Abstract
Extracellular vesicles (EV) are sub-micron circulating vesicles found in all bodily fluids and in all species so far tested. They have also recently been identified in seawater and it has further been shown that they are released from microorganisms and may participate in interspecies communication in the gut. EV are typically composed of a lipid bilayer formed from the plasma membrane and which encases a cargo that can include genetic material, proteins, and lipids. At least two different processes of formation and release have been described in mammalian cells. The exosome population (50 to 150nm size) are produced via a lyso-endosomal pathway, while microvesicles (100 to 1000nm) are formed by budding of the plasma membrane in a calcium dependent process. Both pathways are highly regulated and appear to be conserved amongst different species. EV release has been shown to be upregulated in a number of human chronic diseases including cardiovascular disease, metabolic disorders, obesity, and cancer; evaluation of their presence in veterinary samples may aid diagnosis in the future. This review will provide insight into the formation of EV and their detection in bodily fluids from different veterinary species and how they may provide a novel addition to the veterinary toolkit of the future.
Keywords: extracellular vesicles, exosomes, equine, bovine, canine, feline
Introduction
Extracellular vesicles (EV) are a heterogeneous population of cell-derived circulating vesicles that are largely characterized by the presence of a phospholipid bilayer and an internal cargo that can include DNA, mRNA, miRNA, enzymes, growth factors, and lipids. EV have been isolated from plasma, urine, tears, sweat, milk, seminal fluid, cerebrospinal fluid, as well as from plants and microorganisms, and have also been recently identified in seawater. Their cargo can be transferred to distant cells within the body, or to other organisms which may be from the same or different species. Thus, they are increasingly regarded as a novel delivery route, enabling intercellular communication via delivery of genetic material, protein, and lipid mediators. EV are increasingly being targeted for potential as non-invasive biomarkers in a range of diseases, since samples can be collected with minimal distress to the patient, particularly when measured in bodily fluids such as saliva or urine. A small sample volume is used, they require relatively little processing and often don’t need any prior staining. Some veterinary diseases can be hard to diagnose in the early stages due to lack of availability of species-specific diagnostic tools, added to which is the challenge of collecting sufficient biological sample material from some smaller domestic species. The properties of EV make them a particularly attractive proposition for the development of new tests for diagnosis and monitoring.
Formation
Extracellular vesicles were first identified several decades ago in a number of different physiological systems; the pro-coagulant platelet derived particles described by [1], later referred to as platelet dust by [2], and the matrix particles identified by [3], were followed by several different studies that identified particles released from adenoma cells, virus like particles released from mammalian cells and prostasomes released in seminal fluid [4]. In the next decade came the first description of the role of multivesicular bodies (MVB) during the formation of exosomes, the smallest of the classes of circulating EV to be described so far [5,6]. In the mid-1990s, interest was once more focused on these populations of circulating vesicles, and the discovery that they contain a range of cargo that includes genetic material such as miRNA has led to renewed attention and a rapid increase in publications [7]. Until recently there has been no standardized nomenclature to categorize the different populations of EV, although the International Society for Extracellular Vesicles (ISEV) has recently published a position paper for the benefit of the growing EV community [8]. EV have been classified both by size and by the mechanism by which they are produced within cells and have been given several different names in the literature. The smallest sized (50-150nm population) are generally known as exosomes, whilst those in the range of 100-1000nm have been variously described as microparticles, ectosomes and more recently described as microvesicles (MV). The third, larger population (> 2000nm) that has been described are apoptotic bodies (AB) and are released during cell death. AB are the most heterogeneous population. For the remainder of this review we will focus on exosomes and microvesicles.
Exosomes are produced via a lyso-endosomal pathway. This involves invagination of the plasma membrane to form an intracellular endosome, followed by further inward budding of this within the cytoplasm to form a multivesicular body (MVB) containing multiple intraluminal vesicles (ILV) within. (Figure 1). Exosomes characteristically bear CD63, CD9 and CD81 tetraspanin proteins, which have been shown to be required during assembly of the vesicles [9-12]. The tetraspanins were previously thought to be specific markers of exosomes, enabling characterization after isolation, however, it has recently been shown that they may also be identified in MV. Exosomes may also carry major histocompatibility complex (MHC) class II and heat shock proteins. Tsg101 and Alix have also been identified in multiple studies [11,12].
Figure 1.
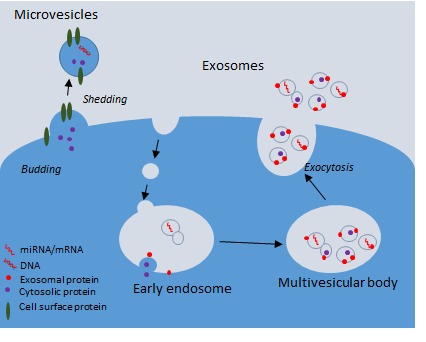
Mechanisms of extracellular vesicle production and release from cells. Extracellular vesicles include exosomes, microvesicles, and apoptotic bodies (not shown on this diagram). A. Microvesicles (MVs) are approximately 100nm to 1 µm in size, whilst exosomes are 50-150nm in diameter. MVs are released by membrane budding and are decorated with a number of cell surface molecules specific to the parent cell from which they were released and may also have exposed phosphatidyl serine on their outer membrane. B. Exosomes are formed within multivesicular bodies (MVBs) and are released following the fusion of MVB with the plasma membrane. Exosomes have surface markers including CD81, CD63. EV carry cargo including protein, lipids, miRNAs, and mRNAs, which they can deliver to their target cells.
In contrast, formation of microvesicles occurs directly at the plasma membrane via a calcium-regulated pathway. Less is understood about this process, however, there is a requirement for lipid raft formation where the membrane is pinched or budded off. Enrichment of lipids such as sphingomyelin has been demonstrated in synctiotrophoblast MV in the placenta [13]. Since tetraspanin proteins (CD9, CD63, CD81) have also been identified in MV, it is suggested that they are important for the budding and fusion of the membrane [14]. MV form a more heterogeneous population of circulating vesicles that bear receptors and surface markers from the parental cell. This is likely to be related to their role in intercellular communication and also enables their identification in the laboratory.
Phosphatidyl serine (PS) is a phospholipid usually found on the inner leaflet of the plasma membrane. During formation and release from the cell surface the plasma membrane may “flip” as the membrane asymmetry is lost, similar to that seen during apoptosis. This leaves PS exposed on the outer membrane, which may be important for intercellular communication and is also useful during identification.
Measurement
A range of different methods have been described to measure EV. These can broadly be grouped into optical and non-optical techniques. Electron microscopy (EM) can be considered the gold standard, and the first EV to be described from platelets were observed using this technique [2]. While EM can enable accurate measurement of size and morphological characteristics of different classes of EV within the same fraction, the technique is time consuming, requires specialist expertise which may not be easily available, is not quantitative and cannot be used to further phenotype different vesicle populations (review of methods see [15]). Additionally, the processing required (centrifugation, dehydration, and fixation) alters the size and morphology, producing some artefacts, including the characteristic “cup shape” morphology of exosomes [16,17]. Atomic force microscopy is a newer technique that has also been used to identify EV [15]. This technique does not require chemical fixation [18]. It also enables accurate sizing of vesicles and is advantageous in that it can be used in conjunction with antibody labelling. However, it is also time consuming and as concentration of the sample is required, the technique is not quantitative by nature.
A range of optical methods have been described which include flow cytometry (FCM), which is suitable for measurement of larger (> 200nm) MV, but not usually suitable for smaller exosome detection, although some protocols have been described using latex beads and exosome specific conjugated antibodies [17]. FCM is now accessible in many clinical and research laboratories and so this is probably the most widely reported detection method used to date. One of the benefits of FCM for analysis of MV is that there are a large number of fluorochrome-conjugated antibodies available to enable phenotyping of mixed populations of EV in samples from bodily fluids. Flow cytometers are usually capable of multicolor analysis, so surface marker detection can be coupled with detection of externalized PS using Annexin V reagents, which are available conjugated to many different fluorochromes. This may be important for biomarker detection and especially for veterinary samples where there may be fewer cross-reactive antibodies available to choose from. Standardized guidelines have been published for optimized collection of plasma for later MV detection [19] and there are several protocols to enable differentiation of MV from background noise. Recent advances in FCM technology have enabled direct visualization of MVs using Image-Stream technology [20] and special labelling methods have been established using dedicated flow cytometers to detect individual exosomes. There are some important caveats to detection of MV by FCM, namely that small particles (in older machines this could be anything < 340nm) can scatter light in different ways to the much larger cell populations for which the technique was originally developed. These small particles may also be subject to swarm effect, where several particles below the lower limit of detection are clustered together, and are therefore seen as one larger particle by the laser, which may lead to inaccuracies in the measurements [21]. This can be overcome to some extent by use of pre-calibrated counting and sizing beads as internal controls, which can help with standardization of samples. This could be important to calibrate samples within the same study either on the same machine or for multicenter studies, which may require use of different methodologies [22]. Another key advantage of FCM is that often MV can be stained in native samples (e.g. whole blood [23]) without purification. This can be important where only small volumes are available, such as those that have been collected and are surplus to requirements for clinical diagnosis.
A range of additional optical techniques have been developed and optimized for measurement of the smaller exosome population (50 to 150 nm). These include dynamic light scattering (DLS), tunable resistive pulse sensing (TRPS), and nanoparticle tracking analysis (NTA) [15]. All these methods have a much lower limit of detection (below 50nm size) and so enable quantification of exosomes. They are also much more efficient in terms of both time and cost per sample than either EM or atomic force microscopy. DLS should be approached with particular caution. Although a very time efficient method, the technique has been optimized for use with monodispersive particles, i.e., particles of one particular size in a homogenous population, whereas there are usually a range of vesicles types and sizes present in biological fluids (so called polydispersive samples). It is then important to develop an accurate mathematical model because larger particles scatter light more effectively so any measurements are skewed towards smaller numbers of large particles (for review [21]). The major limitation with NTA and TRPS (although this may change in the next few years) is that they lack the multiple lasers seen in most flow cytometers, so they are not suitable for phenotyping using multicolor analysis with a range of antibodies. Additionally, multiple purification steps may be required for some of the instruments to ensure that only exosomes are counted. Confirmatory techniques such as western blotting and immunodetection for characteristic exosome proteins may be required as they are not able to reliably distinguish between protein aggregates and exosomes [7]. For veterinary samples this may require the optimization of cross-reactive antibodies.
Comparative Biology of EV
Release of EV appears to be an evolutionarily conserved process and therefore we hypothesized some time ago that they could be detected in bodily fluids from veterinary and non-mammalian laboratory species and that they might be useful as non-invasive biomarkers for a range of diseases of veterinary importance. One key difficulty with development of any new diagnostic or research test for use on veterinary samples is the lack of available antibodies, meaning that analyses that do not necessarily require species-specific reagents can be invaluable. We have now identified MV in equine and feline plasma, as well as from bovine milk (Figure 2; for methods please see Appendix A: supplemental methods), from zebrafish embryos (5dpf) and insect cell cultures by flow cytometry and from human and rodent counterparts (data not shown and [24-26]). In addition, we have identified exosomes from bovine milk and zebrafish embryos (5dpf) by NTA (Figure 3; for methods please see Appendix A: supplemental methods). Using a candidate based approach we have identified cross-reactive antibodies to feline antigens to identify platelet (CD61) and erythrocyte (CD235a) MV in plasma (Figure 2). A number of other groups have also identified equine and canine extracellular vesicles, both from plasma and from tissue culture medium using similar techniques to those outlined above [27-30].
Figure 2.
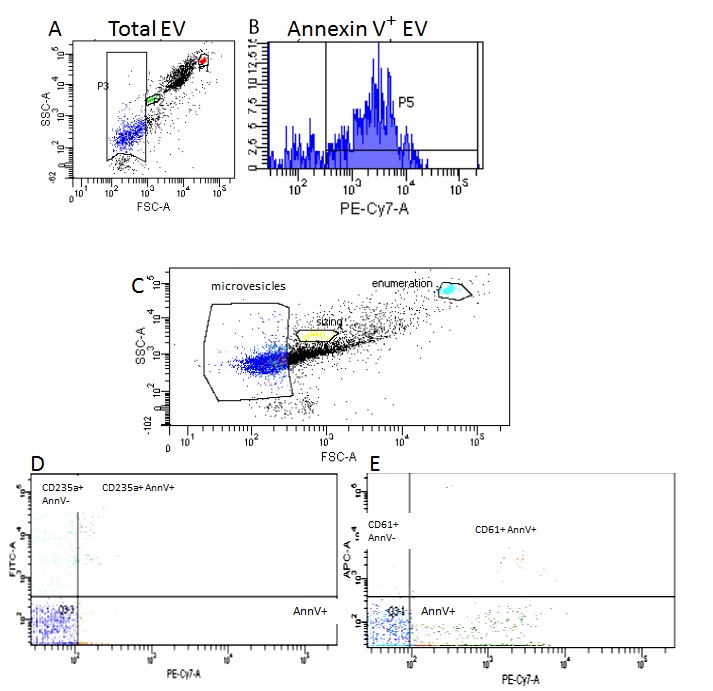
Measurement of microvesicles in plasma using flow cytometry. A. and B. Measurement of MV in equine plasma. A. Gate P1 (red) Commercially available enumeration beads are added to samples to enable accurate quantification of MV number; Gate P2 (green) 1micron latex beads are added to samples to facilitate sizing of MV up to 1000nm diameter; Gate P3 (blue) total MV count in plasma sample. B. Gate P5 (blue) shows MV counted in Gate P3 (panel A) that bind to PE-Cy7.7 labelled annexin V (binds to phosphatidyl serine on the surface of MV). C, D, E Measurement of MV in feline plasma. C. Gate P1 (light blue) enumeration beads; Gate P2 (yellow) 1micron sizing beads; Gate P3 (blue) total MV count in plasma sample. D. Dual florescence analysis of the MV gate for erythrocyte-derived MV. Events in 235+/AnnV+ bind CD235 and Annexin V, indicating erythrocyte cell surface receptors and PS expression. Events in AnnV+ contain nonerythrocyte MV. Events in 235+/AnnV- express erythrocyte cell surface receptors, but do not have PS expression. E. Dual florescence analysis of the MV gate for platelet-derived MV. Events in 61+/AnnV+ bind CD61 and Annexin V, indicating platelet cell surface receptors and PS expression. Events in AnnV++ contain nonplatelet MV. Events in 61+/AnnV+ express platelet cell surface receptors, but do not have PS expression. In both D and E quadrant 3 (Q3) represents debris, machine noise, and MV that did not bind either marker. All analysis was carried out using a Becton Dickinson FACS CANTO II and Diva analysis software (Becton Dickinson, Oxford UK). SCC-A= side scatter; FSC-A= forward scatter; AnnV= Annexin; PE-Cy7-A=Phycoerythrin labelled Annexin V; FITC- fluorescein isothiocyanate labelled anti-CD235a to measure erythrocyte derived MV; APC=Allophycocyanin labelled anti-CD61 to measure platelet-derived MV.
Figure 3.
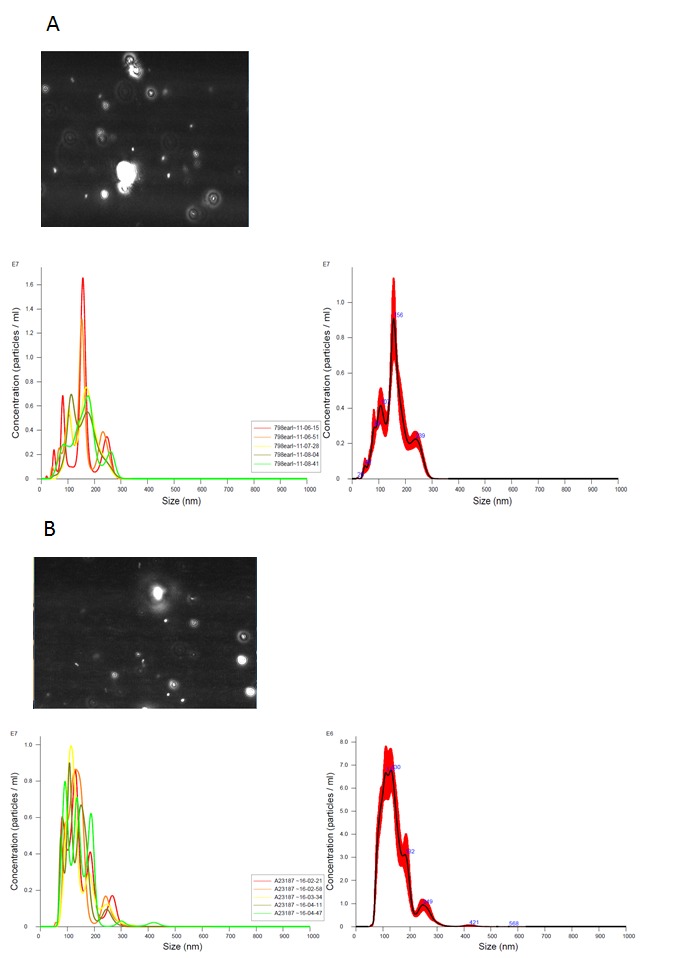
Measurement of exosomes using nanoparticle tracking analysis. A. measurement of exosomes purified from raw cow’s milk, exosomes were isolated using a modification of the method in (Thery 2006 for methods please see Appendix A: supplemental methods); B. measurement of exosomes in supernatant from dispersed cells collected from zebrafish embryos at 96h post fertilization. A and B, left panel, Brownian motion of individual particles is recorded for 60 seconds; middle panel, overlaid histograms of the concentration of particles at different sizes taken from multiple measurements; right panel, smoothed histogram from middle panel showing average concentration and size. Nanosight LM10 instrument (Malvern Instruments Ltd, Malvern, UK) was used for all analysis.
Relevance to Disease Processes as Biomarkers and the Advantages for Veterinary Species over Other Biomarkers
One important aspect that has become clear as the field has grown is that EV have great potential as biomarkers for a range of different diseases, and this may be translatable to common diseases of veterinary species. They have been identified in plasma from patients with a range of different cancers [31] as well as cardiovascular disease including valve disease and heart failure [32,33] and metabolic disorders including obesity, insulin resistance, and type II diabetes [7,34]. Some of the methodology outlined above enables measurement without pre-staining with antibodies (NTA, TRPS) and a number of sophisticated protocols have been developed enabling isolation of genetic material, in particular miRNA, from different populations of EV. These techniques are gradually becoming more affordable and less time consuming/higher throughput. At the same time, the feline, canine, equine, and bovine genomes are becoming better annotated and more is being understood about the role of different miRNAs in regulation of gene expression. Circulating miRNA has been identified in canine plasma, serum and urinary exosomes [35,36], feline plasma [37,38], equine serum and ovarian follicular fluid [39,40], and bovine plasma and milk [41,42]. Similar to findings in humans and rodent models, circulating miRNA in different bodily fluids reflects different disease or physiological states in veterinary species. Thus, the ability to collect this important information regarding the cargo of EV will enable development of new biomarker assays in the clinic and give further insight into the role of EV in different (patho)physiological processes in veterinary species.
In summary, the study of extracellular vesicles is providing us with new insights into how cells communicate both within the individual and also between organisms of the same or different species. Understanding more about their biology enables the generation of new tools and techniques to evaluate their cargo and function and gives us exciting opportunities to develop novel assays for clinical use. This is particularly important for veterinary diseases where simple and robust diagnostic tests are often lacking in the clinic. Development of assays to measure EV from veterinary species will enable high quality care and enhanced welfare for our patients.
Glossary
- AB
apoptotic bodies
- DLS
dynamic light scattering
- EM
electron microscopy
- EV
extracellular vesicles
- FCM
flow cytometry
- ISEV
International Society for Extracellular Vesicles
- ILV
intraluminal vesicles
- MHC
major histocompatibility complex
- miRNA
micro RNA
- MV
microvesicles
- MVB
multivesicular bodies
- NTA
nanoparticle tracking analysis
- PS
phosphatidylserine
- TRPS
tunable resistive pulse sensing
Appendix A.
Author Contributions
CL devised and conducted experiments, carried out analysis and wrote the manuscript; DK, EF, EU devised and conducted experiments, carried out analysis; VLF devised experiments and carried out analysis.
References
- Chargaff E, West R. The biological significance of the thromboplastic protein of blood. J Biol Chem. 1946;166(1):189–97. [PubMed] [Google Scholar]
- Wolf P. The nature and significance of platelet products in human plasma. Br J Haematol. 1967;13(3):269–88. [DOI] [PubMed] [Google Scholar]
- Anderson HC. Vesicles associated with calcification in the matrix of epiphyseal cartilage. J Cell Biol. 1969;41(1):59–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody I, Ronquist G, Gottfries A. Ultrastructural localization of the prostasome - an organelle in human seminal plasma. Ups J Med Sci. 1983;88(2):63–80. [DOI] [PubMed] [Google Scholar]
- Orr L, Adam M, Johnstone RM. Externalization of membrane-bound activities during sheep reticulocyte maturation is temperature and ATP dependent. Biochem Cell Biol. 1987;65(12):1080–90. [DOI] [PubMed] [Google Scholar]
- Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33(3):967–78. [DOI] [PubMed] [Google Scholar]
- Lawson C, Vicencio JM, Yellon DM, Davidson SM. Microvesicles and exosomes: new players in metabolic and cardiovascular disease. J Endocrinol. 2016;228(2):R57–71. [DOI] [PubMed] [Google Scholar]
- Lotvall J, Hill AF, Hochberg F, Buzas EI, Di Vizio D, Gardiner C, et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles. 2014;3:26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charrin S, Jouannet S, Boucheix C, Rubinstein E. Tetraspanins at a glance. J Cell Sci. 2014;127(Pt 17):3641–8. [DOI] [PubMed] [Google Scholar]
- Yanez-Mo M, Siljander PR, Andreu Z, Zavec AB, Borras FE, Buzas EI, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreu Z, Yanez-Mo M. Tetraspanins in extracellular vesicle formation and function. Front Immunol. 2014;5:442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DK, Lee J, Kim SR, Choi DS, Yoon YJ, Kim JH, et al. EVpedia: a community web portal for extracellular vesicles research. Bioinformatics. 2015;31(6):933–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baig S, Lim JY, Fernandis AZ, Wenk MR, Kale A, Su LL, et al. Lipidomic analysis of human placental syncytiotrophoblast microvesicles in adverse pregnancy outcomes. Placenta. 2013;34(5):436–42. [DOI] [PubMed] [Google Scholar]
- Perez-Hernandez D, Gutierrez-Vazquez C, Jorge I, Lopez-Martin S, Ursa A, Sanchez-Madrid F, et al. The intracellular interactome of tetraspanin-enriched microdomains reveals their function as sorting machineries toward exosomes. J Biol Chem. 2013;288(17):11649–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Pol E, Hoekstra AG, Sturk A, Otto C, van Leeuwen TG, Nieuwland R. Optical and non-optical methods for detection and characterization of microparticles and exosomes. J Thromb Haemost. 2010;8(12):2596–607. [DOI] [PubMed] [Google Scholar]
- Gyorgy B, Szabo TG, Pasztoi M, Pal Z, Misjak P, Aradi B, et al. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci. 2011;68(16):2667–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thery C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006;Chapter 3:Unit 3 22. [DOI] [PubMed] [Google Scholar]
- Yuana Y, Koning RI, Kuil ME, Rensen PC, Koster AJ, Bertina RM, et al. Cryo-electron microscopy of extracellular vesicles in fresh plasma. J Extracell Vesicles. 2013:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix R, Judicone C, Poncelet P, Robert S, Arnaud L, Sampol J, et al. Impact of pre-analytical parameters on the measurement of circulating microparticles: towards standardization of protocol. J Thromb Haemost. 2012;10(3):437–46. [DOI] [PubMed] [Google Scholar]
- Headland SE, Jones HR, D’Sa AS, Perretti M, Norling LV. Cutting-edge analysis of extracellular microparticles using ImageStream(X) imaging flow cytometry. Sci Rep. 2014;4:5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Pol E, van Gemert MJ, Sturk A, Nieuwland R, van Leeuwen TG. Single vs. swarm detection of microparticles and exosomes by flow cytometry. J Thromb Haemost. 2012;10(5):919–30. [DOI] [PubMed] [Google Scholar]
- Pollott G, Brito A, Gardiner C, Lawson C. A Comparison of Different Methodologies for the Measurement of Extracellular Vesicles and Milk-derived Particles in Raw Milk from Cows. Biomark Insights. 2016;11:147–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey MG, Wolf SI, Wheeler-Jones CP, Lawson C. Expression of blood coagulation factors on monocytes after exposure to TNF-treated endothelium in a novel whole blood model of arterial flow. J Immunol Methods. 2009;350(1-2):133–41. [DOI] [PubMed] [Google Scholar]
- Heinrich LF, Andersen DK, Cleasby ME, Lawson C. Long-term high fat feeding of rats results in increased numbers of circulating microvesicles with pro-inflammatory effects on endothelial cells. Br J Nutr. 2015;113(11):1704–11. [DOI] [PubMed] [Google Scholar]
- Holtom E, Usherwood JR, Macey MG, Lawson C. Microparticle formation after co-culture of human whole blood and umbilical artery in a novel in vitro model of flow. Cytometry A. 2012;81(5):390–9. [DOI] [PubMed] [Google Scholar]
- Macey MG, Wolf SI, Lawson C. Microparticle formation after exposure of blood to activated endothelium under flow. Cytometry A. 2010;77(8):761–8. [DOI] [PubMed] [Google Scholar]
- Helmond SE, Catalfamo JL, Brooks MB. Flow cytometric detection and procoagulant activity of circulating canine platelet-derived microparticles. Am J Vet Res. 2013;74(2):207–15. [DOI] [PubMed] [Google Scholar]
- Herring JM, Smith SA, McMichael MA, O’Brien M, Ngwenyama TR, Corsi R, et al. Microparticles in stored canine RBC concentrates. Vet Clin Pathol. 2013;42(2):163–9. [DOI] [PubMed] [Google Scholar]
- Springer NL, Smith E, Brooks MB, Stokol T. Flow cytometric detection of circulating platelet-derived microparticles in healthy adult horses. Am J Vet Res. 2014;75(10):879–85. [DOI] [PubMed] [Google Scholar]
- Marędziak M, Marycz K, Lewandowski D, Siudzińska A, Śmieszek A. Static magnetic field enhances synthesis and secretion of membrane-derived microvesicles (MVs) rich in VEGF and BMP-2 in equine adipose-derived stromal cells (EqASCs)—a new approach in veterinary regenerative medicine. In Vitro Cell Dev Biol Anim. 2015;51(3):230–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki J, Ochiya T. Circulating microRNAs and extracellular vesicles as potential cancer biomarkers: a systematic review. Int J Clin Oncol. 2017;22(3):413-420. [DOI] [PubMed] [Google Scholar]
- Boulanger CM, Loyer X, Rautou PE, Amabile N. Extracellular vesicles in coronary artery disease. Nat Rev Cardiol. 2017;14(5):259-272. [DOI] [PubMed] [Google Scholar]
- Davidson SM, Takov K, Yellon DM. Exosomes and Cardiovascular Protection. Cardiovasc Drugs Ther. 2017;31(1):77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng F, Wang S, Zhang L. Endothelial Microparticles Act as Novel Diagnostic and Therapeutic Biomarkers of Diabetes and Its Complications: A Literature Review. Biomed Res Int. 2016;2016:9802026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichii O, Ohta H, Horino T, Nakamura T, Hosotani M, Mizoguchi T, et al. Urinary exosome-derived microRNAs reflecting the changes of renal function and histopathology in dogs. Sci Rep. 2017;7:40340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enelund L, Nielsen LN, Cirera S. Evaluation of microRNA stability in plasma and serum from healthy dogs. MicroRNA. 2017; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Fleischhacker SN, Bauersachs S, Wehner A, Hartmann K, Weber K. Differential expression of circulating microRNAs in diabetic and healthy lean cats. Vet J. 2013;197(3):688–93. [DOI] [PubMed] [Google Scholar]
- Weber K, Rostert N, Bauersachs S, Wess G. Serum microRNA profiles in cats with hypertrophic cardiomyopathy. Mol Cell Biochem. 2015;402(1-2):171–80. [DOI] [PubMed] [Google Scholar]
- da Silveira JC, Veeramachaneni DN, Winger QA, Carnevale EM, Bouma GJ. Cell-secreted vesicles in equine ovarian follicular fluid contain miRNAs and proteins: a possible new form of cell communication within the ovarian follicle. Biol Reprod. 2012;86(3):71. [DOI] [PubMed] [Google Scholar]
- Pacholewska A, Mach N, Mata X, Vaiman A, Schibler L, Barrey E, et al. Novel equine tissue miRNAs and breed-related miRNA expressed in serum. BMC Genomics. 2016;17(1):831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannidis J, Donadeu FX. Changes in circulating microRNA levels can be identified as early as day 8 of pregnancy in cattle. PLoS One. 2017;12(4):e0174892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zempleni J, Aguilar-Lozano A, Sadri M, Sukreet S, Manca S, Wu D, et al. Biological Activities of Extracellular Vesicles and Their Cargos from Bovine and Human Milk in Humans and Implications for Infants. J Nutr. 2017;147(1):3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]


