Abstract
Background
Periprosthetic joint infection (PJI) is a complication of total joint arthroplasty (TJA). The leukocyte esterase (LE) strip test and histology are diagnostic methods for PJI. The aims of this study were to determine the sensitivity and specificity of the LE strip test and to compare it with histology in the diagnosis of PJI.
Material/Methods
Between January and December 2015, 93 patients who underwent TJA with PJI were enrolled in the study. Synovial fluid samples were tested with an LE strip, and three synovial tissue samples from each patient underwent frozen section and formalin-fixed histology. Recent criteria from the Musculoskeletal Infection Society (MSIS) were used for the diagnosis of PJI.
Results
Ninety-three patients studied included 38 cases of PJI and 55 non-infected cases. Sensitivity and specificity of the LE strip test were 92.1% (95% CI, 77.5–97.9%) and 96.4% (95% CI, 86.4–99.4%), respectively. There was no significant difference in sensitivity (p=0.249) or specificity (p=0.480) between frozen and paraffin sections for histology; the two methods were strongly correlated (ϕ=0.892). Comparison of the LE test results with histology showed a strong correlation (ϕ=0.758, and ϕ=0.840).
Conclusions
The findings of this preliminary study have shown that the LE strip test on synovial fluid showed similar sensitivity and specificity as histology for the diagnosis of PJI, indicating that that further controlled clinical studies should be performed to investigate the role of the LE strip test for the early diagnosis of PJI.
MeSH Keywords: Arthroplasty, Replacement, Hip; Arthroplasty, Replacement, Knee; Diagnosis; Frozen Sections; Leukocytes; Prosthesis-Related Infections
Background
Periprosthetic joint infection (PJI) is a complication of total joint arthroplasty (TJA). PJI is a serious complication of TJA that requires early, rapid, and accurate diagnosis. However, the diagnosis of PJI relies on a series of combined criteria and association, and currently, there is no single test that has shown satisfactory sensitivity and specificity in the early diagnosis of PJI [1–3]. The leukocyte esterase (LE) strip test of synovial fluid and histology of the synovium are among the diagnostic tests for PJI [1–3]. Because the diagnosis of PJI remains one of the most challenging tasks for orthopedic surgeons, it is important to evaluate methods for the diagnosis of PJI [1,4,5].
Synovial histology is a commonly used method for the diagnosis of PJI as neutrophils can be seen histologically as a response to the infected joint, and the presence of PJI can be determined by recording the neutrophil counts per high-power field [6]. A recent meta-analysis confirmed that histopathology could be a valuable part of the diagnostic workup performed on patients undergoing revision arthroplasty [6]. Recent criteria from the Musculoskeletal Infection Society (MSIS) MSIS have included histopathological examination as a minor diagnostic criterion in their guidelines [3]. However, a major shortcoming of histological tests is that specimens can only be collected during surgery. Thus, histological results cannot effectively guide pre-operative treatment.
Leukocyte esterase (LE) is an enzyme that is primarily secreted by neutrophils [7]. The LE strip test shows the LE levels through a color change, and the LE strip test has been used for the diagnosis of urinary tract infections (UTIs) since the early 1980s [8–10], and has been shown to have good sensitivity and specificity in the diagnosis of UTI [11]. The concentration of LE that is measured in synovial fluid is a reflection of the number of neutrophils that in an infected joint [8-10]. In 2011, Parvizi and colleagues were the first to show that the LE strip test demonstrates high sensitivity and specificity for the diagnosis of PJI in the knee joint [7].
The main advantage of the LE strip test is that it is convenient and rapid, with results that can be obtained in two or three minutes following the collection of synovial fluid [7,12]. Several studies have now confirmed the reliability of the LE strip test [12–17]. However, further studies are still required to determine the clinical utility of the LE strip test for the diagnosis of PJI, as many of the early studies were based on criteria provided by the individual center running the study, and not on those recommended by MSIS [2–4,7,12]. For example, some previous studies included primary arthroplasty cases [17], and other studies did not indicate the inclusion or exclusion of rheumatic autoimmune diseases [14,15]. Also, some individual studies were retrospective studies that used frozen and stored synovial fluid [16]. These differences are likely to have affected the results of these previous studies.
In 2014, the modified diagnostic criteria of the MSIS considered the LE test to be a minor criterion, equivalent to the synovial white blood cell (WBC) count [3]. However, the relationship between LE strip test and histological examination, which is also based on the concentrations of neutrophils, has not yet been studied. It is possible that LE detection could improve the early diagnosis of PJI, as a synovial biopsy is not required for this test [3].
The aims of this study were to determine the sensitivity and specificity of the LE strip test and to compare the LE test of synovial fluid with synovial histology in the diagnosis of PJI.
Material and Methods
The Council of Ethics of our institution approved this study on periprosthetic joint infection (PJI).
From January 2015 to December 2015, a total of 114 patients received hip or knee revisions in the orthopedic ward of our hospital. In this study, hip or knee joint revision was defined as a need to replace components of the joint, or the use of an entire joint replacement prosthesis, or the need to remove a prosthesis and introduce a spacer. Cases of joint revision to remove an existing spacer were excluded. Cases involving an existing implant other than a joint prosthesis, such as screws and steel plates, were excluded.
Of the initial 114 patients studied, two cases had undergone simultaneous bilateral joint revision, there was one case of simultaneous hip and knee revision, and 18 cases (included the only case of acute PJI out of the 114 patients) with incomplete clinical data (missing histology data or cases in which no synovial fluid could be obtained), and these were also excluded. Ninety-three patients studied included 38 cases of PJI and 55 non-infected cases.
Following a pre-operative diagnostic synovial fluid aspiration, a small amount of synovial fluid (one drop) was immediately applied to a leukocyte esterase (LE) test strip (Aution Sticks 10PA, Arkray, Japan). The LE test strip was read within 2–3 min after the application of the synovial fluid. A strip that turned dark violet, equivalent to the “500” level on the colorimetric card (Figure 1) was determined to be positive; otherwise, the sample was considered to be negative. All test strip results were read by three individual physicians. When interpretations were inconsistent, the final result was determined following consensus.
Figure 1.

Interpretation of the test results for leukocyte esterase. The LE color change can be divided into four degrees: “25”, “75”, “250”, and “500”. A positive result occurs when the color of the LE strip matches the “500” degree result. Otherwise, we consider the result to be negative.
If the sample was mixed with blood that affected the color interpretation, the sample was centrifuged at 5,000 rpm for 2–3 min using a portable centrifuge (SCILOGEX D1008E), and the supernatant was then applied to the LE strip for interpretation [18].
There is no clear guidance on the appropriate number of synovial tissue blocks to use in histological analysis of PJI, and this number ranges from two to five blocks in previous studies [19–21]. Based on methods used at our center, three tissue blocks were submitted for both intraoperative frozen section and postoperative histological paraffin section analysis. After the intraoperative incision of the articular capsule to expose the prosthesis, specimens were collected and submitted to the Department of Pathology for frozen and paraffin sectioning.
The number of neutrophils per high-powered field at ×400 magnification was counted by a pathologist. The results from intraoperative rapid frozen sections were returned approximately half an hour after submission, and those from paraffin sections were returned 1–2 days after surgery.
For frozen histological analysis, if one out of the three tissue blocks had a neutrophil count of >5 per high power field (HPF), the results were considered positive; if all three tissue blocks showed ≤5 neutrophils per HPF, the results were considered to be negative. We also considered a combination of frozen and paraffin section histological analysis to detect PJI, in which any block of tissue with >5 neutrophils per HPF was considered positive. If all six tissue blocks showed ≤5 neutrophils per HPF, the results were considered negative. The LE strip test and histological analysis results were compared between PJI and aseptic joints based on a modified MSIS definition (Table 1) [3].
Table 1.
Modified MSIS criteria (2014).
| PJI is present when one of the major criteria exists or three out of five minor criteria exist |
| Two positive periprosthetic cultures with phenotypically identical organisms, OR A sinus tract communicating with the joint, OR |
|
MSIS – Musculoskeletal Infection Society; PJI – periprosthetic joint infection.
Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for the results of the LE strip test and two methods of histological analysis were calculated. Correlation analysis of the two methods was analyzed by the phi coefficient (ϕ). A comparison of the sensitivity and specificity of the two methods was analyzed using the chi square test. A p-value of <0.05 was considered to be statistically significant.
Results
Of the 93 cases of total joint arthroplasty (TJA) studied, 38 cases showed signs of periprosthetic joint infection (PJI) (18 cases of the knee and 20 cases of the hip). These cases included 18 men and 20 women with an age range of 22–84 (63.13±15.51) years and a body mass index (BMI) range of 15.62–6.49 (25.02±4.24) kg/m2. Concomitant diseases included rheumatoid in three cases, ankylosing spondylitis in two cases and tumors in three cases (after bladder tumor surgery, after lung cancer and with a history of non-Hodgkin’s lymphoma). There were 55 non-infected cases (11 cases of the knee and 44 cases of the hip). These cases included 16 males and 39 females with an age range of 29–89 (61.04±11.77) years and a BMI range of 15.81–37.11 (25.26±4.42) kg/m2. Concomitant diseases included rheumatoid arthritis in one case, ankylosing spondylitis in one case, a tumor in one case (after breast cancer surgery), purpura nephritis in one case and uremia upon dialysis in one case.
Diagnosis of LE strip tests from 93 patients was successful in 89 cases.
The color changes in four cases studied were in between the positive (dark violet) and the negative (light violet) areas of the LE strip, leading to inconsistent interpretation of the results by three physicians. The final result was determined by majority decision, with a final judgment of two positive cases and two negative cases. There were 25 cases in which synovial fluid was mixed with blood and required centrifugation, and readings were available after centrifugation.
The sensitivity, specificity, positive-predictive value (PPV) and negative-predictive value (NPV) were 92.1% (95% CI, 77.5–97.9%), 96.4% (95% CI, 86.4–99.4%), 94.6% (95% CI, 80.5–99.1%) and 94.6% (95% CI, 84.2–98.6%), respectively. The sensitivity, specificity, PPV and NPV of intraoperative frozen section analysis were 89.5% (95% CI, 74.3–96.6%), 89.1% (95% CI, 77.1–95.5%), 85% (95% CI, 69.5–93.8%) and 92.5% (95% CI, 80.9–97.6%), respectively. The sensitivity, specificity, PPV and NPV of paraffin section analysis were 97.4% (95% CI, 84.6–99.9%), 85.5% (95% CI, 72.8–93.1%), 82.2% (95% CI, 67.4–91.5%) and 97.9% (95% CI, 87.5–99.9%), respectively. There were no significant difference in sensitivity (p=0.249) or specificity (p=0.480) between frozen and paraffin section histological analysis.
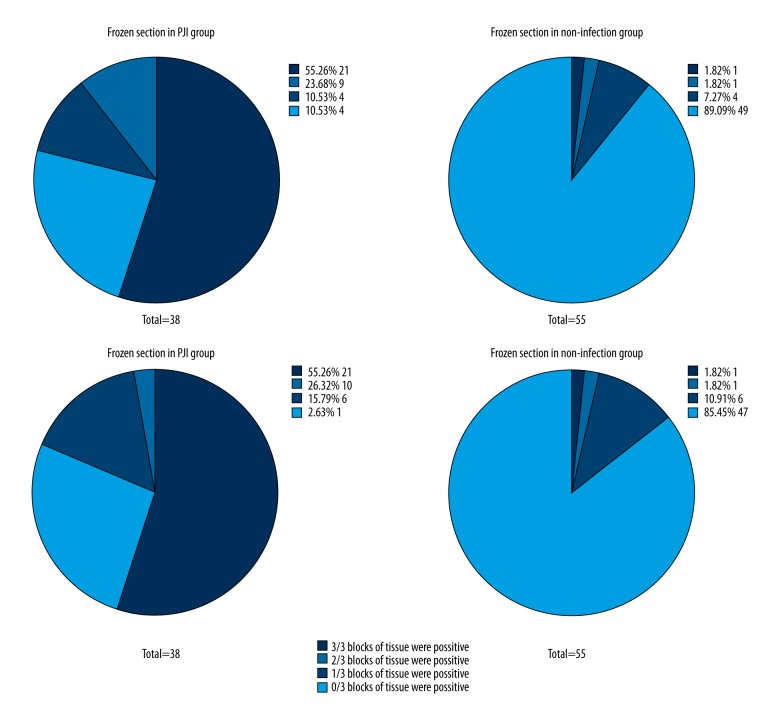
The two methods were also strongly correlated (ϕ=0.892). By coincidence, the sensitivity, specificity, PPV and NPV of the combined histological analysis were also 97.4% (95% CI, 84.6–99.9%), 85.5% (95% CI, 72.8–93.1%), 82.2% (95% CI, 67.4–91.5%) and 97.9% (95% CI, 87.5–99.9%), respectively. Internal results analysis of three tissue blocks revealed internal inconsistency among both frozen and paraffin sections. Thirteen cases (34.21%) in the infection group and five cases (9.09%) in the non-infected group showed inconsistent histological results among three frozen sections.
For paraffin sections, sixteen cases (42.11%) in the infection group and seven cases (12.73%) in the non-infected group showed inconsistent histological results. (Figure 2) In three cases from the infection group, frozen sections produced negative results but combined histological analysis produced positive results. In the non-infection group, two cases produced the same pattern of contradictory results. In a comparison of the results from LE strip tests with the results from frozen section histology, there were no significant differences in sensitivity (p=1.000) or specificity (p=0.221) between the two methods. The two methods were also strongly correlated (ϕ=0.758). In a comparison of LE strip tests and results from combined histological analysis, there was no significant difference in sensitivity (p=0.480), but there was a statistically significant difference in specificity (p=0.041), with LE demonstrating higher specificity. However, the correlation between the two methods was still high (ϕ=0.840) (Table 2).
Figure 2.
Internal consistency of histological analysis. As it was shown in the figures, there was an internal inconsistency in both frozen sections or paraffin sections. Thirteen cases (34.21%) in the infection group and five cases (9.09%) in the non-infected group showed inconsistent histological results among the three frozen sections. And for paraffin section, sixteen cases (42.11%) in the infection group and seven cases (12.73%) in the non-infected group showed inconsistent histological.
Table 2.
Comparison of LE strip tests and histological analysis.
| Comparison | Advantage on a chi square test of sensitivity | Advantage on a chi square test of specificity | Φ (Phi coefficient) |
|---|---|---|---|
| Frozen section vs. paraffin section | χ2=1.33, P=0.249 | χ2=0.50, P=0.480 | ϕ=0.892 |
| LE vs. frozen section | χ2=0.000, P=1.000 | χ2=1.50, P=0.221 | ϕ=0.758 |
| LE vs. combined histology analysis | χ2=0.50, P=0.480 | χ2=4.17, P=0.041 | ϕ=0.840 |
Discussion
Periprosthetic joint infection (PJI) following total joint arthroplasty (TJA) can be diagnosed using the leukocyte esterase (LE) strip test, as LE is an enzyme present in neutrophils, as in patients with PJI, neutrophils will secrete LE around an infected joint [7,12]. The findings of this preliminary study have shown that the LE strip test on synovial fluid showed similar sensitivity and specificity as histology for the diagnosis of PJI. This study was undertaken with reference to the recent criteria from the Musculoskeletal Infection Society (MSIS) [3].
The LE strip test is used for synovial fluid samples and was first described as a diagnostic method for PJI by Parvizi et al., who confirmed the degree of sensitivity and specificity of the LE test using synovial fluid [7]. A positive LE strip test result occurs when the color change of ++ of the LE strip pad matches the final dark purple color, equivalent to “500” in this study, and the sensitivity and specificity are 80.6% (95% CI, 61.9–91.9%) and 100% (95% CI, 94.5–100.0%), respectively [7].
In the present study, the LE strip test had a sensitivity of 92.1% (95% CI, 77.5–97.9%) and a specificity of 96.4% (95% CI, 86.4–99.4%). This finding was supported by two recently reported studies [14,22]. In the present study, the sensitivity of the LE test ranged from 92.9–100% and the specificity ranged from 92.9–97%. Our results further confirmed the accuracy and the reliability of the LE strip test. In the present study, the frozen section synovial histological analysis alone had a sensitivity of 89.5% (95% CI, 74.3–96.6%) and a specificity of 89.1% (95% CI, 77.1–95.5%). Paraffin section synovial histology alone had a sensitivity of 97.4% (95% CI, 84.6–99.9%) and a specificity of 85.5% (95% CI, 72.8–93.1%).
In this study, there were no significant differences in sensitivity (p=0.249) or specificity (p=0.480) between frozen section and formalin-fixed, paraffin section histology, which is similar to previously published results [23–25]. However, a comparison of three tissue blocks corresponding to each type of histological analysis demonstrated internal inconsistency. Thirteen cases (34.21%) in the infection group and five cases (9.09%) in the non-infected group showed inconsistent histological results among the three frozen sections. Also, three cases had different results from the combined analysis; the histological result from a frozen section was negative, but after paraffin section histological analysis was reported, the final result was positive. In one case with inconsistent results, the diagnosis of PJI changed according to the MSIS criteria [3]. In this one case, surgery was complete, and the duration of antibiotic use was extended to six weeks of intravenous antibiotics and six weeks of oral antibiotics, which was the same antibiotic course usually used following a one-stage revision. The patient has reported positive outcomes to date (14 months). We believe that these internally inconsistent results were due to a lack of collection of representative synovial tissue for PJI evaluation.
This study confirmed that the presence of neutrophils in synovial tissue samples, indicating PJI, depends on the surgeon’s expertise, and that combined histological analysis is more likely to detect a positive histological result. Synovial tissue sample processing and histological analysis are more likely to show the changes of PJI, and increasing the number of intraoperative tissue samples taken might improve the accuracy of histological testing. However, the most appropriate number of tissue blocks remains to be determined. These diagnostic limitations were not found when using the LE strip test, which produced relatively stable results due to the lack of error in tissue sampling for histological analysis. The LE strip test displayed similar sensitivity and specificity to frozen sections and better specificity than combined histological analysis. The results of the LE strip test were significantly correlated with those of both frozen sections (ϕ=0.758) and combined histological analysis (ϕ=0.840). We believe this finding was because both the LE strip test and histological analysis are based on detection of neutrophils.
From the findings of this study, in addition to using a synovial neutrophil count [3], the LE test could replace synovial histological analysis for PJI. The LE strip test can provide results before surgery rather than during or after surgery and requires only a drop of synovial fluid for each test, to achieve a diagnosis of PJI even in patients with a very small amount of synovial fluid. The LE test is rapid, and can be completed within minutes, unlike histological analysis. The LE test strip for each test costs only approximately 2.2 yuan (approximately 29.4 cents) and 220 yuan (approximately US$29.40) per bottle, with 100 strips per bottle, which reduces the cost of diagnosis. The required materials and equipment are only an LE strip and a portable centrifuge, and no large-scale equipment is required. Therefore, the test can be performed in both large specialized hospitals and community clinics.
There were several limitations of the present study. First, while conducting the study, we did not collect assess the optimal number of synovial tissue samples to analyze. Second, the minor MSIS diagnostic criteria for PJI were used as the basis for this study [3]. This study was small, and preliminary, and was conducted in a single center, which may have introduced study or analysis bias. Finally, although the LE strip test only requires a minimal amount of synovial fluid, a small proportion of patients were excluded from this study due to the lack of availability of synovial fluid.
Conclusions
The findings of this study have shown that histology of frozen sections and paraffin sections of synovium are both good methods for the diagnosis of periprosthetic joint infection (PJI), which is a complication of total joint arthroplasty (TJA), but with no statistically significant differences in the results. The leukocyte esterase (LE) strip test was found to have a similar sensitivity and specificity to histological analysis. Because the LE strip test is a more rapid test that is less costly, it could be used as a substitute for histological analysis and for the earlier diagnosis of PJI.
Footnotes
Source of support: Departmental sources
Conflicts of interest
None.
References
- 1.Della Valle C, Parvizi J, Bauer TW, et al. Diagnosis of periprosthetic joint infections of the hip and knee. J Am Acad of Orthop Surg. 2010;18(12):760–70. doi: 10.5435/00124635-201012000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Osmon DR, Berbari EF, Berendt AR, et al. Diagnosis and management of prosthetic joint infection: Clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56(1):e1–e25. doi: 10.1093/cid/cis803. [DOI] [PubMed] [Google Scholar]
- 3.Parvizi J, Gehrke T. Definition of periprosthetic joint infection. J Arthroplasty. 2014;29(7):1331. doi: 10.1016/j.arth.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Parvizi J. New definition for periprosthetic joint infection. Am J Orthop. 2011;40(12):614–15. [PubMed] [Google Scholar]
- 5.Nodzo SR, Bauer T, Pottinger PS, et al. Conventional diagnostic challenges in periprosthetic joint infection. J Am Acad Orthop Surg. 2015;23(Suppl):S18–25. doi: 10.5435/JAAOS-D-14-00385. [DOI] [PubMed] [Google Scholar]
- 6.Tsaras G, Maduka-Ezeh A, Inwards CY, et al. Utility of intraoperative frozen section histopathology in the diagnosis of periprosthetic joint infection: A systematic review and meta-analysis. J Bone Joint Surg Am. 2012;94(18):1700–11. doi: 10.2106/JBJS.J.00756. [DOI] [PubMed] [Google Scholar]
- 7.Parvizi J, Jacovides C, Antoci V, Ghanem E. Diagnosis of periprosthetic joint infection: the utility of a simple yet unappreciated enzyme. J Bone Joint Surg Am. 2011;93(24):2242–48. doi: 10.2106/JBJS.J.01413. [DOI] [PubMed] [Google Scholar]
- 8.Perry JL, Matthews JS, Weesner DE. Evaluation of leukocyte esterase activity as a rapid screening technique for bacteriuria. J Clin Microbiol. 1982;15(5):852–54. doi: 10.1128/jcm.15.5.852-854.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smalley DL, Dittmann AN. Use of leukocyte esterase-nitrate activity as predictive assays of significant bacteriuria. J Clin Microbiol. 1983;18(5):1256–57. doi: 10.1128/jcm.18.5.1256-1257.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chernow B, Zaloga GP, Soldano S, et al. Measurement of urinary leukocyte esterase activity: A screening test for urinary tract infections. Ann Emerg Med. 1984;13(3):150–54. doi: 10.1016/s0196-0644(84)80603-4. [DOI] [PubMed] [Google Scholar]
- 11.Berger RE. The urine dipstick test useful to rule out infections. A meta-analysis of the accuracy. J Urol. 2005;174(3):941–42. [PubMed] [Google Scholar]
- 12.Wetters NG, Berend KR, Lombardi AV, et al. Leukocyte esterase reagent strips for the rapid diagnosis of periprosthetic joint infection. J Arthroplasty. 2012;27(8 Suppl):8–11. doi: 10.1016/j.arth.2012.03.037. [DOI] [PubMed] [Google Scholar]
- 13.Tischler EH, Cavanaugh PK, Parvizi J. Leukocyte esterase strip test: Matched for musculoskeletal infection society criteria. J Bone Joint Surg Am. 2014;96(22):1917–20. doi: 10.2106/JBJS.M.01591. [DOI] [PubMed] [Google Scholar]
- 14.Colvin OC, Kransdorf MJ, Roberts CC, et al. Leukocyte esterase analysis in the diagnosis of joint infection: Can we make a diagnosis using a simple urine dipstick? Skeletal Radiol. 2015;44(5):673–77. doi: 10.1007/s00256-015-2097-5. [DOI] [PubMed] [Google Scholar]
- 15.Shafafy R, McClatchie W, Chettiar K, et al. Use of leucocyte esterase reagent strips in the diagnosis or exclusion of prosthetic joint infection. Bone Joint J. 2015;97-b(9):1232–36. doi: 10.1302/0301-620X.97B9.34910. [DOI] [PubMed] [Google Scholar]
- 16.Deirmengian C, Kardos K, Kilmartin P, et al. The alpha-defensin test for periprosthetic joint infection outperforms the leukocyte esterase test strip. Clin Orthop Relat Res. 2015;473(1):198–203. doi: 10.1007/s11999-014-3722-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guenther D, Kokenge T, Jacobs O, et al. Excluding infections in arthroplasty using leucocyte esterase test. Int Orthop. 2014;38(11):2385–90. doi: 10.1007/s00264-014-2449-0. [DOI] [PubMed] [Google Scholar]
- 18.Aggarwal VK, Tischler E, Ghanem E, Parvizi J. Leukocyte esterase from synovial fluid aspirate: A technical note. J Arthroplasty. 2013;28(1):193–95. doi: 10.1016/j.arth.2012.06.023. [DOI] [PubMed] [Google Scholar]
- 19.Della Valle CJ, Bogner E, Desai P, et al. Analysis of frozen sections of intraoperative specimens obtained at the time of reoperation after hip or knee resection arthroplasty for the treatment of infection. J Bone Joint Surg Am. 1999;81(5):684–89. doi: 10.2106/00004623-199905000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Fink B, Gebhard A, Fuerst M, et al. High diagnostic value of synovial biopsy in periprosthetic joint infection of the hip. Clin Orthop Rel Res. 2013;471(3):956–64. doi: 10.1007/s11999-012-2474-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buttaro MA, Martorell G, Quinteros M, et al. Intraoperative synovial C-reactive protein is as useful as frozen section to detect periprosthetic hip infection. Clin Orthop Rel Res. 2015;473(12):3876–8. doi: 10.1007/s11999-015-4340-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Vecchi E, Villa F, Bortolin M, et al. Leucocyte esterase, glucose and C-reactive protein in the diagnosis of prosthetic joint infections: A prospective study. Clin Microbiol Infection. 2016;22(6):555–60. doi: 10.1016/j.cmi.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 23.Frances Borrego A, Martinez FM, Cebrian Parra JL, et al. Diagnosis of infection in hip and knee revision surgery: Intraoperative frozen section analysis. Int Orthop. 2007;31(1):33–37. doi: 10.1007/s00264-005-0069-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tohtz SW, Muller M, Morawietz L, et al. Validity of frozen sections for analysis of periprosthetic loosening membranes. Clin Orthop Rel Res. 2010;468(3):762–68. doi: 10.1007/s11999-009-1102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stroh DA, Johnson AJ, Naziri Q, Mont MA. How do frozen and permanent histopathologic diagnoses compare for staged revision after periprosthetic hip infections? J Arthroplasty. 2012;27(9):1663–8.e1. doi: 10.1016/j.arth.2012.03.035. [DOI] [PubMed] [Google Scholar]