Abstract
Neoatherosclerosis is a form of accelerated atherosclerosis that occurs within stented segments of the coronary vessel late or very late after drug-eluting stent (DES) implantation via percutaneous coronary intervention (PCI). This proliferation of neointima with a formation of new atheromatous plaque within stent struts lacking re-endothelialization can provoke thrombotic occlusion and lead to catastrophic acute coronary events. Knowing that coronary artery disease is the leading single cause of mortality worldwide and that there is a constant trend of increase in PCI procedures, it is reasonable to conclude that late thrombotic events and neoatherosclerosis post-PCI remain an important therapeutic challenge. For these reasons, early identification of patients at risk through the means of advanced imaging methods or preventive solutions available through novel technological solutions in DES design that target pro-inflammatory pathways and enable optimized arterial healing are central strategies in prevention and treatment of in-stent neoatherosclerosis and thrombosis. Due to this, pre-clinical studies performed on animal models are crucial building blocks that enable the objective and scientific assessment of innovative technological and therapeutic solutions before they are introduced to early stages of human clinical trials. A comparative medicine approach allows designing and executing experiments in animal models with a high degree of similarity with human coronary anatomy possibly promising the translation of encouraging findings to human clinical studies. The aim of this review is to provide contemporary insights on the pathophysiology of neoatherosclerosis and in-stent thrombosis and emergence of novel biomedical and technological solutions used to counter them.
Keywords: coronary artery disease; coronary restenosis; models, animal; neoatherosclerosis; percutaneous coronary intervention
Introduction
Atherosclerosis is a chronic process caused by endothelial injury that occurs in native blood vessels over decades and is marked by proliferation of intimal smooth-muscle cells (SMC) and infiltration of monocytes and foam cells supporting chronic inflammation and lipid retention within the arterial wall [1,2]. Percutaneous coronary intervention (PCI) today is the well-established standard for the treatment of significant and symptomatic coronary lesions due to coronary artery disease (CAD) while more severe multivessel CAD cases accompanied with heavily impaired left-ventricular function are still managed by coronary artery bypass grafting (CABG) [3]. The first PCI by plain old balloon angioplasty and transluminal dilatation of coronary artery stenosis was performed by Andreas R. Grüntzig in 1977 while Ulrich Sigwart and colleagues introduced the first restoration of coronary lumen by self-expandable stainless-steel stent in 1987 [4,5]. The lumen diameter reduction post-PCI is termed “restenosis” and can occur because of remodeling/recoil of the vessel due to mechanical stress caused by balloon dilatation or in a case of stent implantation, to the neointimal SMC proliferation with intense formation of new atherosclerotic plaque within the stent struts, in-stent restenosis (ISR) [6]. Stent deployment after balloon dilatation causes denudation of endothelial cells within the stented segment resulting in the healing arterial ulcers promoting ISR and thrombosis by interfering with local endothelial repair processes and by perturbation of local vessel flow hemodynamics [7-9]. In the mid-1990s, the era of bare-metal stent (BMS) implantation began and was in later decades surpassed by drug-eluting stents (DES) that had significantly more success in reducing the rates of ISR and risk of reinterventions, especially in short-to-medium term period, however, were more frequently associated with late and/or very late in-stent thrombotic events and neoatherosclerosis [10-14].
Atherosclerosis is a chronic process caused by endothelial injury that occurs in native blood vessels over decades and is marked by proliferation of intimal smooth-muscle cells (SMC) and infiltration of monocytes and foam cells supporting chronic inflammation and lipid retention within the arterial wall [1,2]. Percutaneous coronary intervention (PCI) today is the well-established standard for the treatment of significant and symptomatic coronary lesions due to coronary artery disease (CAD) while more severe multivessel CAD cases accompanied with heavily impaired left-ventricular function are still managed by coronary artery bypass grafting (CABG) [3]. The first PCI by plain old balloon angioplasty and transluminal dilatation of coronary artery stenosis was performed by Andreas R. Grüntzig in 1977 while Ulrich Sigwart and colleagues introduced the first restoration of coronary lumen by self-expandable stainless-steel stent in 1987 [4,5]. The lumen diameter reduction post-PCI is termed “restenosis” and can occur because of remodeling/recoil of the vessel due to mechanical stress caused by balloon dilatation or in a case of stent implantation, to the neointimal SMC proliferation with intense formation of new atherosclerotic plaque within the stent struts, in-stent restenosis (ISR) [6]. Stent deployment after balloon dilatation causes denudation of endothelial cells within the stented segment resulting in the healing arterial ulcers promoting ISR and thrombosis by interfering with local endothelial repair processes and by perturbation of local vessel flow hemodynamics [7-9]. In the mid-1990s, the era of bare-metal stent (BMS) implantation began and was in later decades surpassed by drug-eluting stents (DES) that had significantly more success in reducing the rates of ISR and risk of reinterventions, especially in short-to-medium term period, however, were more frequently associated with late and/or very late in-stent thrombotic events and neoatherosclerosis [10-14].
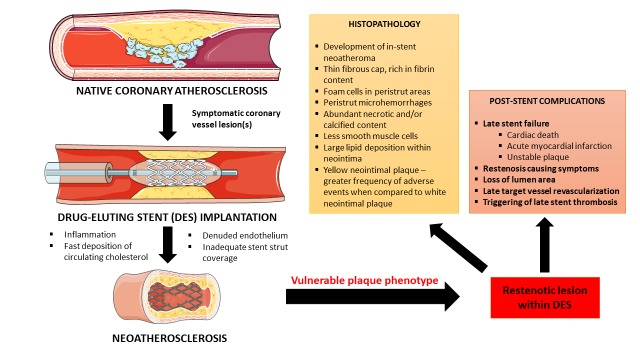
Neoatherosclerosis, on the other hand, is the accelerated form of atherosclerosis of multifactorial etiology that presents a real concern in the contemporary use of DES implants (Figure 1) [15]. It is characterized by the neointimal SMC proliferation with a presence of lipids or calcifications in the stented segment of the coronary vessel, usually late (> 12 months) or very late (> 36 months) post-DES implantation leading to late stent failure [13,16,17]. Mechanistically, it is hypothesized that anti-proliferative and immunosuppressive actions of drugs delivered by the DES prevent restenosis but cause leaky endothelial coverage that gives a rise to accelerated transmigration of low-density lipoproteins, foam cells and inflammatory cells within the stent creating a neoatherosclerotic lesion [15]. Factors such as the delayed endothelialization, peristrut microhemorrhages, polymer coating type, chronic inflammation, localized hypersensitivity reaction, and endothelial dysfunction all have putative roles in the pathogenesis of neoatherosclerosis and in-stent thrombosis [15,18-21]. Neoatherosclerosis is usually confirmed by the advanced imaging methods including intracoronary optical coherence tomography (OCT) and intravascular ultrasound (IVUS) while the analyses of lesion specimens obtained through biopsy provide histopathological characterization [22,23]. The increased risk of ISR after DES implantation advocated for the longer use of dual anti-platelet therapy (DAPT) in patients with DES compared to those with BMS [24]. Similarly, the degree of endothelialization within stented segment of the vessel has a significant impact on the duration of DAPT usage while DAPT discontinuation is associated with an increased rate of acute thrombotic events late after DES implantation [10,11,25]. Clinically, neoatherosclerosis is an important therapeutic challenge since late/very late ISR of neoatherosclerotic etiology bears a high risk for development of acute coronary syndromes (ACS) and is generally associated with poor survival prognosis and major adverse cardiac events (MACE) in these patients [23,26,27].
Figure 1.
Overview of neoatherosclerosis that occurs late after drug-eluting stent implantation. Constructed by the authors using illustration elements that were kindly provided by the Servier. Servier Medical Art is licensed under a Creative Commons Attribution 3.0 Unported License.
Therefore, the aim of this review is to provide answers on how can the latest advancements in interventional cardiology such as bioresorbable vascular scaffolds (BVS), molecular-based therapies, systemic therapies and dual-therapy stents affect patient care and clinical outcomes, and how can studying animal models and comparative medicine approach facilitate our understanding and therapeutic and/or preventive approaches towards neoatherosclerosis.
The Role of Pre-clinical Models of In-stent Restenosis and its Translational Value
In the last fifteen years, we have observed a significant rise in the allocation of National Institutes of Health (NIH) funding for animal-based research (72.7 percent rise) aimed towards modeling of human diseases in experimental animals [28]. One of the key issues in translational and comparative medicine is how and to what degree can we translate findings obtained from pre-clinical models of the disease to human biology? The value of animal models remains questionable since a substantial number of promising experiments performed in animals fail to translate with similar efficacy in human-based clinical trials [29,30]. Hackam and colleagues concluded that approximately one-third of highly cited animal research is successfully translated at the level of randomized controlled trials (RCTs) in humans [29]. Moreover, a recent study showed that publication bias in reports on animal stroke models accounted for the 30 percent overstatements of efficacy in interventions that were performed on animals [31]. Thereupon, some authors suggest that systematic review and meta-analysis of animal studies might be useful in selecting those intervention strategies that are most promising for translation to human RCTs [30]. Furthermore, an early translation from pre-clinical studies to phase I/II human trials should be carried out in the form of large-scale multicenter RCTs that are powered for long-term “hard” end-points [32].
In terms of experimental animal model use in coronary intervention, it should be noted that most of the information on coronary ISR and DES usage have been obtained from porcine models [33-35]. Comparatively, porcine coronary models of ISR, nowadays, provide the highest correlation with human cardiovascular anatomy, hemodynamic and coagulation physiology, vasa vasorum development, and collateral arterial supply [36]. The fundamental principle of an animal model of ISR consists of experimental induction of atherosclerotic lesion within the coronary segment through vascular injury, consequently eliciting localized healing responses and neointimal proliferation that mimic ISR [37]. In 1992, Schwartz and colleagues demonstrated that the degree of vessel injury after PCI strongly correlated with neointimal proliferation and percent diameter stenosis in a porcine model of purposely overexpanded stent implantation [38]. Also, an adventitial contraction known as remodeling along with persistent inflammation, intimal and medial dissections post-PCI, and elastic recoil upon balloon dilatation all contribute to restenosis [39,40]. While the efficacy of interventional coronary treatments in humans might not closely follow those obtained in swine models, it should be highlighted that animal models are useful in testing critical hypotheses and providing mechanistic insights into fundamental biological processes and responses to pathology that occur within a coronary vessel [41]. Expert recommendations regarding the pre-clinical evaluation of DES suggest that the iliac arteries in rabbits and coronary arteries in domestic swine models are appropriate for the testing of novel DES solutions and show a substantial degree of correlation with clinical applications of such devices in humans [42]. The standardization of histopathological analyses after DES implantation in animal models should include: determination of injury and inflammation score, stent strut positioning, degree of endothelialization and condition of adjacent tissues, stent design (drug used, polymer coating type), vascular response and healing, potential overlapping of the stent, stent fractures and histopathologic sampling of in-stent tissue samples at multiple time points [41]. The latter is of a particular concern in late in-stent thrombosis and neoatherosclerosis while data show that the endothelial healing process post-DES implantation is comparatively different between animal models and humans [41,43-45]. Therefore, Nakazawa and colleagues emphasized that due to the multifactorial nature of arterial healing, next generation of DESs should undergo extensive pre-clinical testing that will assess the degree of endothelialization, inflammation, drug release kinetics and neointimal reduction to determine the safety level of these devices before they are introduced to respective RCTs in humans [43].
Use of BVS and Biodegradable Polymers to Attenuate and Prevent Neoatherosclerosis
Bioresorbable vascular scaffolds (BVS) or bioresorbable stents (BRS) are the new tool in the PCI armamentarium, aimed to acutely prevent vessel closure and to deliver anti-proliferative drugs into the stented segments that inhibit neointimal hyperplasia, followed by their complete biological resorption and long-term integration with the vessel wall [46,47]. In this way, bioresorbable devices support the transient nature of vascular scaffolding and offer more optimized vessel healing while avoiding permanent vessel caging and thus decrease the risk for later ISR and thrombotic occlusion [48,49]. Although these devices are conceptually an attractive design, their efficacy, safety, and cost-effectiveness in the long-term period and comparatively with third-generation DES and BMS still need to be confirmed and validated in carefully designed RCTs [50]. Due to this, translational and comparative studies exploring polymer chemistry, biotechnical solutions, as well as in-vitro and in-vivo testing in animal models with relevant histopathologic characterization are necessary [51]. A recent study by Nakazawa et al. performed on rabbit iliac artery model showed that biodegradable polymer-coated thin-strut DES significantly diminished foamy macrophage infiltration within neointima (neoatherosclerosis) and showed faster stent strut neointimal coverage in comparison to durable polymer-based stent [52]. Transitioning to clinical practice, the bioresorbable polymer-coated sirolimus-eluting stent was associated with low and stable in-stent late lumen loss (LLL), complete strut coverage and no in-stent thrombosis at 18-month follow-up after implantation showing that DES with absorbable polymer is a safe and efficacious in lowering long-term risks of inflammation, delayed healing, and adverse effects in humans [53]. Similarly, Wang et al. demonstrated that biodegradable polymer sirolimus-eluting stents were associated with rapid endothelialization and low LLL rates at 4 and 12 months follow-up [54]. Finally, the wide spectrum of available technology and stent types will enable selection of a stent device and therapeutic tailoring towards each individual patient, affirming the postulates of precision medicine in which “one size fits all” approach no longer has justification.
Neoatherosclerosis and Systemic Pharmacotherapy
An in-stent neoatherosclerosis is a local inflammatory process that interferes with vascular biology and endothelialization of the coronary vessel [55]. In that respect, a recent pre-clinical study showed that systemic intravenous (IV) use of methotrexate (MTX) combined with DES in a rabbit model decreased in-stent neoatherosclerosis as assessed by in-vivo OCT imaging and histological analyses [56]. MTX-treated animals showed decreased rates of lipid-rich intima and reduced neointimal thickness along with larger fibrous cap thickness and smaller lumen areas in comparison to neoatheroslerotic animals that received placebo. Furthermore, there was a marked reduction in the serum levels of pro-inflammatory cytokines (IL-6, IL-12, MCP-1, TNF-α), adhesion molecules (ICAM-1 and VCAM-1), and nuclear factor-κB p65 with an increase of IL-10 in animals receiving systemic MTX along with DES implantation. MTX exerts the anti-inflammatory effect by blocking NF-κB signaling and this, in turn, decreases expression and secretion of pro-inflammatory cytokines [57,58]. Results of the study by Zhang and colleagues clearly suggested that there is an intimate relationship between inflammatory cells, pro-inflammatory cytokines, and vascular healing responses within stented segments of coronary vessels, as previously suggested [59-61]. In conclusion, pharmacologic targeting of pro-inflammatory pathways might be a feasible therapeutic strategy to prevent ISR and reduce long-term risk for late thrombotic events.
Molecular Therapy for Accelerated Endothelial Recovery of the Vessel Post-PCI
Rapid and complete endothelialization of the coronary artery after an injury caused by DES implantation should theoretically prevent short to long-term complications such as restenosis, thrombosis, and neotherosclerosis [62]. Magnetically targeted delivery of therapeutic agents to injured blood vessels is a novel approach in the prevention of in-stent restenosis that might enable site-specific delivery of small-molecule proteins, drugs, gene vectors and cells to the lesion of interest [63]. In this respect, Adamo and colleagues performed an experiment in stented rat carotid arteries in which endothelial cells (ECs) were magnetically guided and delivered along with polylactide-based biodegradable magnetic nanoparticles (MNP) that served as carrier vehicle to the site of the lesion [64]. Results showed that accelerated re-endothelialization of the stented segment of the artery was achieved, with marked enhancement of endothelial growth and cellular propagation of MNP-functionalized ECs. Similar results were observed in rat carotid artery stent angioplasty model where localization of ECs exhibited significant protection from stenosis at the distal part of the stent in the cell therapy group compared to both the proximal part of the stent in the cell therapy group and the control [65]. Namely, there was a 1.7-fold less reduction in lumen diameter, 2.3-fold less reduction in the ratios of peak systolic velocities and 2.1-fold attenuation of stenosis as assessed by end-point histomorphometric analyses in a group treated with cell therapy. Furthermore, a site-specific vascular gene delivery to stented arteries by using magnetically guided nanoparticles loaded with adenoviral vectors showed that transduction of cultured endothelial and smooth muscle cells under magnetic conditions was achieved and injured arteries showed a significant increase in localized vascular gene expression [66]. A previous study showed that vascular stent in porcine coronary arteries was an appropriate platform for highly localized and efficient viral vector delivery system without systematic spreading of the vector to other organs or in the downstream segments of the stented arteries [67]. These therapies might provide accelerated endothelial cell repopulation within stented segments of blood vessels following PCI in humans, however, a translational value of such strategies is yet to be confirmed in further pre-clinical studies in other animal models to allow for comparative analyses of designated end-points. Of concern are the further improvements in MNP design and cell treatment protocols that would provide suitable magnetic properties without excessive compromise in cell viability and function [68].
Dual-therapy Endothelial Progenitor Cell-capturing DES
“Pro-healing” approach towards the stented coronary lesion has been recently exemplified in a clinical trial (The EGO-Combo Study) examining the effects of dual-therapy with endothelial progenitor cell-capturing technology combined with sirolimus-eluting stent (Combo stent) showing that this device provided unique late neointimal regression (9 to 24 month follow-up) that was not observed with any other commercially available stent and these results were even extended to 36 months displaying minimal restenosis and no late stent thrombosis in humans [69]. This technology is based on reducing the neointimal hyperplasia through an abluminal bioresorbable polymer eluting sirolimus while luminally immobilized anti-CD34+ antibodies are simultaneously capturing circulating endothelial progenitor cells, therefore, countering delayed arterial healing, hypersensitivity, late stent thrombosis and neoatherosclerosis [70,71]. Favorable effects of this innovative stent design have been first observed and obtained from experiments performed in pigs, once more showing that biotechnical solutions accomplished in comparative animal models are crucial for a successful translation of these designs in humans [72-74]. Finally, in an important REMEDEE clinical trial, Combo stent showed noninferiority in primary angiographic end-point in comparison to Xience-V stent and was safe with no stent thrombosis up to 12 months [75].
Conclusions and Outlook
Pre-clinical studies performed on animals and comparative medicine approach are crucial for the successful translation of novel pharmacologic and device solutions that are targeted against in-stent neoatherosclerosis and thrombosis in humans with CAD requiring PCI. Porcine and rabbit animal models comparatively remain the gold standard in this area of research with the highest degree of similarity with human coronary and cardiovascular anatomy/physiology and best translational value from bench to bedside. It is expected that the wide array of approved novel devices and drugs on the market will offer highly individualized precision medicine treatment for patients with CAD requiring PCI. Future treatment modalities will encompass BVS, dual-therapy bioresorbable stents, systemic anti-inflammatory chemotherapeutics, cell-based and gene vector-based therapies that will promote uninterrupted arterial healing, suppression of inflammation, and prevention of restenosis and thrombotic events.
Acknowledgments
This article celebrates 40 years of PCI-percutaneous coronary intervention (1977-2017) and is dedicated by the authors to the memory of late Andreas R. Grüntzig (1939-1985), father of modern interventional cardiology.
Glossary
- ACS
Acute Coronary Syndromes
- BMS
Bare Metal Stents
- BVS
Bioresorbable Vascular Scaffold
- CAD
Coronary Artery Disease
- DAPT
Dual Anti-Platelet Therapy
- DES
Drug-Eluting Stents
- ISR
In-Stent Restenosis
- IVUS
Intravascular Ultrasound
- OCT
Optical Coherence Tomography
- PCI
Percutaneous Coronary Intervention
- SMC
Smooth Muscle Cell
- STEMI
ST-segment elevation myocardial infarction
Author Contributions
Original conception and design of the manuscript: JAB; Drafting the article: JAB; Revising it critically for important intellectual content: JAB, DD’A and GN; Approved final version of the manuscript: JAB, DD’A and GN.
References
- Lusis AJ. Atherosclerosis. Nature. 2000;407(6801):233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P, Ridker PM, Hansson GK. on Atherothrombosis LTN. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009;54(23):2129–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grech ED. Percutaneous coronary intervention. I: history and development. BMJ. 2003;326(7398):1080–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruntzig A. Transluminal dilatation of coronary-artery stenosis. Lancet. 1978;1(8058):263. [DOI] [PubMed] [Google Scholar]
- Sigwart U, Puel J, Mirkovitch V, Joffre F, Kappenberger L. Intravascular stents to prevent occlusion and restenosis after transluminal angioplasty. N Engl J Med. 1987;316(12):701–706. [DOI] [PubMed] [Google Scholar]
- Buccheri D, Piraino D, Andolina G, Cortese B. Understanding and managing in-stent restenosis: a review of clinical data, from pathogenesis to treatment. J Thorac Dis. 2016;8(10):E1150–e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Heiden K, Gijsen FJ, Narracott A, Hsiao S, Halliday I, Gunn J, et al. The effects of stenting on shear stress: relevance to endothelial injury and repair. Cardiovasc Res. 2013;99(2):269–275. [DOI] [PubMed] [Google Scholar]
- Hsiao ST, Spencer T, Boldock L, Prosseda SD, Xanthis I, Tovar-Lopez FJ, et al. Endothelial repair in stented arteries is accelerated by inhibition of Rho-associated protein kinase. Cardiovasc Res. 2016;112(3):689–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald A, Iruela-Arispe ML. Healing arterial ulcers: endothelial lining regeneration upon vascular denudation injury. Vascul Pharmacol. 2015;72:9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden EP, Stabile E, Regar E, Cheneau E, Ong ATL, Kinnaird T, et al. Late thrombosis in drug-eluting coronary stents after discontinuation of antiplatelet therapy. Lancet. 2004;364(9444):1519–1521. [DOI] [PubMed] [Google Scholar]
- Pfisterer M, Brunner-La Rocca HP, Buser PT, Rickenbacher P, Hunziker P, Mueller C, et al. Late Clinical Events After Clopidogrel Discontinuation May Limit the Benefit of Drug-Eluting Stents: An Observational Study of Drug-Eluting Versus Bare-Metal Stents. J Am Coll Cardiol. 2006;48(12):2584–2591. [DOI] [PubMed] [Google Scholar]
- Kastrati A, Dibra A, Spaulding C, Laarman GJ, Menichelli M, Valgimigli M, et al. Meta-analysis of randomized trials on drug-eluting stents vs. bare-metal stents in patients with acute myocardial infarction. Eur Heart J. 2007;28(22):2706–2713. [DOI] [PubMed] [Google Scholar]
- Nakazawa G, Otsuka F, Nakano M, Vorpahl M, Yazdani SK, Ladich E, et al. The pathology of neoatherosclerosis in human coronary implants bare-metal and drug-eluting stents. J Am Coll Cardiol. 2011;57(11):1314–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmerini T, Biondi-Zoccai G, Riva DD, Stettler C, Sangiorgi D, D’Ascenzo F, et al. Stent thrombosis with drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis. Lancet. 2012;379(9824):1393–1402. [DOI] [PubMed] [Google Scholar]
- Yahagi K, Kolodgie FD, Otsuka F, Finn AV, Davis HR, Joner M, et al. Pathophysiology of native coronary, vein graft, and in-stent atherosclerosis. Nat Rev Cardiol. 2016;13(2):79–98. [DOI] [PubMed] [Google Scholar]
- Park SJ, Kang SJ, Virmani R, Nakano M, Ueda Y. In-stent neoatherosclerosis: a final common pathway of late stent failure. J Am Coll Cardiol. 2012;59(23):2051–2057. [DOI] [PubMed] [Google Scholar]
- Amabile N, Souteyrand G, Ghostine S, Combaret N, Slama MS, Barber-Chamoux N, et al. Very late stent thrombosis related to incomplete neointimal coverage or neoatherosclerotic plaque rupture identified by optical coherence tomography imaging. Eur Heart J Cardiovasc Imaging. 2014;15(1):24–31. [DOI] [PubMed] [Google Scholar]
- Taniwaki M, Radu MD, Zaugg S, Amabile N, Garcia-Garcia HM, Yamaji K, et al. Mechanisms of Very Late Drug-Eluting Stent Thrombosis Assessed by Optical Coherence Tomography. Circulation. 2016;133(7):650–660. [DOI] [PubMed] [Google Scholar]
- Terzian Z, Gasser TC, Blackwell F, Hyafil F, Louedec L, Deschildre C, et al. Peristrut microhemorrhages: a possible cause of in-stent neoatherosclerosis? Cardiovasc Pathol. 2017;26:30–38. [DOI] [PubMed] [Google Scholar]
- Yamaji K, Kubo S, Inoue K, Kadota K, Kuramitsu S, Shirai S, et al. Association of localized hypersensitivity and in-stent neoatherosclerosis with the very late drug-eluting stent thrombosis. PLoS One. 2014;9(11):e113870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne R, Joner M, Kastrati A. Polymer coatings and delayed arterial healing following drug-eluting stent implantation. Minerva Cardioangiol. 2009;57(5):567–584. [PubMed] [Google Scholar]
- Otsuka F, Byrne RA, Yahagi K, Mori H, Ladich E, Fowler DR, et al. Neoatherosclerosis: overview of histopathologic findings and implications for intravascular imaging assessment. Eur Heart J. 2015;36(32):2147–2159. [DOI] [PubMed] [Google Scholar]
- Lee S-Y, Hur S-H, Lee S-G, Kim S-W, Shin D-H, Kim J-S, et al. Optical Coherence Tomographic Observation of In-Stent Neoatherosclerosis in Lesions With More Than 50% Neointimal Area Stenosis After Second-Generation Drug-Eluting Stent Implantation. Circ Cardiovasc Interv. 2015;8(2):e001878. [DOI] [PubMed] [Google Scholar]
- Kereiakes DJ, Yeh RW, Massaro JM, Driscoll-Shempp P, Cutlip DE, Steg PG, et al. Stent Thrombosis in Drug-Eluting or Bare-Metal Stents in Patients Receiving Dual Antiplatelet Therapy. JACC Cardiovasc Interv. 2015;8(12):1552–1562. [DOI] [PubMed] [Google Scholar]
- Habib A, Finn AV. Endothelialization of Drug Eluting Stents and its Impact on Dual Anti-platelet Therapy Duration. Pharmacol Res. 2015;93:22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SJ, Lee CW, Song H, Ahn JM, Kim WJ, Lee JY, et al. OCT analysis in patients with very late stent thrombosis. JACC Cardiovasc Imaging. 2013;6(6):695–703. [DOI] [PubMed] [Google Scholar]
- Virmani R, Kolodgie FD, Farb A, Lafont A. Drug eluting stents: are human and animal studies comparable? Heart. 2003;89(2):133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman J, Chandna A, Roe K. Trends in animal use at US research facilities. J Med Ethics. 2015;41(7):567–569. [DOI] [PubMed] [Google Scholar]
- Hackam DG, Redelmeier DA. Translation of research evidence from animals to humans. JAMA. 2006;296(14):1731–1732. [DOI] [PubMed] [Google Scholar]
- van der Worp HB, Howells DW, Sena ES, Porritt MJ, Rewell S, O’Collins V, et al. Can animal models of disease reliably inform human studies? PLoS Med. 2010;7(3):e1000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sena ES, van der Worp HB, Bath PM, Howells DW, Macleod MR. Publication bias in reports of animal stroke studies leads to major overstatement of efficacy. PLoS Biol. 2010;8(3):e1000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCafferty K, Forbes S, Thiemermann C, Yaqoob MM. The challenge of translating ischemic conditioning from animal models to humans: the role of comorbidities. Dis Model Mech. 2014;7(12):1321–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldman AW, Cheng L, Jenkins GM, Heller PF, Kim DW, Ware M, Jr, et al. Paclitaxel stent coating inhibits neointimal hyperplasia at 4 weeks in a porcine model of coronary restenosis. Circulation. 2001;103(18):2289–2295. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Kopia G, Hayashi S, Bailey LR, Llanos G, Wilensky R, et al. Stent-based delivery of sirolimus reduces neointimal formation in a porcine coronary model. Circulation. 2001;104(10):1188–1193. [DOI] [PubMed] [Google Scholar]
- Gallo R, Padurean A, Jayaraman T, Marx S, Roque M, Adelman S, et al. Inhibition of intimal thickening after balloon angioplasty in porcine coronary arteries by targeting regulators of the cell cycle. Circulation. 1999;99(16):2164–2170. [DOI] [PubMed] [Google Scholar]
- Lowe HC, Schwartz RS, Mac Neill BD, Jang IK, Hayase M, Rogers C, et al. The porcine coronary model of in-stent restenosis: current status in the era of drug-eluting stents. Catheter Cardiovasc Interv. 2003;60(4):515–523. [DOI] [PubMed] [Google Scholar]
- Perkins LE. Preclinical models of restenosis and their application in the evaluation of drug-eluting stent systems. Vet Pathol. 2010;47(1):58–76. [DOI] [PubMed] [Google Scholar]
- Schwartz RS, Huber KC, Murphy JG, Edwards WD, Camrud AR, Vlietstra RE, et al. Restenosis and the proportional neointimal response to coronary artery injury: results in a porcine model. J Am Coll Cardiol. 1992;19(2):267–274. [DOI] [PubMed] [Google Scholar]
- Schwartz RS. The vessel wall reaction in restenosis. Semin Interv Cardiol. 1997;2(2):83–88. [PubMed] [Google Scholar]
- Schwartz RS, Henry TD. Pathophysiology of coronary artery restenosis. Rev Cardiovasc Med. 2002;3 Suppl 5:S4–S9. [PubMed] [Google Scholar]
- Suzuki Y, Yeung AC, Ikeno F. The pre-clinical animal model in the translational research of interventional cardiology. JACC Cardiovasc Interv. 2009;2(5):373–383. [DOI] [PubMed] [Google Scholar]
- Schwartz RS, Chronos NA, Virmani R. Preclinical restenosis models and drug-eluting stents: still important, still much to learn. J Am Coll Cardiol. 2004;44(7):1373–1385. [DOI] [PubMed] [Google Scholar]
- Nakazawa G, Finn AV, John MC, Kolodgie FD, Virmani R. The significance of preclinical evaluation of sirolimus-, paclitaxel-, and zotarolimus-eluting stents. Am J Cardiol. 2007;100 8b:36m–44m. [DOI] [PubMed] [Google Scholar]
- Kotani J, Awata M, Nanto S, Uematsu M, Oshima F, Minamiguchi H, et al. Incomplete neointimal coverage of sirolimus-eluting stents: angioscopic findings. J Am Coll Cardiol. 2006;47(10):2108–2111. [DOI] [PubMed] [Google Scholar]
- Joner M, Finn AV, Farb A, Mont EK, Kolodgie FD, Ladich E. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006;48(1):193–202. [DOI] [PubMed] [Google Scholar]
- Iqbal J, Onuma Y, Ormiston J, Abizaid A, Waksman R, Serruys P. Bioresorbable scaffolds: rationale, current status, challenges, and future. Eur Heart J. 2014;35(12):765–776. [DOI] [PubMed] [Google Scholar]
- Garg S, Bourantas C, Serruys PW. New concepts in the design of drug-eluting coronary stents. Nat Rev Cardiol. 2013;10(5):248–260. [DOI] [PubMed] [Google Scholar]
- Indolfi C, De Rosa S, Colombo A. Bioresorbable vascular scaffolds - basic concepts and clinical outcome. Nat Rev Cardiol. 2016;13(12):719–729. [DOI] [PubMed] [Google Scholar]
- Cerrato E, Echavarria-Pinto M, Tandjung K, Macaya C, Escaned J. Optimizing vessel healing following drug eluting stent implantation with biodegradable polymer DES. Minerva Cardioangiol. 2014;62(5):407–420. [PubMed] [Google Scholar]
- Charpentier E, Barna A, Guillevin L, Juliard JM. Fully bioresorbable drug-eluting coronary scaffolds: A review. Arch Cardiovasc Dis. 2015;108(6-7):385–397. [DOI] [PubMed] [Google Scholar]
- Sanchez OD, Yahagi K, Byrne RA, Mori H, Zarpak R, Wittchow E, et al. Pathological aspects of bioresorbable stent implantation. EuroIntervention. 2015;11 Suppl V:V159-65. [DOI] [PubMed] [Google Scholar]
- Nakazawa G, Torii S, Ijichi T, Nagamatsu H, Ohno Y, Kurata F, et al. Comparison of Vascular Responses Following New-Generation Biodegradable and Durable Polymer-Based Drug-Eluting Stent Implantation in an Atherosclerotic Rabbit Iliac Artery Model. J Am Heart Assoc. 2016;5(10):e003803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormiston J, Webster M, Stewart J, Vrolix M, Whitbourn R, Donohoe D, et al. First-in-human evaluation of a bioabsorbable polymer-coated sirolimus-eluting stent: imaging and clinical results of the DESSOLVE I Trial (DES with sirolimus and a bioabsorbable polymer for the treatment of patients with de novo lesion in the native coronary arteries). JACC Cardiovasc Interv. 2013;6(10):1026–1034. [DOI] [PubMed] [Google Scholar]
- Wang G, Sun Z, Jin Q, Xu K, Li Y, Wang X, et al. First-in-man study evaluating the safety and efficacy of a second generation biodegradable polymer sirolimus-eluting stent in the treatment of patients with de novo coronary lesions: clinical, Angiographic, and OCT outcomes of CREDIT-1. Catheter Cardiovasc Interv. 2015;85 Suppl 1:744–751. [DOI] [PubMed] [Google Scholar]
- Otsuka F, Finn AV, Yazdani SK, Nakano M, Kolodgie FD, Virmani R. The importance of the endothelium in atherothrombosis and coronary stenting. Nat Rev Cardiol. 2012;9(8):439–453. [DOI] [PubMed] [Google Scholar]
- Zhang R, Chen S, Zhang H, Liu Q, Xing J, Zhao Q, et al. Effects of Methotrexate in a Rabbit Model of In-Stent Neoatherosclerosis: An Optical Coherence Tomography Study. Sci Rep. 2016;6:33657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tak PP, Firestein GS. NF-κB: a key role in inflammatory diseases. J Clin Invest. 2001;107(1):7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar S, Aggarwal BB. Methotrexate Suppresses NF-κB Activation Through Inhibition of IκBα Phosphorylation and Degradation. J Immunol. 2001;167(5):2911–2920. [DOI] [PubMed] [Google Scholar]
- Rogers C, Edelman ER. Endovascular stent design dictates experimental restenosis and thrombosis. Circulation. 1995;91(12):2995–3001. [DOI] [PubMed] [Google Scholar]
- Ribichini F, Joner M, Ferrero V, Finn AV, Crimins J, Nakazawa G, et al. Effects of oral prednisone after stenting in a rabbit model of established atherosclerosis. J Am Coll Cardiol. 2007;50(2):176–185. [DOI] [PubMed] [Google Scholar]
- Farb A, John M, Acampado E, Kolodgie FD, Prescott MF, Virmani R. Oral everolimus inhibits in-stent neointimal growth. Circulation. 2002;106(18):2379–2384. [DOI] [PubMed] [Google Scholar]
- Ong AT, Aoki J, Kutryk MJ, Serruys PW. How to accelerate the endothelialization of stents. Arch Mal Coeur Vaiss. 2005;98(2):123–126. [PubMed] [Google Scholar]
- Chorny M, Fishbein I, Adamo RF, Forbes SP, Folchman-Wagner Z, Alferiev IS. Magnetically targeted delivery of therapeutic agents to injured blood vessels for prevention of in-stent restenosis. Methodist DeBakey Cardiovasc J. 2012;8(1):23–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamo RF, Fishbein I, Zhang K, Wen J, Levy RJ, Alferiev IS, et al. Magnetically enhanced cell delivery for accelerating recovery of the endothelium in injured arteries. J Control Release. 2016;222:169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyak B, Medved M, Lazareva N, Steele L, Patel T, Rai A, et al. Magnetic Nanoparticle-Mediated Targeting of Cell Therapy Reduces In-Stent Stenosis in Injured Arteries. ACS Nano. 2016 [DOI] [PubMed] [Google Scholar]
- Chorny M, Fishbein I, Tengood JE, Adamo RF, Alferiev IS, Levy RJ. Site-specific gene delivery to stented arteries using magnetically guided zinc oleate-based nanoparticles loaded with adenoviral vectors. FASEB J. 2013;27(6):2198–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klugherz BD, Song C, DeFelice S, Cui X, Lu Z, Connolly J, et al. Gene delivery to pig coronary arteries from stents carrying antibody-tethered adenovirus. Hum Gene Ther. 2002;13(3):443–454. [DOI] [PubMed] [Google Scholar]
- Chorny M, Alferiev IS, Fishbein I, Tengood JE, Folchman-Wagner Z, Forbes SP, et al. Formulation and in vitro characterization of composite biodegradable magnetic nanoparticles for magnetically guided cell delivery. Pharm Res. 2012;29(5):1232–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SW, Lam SC, Tam FC, Chan KK, Shea CP, Kong SL, et al. Evaluation of Early Healing Profile and Neointimal Transformation Over 24 Months Using Longitudinal Sequential Optical Coherence Tomography Assessments and 3-Year Clinical Results of the New Dual-Therapy Endothelial Progenitor Cell Capturing Sirolimus-Eluting Combo Stent: The EGO-Combo Study. Circ Cardiovasc Interv. 2016;9(7):e003469. [DOI] [PubMed] [Google Scholar]
- Zarpak R, Sanchez OD, Joner M, Guy LG, Leclerc G, Virmani R. A novel “pro-healing” approach: the COMBO dual therapy stent from a pathological view. Minerva Cardioangiol. 2015;63(1):31–43. [PubMed] [Google Scholar]
- Woudstra P, de Winter RJ, Beijk MA. Next-generation DES: the COMBO dual therapy stent with Genous endothelial progenitor capturing technology and an abluminal sirolimus matrix. Expert Rev Med Devices. 2014;11(2):121–135. [DOI] [PubMed] [Google Scholar]
- Granada JF, Inami S, Aboodi MS, Tellez A, Milewski K, Wallace-Bradley D, et al. Development of a novel prohealing stent designed to deliver sirolimus from a biodegradable abluminal matrix. Circ Cardiovasc Interv. 2010;3(3):257–266. [DOI] [PubMed] [Google Scholar]
- Yang F, Feng SC, Pang XJ, Li WX, Bi YH, Zhao Q, et al. Combination coating of chitosan and anti-CD34 antibody applied on sirolimus-eluting stents can promote endothelialization while reducing neointimal formation. BMC Cardiovasc Disord. 2012;12:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa G, Granada JF, Alviar CL, Tellez A, Kaluza GL, Guilhermier MY, et al. Anti-CD34 antibodies immobilized on the surface of sirolimus-eluting stents enhance stent endothelialization. JACC Cardiovasc Interv. 2010;3(1):68–75. [DOI] [PubMed] [Google Scholar]
- Haude M, Lee SW, Worthley SG, Silber S, Verheye S, Erbs S, et al. The REMEDEE trial: a randomized comparison of a combination sirolimus-eluting endothelial progenitor cell capture stent with a paclitaxel-eluting stent. JACC Cardiovasc Interv. 2013;6(4):334–343. [DOI] [PubMed] [Google Scholar]