Abstract
Background
UCA1 is a long non-coding RNA that has been found to be aberrantly upregulated in various cancers. The aim of this study was to determine the expression level and function of UCA1 in medulloblastoma, the most common malignant brain tumor during childhood.
Material/Methods
Real-time PCR was used to detect the expression of UCA1 in medulloblastoma specimens and cell lines. Lentiviral-mediated expression of a short hairpin RNA (shRNA) targeting UCA1 or a negative control shRNA was also achieved with the medulloblastoma cell line, Daoy. Cell proliferation and cell cycle progression were subsequently characterized with cell counting kit (CCK)-8 and flow cytometry. Cell migration was examined in wound healing and Transwell migration assays.
Results
Levels of UCA1 mRNA were higher in the medulloblastoma specimens (p<0.05) and cell lines (p<0.05) compared to the corresponding nontumor adjacent tissue specimens and a glioblastoma cell line, respectively. For the Daoy cells with silenced UCA1, their proliferation was reduced by 30% compared to the Daoy cells expressing a negative control shRNA (p=0.017). Cell cycle arrest in the G0/G1 phase, resulting in a decreased number of cells in the S phase, as well as reduced cell migration in both wound scratch healing (p=0.001) and Transwell migration assays (p=0.021) were also observed for the Daoy cells with silenced UCA1.
Conclusions
UCA1 was highly expressed in part of medulloblastoma specimens and cell lines examined. In addition, knockdown of UCA1 significantly inhibited the proliferation and migration of medulloblastoma cells in vitro.
MeSH Keywords: Cell Migration Assays; Cell Proliferation; Medulloblastoma; RNA, Long Noncoding
Background
Medulloblastoma was first described by Harvey Cushing and Percival Bailey in 1925 and is a malignant tumor that develops in neuroepithelial tissue in the central nervous system. It is also the most common malignant primary brain tumor that develops in children [1,2]. Despite the development of multiple therapeutic strategies in recent years, including surgical resection, adjuvant chemotherapy, and cranio-spinal irradiation, the prognosis of medulloblastoma remains unsatisfactory. Moreover, the survival rates for patients with medulloblastoma have been found to decrease with patient age, and children have higher rates of survival than adults [3]. Over the past few decades, many genes that mediate the development and prognosis of medulloblastoma have been discovered [3,4]. These efforts have revealed four distinct subgroups (WNT, SHH, Group 3, Group 4) rather than one single morphological entity. Each subgroup has a characteristic survival rate, age demographics, and genetic aberrations [5]. For example, 90% of WNT patients have somatic missense mutations in CTNNB1 [6], while amplification of GLI2 and MYCN, as well as missense mutations in MSO, PTCH, and SUFU, are generally observed in SHH patients [7–10]. In contrast, genome alterations associated with Group 3 are diverse and are often shared with Group 4.
Long non-coding RNAs (lncRNAs) are a group of transcribed RNA molecules with lengths exceeding 200 nucleotides [11,12] that do not encode proteins, yet they contribute to the expression and regulation of various genes [13]. Over the past few years, lncRNAs have been found to have roles in the tumorigenesis, metastasis, and prognosis of various cancers [14]. Human UCA1 is an lncRNA that is composed of three exons that encode a 1.4 kb isoform and a 2.2 kb isoform [15]. UCA1 was first detected in human bladder carcinoma [16] and has since been found to contribute to several epithelial cancers, including bladder, prostate, breast, non-small cell lung, tongue squamous cell, esophageal squamous cell, gastric, colorectal, hepatocellular, melanoma, and ovarian cancers [17–27]. However, the role of UCA1 in medulloblastoma remains to be elucidated. Therefore, in the present study, expression levels of UCA1 in medulloblastoma specimens and cell lines were initially detected with quantitative real-time PCR. We next investigated whether abnormal expression of UCA1 affects the proliferation, migration, apoptosis, and cell cycle progression of medulloblastoma cells.
Material and Methods
Patient samples
Ten medulloblastoma specimens and corresponding nontumor adjacent tissue specimens were obtained from the Department of Neurosurgery, Zhujiang Hospital of Southern Medical University, China, between 2014 and 2016. Each specimen was preserved in liquid nitrogen before being prepared for pathological analysis in the Department of Pathology, Zhujiang Hospital of Southern Medical University, China. Informed consent was obtained after patients were made aware of the aims and sample collection process for this study. This study was approved by the ethics committee of Zhujiang Hospital of Southern Medical University according to the Declaration of Helsinki.
Cell culture
The human medulloblastoma cell line, Daoy, and the human glioma cell line, U251, were obtained from the Cell Bank of Chinese Academy of Sciences (Shanghai, China). Both cell lines were cultured in DMEM supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. The human medulloblastoma cell line, D283, was obtained from American Type Culture Collection (ATCC; Manassas, VA, USA) and maintained in RPMI 1640 supplemented with 15% FBS and 1% penicillin/streptomycin. All of the cells were maintained in a humidified atmosphere containing 5% CO2 at 37°C.
Quantitative real-time PCR
A homogenate of each medulloblastoma specimen was obtained according to a liquid nitrogen grounding method. Total RNA was then extracted from each homogenate with the RNAiso Plus kit (Takara Bio Inc.) according to the manufacturer’s instructions. Reverse transcription was performed with the PrimeScript™ RT reagent kit (Takara Bio Inc.) and Veriti 96-Well Thermal Cycler (Applied Biosystems, Foster City, CA, USA) to obtain cDNA. Quantitative PCR was performed with the ECO™ Real-time PCR System (Illumina, San Diego, CA, USA) and SYBR® Premix Ex Taq™ II (Takara Bio Inc.). Detection of GAPDH and 18S were performed as internal controls. Forward and reverse primers for the following targets were used (Sangon Biotech Co.): human UCA1: 5′-TGTTAGAGGGCTTGGGACAT-3′ and 5′-ATAGGTGTGAGTGGCGGTCT-3′; human GAPDH: 5′-CAGGA GGCATTGCTGATGAT-3′ and 5′-GAAGGCTGGGGCTCATTT-3′; human 18S: 5′-GTAACCCGTTGAACCCCATT-3′ and 5′-CCATCCA ATCGGTAGTAGCG-3′, respectively in each case.
Short hairpin RNA (shRNA) constructs and viral infection
A shRNA targeting UCA1 and a negative control shRNA were designed and synthesized (GenePharma, Shanghai, China). These shRNAs were subsequently inserted into a lentivirus vector (GenePharma, Shanghai, China) and transfected into medulloblastoma cells. Stable expression clones were selected by puromycin according to the manufacturer’s instructions and knockdown efficiency was determined with quantitative real-time PCR assays.
Cell proliferation assay
Cell proliferation was detected with a cell counting kit (CCK)-8 (Dojindo, Japan) as described in previous articles [28]. Briefly, cells were added to a 96-well plate (2,000 cell/well) and incubated for various periods of time (0, 24, 48, and 72 hours) in a humidified atmosphere containing 5% CO2 at 37°C. Then, 10 μL of CCK-8 solution was added to each well. The plates were incubated for an additional 2 hours until absorbance values at 450 nm were recorded with a microplate reader.
Flow cytometry analysis
Medulloblastoma cells were cultured in 6-well plates for a suitable period that cells were grown to reach 70–80% confluence. The cells were then rinsed 3 times with PBS and fixed in 70% ethanol for 2 hours at 4 °C. Each sample was resuspended in 100 uL of PBS containing RNase A (2 mg/mL; Sigma Aldrich, St. Louis, USA) and propidium iodide (100 mg/mL; Sigma Aldrich). Fluorescence activated cell sorting (FACS) analysis was performed to detect the distribution of cells among the various phases of the cell cycle.
Scratch wound healing assay
Cell migration was investigated in scratch wound healing assays. Briefly, cells were cultured in 6-well plates until they reached 90% confluency. Scratch wounds were then made in the bottom of each well with a pipette tip. The medium from each well was subsequently removed and replaced with serum-free culture media. At various time points, the wounds were observed and imaged with an inverted microscope. Then divide the original scratch wound width by average wound width after culture, we get the results of%wound closure.
Transwell migration assay
Cell migration assays were performed with Transwell inserts with 8-μm pore size polycarbonate membranes (Corning, Corning, NY, USA). Briefly, cells in serum-free culture media were added into the upper chambers of each Transwell insert. The lower chamber for each well contained media supplemented with 20% FBS. After 24 hours in a humidified atmosphere of 5% CO2 at 37°C, the cells on the upper membrane surface were removed mechanically with cotton swabs [29]. The filters were then fixed with methanol for 20 minutes and stained with a 0.1% crystal violet solution for 15 minutes. The number of migrating cells observed in five random fields with an inverted microscope was recorded and representative images were acquired with Image-Pro Plus 6.
Statistical analysis
Data are presented as the mean ± standard deviation (SD). All statistical analyses included Student’s t-tests were analyzed using SPSS 19.0 Windows version software (SPSS, Chicago, IL, USA). A p value less than 0.05 was considered statistically significant.
Results
Expression of UCA1 in medulloblastoma specimens and cell lines
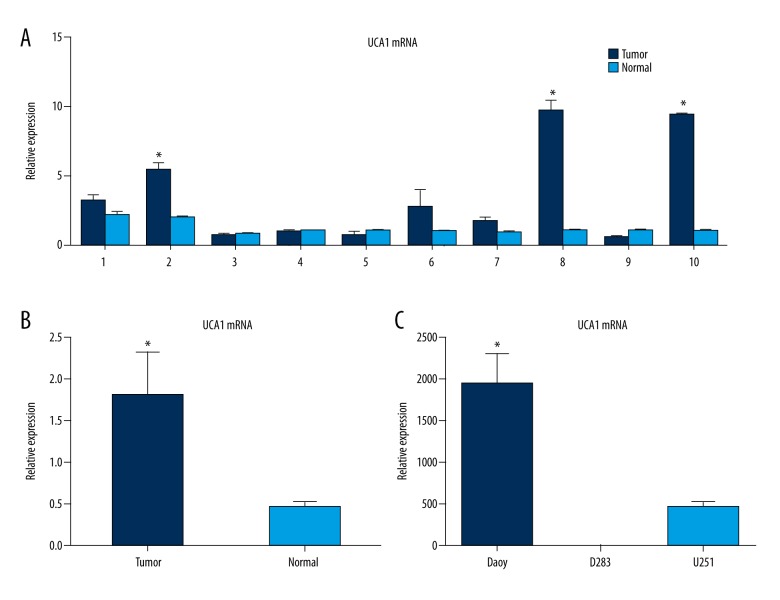
We initially compared the expression levels of UCA1 between 10 pairs of medulloblastoma tissues and corresponding adjacent nontumor normal tissues with RT-PCR. The levels of UCA1 in the medulloblastoma specimens were approximately 30% higher than the levels detected in the nontumor adjacent tissues (p<0.05; Figure 1). The levels of UCA1 expression were also compared with the characteristics of the patients from whom the specimens were resected (Table 1). Among these patients, 8/10 were younger than 10 years of age and 3/10 were female. The resected tumors were located in the fourth ventricle for 5/10 of the patients, and the rest of the tumors were located in the cerebellum. Eight of the patients developed distant metastasis and all 10 patients were diagnosed with stage IV medulloblastoma according to the World Health Organization criteria.
Figure 1.
Levels of UCA1 mRNA detected in the medulloblastoma samples and cell lines examined (t-test). (A) Relative mRNA levels of UCA1 that were detected in ten medulloblastoma specimens and paired nontumor adjacent tissues. (B) The mean levels of UCA1 from the data in (A) (p<0.05). (C) Relative UCA1 expression levels in the medulloblastoma cell lines, Daoy and D283, compared with the glioma cell line, U251 (p<0.05). All data are presented ±SD.
Table 1.
UCA1 Expression and the characteristics of the patients from whom the medulloblastoma specimens and nontumor specimens were resected (n=10).
| Patient no. | Gender | Age (y) | Tumor length (cm) | Tumor location | Distant metastasis | p53 expression | UCA1 expression |
|---|---|---|---|---|---|---|---|
| 1 | Male | 7 | 4.6 | 4th ventricle | − | + | − |
| 2 | Male | 37 | 5.5 | Cerebellum | − | + | − |
| 3 | Female | 2 | 6.3 | Cerebellum | − | + | − |
| 4 | Male | 7 | 4.1 | Cerebellum | − | − | − |
| 5 | Male | 6 | 4.9 | 4th ventricle | − | − | + |
| 6 | Male | 5 | 4.5 | Cerebellum | − | + | − |
| 7 | Female | 2 | 5.7 | 4th ventricle | − | + | + |
| 8 | Male | 6 | 3.8 | 4th ventricle | − | + | − |
| 9 | Male | 8 | 3.6 | 4th ventricle | + | − | + |
| 10 | Female | 34 | 4.0 | Cerebellum | + | + | − |
’+’ – positive; ‘−’ – negative. Positive UCA1 expression was defined as a level of UCA1 expression in the medulloblastoma.
The mRNA levels of UCA1 were also detected in the medulloblastoma cell lines, Daoy and D283, and in the glioma cell line, U251, with quantitative PCR. UCA1 expression was markedly higher in the Daoy cell line compared to the D283 and U251 cell lines (p<0.05; Figure 1C).
Knockdown of UCA1 in medulloblastoma cells by lentiviral shRNA
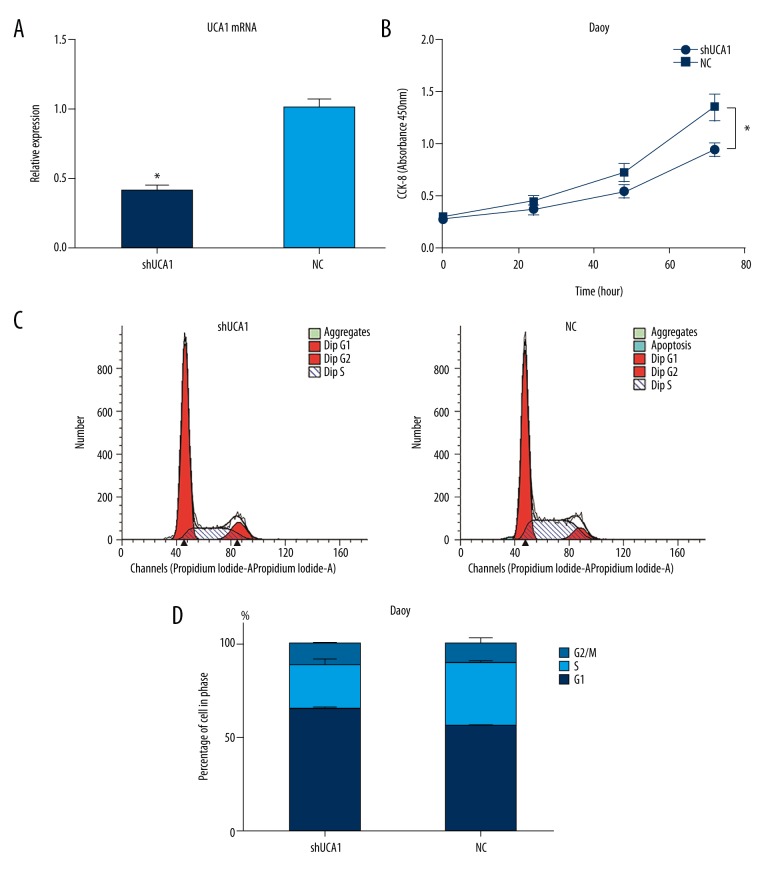
To elucidate the importance of UCA1 in medulloblastoma, stable knockdown expression clones were established in Daoy cells. Briefly, a shRNA designed to target UCA1 was constructed, packaged in a lentivirus vector, and transfected into Daoy cells (referred to as the shUCA1 group). A negative control lentiviral shRNA was generated and transfected in parallel (referred to as the NC group). Quantitative RT-PCR detected a knockdown efficiency of 60% for these shRNAs (p<0.05; Figure 2A).
Figure 2.
Results of the CCK-8 assays and flow cytometry analyses performed following the knockdown of UCA1 in the Daoy cells (t-test). (A) Relative expression levels of UCA1 ±SD in the shUCA1 and NC groups as detected by RT-PCR (p<0.05). (B) OD450 values ±SD for the shUCA1 group were reduced by 30% compared to the NC group in the CCK-8 assays (p<0.05). (C) The distribution of cells among the phases of the cell cycle for the shUCA1 and NC groups as detected by flow cytometry. (D) The percentage of cells that were distributed among the three phases of the cell cycle ±SD in the shUCA1 and NC groups.
Knockdown of UCA1 alters cell proliferation, cell cycle, and migration of medulloblastoma cells in vitro
To detect possible biological roles of UCA1 in the tumorigenicity of medulloblastoma, the proliferation, cell cycle progression, and migration of Daoy cells following knockdown of UCA1 were assayed. In the CCK-8 assays that were performed to detect cell proliferation, the OD450 values for the shUCA1 group were 30% lower compared with the NC group (p<0.05; Figure 2B). In the analysis of cell cycle progression with flow cytometry, silencing of UCA1 in the Daoy cells induced a cell cycle arrest in the G0/G1 phase compared to the NC group. In addition, the number of cells in the S phase significantly decreased (Figure 2C, 2D).
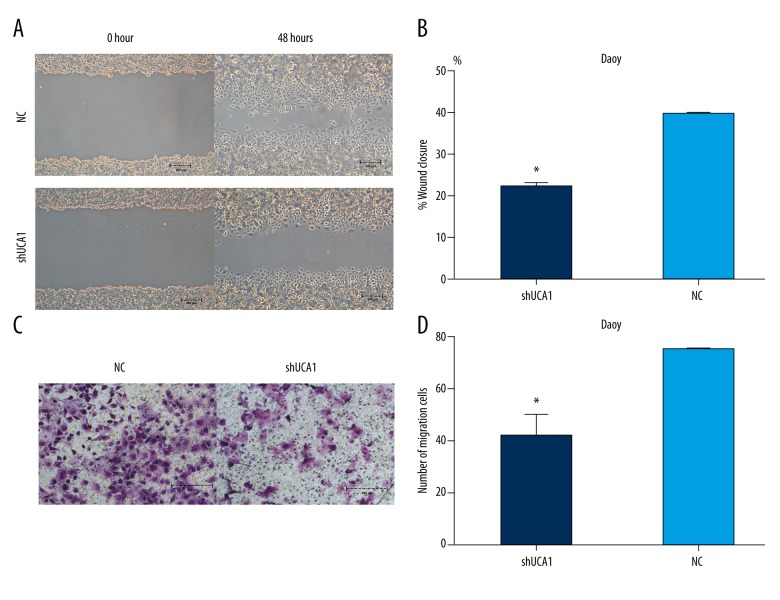
To examine the influence of UCA1 silencing on the migration ability of Daoy cells, scratch wound healing and Transwell migration assays were performed. In the former assays, the cell free area was significantly wider in the shUCA1 group than in the NC group (Figure 3A), with a 56.16% reduction in migration observed for the shUCA1 group versus the NC group (p<0.05; Figure 3B). Similarly, the results of the Transwell migration assays showed that knockdown of UCA1 significantly suppressed migration by 44.64% compared to the NC group (p<0.05; Figure 3C 3D).
Figure 3.
Results of the wound scratch healing and Transwell migration assays (t-test). (A) Representative images of wound scratch healing assays for the shUCA1 group and the NC group 0 hour and 48 hours after the start of the assay (p<0.05). (B) Quantitation of the amount of wound closure measured for the shUCA1 and NC groups ±SD. (C) Representative images of the Transwell migration assay data for the shUCA1 and NC groups 24 hours after the start of the assay. (D) Number of migrating Daoy cells that were counted for shUCA1 group and NC group ±SD (p<0.05).
Discussion
Medulloblastoma is the most common malignant intracranial tumor during childhood, and it currently accounts for approximately 20% of the tumors of the central nervous system in children [30]. With the development of multiple therapeutic strategies, the survival rate of medulloblastoma has improved [31]. However, the quality of life for medulloblastoma survivors is frequently affected by neurocognitive sequelae. Thus, safer and more efficient targeting therapies are needed. Among the long non-coding RNAs that have been identified as having roles in a wide range of biological processes in tumors [14], UCA1 is of particular interest to our laboratory. Based on the diverse oncogenic functions that UCA1 has been associated with in a variety of epithelial cancers, it appears that UCA1 affects the proliferation, migration, invasion, apoptosis, and cell cycle progression of these cancers [15–27]. In addition, UCA1 has been shown to contribute to the WNT, SUFU, and PI3K/AKT pathways as well [32]. Therefore, the oncogenic effect of UCA1 in medulloblastoma was investigated here.
In the present study, expression of UCA1 was detected by RT-PCR and was found to be expressed at markedly higher levels in all ten medulloblastoma samples that were examined compared with the corresponding paired nontumor adjacent tissues. Moreover, for the medulloblastoma cell lines (Daoy and D283) and glioma cell line (U251) that were assayed by the same method, the UCA1 levels detected in the Daoy cell line were significantly higher than the levels detected in the D283 and U251 cell lines. Thus, Daoy cells were confirmed to be a suitable cell line for the knockdown of UCA1 expression via lentiviral shRNA and the selection of stable expression clones. As a result, proliferation of the Daoy cells with silenced UCA1 was found to be reduced due to cell cycle arrest in the G0/G1 phase, and this was accompanied by a marked decrease in the number of cells in the S phase. However, this cell cycle arrest was not accompanied by a significant increase in apoptosis. Furthermore, UCA1 silencing markedly suppressed the migration ability of the Daoy cells compared to the negative control group.
To date, four distinct subgroups of medulloblastoma have been identified, including WNT, SHH, Group 3, and Group 4, and each subgroup has its own genetic features. In the WNT subgroup, the WNT signaling pathway is activated. The WNT pathway has been shown to play an important role in cell fate specification, cell proliferation, and cell migration [33]. In bladder cancer, Fan et al. showed that UCA1 is able to activate the WNT signaling pathway and also upregulate Wnt6 expression [33]. Recent research has also demonstrated that UCA1 can activate the PI3K/AKT signaling pathway in bladder cancer [34]. In the SHH subgroup, the SHH signaling pathway is excessively activated and GLI is overexpressed [33]. In combination, these events facilitate tumor formation. Li et al. further discovered that mTOR is upregulated by UCA1 [35]. Thus, activation of PI3K/AKT and mTOR by UCA1 has the potential to inhibit SUFU, a negative regulatory factor of GLI, thereby promoting activation of the SHH signaling pathway [32]. Meanwhile, SUFU can bind to β-catenin to inhibit binding interactions between β-catenin and Tcf/Lef, thereby inactivating the WNT signaling pathway [36]. All of these interactions represent potential mechanisms by which UCA1 may contribute to the proliferation, migration, and cell cycle progression of the Daoy medulloblastoma cell line, and possibly to medulloblastomas in vivo as well.
There were limitations associated with the present study. First, there were no significant differences observed regarding the possible correlations between UCA1 expression and the clinical features and prognosis of the patients examined. We hypothesize that this is due to the limited number of samples that were examined. Second, although an oncogenic function was demonstrated for UCA1 in medulloblastoma in the present study, the mechanisms and downstream mediators that mediate this function remain to be identified.
Conclusions
To the best of our knowledge, the results of the present study show for the first time that UCA1 is overexpressed in medulloblastomas both in vivo and in vitro. Furthermore, silencing of UCA1 resulted in a significant inhibition of cell proliferation and migration in vitro and induced a G0/G1 phase arrest. Thus, targeting of UCA1 appears to represent a potentially effective approach for treating medulloblastoma and further study is warranted.
Footnotes
Source of support: The authors are grateful for the support provided by The Guangdong Provincial Clinical Medical Center for Neurosurgery [No. 2013B020400005]
References
- 1.Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016;131(6):803–20. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 3.Ramaswamy V, Remke M, Bouffet E, et al. Risk stratification of childhood medulloblastoma in the molecular era: The current consensus. Acta Neuropathol. 2016;131(6):821–31. doi: 10.1007/s00401-016-1569-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang X, Dubuc AM, Ramaswamy V, et al. Medulloblastoma subgroups remain stable across primary and metastatic compartments. Acta Neuropathol. 2015;129(3):449–57. doi: 10.1007/s00401-015-1389-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mabbott DJ, Spiegler BJ, Greenberg ML, et al. Serial evaluation of academic and behavioral outcome after treatment with cranial radiation in childhood. J Clin Oncol. 2005;23(10):2256–63. doi: 10.1200/JCO.2005.01.158. [DOI] [PubMed] [Google Scholar]
- 6.Bourdeaut F, Miquel C, Richer W, et al. Rubinstein-Taybi syndrome predisposing to non-WNT, non-SHH, group 3 medulloblastoma. Pediatr Blood Cancer. 2014;61(2):383–86. doi: 10.1002/pbc.24765. [DOI] [PubMed] [Google Scholar]
- 7.Northcott PA, Shih DJ, Peacock J, et al. Subgroup-specific structural variation across 1,000 medulloblastoma genomes. Nature. 2012;488(7409):49–56. doi: 10.1038/nature11327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones DT, Jager N, Kool M, et al. Dissecting the genomic complexity underlying medulloblastoma. Nature. 2012;488(7409):100–5. doi: 10.1038/nature11284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robinson G, Parker M, Kranenburg TA, et al. Novel mutations target distinct subgroups of medulloblastoma. Nature. 2012;488(7409):43–48. doi: 10.1038/nature11213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pugh TJ, Weeraratne SD, Archer TC, et al. Medulloblastoma exome sequencing uncovers subtype-specific somatic mutations. Nature. 2012;488(7409):106–10. doi: 10.1038/nature11329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kapranov P, Cheng J, Dike S, et al. RNA maps reveal new RNA classes and a possible function for pervasive transcription. Science (New York, NY) 2007;316(5830):1484–88. doi: 10.1126/science.1138341. [DOI] [PubMed] [Google Scholar]
- 12.Katayama S, Tomaru Y, Kasukawa T, et al. Antisense transcription in the mammalian transcriptome. Science (New York, NY) 2005;309(5740):1564–66. doi: 10.1126/science.1112009. [DOI] [PubMed] [Google Scholar]
- 13.Cabili MN, Trapnell C, Goff L, et al. Integrative annotation of human large intergenic noncoding RNAs reveals global properties and specific subclasses. Genes Dev. 2011;25(18):1915–27. doi: 10.1101/gad.17446611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xing YH, Bai Z, Liu CX, et al. Research progress of long noncoding RNA in China. IUBMB Life. 2016;68(11):887–93. doi: 10.1002/iub.1564. [DOI] [PubMed] [Google Scholar]
- 15.Xiao C, Wu CH, Hu HZ. LncRNA UCA1 promotes epithelial-mesenchymal transition (EMT) of breast cancer cells via enhancing Wnt/beta-catenin signaling pathway. Eur Rev Med Pharmacol Sci. 2016;20(13):2819–24. [PubMed] [Google Scholar]
- 16.Wang XS, Zhang Z, Wang HC, et al. Rapid identification of UCA1 as a very sensitive and specific unique marker for human bladder carcinoma. Clin Cancer Res. 2006;12(16):4851–58. doi: 10.1158/1078-0432.CCR-06-0134. [DOI] [PubMed] [Google Scholar]
- 17.Xue M, Pang H, Li X, et al. Long non-coding RNA urothelial cancer-associated 1 promotes bladder cancer cell migration and invasion by way of the hsa-miR-145-ZEB1/2-FSCN1 pathway. Cancer Sci. 2016;107(1):18–27. doi: 10.1111/cas.12844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Na XY, Liu ZY, Ren PP, et al. Long non-coding RNA UCA1 contributes to the progression of prostate cancer and regulates proliferation through KLF4-KRT6/13 signaling pathway. Int J Clin Exp Med. 2015;8(8):12609–16. [PMC free article] [PubMed] [Google Scholar]
- 19.Tuo YL, Li XM, Luo J. Long noncoding RNA UCA1 modulates breast cancer cell growth and apoptosis through decreasing tumor suppressive miR-143. Eur Rev Med Pharmacol Sci. 2015;19(18):3403–11. [PubMed] [Google Scholar]
- 20.Nie W, Ge HJ, Yang XQ, et al. LncRNA-UCA1 exerts oncogenic functions in non-small cell lung cancer by targeting miR-193a-3p. Cancer Lett. 2016;371(1):99–106. doi: 10.1016/j.canlet.2015.11.024. [DOI] [PubMed] [Google Scholar]
- 21.Fang Z, Wu L, Wang L, et al. Increased expression of the long non-coding RNA UCA1 in tongue squamous cell carcinomas: A possible correlation with cancer metastasis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117(1):89–95. doi: 10.1016/j.oooo.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 22.Wang X, Gao Z, Liao J, et al. lncRNA UCA1 inhibits esophageal squamous-cell carcinoma growth by regulating the Wnt signaling pathway. J Toxicol Environ Health A. 2016;79(9–10):407–18. doi: 10.1080/15287394.2016.1176617. [DOI] [PubMed] [Google Scholar]
- 23.Liu YV, Baek JH, Zhang H, et al. RACK1 competes with HSP90 for binding to HIF-1alpha and is required for O(2)-independent and HSP90 inhibitor-induced degradation of HIF-1alpha. Mol Cell. 2007;25(2):207–17. doi: 10.1016/j.molcel.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bian Z, Jin L, Zhang J, et al. LncRNA-UCA1 enhances cell proliferation and 5-fluorouracil resistance in colorectal cancer by inhibiting miR-204-5p. Sci Rep. 2016;6:23892. doi: 10.1038/srep23892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang F, Ying HQ, He BS, et al. Upregulated lncRNA-UCA1 contributes to progression of hepatocellular carcinoma through inhibition of miR-216b and activation of FGFR1/ERK signaling pathway. Oncotarget. 2015;6(10):7899–917. doi: 10.18632/oncotarget.3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tian Y, Zhang X, Hao Y, et al. Potential roles of abnormally expressed long noncoding RNA UCA1 and Malat-1 in metastasis of melanoma. Melanoma Res. 2014;24(4):335–41. doi: 10.1097/CMR.0000000000000080. [DOI] [PubMed] [Google Scholar]
- 27.Zhang L, Cao X, Zhang L, et al. UCA1 overexpression predicts clinical outcome of patients with ovarian cancer receiving adjuvant chemotherapy. Cancer Chemother Pharmacol. 2016;77(3):629–34. doi: 10.1007/s00280-016-2963-4. [DOI] [PubMed] [Google Scholar]
- 28.Hou L, Chen M, Yang H, et al. MiR-940 inhibited cell growth and migration in triple-negative breast cancer. Med Sci Monit. 2016;22:3666–72. doi: 10.12659/MSM.897731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma T, Luan SL, Huang H, et al. Upregulation of CC chemokine receptor 7 (CCR7) enables migration of xenogeneic human adipose-derived mesenchymal stem cells to rat secondary lymphoid organs. Med Sci Monit. 2016;22:5206–17. doi: 10.12659/MSM.902690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Millard NE, De Braganca KC. Medulloblastoma. J Child Neurol. 2016;31(12):1341–53. doi: 10.1177/0883073815600866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bartlett F, Kortmann R, Saran F. Medulloblastoma. Clin Oncol. 2013;25(1):36–45. doi: 10.1016/j.clon.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 32.Xue M, Chen W, Li X. Urothelial cancer associated 1: A long noncoding RNA with a crucial role in cancer. J Cancer Res Clin Oncol. 2016;142(7):1407–19. doi: 10.1007/s00432-015-2042-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fan Y, Shen B, Tan M, et al. Long non-coding RNA UCA1 increases chemoresistance of bladder cancer cells by regulating Wnt signaling. FEBS J. 2014;281(7):1750–58. doi: 10.1111/febs.12737. [DOI] [PubMed] [Google Scholar]
- 34.Yang C, Li X, Wang Y, et al. Long non-coding RNA UCA1 regulated cell cycle distribution via CREB through PI3-K dependent pathway in bladder carcinoma cells. Gene. 2012;496(1):8–16. doi: 10.1016/j.gene.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 35.Li Z, Li X, Wu S, et al. Long non-coding RNA UCA1 promotes glycolysis by upregulating hexokinase 2 through the mTOR-STAT3/microRNA143 pathway. Cancer Sci. 2014;105(8):951–55. doi: 10.1111/cas.12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meng X, Poon R, Zhang X, et al. Suppressor of fused negatively regulates beta-catenin signaling. J Biol Chem. 2001;276(43):40113–19. doi: 10.1074/jbc.M105317200. [DOI] [PubMed] [Google Scholar]