left atrial remodeling in hypertrophic cardiomyopathy and susceptibility markers for atrial fibrillation identified by cardiovascular magnetic resonance. Am J Cardiol. 2014;113(8):1394–400. [DOI] [PubMed] [Google Scholar]
- Maron BJ, Ommen SR, Semsarian C, Spirito P, Olivotto I, Maron MS. Hypertrophic cardiomyopathy: present and future, with translation into contemporary cardiovascular medicine. J Am Coll Cardiol. 2014;64(1):83–99. [DOI] [PubMed] [Google Scholar]
- Maron BJ, Maron MS, Semsarian C. Genetics of hypertrophic cardiomyopathy after 20 years: clinical perspectives. J Am Coll Cardiol. 2012;60(8):705–15. [DOI] [PubMed] [Google Scholar]
- Wess G, Schinner C, Weber K, Kuchenhoff H, Hartmann K. Association of A31P and A74T polymorphisms in the myosin binding protein C3 gene and hypertrophic cardiomyopathy in Maine Coon and other breed cats. J Vet Intern Med. 2010;24(3):527–32. [DOI] [PubMed] [Google Scholar]
- Efthimiadis GK, Pagourelias ED, Gossios T, Zegkos T. Hypertrophic cardiomyopathy in 2013: current speculations and future perspectives. World J Cardiol. 2014;6(2):26–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly M, Semsarian C. Multiple mutations in genetic cardiovascular disease: a marker of disease severity? Circ Cardiovasc Genet. 2009;2(2):182–90. [DOI] [PubMed] [Google Scholar]
- Wei B, Jin JP. TNNT1, TNNT2, and TNNT3: isoform genes, regulation, and structure-function relationships. Gene. 2016;582(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura A, Harada H, Park JE, Nishi H, Satoh M, Takahashi M, et al. Mutations in the cardiac troponin I gene associated with hypertrophic cardiomyopathy. Nat Genet. 1997;16(4):379–82. [DOI] [PubMed] [Google Scholar]
- Watkins H, McKenna WJ, Thierfelder L, Suk HJ, Anan R, O’Donoghue A, et al. Mutations in the genes for cardiac troponin T and alpha-tropomyosin in hypertrophic cardiomyopathy. N Engl J Med. 1995;332(16):1058–64. [DOI] [PubMed] [Google Scholar]
- Satoh M, Takahashi M, Sakamoto T, Hiroe M, Marumo F, Kimura A. Structural analysis of the titin gene in hypertrophic cardiomyopathy: identification of a novel disease gene. Biochem Biophys Res Commun. 1999;262(2):411–7. [DOI] [PubMed] [Google Scholar]
- Geier C, Perrot A, Ozcelik C, Binner P, Counsell D, Hoffmann K, et al. Mutations in the human muscle LIM protein gene in families with hypertrophic cardiomyopathy. Circulation. 2003;107(10):1390–5. [DOI] [PubMed] [Google Scholar]
- Geier C, Gehmlich K, Ehler E, Hassfeld S, Perrot A, Hayess K, et al. Beyond the sarcomere: CSRP3 mutations cause hypertrophic cardiomyopathy. Hum Mol Genet. 2008;17(18):2753–65. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Arimura T, Itoh-Satoh M, Ueda K, Hohda S, Inagaki N, et al. Tcap gene mutations in hypertrophic cardiomyopathy and dilated cardiomyopathy. J Am Coll Cardiol. 2004;44(11):2192–201. [DOI] [PubMed] [Google Scholar]
- Osio A, Tan L, Chen SN, Lombardi R, Nagueh SF, Shete S, et al. Myozenin 2 is a novel gene for human hypertrophic cardiomyopathy. Circ Res. 2007;100(6):766–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegert R, Perrot A, Keller S, Behlke J, Michalewska-Wludarczyk A, Wycisk A, et al. A myomesin mutation associated with hypertrophic cardiomyopathy deteriorates dimerisation properties. Biochem Biophys Res Commun. 2011;405(3):473–9. [DOI] [PubMed] [Google Scholar]
- Landstrom AP, Ackerman MJ. Mutation type is not clinically useful in predicting prognosis in hypertrophic cardiomyopathy. Circulation. 2010;122(23):2441–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain S, Asghar M, Javed Q. Resistin gene promoter region polymorphism and the risk of hypertrophic cardiomyopathy in patients. Transl Res. 2010;155(3):142–7. [DOI] [PubMed] [Google Scholar]
- Friedrich FW, Bausero P, Sun Y, Treszl A, Kramer E, Juhr D, et al. A new polymorphism in human calmodulin III gene promoter is a potential modifier gene for familial hypertrophic cardiomyopathy. Eur Heart J. 2009;30(13):1648–55. [DOI] [PubMed] [Google Scholar]
- McLeod CJ, Ackerman MJ, Nishimura RA, Tajik AJ, Gersh BJ, Ommen SR. Outcome of patients with hypertrophic cardiomyopathy and a normal electrocardiogram. J Am Coll Cardiol. 2009;54(3):229–33. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Avari JN, Rhee EK, Woodard PK, Rudy Y. Hypertrophic cardiomyopathy with preexcitation: insights from noninvasive electrocardiographic imaging (ECGI) and catheter mapping. J Cardiovasc Electrophysiol. 2008;19(11):1215–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maron BJ, Maron MS. Hypertrophic cardiomyopathy. Lancet. 2013;381(9862):242–55. [DOI] [PubMed] [Google Scholar]
- Chan RH, Maron BJ, Olivotto I, Pencina MJ, Assenza GE, Haas T, et al. Prognostic value of quantitative contrast-enhanced cardiovascular magnetic resonance for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation. 2014;130(6):484–95. [DOI] [PubMed] [Google Scholar]
- Sato Y, Taniguchi R, Nagai K, Makiyama T, Okada H, Yamada T, et al. Measurements of cardiac troponin T in patients with hypertrophic cardiomyopathy. Heart. 2003;89(6):659–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGorrian CM, Lyster S, Roy A, Tarrant H, Codd M, Doran P, et al. Use of a highly-sensitive cardiac troponin I assay in a screening population for hypertrophic cardiomyopathy: a case-referent study. BMC Cardiovasc Disord. 2013;13:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coats CJ, Gallagher MJ, Foley M, O’Mahony C, Critoph C, Gimeno J, et al. Relation between serum N-terminal pro-brain natriuretic peptide and prognosis in patients with hypertrophic cardiomyopathy. Eur Heart J. 2013;34(32):2529–37. [DOI] [PubMed] [Google Scholar]
- Maron BJ, Tholakanahalli VN, Zenovich AG, Casey SA, Duprez D, Aeppli DM, et al. Usefulness of B-type natriuretic peptide assay in the assessment of symptomatic state in hypertrophic cardiomyopathy. Circulation. 2004;109(8):984–9. [DOI] [PubMed] [Google Scholar]
- Maass A, Leinwand LA. Animal models of hypertrophic cardiomyopathy. Curr Opin Cardiol. 2000;15(3):189–96. [DOI] [PubMed] [Google Scholar]
- Camacho P, Fan H, Liu Z, He JQ. Small mammalian animal models of heart disease. Am J Cardiovasc Dis. 2016;6(3):70–80. [PMC free article] [PubMed] [Google Scholar]
- Vikstrom KL, Factor SM, Leinwand LA. Mice expressing mutant myosin heavy chains are a model for familial hypertrophic cardiomyopathy. Mol Med. 1996;2(5):556–67. [PMC free article] [PubMed] [Google Scholar]
- Georgakopoulos D, Christe ME, Giewat M, Seidman CM, Seidman JG, Kass DA. The pathogenesis of familial hypertrophic cardiomyopathy: early and evolving effects from an alpha-cardiac myosin heavy chain missense mutation. Nat Med. 1999;5(3):327–30. [DOI] [PubMed] [Google Scholar]
- Miller T, Szczesna D, Housmans PR, Zhao J, de Freitas F, Gomes AV, et al. Abnormal contractile function in transgenic mice expressing a familial hypertrophic cardiomyopathy-linked troponin T (I79N) mutation. J Biol Chem. 2001;276(6):3743–55. [DOI] [PubMed] [Google Scholar]
- Milani-Nejad N, Janssen PM. Small and large animal models in cardiac contraction research: advantages and disadvantages. Pharmacol Ther. 2014;141(3):235–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaragoza C, Gomez-Guerrero C, Martin-Ventura JL, Blanco-Colio L, Lavin B, Mallavia B, et al. Animal models of cardiovascular diseases. J Biomed Biotechnol. 2011;2011:497841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne JR, Brodbelt DC, Luis Fuentes V. Cardiomyopathy prevalence in 780 apparently healthy cats in rehoming centres (the CatScan study). J Vet Cardiol. 2015;17 Suppl 1:S244–57. [DOI] [PubMed] [Google Scholar]
- Paige CF, Abbott JA, Elvinger F, Pyle RL. Prevalence of cardiomyopathy in apparently healthy cats. J Am Vet Med Assoc. 2009;234(11):1398–403. [DOI] [PubMed] [Google Scholar]
- Payne J, Luis Fuentes V, Boswood A, Connolly D, Koffas H, Brodbelt D. Population characteristics and survival in 127 referred cats with hypertrophic cardiomyopathy (1997 to 2005). J Small Anim Pract. 2010;51(10):540–7. [DOI] [PubMed] [Google Scholar]
- Liu SK. Acquired cardiac lesions leading to congestive heart failure in the cat. Am J Vet Res. 1970;31(11):2071–88. [PubMed] [Google Scholar]
- Basso C, Fox PR, Meurs KM, Towbin JA, Spier AW, Calabrese F, et al. Arrhythmogenic right ventricular cardiomyopathy causing sudden cardiac death in boxer dogs: a new animal model of human disease. Circulation. 2004;109(9):1180–5. [DOI] [PubMed] [Google Scholar]
- Rush JE, Freeman LM, Fenollosa NK, Brown DJ. Population and survival characteristics of cats with hypertrophic cardiomyopathy: 260 cases (1990-1999). J Am Vet Med Assoc. 2002;220(2):202–7. [DOI] [PubMed] [Google Scholar]
- Fox PR, Oyama MA, Reynolds C, Rush JE, DeFrancesco TC, Keene BW, et al. Utility of plasma N-terminal pro-brain natriuretic peptide (NT-proBNP) to distinguish between congestive heart failure and non-cardiac causes of acute dyspnea in cats. J Vet Cardiol. 2009;11 Suppl 1:S51–61. [DOI] [PubMed] [Google Scholar]
- Maron BJ, Fox PR. Hypertrophic cardiomyopathy in man and cats. J Vet Cardiol. 2015;17 Suppl 1:S6–9. [DOI] [PubMed] [Google Scholar]
- Liu SK, Roberts WC, Maron BJ. Comparison of morphologic findings in spontaneously occurring hypertrophic cardiomyopathy in humans, cats and dogs. Am J Cardiol. 1993;72(12):944–51. [DOI] [PubMed] [Google Scholar]
- Fox PR, Liu SK, Maron BJ. Echocardiographic assessment of spontaneously occurring feline hypertrophic cardiomyopathy. An animal model of human disease. Circulation. 1995;92(9):2645–51. [DOI] [PubMed] [Google Scholar]
- Abbott JA, MacLean HN. Two-dimensional echocardiographic assessment of the feline left atrium. J Vet Intern Med. 2006;20(1):111–9. [DOI] [PubMed] [Google Scholar]
- Olivotto I, Cecchi F, Gistri R, Lorenzoni R, Chiriatti G, Girolami F, et al. Relevance of coronary microvascular flow impairment to long-term remodeling and systolic dysfunction in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2006;47(5):1043–8. [DOI] [PubMed] [Google Scholar]
- Maron MS, Olivotto I, Betocchi S, Casey SA, Lesser JR, Losi MA, et al. Effect of left ventricular outflow tract obstruction on clinical outcome in hypertrophic cardiomyopathy. N Engl J Med. 2003;348(4):295–303. [DOI] [PubMed] [Google Scholar]
- Kittleson MD, Meurs KM, Munro MJ, Kittleson JA, Liu SK, Pion PD, et al. Familial hypertrophic cardiomyopathy in maine coon cats: an animal model of human disease. Circulation. 1999;99(24):3172–80. [DOI] [PubMed] [Google Scholar]
- Godiksen MT, Granstrom S, Koch J, Christiansen M. Hypertrophic cardiomyopathy in young Maine Coon cats caused by the p.A31P cMyBP-C mutation—the clinical significance of having the mutation. Acta Vet Scand. 2011;53:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittleson MD, Meurs KM, Harris SP. The genetic basis of hypertrophic cardiomyopathy in cats and humans. J Vet Cardiol. 2015;17 Suppl 1:S53–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlos Sampedrano C, Chetboul V, Mary J, Tissier R, Abitbol M, Serres F, et al. Prospective echocardiographic and tissue Doppler imaging screening of a population of Maine Coon cats tested for the A31P mutation in the myosin-binding protein C gene: a specific analysis of the heterozygous status. J Vet Intern Med. 2009;23(1):91–9. [DOI] [PubMed] [Google Scholar]
- Borgeat K, Casamian-Sorrosal D, Helps C, Luis Fuentes V, Connolly DJ. Association of the myosin binding protein C3 mutation (MYBPC3 R820W) with cardiac death in a survey of 236 Ragdoll cats. J Vet Cardiol. 2014;16(2):73–80. [DOI] [PubMed] [Google Scholar]
- Ripoll Vera T, Monserrat Iglesias L, Hermida Prieto M, Ortiz M, Rodriguez Garcia I, Govea Callizo N, et al. The R820W mutation in the MYBPC3 gene, associated with hypertrophic cardiomyopathy in cats, causes hypertrophic cardiomyopathy and left ventricular non-compaction in humans. Int J Cardiol. 2010;145(2):405–7. [DOI] [PubMed] [Google Scholar]
- Connolly DJ, Cannata J, Boswood A, Archer J, Groves EA, Neiger R. Cardiac troponin I in cats with hypertrophic cardiomyopathy. J Feline Med Surg. 2003;5(4):209–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langhorn R, Tarnow I, Willesen JL, Kjelgaard-Hansen M, Skovgaard IM, Koch J. Cardiac troponin I and T as prognostic markers in cats with hypertrophic cardiomyopathy. J Vet Intern Med. 2014;28(5):1485–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangster JK, Panciera DL, Abbott JA, Zimmerman KC, Lantis AC. Cardiac biomarkers in hyperthyroid cats. J Vet Intern Med. 2014;28(2):465–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox PR, Rush JE, Reynolds CA, Defrancesco TC, Keene BW, Atkins CE, et al. Multicenter evaluation of plasma N-terminal probrain natriuretic peptide (NT-pro BNP) as a biochemical screening test for asymptomatic (occult) cardiomyopathy in cats. J Vet Intern Med. 2011;25(5):1010–6. [DOI] [PubMed] [Google Scholar]
- Knowlen GG, Weller RE, Perry RL, Baer JF, Gozalo AS. Hypertrophic cardiomyopathy in owl monkeys (Aotus spp.). Comp Med. 2013;63(3):279–87. [PMC free article] [PubMed] [Google Scholar]
- Seiler BM, Dick EJ, Jr, Guardado-Mendoza R, VandeBerg JL, Williams JT, Mubiru JN, et al. Spontaneous heart disease in the adult chimpanzee (Pan troglodytes). J Med Primatol. 2009;38(1):51–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reader JR, Canfield DR, Lane JF, Kanthaswamy S, Ardeshir A, Allen AM, et al. Left Ventricular Hypertrophy in Rhesus Macaques (Macaca mulatta) at the California National Primate Research Center (1992-2014). Comp Med. 2016;66(2):162–9. [PMC free article] [PubMed] [Google Scholar]
- Maron BJ, Wolfson JK, Roberts WC. Relation between extent of cardiac muscle cell disorganization and left ventricular wall thickness in hypertrophic cardiomyopathy. Am J Cardiol. 1992;70(7):785–90. [DOI] [PubMed] [Google Scholar]
- Marian AJ, Wu Y, Lim DS, McCluggage M, Youker K, Yu QT, et al. A transgenic rabbit model for human hypertrophic cardiomyopathy. J Clin Invest. 1999;104(12):1683–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bers DM. Cardiac Na/Ca exchange function in rabbit, mouse and man: what’s the difference? J Mol Cell Cardiol. 2002;34(4):369–73. [DOI] [PubMed] [Google Scholar]
- James J, Zhang Y, Osinska H, Sanbe A, Klevitsky R, Hewett TE, et al. Transgenic modeling of a cardiac troponin I mutation linked to familial hypertrophic cardiomyopathy. Circ Res. 2000;87(9):805–11. [DOI] [PubMed] [Google Scholar]
- Sanbe A, James J, Tuzcu V, Nas S, Martin L, Gulick J, et al. Transgenic rabbit model for human troponin I-based hypertrophic cardiomyopathy. Circulation. 2005;111(18):2330–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Stainier DY. Zebrafish in the study of early cardiac development. Circ Res. 2012;110(6):870–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JR, Deo RC, Werdich AA, Panakova D, Coy S, MacRae CA. Human cardiomyopathy mutations induce myocyte hyperplasia and activate hypertrophic pathways during cardiogenesis in zebrafish. Dis Model Mech. 2011;4(3):400–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SK, Chiu YT, Shyu JJ, Factor SM, Chu R, Lin JH, et al. Hypertrophic cardiomyopathy in pigs: quantitative pathologic features in 55 cases. Cardiovasc Pathol. 1994;3(4):261–8. [DOI] [PubMed] [Google Scholar]
- Lin JH, Huang SY, Lee WC, Liu SK, Chu RM. Echocardiographic features of pigs with spontaneous hypertrophic cardiomyopathy. Comp Med. 2002;52(3):238–42. [PubMed] [Google Scholar]
- Huang SY, Tsou HL, Chiu YT, Shyu JJ, Wu JJ, Lin JH, et al. Heritability estimate of hypertrophic cardiomyopathy in pigs (Sus scrofa domestica). Lab Anim Sci. 1996;46(3):310–4. [PubMed] [Google Scholar]
- Chiu YT, Liu SK, Liu M, Chen SP, Lin YH, Mao SJ, et al. Characterization and quantitation of extracellular collagen matrix in myocardium of pigs with spontaneously occurring hypertrophic cardiomyopathy. Cardiovasc Pathol. 1999;8(3):169–75. [DOI] [PubMed] [Google Scholar]
- Dai KS, Chu LC, Liu CY, Yang PC, Chen SP, Mao SJ. Collagen in naturally occurring hypertrophied porcine myocardium. J Submicrosc Cytol Pathol. 1996;28(1):81–5. [PubMed] [Google Scholar]
- Dai KS, Liang CS, Ch’iu YT, Yang PC, Cheng IC. Altered adenosine triphosphatase activities in pigs with naturally occurring hypertrophic cardiomyopathy. Vet Res Commun. 1995;19(2):115–25. [DOI] [PubMed] [Google Scholar]
- Liu SK, Maron BJ, Tilley LP. Hypertrophic cardiomyopathy in the dog. Am J Pathol. 1979;94(3):497–508. [PMC free article] [PubMed] [Google Scholar]
- Watkins H, Seidman CE, Seidman JG, Feng HS, Sweeney HL. Expression and functional assessment of a truncated cardiac troponin T that causes hypertrophic cardiomyopathy. Evidence for a dominant negative action. J Clin Invest. 1996;98(11):2456–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyska MJ, Hayes E, Giewat M, Seidman CE, Seidman JG, Warshaw DM. Single-molecule mechanics of R403Q cardiac myosin isolated from the mouse model of familial hypertrophic cardiomyopathy. Circ Res. 2000;86(7):737–44. [DOI] [PubMed] [Google Scholar]
- Lowey S. Functional consequences of mutations in the myosin heavy chain at sites implicated in familial hypertrophic cardiomyopathy. Trends Cardiovasc Med. 2002;12(8):348–54. [DOI] [PubMed] [Google Scholar]
- Guinto PJ, Haim TE, Dowell-Martino CC, Sibinga N, Tardiff JC. Temporal and mutation-specific alterations in Ca2+ homeostasis differentially determine the progression of cTnT-related cardiomyopathies in murine models. Am J Physiol Heart Circ Physiol. 2009;297(2):H614–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudenbacher F, Schober T, Pinto JR, Sidorov VY, Hilliard F, Solaro RJ, et al. Myofilament Ca2+ sensitization causes susceptibility to cardiac arrhythmia in mice. J Clin Invest. 2008;118(12):3893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnava AM, Elliott PM, Baboonian C, Davison F, Davies MJ, McKenna WJ. Hypertrophic cardiomyopathy: histopathological features of sudden death in cardiac troponin T disease. Circulation. 2001;104(12):1380–4. [DOI] [PubMed] [Google Scholar]
- Varnava AM, Elliott PM, Mahon N, Davies MJ, McKenna WJ. Relation between myocyte disarray and outcome in hypertrophic cardiomyopathy. Am J Cardiol. 2001;88(3):275–9. [DOI] [PubMed] [Google Scholar]
- Teekakirikul P, Eminaga S, Toka O, Alcalai R, Wang L, Wakimoto H, et al. Cardiac fibrosis in mice with hypertrophic cardiomyopathy is mediated by non-myocyte proliferation and requires Tgf-beta. J Clin Invest. 2010;120(10):3520–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hanlon R, Grasso A, Roughton M, Moon JC, Clark S, Wage R, et al. Prognostic significance of myocardial fibrosis in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2010;56(11):867–74. [DOI] [PubMed] [Google Scholar]
- Ashrafian H, Redwood C, Blair E, Watkins H. Hypertrophic cardiomyopathy:a paradigm for myocardial energy depletion. Trends Genet. 2003;19(5):263–8. [DOI] [PubMed] [Google Scholar]
- Belus A, Piroddi N, Scellini B, Tesi C, D’Amati G, Girolami F, et al. The familial hypertrophic cardiomyopathy-associated myosin mutation R403Q accelerates tension generation and relaxation of human cardiac myofibrils. J Physiol. 2008;586(15):3639–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey N, Brixius K, Schwinger RH, Benis T, Karpowski A, Lorenzen HP, et al. Alterations of tension-dependent ATP utilization in a transgenic rat model of hypertrophic cardiomyopathy. J Biol Chem. 2006;281(40):29575–82. [DOI] [PubMed] [Google Scholar]
- Green EM, Wakimoto H, Anderson RL, Evanchik MJ, Gorham JM, Harrison BC, et al. A small-molecule inhibitor of sarcomere contractility suppresses hypertrophic cardiomyopathy in mice. Science. 2016;351(6273):617–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern JA, Markova S, Ueda Y, Kim JB, Pascoe PJ, Evanchik MJ, et al. A Small Molecule Inhibitor of Sarcomere Contractility Acutely Relieves Left Ventricular Outflow Tract Obstruction in Feline Hypertrophic Cardiomyopathy. PLoS One. 2016;11(12):e0168407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson TM, Doan TP, Kishimoto NY, Whitby FG, Ackerman MJ, Fananapazir L. Inherited and de novo mutations in the cardiac actin gene cause hypertrophic cardiomyopathy. J Mol Cell Cardiol. 2000;32(9):1687–94. [DOI] [PubMed] [Google Scholar]
- Arad M, Penas-Lado M, Monserrat L, Maron BJ, Sherrid M, Ho CY, et al. Gene mutations in apical hypertrophic cardiomyopathy. Circulation. 2005;112(18):2805–11. [DOI] [PubMed] [Google Scholar]
- Monserrat L, Hermida-Prieto M, Fernandez X, Rodriguez I, Dumont C, Cazon L, et al. Mutation in the alpha-cardiac actin gene associated with apical hypertrophic cardiomyopathy, left ventricular non-compaction, and septal defects. Eur Heart J. 2007;28(16):1953–61. [DOI] [PubMed] [Google Scholar]
- Chiu C, Bagnall RD, Ingles J, Yeates L, Kennerson M, Donald JA, et al. Mutations in alpha-actinin-2 cause hypertrophic cardiomyopathy: a genome-wide analysis. J Am Coll Cardiol. 2010;55(11):1127–35. [DOI] [PubMed] [Google Scholar]
- Girolami F, Iascone M, Tomberli B, Bardi S, Benelli M, Marseglia G, et al. Novel alpha-actinin 2 variant associated with familial hypertrophic cardiomyopathy and juvenile atrial arrhythmias: a massively parallel sequencing study. Circ Cardiovasc Genet. 2014;7(6):741–50. [DOI] [PubMed] [Google Scholar]
- Chiu C, Tebo M, Ingles J, Yeates L, Arthur JW, Lind JM, et al. Genetic screening of calcium regulation genes in familial hypertrophic cardiomyopathy. J Mol Cell Cardiol. 2007;43(3):337–43. [DOI] [PubMed] [Google Scholar]
- Landstrom AP, Weisleder N, Batalden KB, Bos JM, Tester DJ, Ommen SR, et al. Mutations in JPH2-encoded junctophilin-2 associated with hypertrophic cardiomyopathy in humans. J Mol Cell Cardiol. 2007;42(6):1026–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita Y, Furukawa T, Kasanuki H, Nishibatake M, Kurihara Y, Ikeda A, et al. Mutation of junctophilin type 2 associated with hypertrophic cardiomyopathy. J Hum Genet. 2007;52(6):543–8. [DOI] [PubMed] [Google Scholar]
- Watkins H, Conner D, Thierfelder L, Jarcho JA, MacRae C, McKenna WJ, et al. Mutations in the cardiac myosin binding protein-C gene on chromosome 11 cause familial hypertrophic cardiomyopathy. Nat Genet. 1995;11(4):434–7. [DOI] [PubMed] [Google Scholar]
- Carrier L, Bonne G, Bahrend E, Yu B, Richard P, Niel F, et al. Organization and sequence of human cardiac myosin binding protein C gene (MYBPC3) and identification of mutations predicted to produce truncated proteins in familial hypertrophic cardiomyopathy. Circ Res. 1997;80(3):427–34. [PubMed] [Google Scholar]
- Moolman JA, Reith S, Uhl K, Bailey S, Gautel M, Jeschke B, et al. A newly created splice donor site in exon 25 of the MyBP-C gene is responsible for inherited hypertrophic cardiomyopathy with incomplete disease penetrance. Circulation. 2000;101(12):1396–402. [DOI] [PubMed] [Google Scholar]
- Waldmuller S, Sakthivel S, Saadi AV, Selignow C, Rakesh PG, Golubenko M, et al. Novel deletions in MYH7 and MYBPC3 identified in Indian families with familial hypertrophic cardiomyopathy. J Mol Cell Cardiol. 2003;35(6):623–36. [DOI] [PubMed] [Google Scholar]
- Xin B, Puffenberger E, Tumbush J, Bockoven JR, Wang H. Homozygosity for a novel splice site mutation in the cardiac myosin-binding protein C gene causes severe neonatal hypertrophic cardiomyopathy. Am J Med Genet A. 2007;143A(22):2662–7. [DOI] [PubMed] [Google Scholar]
- Ingles J, Doolan A, Chiu C, Seidman J, Seidman C, Semsarian C. Compound and double mutations in patients with hypertrophic cardiomyopathy: implications for genetic testing and counselling. J Med Genet. 2005;42(10):e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajsharghi H, Leren TP, Abdul-Hussein S, Tulinius M, Brunvand L, Dahl HM, et al. Unexpected myopathy associated with a mutation in MYBPC3 and misplacement of the cardiac myosin binding protein C. J Med Genet. 2010;47(8):575–7. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wang Z, Yang Q, Zou Y, Zhang H, Yan C, et al. Autosomal recessive transmission of MYBPC3 mutation results in malignant phenotype of hypertrophic cardiomyopathy. PLoS One. 2013;8(6):e67087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calore C, De Bortoli M, Romualdi C, Lorenzon A, Angelini A, Basso C, et al. A founder MYBPC3 mutation results in HCM with a high risk of sudden death after the fourth decade of life. J Med Genet. 2015;52(5):338–47. [DOI] [PubMed] [Google Scholar]
- Geisterfer-Lowrance AA, Kass S, Tanigawa G, Vosberg HP, McKenna W, Seidman CE, et al. A molecular basis for familial hypertrophic cardiomyopathy: a beta cardiac myosin heavy chain gene missense mutation. Cell. 1990;62(5):999–1006. [DOI] [PubMed] [Google Scholar]
- Perryman MB, Yu QT, Marian AJ, Mares A, Jr, Czernuszewicz G, Ifegwu J, et al. Expression of a missense mutation in the messenger RNA for beta-myosin heavy chain in myocardial tissue in hypertrophic cardiomyopathy. J Clin Invest. 1992;90(1):271–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair E, Price SJ, Baty CJ, Ostman-Smith I, Watkins H. Mutations in cis can confound genotype-phenotype correlations in hypertrophic cardiomyopathy. J Med Genet. 2001;38(6):385–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anan R, Greve G, Thierfelder L, Watkins H, McKenna WJ, Solomon S, et al. Prognostic implications of novel beta cardiac myosin heavy chain gene mutations that cause familial hypertrophic cardiomyopathy. J Clin Invest. 1994;93(1):280–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi H, Kimura A, Harada H, Adachi K, Koga Y, Sasazuki T, et al. Possible gene dose effect of a mutant cardiac beta-myosin heavy chain gene on the clinical expression of familial hypertrophic cardiomyopathy. Biochem Biophys Res Commun. 1994;200(1):549–56. [DOI] [PubMed] [Google Scholar]
- Jeschke B, Uhl K, Weist B, Schroder D, Meitinger T, Dohlemann C, et al. A high risk phenotype of hypertrophic cardiomyopathy associated with a compound genotype of two mutated beta-myosin heavy chain genes. Hum Genet. 1998;102(3):299–304. [DOI] [PubMed] [Google Scholar]
- Moolman-Smook JC, De Lange WJ, Bruwer EC, Brink PA, Corfield VA. The origins of hypertrophic cardiomyopathy-causing mutations in two South African subpopulations: a unique profile of both independent and founder events. Am J Hum Genet. 1999;65(5):1308–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard P, Isnard R, Carrier L, Dubourg O, Donatien Y, Mathieu B, et al. Double heterozygosity for mutations in the beta-myosin heavy chain and in the cardiac myosin binding protein C genes in a family with hypertrophic cardiomyopathy. J Med Genet. 1999;36(7):542–5. [PMC free article] [PubMed] [Google Scholar]
- Tanjore RR, Sikindlapuram AD, Calambur N, Thakkar B, Kerkar PG, Nallari P. Genotype-phenotype correlation of R870H mutation in hypertrophic cardiomyopathy. Clin Genet. 2006;69(5):434–6. [DOI] [PubMed] [Google Scholar]
- Poetter K, Jiang H, Hassanzadeh S, Master SR, Chang A, Dalakas MC, et al. Mutations in either the essential or regulatory light chains of myosin are associated with a rare myopathy in human heart and skeletal muscle. Nat Genet. 1996;13(1):63–9. [DOI] [PubMed] [Google Scholar]
- Flavigny J, Richard P, Isnard R, Carrier L, Charron P, Bonne G, et al. Identification of two novel mutations in the ventricular regulatory myosin light chain gene (MYL2) associated with familial and classical forms of hypertrophic cardiomyopathy. J Mol Med (Berl). 1998;76(3-4):208–14. [DOI] [PubMed] [Google Scholar]
- Kabaeva ZT, Perrot A, Wolter B, Dietz R, Cardim N, Correia JM, et al. Systematic analysis of the regulatory and essential myosin light chain genes: genetic variants and mutations in hypertrophic cardiomyopathy. Eur J Hum Genet. 2002;10(11):741–8. [DOI] [PubMed] [Google Scholar]
- Olson TM, Karst ML, Whitby FG, Driscoll DJ. Myosin light chain mutation causes autosomal recessive cardiomyopathy with mid-cavitary hypertrophy and restrictive physiology. Circulation. 2002;105(20):2337–40. [DOI] [PubMed] [Google Scholar]
- Caleshu C, Sakhuja R, Nussbaum RL, Schiller NB, Ursell PC, Eng C, et al. Furthering the link between the sarcomere and primary cardiomyopathies: restrictive cardiomyopathy associated with multiple mutations in genes previously associated with hypertrophic or dilated cardiomyopathy. Am J Med Genet A. 2011;155A(9):2229–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggiero A, Chen SN, Lombardi R, Rodriguez G, Marian AJ. Pathogenesis of hypertrophic cardiomyopathy caused by myozenin 2 mutations is independent of calcineurin activity. Cardiovasc Res. 2013;97(1):44–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Li Z, Wang J, Sun K, Cui Q, Song L, et al. Mutations in NEXN, a Z-disc gene, are associated with hypertrophic cardiomyopathy. Am J Hum Genet. 2010;87(5):687–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamisawa S, Sato Y, Tatsuguchi Y, Fujino T, Imamura S, Uetsuka Y, et al. Mutation of the phospholamban promoter associated with hypertrophic cardiomyopathy. Biochem Biophys Res Commun. 2003;304(1):1–4. [DOI] [PubMed] [Google Scholar]
- Medin M, Hermida-Prieto M, Monserrat L, Laredo R, Rodriguez-Rey JC, Fernandez X, et al. Mutational screening of phospholamban gene in hypertrophic and idiopathic dilated cardiomyopathy and functional study of the PLN -42 C>G mutation. Eur J Heart Fail. 2007;9(1):37–43. [DOI] [PubMed] [Google Scholar]
- Blair E, Redwood C, Ashrafian H, Oliveira M, Broxholme J, Kerr B, et al. Mutations in the gamma(2) subunit of AMP-activated protein kinase cause familial hypertrophic cardiomyopathy: evidence for the central role of energy compromise in disease pathogenesis. Hum Mol Genet. 2001;10(11):1215–20. [DOI] [PubMed] [Google Scholar]
- Arad M, Benson DW, Perez-Atayde AR, McKenna WJ, Sparks EA, Kanter RJ, et al. Constitutively active AMP kinase mutations cause glycogen storage disease mimicking hypertrophic cardiomyopathy. J Clin Invest. 2002;109(3):357–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly BP, Russell MW, Hennessy JR, Ensing GJ. Severe hypertrophic cardiomyopathy in an infant with a novel PRKAG2 gene mutation: potential differences between infantile and adult onset presentation. Pediatr Cardiol. 2009;30(8):1176–9. [DOI] [PubMed] [Google Scholar]
- Laforet P, Richard P, Said MA, Romero NB, Lacene E, Leroy JP, et al. A new mutation in PRKAG2 gene causing hypertrophic cardiomyopathy with conduction system disease and muscular glycogenosis. Neuromuscul Disord. 2006;16(3):178–82. [DOI] [PubMed] [Google Scholar]
- Bos JM, Poley RN, Ny M, Tester DJ, Xu X, Vatta M, et al. Genotype-phenotype relationships involving hypertrophic cardiomyopathy-associated mutations in titin, muscle LIM protein, and telethonin. Mol Genet Metab. 2006;88(1):78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Y, Pinto JR, Gomes AV, Xu Y, Wang Y, Wang Y, et al. Functional consequences of the human cardiac troponin I hypertrophic cardiomyopathy mutation R145G in transgenic mice. J Biol Chem. 2008;283(29):20484–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier A, Judge DP, Schulman SP, Johnson N, Holmes KW, Murphy AM. Familial hypertrophic cardiomyopathy associated with cardiac beta-myosin heavy chain and troponin I mutations. Pediatr Cardiol. 2008;29(4):846–50. [DOI] [PubMed] [Google Scholar]
- Thierfelder L, Watkins H, MacRae C, Lamas R, McKenna W, Vosberg HP, et al. Alpha-tropomyosin and cardiac troponin T mutations cause familial hypertrophic cardiomyopathy: a disease of the sarcomere. Cell. 1994;77(5):701–12. [DOI] [PubMed] [Google Scholar]
- Menon SC, Michels VV, Pellikka PA, Ballew JD, Karst ML, Herron KJ, et al. Cardiac troponin T mutation in familial cardiomyopathy with variable remodeling and restrictive physiology. Clin Genet. 2008;74(5):445–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anan R, Shono H, Kisanuki A, Arima S, Nakao S, Tanaka H. Patients with familial hypertrophic cardiomyopathy caused by a Phe110Ile missense mutation in the cardiac troponin T gene have variable cardiac morphologies and a favorable prognosis. Circulation. 1998;98(5):391–7. [DOI] [PubMed] [Google Scholar]
- Watkins H, Anan R, Coviello DA, Spirito P, Seidman JG, Seidman CE. A de novo mutation in alpha-tropomyosin that causes hypertrophic cardiomyopathy. Circulation. 1995;91(9):2302–5. [DOI] [PubMed] [Google Scholar]
- Karibe A, Tobacman LS, Strand J, Butters C, Back N, Bachinski LL, et al. Hypertrophic cardiomyopathy caused by a novel alpha-tropomyosin mutation (V95A) is associated with mild cardiac phenotype, abnormal calcium binding to troponin, abnormal myosin cycling, and poor prognosis. Circulation. 2001;103(1):65–71. [DOI] [PubMed] [Google Scholar]
- Herman DS, Lam L, Taylor MR, Wang L, Teekakirikul P, Christodoulou D, et al. Truncations of titin causing dilated cardiomyopathy. N Engl J Med. 2012;366(7):619–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasile VC, Ommen SR, Edwards WD, Ackerman MJ. A missense mutation in a ubiquitously expressed protein, vinculin, confers susceptibility to hypertrophic cardiomyopathy. Biochem Biophys Res Commun. 2006;345(3):998–1003. [DOI] [PubMed] [Google Scholar]
- Longeri M, Ferrari P, Knafelz P, Mezzelani A, Marabotti A, Milanesi L, et al. Myosin-binding protein C DNA variants in domestic cats (A31P, A74T, R820W) and their association with hypertrophic cardiomyopathy. J Vet Intern Med. 2013;27(2):275–85. [DOI] [PMC free article] [PubMed] [Google Scholar]