ABSTRACT
Tocilizumab (TCZ), is a recombinant humanized anti-interleukin-6 receptor (IL-6R) monoclonal antibody which has a main use in the treatment of rheumatoid arthritis, systemic juvenile idiopathic arthritis (sJIA) and polyarticular juvenile idiopathic arthritis (pJIA). This article provides an overview of TCZ including looking into the past at the discovery of interleukin-6 (IL-6) as a pro-inflammatory cytokine. It also looks at how tocilizumab was developed, manufactured and tested to ensure both safety and efficacy in a human population. The article then explores the advantages and disadvantages of using TCZ when compared to other biologics approved in RA, sJIA and pJIA and finally looks ahead to the future and the emerging role of IL-6 and its blockade by TCZ as a treatment for giant cell arteritis (GCA), polymyalgia rheumatica (PMR) and large vessel vasculitis (LVV).
KEYWORDS: giant cell arteritis, interleukin-6, monoclonal antibody, rheumatoid arthritis, tocilizumab, vasculitis
Introduction
Tocilizumab (TCZ), is a recombinant humanized, anti-human monoclonal antibody of the immunoglobulin G1k subclass directed against soluble and membrane-bound interleukin 6 receptors (IL-6R).1 It is marketed in the European Union (EU) under the trade name RoActemra and in the United States as Actemra.2,3 TCZ was first approved in 2005 as an orphan drug in Japan for the treatment of Castleman's disease, a rare lymphoproliferative disease involving expansion of plasma cell numbers.4 TCZ is now licensed in the EU for use alone or in combination with disease-modifying anti-rheumatic drugs (DMARDs) to treat adult patients with moderate to severely active rheumatoid arthritis (RA), children over 2 y of age with the systemic form of juvenile idiopathic arthritis (sJIA) or children over 2 y of age with the polyarticular form of juvenile idiopathic arthritis (pJIA).5 It has also been studied as a treatment of other conditions such as Crohn's disease,6 systemic lupus erythematosus,7 Takayasu arteritis (TA),8 giant cell arteritis (GCA)9 polymyalgia rheumatica (PMR)10,11 and refractory adult-onset Still disease12-14 but has not yet received licenses in these indications.
Origin of TCZ and the role of IL-6 in the inflammatory pathway
TCZ was first introduced in 2008 in Japan followed by the EU in 2009 and the US in 2010.15 The molecule is a genetically engineered humanized monoclonal antibody that is produced by grafting the complementarity determining region of mouse anti-human IL-6 receptor to human IgG1.16 TCZ inhibits the binding of IL-6 to its receptors, in doing this it reduces this cytokine's pro-inflammatory activity by competing with both the soluble and membrane-bound forms of the human IL-6 receptor (IL-6R).17 The human IL-6 gene has been mapped to chromosome 7p21 and consists of 184 amino-acids with 2 N-glycosylation sites and 2 cysteine-cysteine bridges in the molecule.18 IL-6 is referred to as B-cell stimulatory factor-2 and it plays an essential role in the final differentiation of B cells into immunoglobulin-secreting cells. IL-6 exerts its biologic activities through 2 molcules: IL-6R (also known as IL-6Rα, gp80 or CD126) and the trans membrane protein gp130.19 On target cells, IL-6 binds to IL-6R. Signaling occurs when the IL-6/IL-6R complex associates with the 130 kDa transmembrane protein gp13020. Dimerization of gp130 leads to activation of Janus kinases (JAKs) which phosphorylate the cytoplasmic portion of gp130.20 Gp130 activation leads to the expression of acute phase protein genes.21 Many cells in the body can synthesize IL-6 but not many express the IL-6R so therefore are not responsive to cytokines.16 The soluble form of IL-6R is generated by limited proteolysis of the membrane-bound IL-6R or by translation from an alternatively spliced mRNA.22 The complex of IL-6 and the soluble IL-6R can also stimulate cells that do not express the IL-6R and this signaling mode is called IL-6 trans-signaling whereas signaling via the membrane-bound IL-6R is referred as IL-6 classic signaling.22
Following animal and in vivo models, a fusion protein of the soluble extracellular portion of gp130 with the constant portion of the human IgG1 antibody (sgp130Fc) were shown to exclusively block IL-6 trans-signaling without affecting the classic pathway.19,22 Inhibition of the trans-signaling pathway was sufficient to block inflammation without compromising the immune defense against bacterial infection.15 These findings led to the conclusion that the IL-6 classic pathway is important for regenerative and protective function whereas the IL-6 trans-signaling pathway constitutes the pro-inflammatory activity of IL-6.23
IL-6 is referred to as a pleiotropic cytokine. It is a T-cell derived factor inducing B cells to differentiate into antibody-producing cells and is also known to influence numerous cell types with several biologic activities.24 One important aspect is the secretion of IL-6 by cells of the immune system such as neutrophils and monocytes or macrophages upon stimulation.25 Upon IL-6 secretion endothelial cells release chemokines which recruit more immune cells and hepatocytes synthesize and secrete acute-phase proteins.16
IL-6 is also known to have an important role in the differentiation of T helper cells and in regulating the balance between interleukin 17 (IL-17) producing T helper 17 cells (Th17) and regulatory T-cells (Treg).26
IL-6 and transforming growth factor β (TGF-β) results in Th17 cell differentiation, an identified T-helper subset. It has also been described that human T-cells upon T-cell receptor activation cleave their membrane-bound IL-6R and release soluble IL-6R.26 As a result, these T-cells are no longer responsive to IL-6 but require soluble IL-6R for differentiation into Th17 cells. Therefore, an initial stimulation with IL-6 needs to be followed by IL-6 and sIL-6R stimulation for the differentiation of Th17 cells to be effective. The stimulation with TGF-β and the IL-6/soluble IL-6R complex causes a more sustained and long-term generation of Th17 cells which has been recognized to be important for inflammatory states. IL-6 seems to be an important mediator regarding vascular wall tissues and immune cells and is critical in polarizing T-cells into T helper 17 cells, believed to be important in the pathogenesis of vasculitis.15
On the other hand, IL-6 acts directly on osteoblast precursor cells and suppresses their differentiation by regulating the transcription of specific genes related to Mitogen-activated protein kinases (MAPK) phosphatases and the ubiquitin pathway.20 IL-6 induces the production of matrix metalloproteinase (MMP-1, MMP-3 and MMP-13) and a disintegrin and metalloprotease with thrombospondin motifs (ADAMTS-4) from chondrocytes and synovial cells. MMP-1 and MMP-13 are capable of cleaving collagen type II, whereas MMP-3 is active against other components of the extracellular matrix, such as fibronectin and laminin.25 Also, when IL-6 is generated in bone marrow stromal cells it stimulates the receptor activator of the nuclear factor kappa B ligand (RANKL) and subsequently induces the differentiation and activation of osteoclasts.27
IL-6 induces hepcidin production in liver cells. Hepcidin is a master regulator of iron homeostasis in humans and other mammals.20 It inhibits the absorption of iron in the small intestine and the release of recycled iron from macrophages, effectively decreasing the delivery of iron to maturing erythrocytes in the bone marrow causing anaemia.20
IL-6 has also been shown to indirectly induce angiogenesis by inducing Vascular endothelial growth factor (VEGF) expression.28 Angiogenesis is an essential component of inflammation and its resolution. Inflammatory cells, such as monocytes/macrophages, T-cells and monocytes, fully participate in the angiogenic process by secreting pro- and anti-inflammatory cytokines.
A few key observations have played a major role in the interest in IL-6 as a target. One was the observation that patients having Castleman's disease, in which benign tumors overproduce IL-6, exhibit the same symptoms as those with RA (RA).19,20 The pathological significance of IL-6 was first demonstrated in a case of undifferentiated connective tissue disease when a large quantity of IL-6 was identified in the histological tissue of the patient's cardiac myxoma.29,30 It was later observed from murine models that IL-6-deficient mice were incapable of producing an inflammatory response.
Specifically, IL-6 in RA promotes osteoclast activation as RANKL expression is regulated by pro-inflammatory cytokines; it leads to neutrophil recruitment in synovial tissues by directly acting on them through membrane-bound IL-6R. This contributes to inflammation and joint destruction by secreting proteolytic enzymes and reactive oxygen intermediates, pannus formation via promotion of VEGF production locally.31,32 IL-6 also has 2 other local effects in RA; B-cell proliferation with subsequent antibody production and T-cell proliferation and differentiation both leading to a feedback loop for additional T-cell, macrophages and B-cell interactions.32 IL-6 has a major effect on acute phase production which may exacerbate disease-related tissue damage and contribute to the development of cardiovascular disease. IL-6 from synovial tissue amends the function of adipose tissue, skeletal muscle, liver and vascular endothelium, causing insulin resistance, dyslipidaemia, increased global oxidative activity and endothelial dysfunction. In IL-6 transgenic mice, where high circulating levels of IL-6 are present, increased osteoclastogenesis and reduced bone formation by decreased osteoblast activity was noted causing osteopenia and subsequently osteoporosis. Hypothalamic-pituitary-adrenal axis and the stress system are associated with fatigue and depression in case reports in patients with RA and are primarily mediated by the upregulation of cytokines where IL-6 plays again a major role.32
IL-6 is associated with many of the manifestations of sJIA such as fever, rash, lymphadenopathy, hepatosplenomegaly, anaemia, and poor growth as it interacts with various cells such as T-cells, B-cells, lymphocytes, monocytes, and fibroblasts, driving synovial proliferation, inflammation, autoimmunity, and destruction of articular structures through the activation of osteoclasts. It appears that sJIA follows a fairly distinct cytokine pattern as indicated by the clinical studies.33
IL-6 inhibits differentiation, mineralization and may increase apoptosis of human osteoblast T-cells cultures, and inflammatory cytokines in pJIA.34
TCZ as neutralising antibody against IL-6 and IL-6R blocks both classic and trans-signaling pathways. TCZ can dissociate the IL-6–sIL-6R complex, but not the IL-6–sIL-6R–sgp130 complex, suggesting that the IL-6–sIL-6R complex is less rigid than the IL-6–sIL-6R–sgp130 complex.20,23 IL-6 trans-signaling is pro-inflammatory whereas classic IL-6 signaling via the membrane bound IL-6R is needed for regenerative or anti-inflammatory activities of the cytokine. This detailed knowledge of IL-6 biology has important consequences for therapeutic strategies aimed at the blockade of the cytokine IL-6.
Manufacturing, analytical and regulatory processes
Production of tocilizumab
TCZ is a humanised recombinant monoclonal antibody of the IgG1 k subclass produced using recombinant DNA technology.1 The antibody is composed of 2 heavy and 2 light chains with 12 intra-chain and 4 inter-chain disulphide bonds with a total molecular weight of 149 kDa.1 The steps involved in this process are highlighted in Fig. 1.
Figure 1.
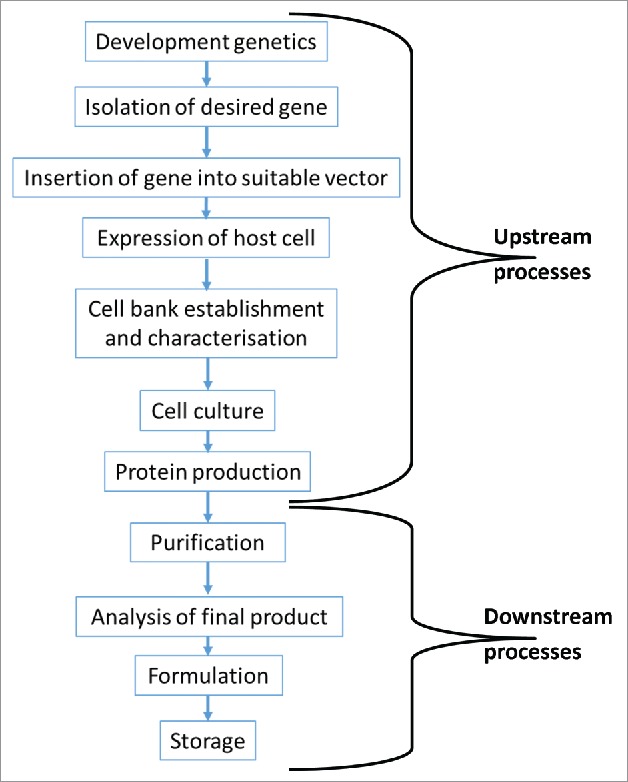
Overview of the process required to produce a biologic medicinal product. The production process is broken down into 2 main parts. The upstream processes are responsible for genetic manipulation of a host cell and the subsequent large scale production of the drug substance. The downstream processes are responsible for the purification of the drug substance and its formulation into a drug product.
To produce a cell line which can then be cultured, the genes that code for TCZ production must be inserted into a host T-cell.1 The process by which this is achieved using Chinese hamster ovary cells is summarised in Fig. 2.
Figure 2.
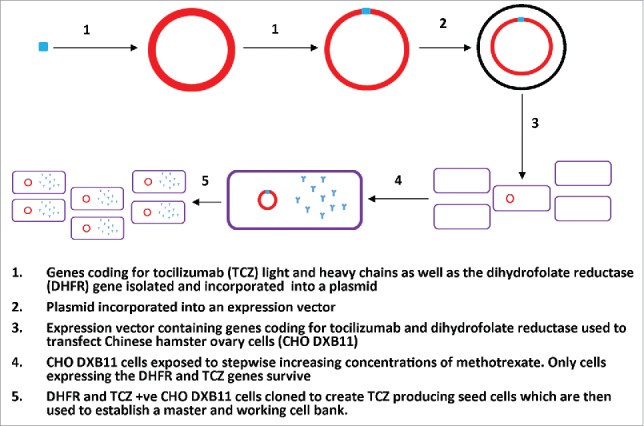
The development and isolation of tocilizumab producing seed cells. This figure adapted from the production section of the IV tocilizumab EPAR, details the insertion of the gene coding for tocilizumab production into a host CHO cell and the subsequent processes to isolate and culture the tocilizumab producing CHO cell into a viable master and working cell bank.
Once the isolation of TCZ producing seed cells (CHO V4) is complete they are cultured into a master and working cell bank which can be indefinitely used as the starting point in the production of TCZ. The CHO V4 cells are then adapted to enable growth in suspension culture in a serum free medium.1
To increase the number of cells able to produce TCZ a vial of the working cell bank is thawed and the number of cells expanded using spinner flasks with serum free growth medium. The resulting inoculum is then expanded using a series of bioreactors with increasing volumes. This process enables the generation of sufficient CHO cells to inoculate several production bioreactors which can produce the large scale batches of TCZ required to enable commercial supply. Following this process, the contents of the bioreactor are harvested using tangential flow filtration enabling the removal of cells from their culture medium.1
Once the culture medium has been removed it undergoes a series of purification processes. These include protein A chromatography, viral inactivation, anion exchange chromatography, mixed-mode ion exchange chromatography, ultra diafiltration and nanofiltration. TCZ is formulated to prevent protein aggregation by adding sucrose, polysorbate 80, disodium phosphate dodecahydrate, sodium dihydrogen phosphate dihydrate and water for injections. Once formulated it is presented in a type 1 glass vial at strengths of 80mg, 200 mg and 400mg. The final product then undergoes sterile filtration, aseptic filling into vials, stoppering and finally capping.1
Assays for releasing and characterizing the final product
All monoclonal antibodies require extensive characterization to gain approval to move to the clinical trial stage and ultimately gain a marketing authorisation.35 In 2009 the European Medicines Agency (EMA) published guidelines stating that analysis should be performed to ascertain the structural characterization including analysis of the physicochemical properties, biologic activity and immunochemical properties of the biologic drug substance seeking approval.36 The purity of the drug substance is also assessed along with an analysis of the process related and product related impurities which can occur either as part of the biologic manufacturing or storage process.
Characterization of the TCZ drug substance
The amino acid sequence of TCZ was identified and from this the primary, secondary and tertiary structure of TCZ was analyzed to establish how structurally comparable the antibody was to a molecule of human IgG11. This analysis demonstrated that the disufide linkages and the types and amounts of monosaccharaides identified match what was known to occur in an IgG1 molecule.1 The oligosaccharide composition was also analyzed. It was established that the major glycostructures consisted of core-fucosylated biantennary complex-type oligosaccharide structures with different amounts of terminal galactosylation.1 It was also established that as well as the major glycostructures, TCZ contained low levels of high mannose type and afucosylated structures. The presence of several isoforms was revealed using ion exchange chromatography. These isoforms were found to mainly occur as a result of heterogeneity at the C- and N- terminal of the heavy chain on the TCZ molecule but also as a result of incomplete cleavage of the signal sequence form the N terminus on the light chain. Analysis was also conducted to detect charge based isoforms and to investigate the structural integrity of TCZ. The size distribution of the TCZ molecule was analyzed using size exclusion chromatography from which 2 peaks were detected which were found to represent the monomer and the diamer of the TCZ molecule.1
A cell-based bioassay was conducted in which both TCZ and IL-6 were added to cells. This was to allow them to compete for both the membrane bound and soluble interleukin 6 receptors and subsequently analyze the growth inhibiting activity and binding activities of TCZ. The data from these studies conducted In vitro confirmed that TCZ had minimal or no compliment dependent cytotoxicity or antibody-dependant-cellular cytotoxicity.1
Product related substances were detected by ion exchange chromatography corresponding to the isoform peaks and size exclusion chromatography corresponding to the diamer and degradation peaks observed in the TCZ drug substance.1 Other impurities detected included cell substrate derived (DNA, host-cell proteins) downstream derived (leached protein A) and other impurities such as endotoxin or bioburden.1
The risk of contamination with adventitious agents during production was minimised by only using fish or milk derived raw materials during the fermentation process.1 Viral screening revealed the presence of intracellular type A and C retroviral particles however this was considered acceptable as such particles are known to be present in CHO cells and there was sufficient capacity in the production process for reduction of these particles.1 Viral safety was demonstrated and the purification process included several steps for inactivation/removal of enveloped viruses.1
Analysis of the final TCZ drug product
While it is recognized that absolute replication of a biologic drug product like tocilizumab is impossible, strict process controls are put in place to ensure pharmacopoeial and non pharmacopoeial methods demonstrate lot to lot consistency with respect to the purity, quantity, potency and identity.
While the analysis of the drug substance looks at characterizing the structure and assessing the heterogeneity profile with respect to both the drug substance and impurities, analysis and final release testing of the final formulated drug product centers around ensuring that the batch produced does not significantly deviate from the initial product used in pre-clinical and clinical studies. The drug product is analyzed throughout the manufacturing process to ensure that it conforms to certain acceptance criteria. These tests typically involve pharmacopoeial analysis of sterility, microbial limits, particulate matter, uniformity of dosing limits and volume in the container. Other tests are conducted including a description of the appearance of the final product, qualitative analysis of the identity, analysis of the purity and impurities, potency and quantity produced.35
The TCZ EPAR documents that the manufacturing process and in process controls were adequately detailed and the limits applied were deemed acceptable. Structural, physicochemical and biologic analysis were conducted before and after each new generation of the manufacturing process. The process controls for the sterile filtration and aseptic filling of the reconstituted product were well documented and deemed robust enough to enable lot to lot consistency. All manufacturing processes involved in the production of the TCZ drug product were demonstrated to comply with good manufacturing practice and the consistency of the final drug product was demonstrated in both small scale and large scale batches. Both real time and accelerated stability testing conducted on the final drug product based on ICH guidelines enabled it to receive a 30 month shelf life when stored between temperatures of 2–8°C.1
Regulatory issues
In Europe all biotechnology derived active substances are approved centrally by the EMA or more specifically by the Committee for Medicinal Products for Human Use (CHMP). Once a marketing authorisation is granted by the CHMP the biologic medication can be adopted as approved by all EU member states. To obtain a marketing authorisation the company responsible for manufacturing the biopharmaceutical must provide documented evidence to the CHMP, which forms the basis of an assessment report, or EPAR. Table 1 contains an overview of the information contained within the EPAR document for RoActemra.
Table 1.
Overview and summary of the contents of the tocilizumab European Public Assessment Report (EPAR).
| Section | Content |
|---|---|
| Quality Aspects | • Description of the active substance, physicochemical structure, molecular weight and a description of where the product is manufactured formulated and packaged. |
| • Description of the process followed to produce a master cell bank and a working cell bank, fermentation process used to expand the cell mass and the process undertaken to purify the expanded cells to ensure homogeneity. | |
| • Description of the manufacturing process including both small and large scale process evaluation studies to ensure that each step of the manufacturing process is appropriately designed to produce tocilizumab. | |
| • Description of the process verification studies to ensure that the manufacturing process is able to effectively and reproducibly produce both intermediate compounds and the final product in multiple consecutive batches. | |
| • A description on how the final product is characterized to elicit the physicochemical and biologic characteristics and also to determine the presence of any impurities or any adventitious agents. | |
| • A description of the stability profile of both the monoclonal antibody alone and the final formulated injection. | |
| Non-clinical aspects | • Description of the in vitro and in vivo animal based non-clinical studies performed to determine the safety and toxicology, pharmacodynamic and pharmacokinetic profiles of tocilizumab. |
| Clinical aspects | • Description of human studies and analyses conducted to elicit the pharmacodynamic and pharmacokinetic profile of tocilizumab. |
| • Description of the pivotal human studies conducted to establish the efficacy and side effect profile of tocilizumab in patients with RA, sJIA and pJIA. | |
| Pharmacovigilance | • Description of all measures to be taken to maintain appropriate future pharmacovigilance to monitor each adverse event identified in clinical studies. |
| Overall conclusions | • Analysis of overall risks and benefits associated with tocilizumab use |
| • Summary of findings from previous sections and overall recommendation regarding the granting of a marketing authorisation |
The EMA dictates that once a biologic product has obtained a marketing authorization, any variations to this marketing authorization must be classified and their effect on the final product analyzed in accordance with EU regulations. Variations range from high risk variations like a change in the manufacturing process to low risk variations such as change of the marketing authorisation holders address. Higher risk category variations carry a greater chance of affecting either the physicochemical or biochemical characteristics of the final product which in turn may affect its efficacy, safety or immunogenicity profile. The EMA mandates the undertaking of a comparability exercise to determine whether the biologic product pre and post variation are highly similar. The actual tests undertaken and level of evidence required varies based on the risk category of the variation with the higher risk variations requiring more evidence of comparability than the lower risk variations. The combined results of these tests should aim to prove that the variation has no adverse impact on the efficacy, safety and immunogenicity profiles of the biologic product in question.37
A review by Vezér et al published in 2016 reported that since its original approval TCZ has undergone 7 manufacturing changes overall.38 These have comprised of 1 high risk change, 4 moderate risk changes and 2 low risk changes and none of these variations have led to the marketing authorization being withdrawn.38 Since the original EU approval of TCZ, Roche has submitted 6 new EPARs to gain further marketing authorisations for the use of TCZ within the EU. The timeline of these authorisations has been summarised in Table 2.
Table 2.
Overview of all the marketing authorisations granted for tocilizumab.
| Date | Details of marketing authorisation granted |
|---|---|
| November 2008 | Treatment of rheumatoid arthritis either as a first line biologic or after the failure of a TNF inhibitor with or without methotrexate |
| April 2010 | RA marketing authorization terms modified to state that tocilizumab improves physical function and slows the X-ray progression of joint damage when taken with methotrexate. |
| May 2011 | Treatment of the active systemic form of Juvenile idiopathic arthritis (sJIA) in patients 2 y or older who have not responded to previous therapy with NSAIDs or steroids |
| April 2013 | Treatment of polyarticular juvenile idiopathic arthritis (pJIA) |
| December 2013 | New subcutaneous formulation of RoActemra for use in the same indications already approved for intravenous use |
| February 2014 | Following syringe mechanical problems, marketing authorisation for use of the subcutaneous formulation of RoActemra was reinstated in the same indications already approved for intravenous use |
| July 2014 | Treatment of patients with severe, active and progressive RA not previously treated with methotrexate. |
Pre-clinical and clinical studies
Pre-clinical efficacy studies
In preclinical pharmacodynamic studies TCZ was shown to specifically block both membrane bound and soluble IL-6R with an equal affinity.1 TCZ binding to IL-6R has been shown to be dose dependent and it has the ability to displace IL6 already bound to IL6 receptors. In vitro studies have demonstrated that TCZ has no inhibitory effect on TNF-α, IL-1β, IL-15 or IL-21.
Cynomolgus monkeys were chosen as the most pharmacologically relevant species test bed for TCZ as it was shown both In vitro and In vivo that TCZ cross reacts with monkey IL-6R.1 In cynomolgus monkeys with collagen-induced arthritis it was demonstrated that TCZ prevents inflammation both locally in joints and systemically.1 It was also demonstrated that inhibition of IL-6 causes normalization of the inflammation driven osteoclastic bone destruction and that normal bone homeostasis returns with the continuous IL-6 inhibition caused by TCZ.1
Pre-clinical toxicity studies
The overall toxicity profile of TCZ in pre-human testing was established using single and multiple dose studies in cynomolgus monkeys and the rodent analog MR16–1, up to a duration of 6 months.1 TCZ was shown to be well tolerated in single IV doses up to 100mg/kg and in multiple IV doses up to 50mg/kg/day for 4 weeks and doses up to 100mg/kg/week for 6 months in cynomolgus monkeys.1 It should be noted that the minimum systemic plasma steady-state concentration of TCZ in these monkeys was 8 to 10 times greater than that seen in any human clinical trials.1
The immunomodulatory consequences of IL-6R inhibition was studied using MR16–1 mice where it was shown that IL6 inhibition does not affect primary response of antibodies to a T-cell dependent antigen. The delayed type hypersensitivity reaction was reduced when IL6 was inhibited during initiation phase suggesting that inhibition of IL-6R affected T-cell priming rather than T-cell differentiation, the development of T-cell memory or T helper cell activity.1
Human TCZ was observed to be immunogenic in cynomolgus monkey studies due to differences in the polypeptide structure of both the heavy and light chains between human and cynomolgus monkeys. In these studies an inverse dose response was seen with respect to the presence of anti-TCZ antibodies, a phenomenon common to molecules of this type.
There were observed changes in absolute neutrophil count (ANC) in both the 2-week and 4 week daily treatment toxicity studies in cynomolgus monkeys.1 In these studies there was no bone marrow myeloid hypo or hyperplasia which along with the lack of neutrophil morphological changes suggests that incomplete granulopoiesis or peripheral sequestration is responsible for the low neutrophil levels seen.1
In vivo studies in cynomolgus monkeys have demonstrated that TCZ had no effect on electrophysiological performance, blood pressure, cardiac tissue integrity or pro-thrombotic activity in IV doses up to 50mg/kg1. Safety studies demonstrate that continuous inhibition of IL-6 does not affect normal bone homeostasis.1
Pre-clinical studies have demonstrated conflicting evidence on what effect TCZ can have on the liver. In some studies mild to moderate elevations of hepatic transaminases have been seen post TCZ treatment.1 Although there is no evidence that this progresses to serious hepatic injury, transaminase elevation was more common with concomitant use of other hepatotoxic drugs.1 Studies on cynomolgus monkeys with induced inflammation treated with TCZ did not observe the development of any raised hepatic transaminases.1
No specific studies into carcinogenicity with TCZ use were performed, as IgG1 monoclonal antibodies were not deemed to have carcinogenic potential.39 All available non-clinical data from 6-month toxicity studies in cynomolgus monkeys and IL6 knockout mice do not suggest any carcinogenic risk associated with TCZ.39 Studies in cynomolgus monkeys demonstrated that foetal plasma concentrations of TCZ were 39% and 60% of maternal plasma concentrations after doses of 10 mg and 50mg/kg/day respectively.1 This suggests that TCZ is able to cross the placental barrier, due to its IgG structure.1 Despite this the data from non-clinical studies does not suggest that there is an effect on fertility in cynomolgus monkeys.1 There were no effects on the endocrine or reproductive organsand there is no preclinical evidence that IL-6 signaling is in any way involved in the reproductive process.1 Studies in IL-6 deficient mice have not demonstrated any alteration in reproductive performanceand neither IL-6 knockout mice nor monkeys exposed to TCZ for more than 6 months have demonstrated any morphological alterations in either their primary or secondary tissues of the immune system or any other organs or tissues.1 The only study that demonstrated a slight increase in abortion/embryo-foetal deaths in cynomolgus monkeys exposed them to greater than 100 times the maximal dose of TCZ used in human studies.1
Studies of Human Pharmacokinetics
Studies in human subjects have demonstrated that TCZ has a non-linear pharmacokinetic profile.39 As the dose is increased the maximum concentration of TCZ in the plasma (Cmax) increases proportionally however, the area under the curve (AUC) increases disproportionately.40 Analysis of the TCZ dose response curves showed that they flattened with increasing exposure and because of this doses greater than 800 mg are not recommended due to a lack of increase in clinical efficacy.5
Analyses conducted with TCZ in human studies have not shown gender, age, ethnicity, mild renal impairment, treatment with methotrexate, treatment with NSAIDs or treatment with corticosteroids to have an effect on the pharmacokinetics of TCZ.40,5 There have not been studies conducted on the pharmacokinetics of TCZ in patients with moderate to severe renal impairment or in patients with hepatic impairment of any form.5
Chromatographic studies have shown that TCZ mainly exists in the plasma unchanged. In vitro studies of hepatocytes have shown that TCZ inhibits the down regulation of the cytochrome P450 enzyme (CYP) system caused by IL61. The enzymes in which expression is inhibited by IL6 include CYP1A2, CYP2C9, CYP2C19 and CYP3A45. The relevance of this finding is unclear as this down regulation has only been demonstrated at very high concentrations. However, it would be prudent to consider this interaction in the presence of another drug metabolised by these enzymes. Particular care should be exercised with concomitant medication that has a narrow therapeutic index such as theophylline, warfarin, phenprocoumon, phenytoin or ciclosporin.5 It should also be noted that due to the long half-life of TCZ monitoring of this interaction may be necessary for 1 to 2 months after the TCZ is discontinued.5
The elimination of TCZ has been shown to be relatively slow and concentration dependent. After saturation of IL6 receptors, clearance medicated by the mononuclear phagocyte system becomes linear. Increases in the dose have been shown to lead to prolongation of the half-life suggesting that the elimination of TCZ is capacity limited.40
Efficacy from pivotal studies
Intravenous (IV) TCZ, together with Methotrexate (MTX) is recommended for the treatment of RA in adult patients, who are inadequate responders to previous therapy with DMARDs or a TNF∝ inhibitor and in whom rituximab is contraindicated or if the patient has had a previous adverse event (AE) to rituximab.5 Furthermore, it is indicated in patients who are inadequate responders to one or more TNF inhibitors and to rituximab.5 The evidence for is use within this population has come from the RADIATE,41 OPTION,42 AMBITION,43 LITHE44 and TOWARD45 trials (Table 3). TCZ was first developed as an IV infusion however in October 2013 the US FDA approved a subcutaneous (SC) formulation of TCZ for use in RA. The data used for the approval of TCZ SC, came from 2 phase III clinical trials, SUMMACTA46 and BREVACTA47 (Table 3). Finally, TCZ was given a marketing authorisation for use in systemic juvenile idiopathic arthritis (sJIA) in 2011 and polyarticular juvenile idiopathic arthritis (pJIA) in 2013 using data from the TENDER48 and CHERISH49 studies respectively (Table 4).
Table 3.
Description and results from the pivotal clinical trials involving tocilizumab use in patients with RA.
| Study | Sample Size (n) | Design | Endpoint | Posology | Results |
|---|---|---|---|---|---|
| AMBITION | 673 | Randomized double blinded placebo controlled | ACR20 response at week 24 | Group 1 | Group 1 |
| IV TCZ (8mg/kg) every 4 weeks | • 70% ACR20 response | ||||
| Inclusion criteria: moderate or severe RA | • DAS28 reduced by 3.31 | ||||
| • Patients 5 times more likely than Group 2 to achieve remission | |||||
| Exclusion criteria: previously failed MTX or biologics | |||||
| Group 2 | Group 2 | ||||
| MTX 7.5–20 mg every week | • 52% ACR20 response | ||||
| • DAS28 reduced by 2.05 | |||||
| LITHE | 1196 | Randomized double blinded placebo controlled | Genant-modified Sharp score, | Group 1 | Group 1 |
| Inclusion criteria: RA <5 years, inadequate response to MTX and no previous biologics | IV TCZ (4mg/kg) every 4 weeks & MTX | • 56% ACR20 response | |||
| HAQI | • 74% structural damage reduction | ||||
| • >0.3 UI HAQI improvement | |||||
| ACR20 response at week 52 | Group 2 | Group 2 | |||
| IV TCZ (8mg/kg) | • 51% ACR20 response | ||||
| every 4 weeks & MTX | • 70% structural damage reduction | ||||
| Group 3 | Group 3 | ||||
| Placebo & MTX | • Twenty-seven% ACR20 response | ||||
| OPTION | 623 | Randomized double blinded parallel group | ACR20 response at week 24 | Group 1 | Group 1 |
| Inclusion criteria: moderate or severe RA, inadequate response to MTX | IV TCZ (4mg/kg) every 4 weeks & MTX | • 59% ACR20 response | |||
| • Twenty-seven% DAS28 remission | |||||
| Group 2 | Group 2 | ||||
| IV TCZ (8mg/kg) every 4 weeks & MTX | • 48% ACR20 response | ||||
| • Thirteen% DAS28 remission | |||||
| Group 3 | Group 3 | ||||
| Placebo & MTX | • Twenty-six% ACR20 response | ||||
| • 0.8% DAS28 remission | |||||
| TOWARD | 1220 | Randomized (in a 2:1 manner) double blinded placebo controlled | ACR20, ACR50, ACR70 response at week 24 | Group 1 | Group 1 |
| Conducted in multiple centers / countries | IV TCZ (4mg/kg) every 4 weeks and conventional DMARDs | • 61% ACR20 response | |||
| Inclusion criteria: moderate or severe RA, multiple DMARDs | • Thirty% DAS28 remission | ||||
| Group 2 | Group 2 | ||||
| Placebo and conventional DMARDs | • Twenty-five% ACR20 response | ||||
| • Three% DAS28 remission | |||||
| RADIATE | 499 | Double blinded placebo controlled | ACR20 response at week 24 | Group 1 | Group 1 |
| Inclusion criteria: RA refractory to anti-TNF therapy | IV TCZ (4mg/kg) every 4 weeks & MTX | • Thirty.4% ACR20 response | |||
| • Seven.6% DAS28 remission | |||||
| Group 2 | Group 2 | ||||
| IV TCZ (8mg/kg) every 4 weeks & MTX | • 50% ACR20 response | ||||
| • Thirty.1% DAS28 remission | |||||
| Group 3 | Group 3 | ||||
| Placebo & MTX | • Ten.1% ACR20 response | ||||
| • One.6% DAS28 remission | |||||
| ADACTA | 325 | Randomized double blinded parallel group phase 4 | DAS28, ACR20, ACR50 and ACR70 response at week 24 | Group 1 | Group 1 |
| Inclusion criteria: intolerant or inappropriate to be on MTX | IV TCZ (8mg/kg) every 4 weeks and placebo | • 39.9% DAS28 remission | |||
| • DAS28 reduced by 3.3 | |||||
| Group 2 | Group 2 | ||||
| Adalimumab 40 mg every 2 weeks and placebo | • Ten.5% DAS28 remission | ||||
| • DAS28 reduced by 1.8 | |||||
| FUNCTION | 1162 | Double blinded placebo controlled | DAS28 remission at week 24 | Group 1 | Group 1 |
| Inclusion criteria: early progressive RA, MTX naive | IV TCZ 4mg/kg every 4 weeks & MTX | • 40% DAS28 remission | |||
| Evaluation of radiographic and physical function outcomes at week 52 | Group 2 | Group 2 | |||
| IV TCZ 8 mg/kg every 4 weeks & MTX | • 45% DAS28 remission | ||||
| • 0.08 Van de Heijde-modified total Sharp score | |||||
| • 0.81 UI reduction in HAQI | |||||
| Group 3 | Group 3 | ||||
| IV TCZ 8 mg/kg every 4 weeks and placebo | • 39% DAS28 remission | ||||
| Group 4 | Group 4 | ||||
| Placebo & MTX | • Fifteen% DAS28 remission | ||||
| • One.14 Van de Heijde-modified total Sharp score | |||||
| • 0.64 UI reduction in HAQI | |||||
| SUMMACTA | 1262 | Randomized, double-blind, active controlled, parallel group, multicentre study | ACR20 response at week 24 | Group 1 | Group 1 |
| TCZ-SC 162 mg weekly + placebo-IV every 4 weeks | 69% ACR20 response | ||||
| Patients with moderate to severely active RA on concomitant MTX treatment | |||||
| Group 2 | Group 2 | ||||
| TCZ-IV 8mg/kg every 4 weeks + placebo SC weekly | 73% ACR 20 response | ||||
| Similar safety profile observed between the 2 groups, apart from more injection site reactions with TCZ-SC. | |||||
| BREVACTA | 656 | Randomized, double-blind, placebo controlled, parallel group study | ACR20 response at week 24 | Group 1 | Group 1 |
| TCZ-SC 162 mg fortnightly + DMARDs | 61% ACR20 response | ||||
| Group 2 | Group 2 | ||||
| Placebo-SC fortnightly + DMARDs | 32% ACR 20 response | ||||
| (escape therapy with TCZ-SC 162 mg offered from week 12 if inadequate responders) | Significantly less structural joint damage progression demonstrated in the TCZ-SC fortnightly plus DMARDs group as compared with the placebo plus DMARDs group. |
Table 4.
Description and results from the pivotal clinical trials involving tocilizumab use in patients with sJIA and pJIA.
| Study | Sample Size (n) | Design | Endpoint | Posology | Results |
|---|---|---|---|---|---|
| CHERISH | 188 | Randomized double blinded placebo controlled withdrawal 3-part study with 16 weeks open lead-in phase and 64 weeks open label follow up | % ACR30 flare in Part II of the study | Part I | |
| IV TCZ 8 or 10 mg/kg every 4 weeks for 16 weeks (n = 188) | |||||
| Inclusive criteria: active pJIA with inadequate response or intolerant to MTX | Part II | 25.6% in TCZ group, 48.1 % in placebo group | |||
| IV TCZ 8 or 10 mg/kg every 4 weeks for 16 weeks (n = 82) | |||||
| OR | |||||
| Placebo every 4 weeks for 16 weeks (n = 84) | |||||
| Part III | |||||
| SC TCZ 162 mg every 4 weeks for up to 64 weeks (n = 160) | |||||
| TENDER | 112 | Part I | Part I | Part I | Part I |
| Randomized double blinded placebo controlled parallel group 2-arm study for 12 weeks | % of participants with ≥ 30% improvement in JIA ACR and absence of fever | IV TCZ (8mg/kg) every 2 weeks (n = 37) | 85.3 and 24% for IV TCZ and placebo groups, respectively | ||
| Inclusive criteria: active pJIA | OR | ||||
| IV TCZ (12mg/kg) every 2 weeks (n = 38) | |||||
| OR | |||||
| IV Placebo every 2 weeks (n = 37) | |||||
| Part II | Part II | Part II | Part II | ||
| Single arm open-label extension study for 92weeks | % of participants with decrease dose of oral corticosteroids at week 104 | IV TCZ (8mg/kg) every 2 weeks (n = 52) | • 76% with ≥ 20% decrease | ||
| OR | • 73% with ≥ 50% decrease | ||||
| IV TCZ (12mg/kg) every 2 weeks (n = 40) | • 62% with ≥ 75% decrease | ||||
| OR | • 47% with ≥ 90% decrease | ||||
| TCZ switchers (n = 20) | |||||
| Part III | Part III | Part III | Part III | ||
| Open label continuation study for 3 y | Long term safety from week 104 to week 260 | IV TCZ 8 mg/kg every 2 weeks for up to 240 weeks (n = 53) | ACR responses at week 104 | ||
| OR | • ACR30: 100% | ||||
| Long term efficacy (JIA ACR30,50,70 and 90) | IV TCZ 12 mg/kg every 2 weeks for up to 240 weeks (n = 59) | • ACR50: 100% | |||
| OR | • ACR70: 94.4% | ||||
| IV TCZ 8 or 12 mg/kg every 2 weeks for up to 156 weeks (n = 112) | • ACR90: 76.4%. | ||||
| ACR responses at week 260 | |||||
| • ACR30 96.7% | |||||
| • ACR50 93.3% | |||||
| • ACR70 90% | |||||
| • ACR90 63.3% |
Long-term efficacy and safety
The major considerations when using TCZ are its immunosuppressive effects, which lead to increased risk of infection and its negative influence on lipid profile. The 5-year extension STREAM study shows that TCZ has a sustained long-term efficacy and good safety profile.50 Another study shows that a tendency toward a deteriorating lipid profile the first 3 months of staring TCZ is evident but there was no statically significant difference in lipid profile in the long run.51 A 52-week study of TCZ in sJIA patients, from 2 to 17 y old, reported 33 serious adverse effects in 25 patients, 12 were considered related to TCZ; all of those resolved and none led to discontinuation from the study.52 Another 3 and 4.6 y follow up study respectively concluded that the safety profile of TCZ was consistent over time in patients treated for RA.53,54 RA has been associated with an increased risk for cardiovascular (CV) diseases despite the fact that high levels IL-6 are associated with lower cholesterol levels. Reducing the inflammatory burden of RA by reducing the levels of C reactive protein (CRP) and IL-6 may reduce CV risk in RA patients. As TCZ may suppress the effect of IL-6 all patients should have a baseline fasting lipid profile and repeat fasting lipid profile at least 3 months after initiation of the treatment.30
As described previously no dosage recommendations are provided in hepatic or renal impairment.5 Recommendations for dealing with hepatotoxicity, nephrotoxicity or neutropenia during treatment are detailed in Table 5. The safety and efficacy of TCZ has not been established in children below 2 y of age. The only absolute contraindication of TCZ is hypersensitivity to TCZ or any component included in the formulation of TCZ. No live vaccines should be administered as no data are available regarding patients receiving TCZ IV or SC with or without DMARDs.5
Table 5.
Description of the most common side effects associated with tocilizumab use and how to manage them.
| Side effects | Action |
|---|---|
| Skin/soft tissue infections | • Antibiotic therapy |
| • Omit infusion for 4 weeks and the restart when infection resolved | |
| Liver function tests | • Monitor Monthly for first 6 months |
| • No action if < 3 times Upper limit of normal range (ULN) – Consider modifying MTX dose | |
| • Omit for 4 weeks if 3–5 times the ULN | |
| • Cease if > 5 times the ULN | |
| Hypercholesterolemia | • Baseline fasting lipid profile and monitor 2 to 3 times in first 6 months, then annually. |
| • Treatment with statins if elevated according to local guidelines | |
| Neutropenia | • Monitor monthly for first 6 months |
| • No action required if greater > 1 × 109/l | |
| • Omit for 4 weeks if 0.5–1 × 109/l | |
| • Cease if < 0.5 × 109/l | |
| Anaphylaxis | • Monitor carefully in first 6 infusions |
| • Do not re-challenge if patient could not complete prior infusion |
Adverse effects
Adverse effects (AEs) have been documented for TCZ monotherapy or in combination with other DMARDs.5 Safety data are not available regarding combination with other biologic therapy.5 The most common AEs reported were infections.5 Skin infections were more common in TCZ group in the AMBITION trial.43 Five serious infections were reported in TOWARD trial: staphylococcus cellulitis, acute pyelonephritis and sepsis in the TCZ group and 2 cases of pneumonia in the control group.45 Infection rates from phase 3 trials (OPTION, TOWARD, LITHE and RADIATE) where patients received both DMARDs and TCZ or placebo, showed no evidence of increased risk with continued TCZ therapy.41,42,44,45 The TENDER trial reports 4 serious AE in the TCZ arm of the study in 3 patients and this included angioedema and urticaria (one), varicella (one), and bacterial arthritis.48
Gastrointestinal disorders included nausea; abdominal pain, mouth ulceration and gastritis were the second most common AEs. Perforations are also reported mostly in patients who have been diagnosed with diverticular disease before. One patient reported to have died in AMBITION trial in the TCZ group because of upper gastrointestinal hemorrhage and perforation.43
Grade 3 or 4 neutropenia occurred in TOWARD, OPTION, AMBITION, RADIATE and LITHE trials in the TCZ groups.41-45 Neutropenia was noted to be transient. A meta-analysis of 6 trials, found that grade 3/4 neutropenia occurred at least once in 6% of patients.55 Elevations in liver enzymes levels are also reported in the TCZ group and rise in total cholesterol and LDL cholesterol reported to TCZ group as compared with patients in the MTX group. Other events occurring more frequently in the TCZ group included headache and hypertension.45
Concerning trials, which included the SC use of TCZ, the SUMMACTA trial in terms of AEs showed a similar safety profile compared with IV TCZ, apart from more injection site reactions with TCZ-SC.46 Infection was the most common AE and serious AE in both groups. In the BREVACTA trial, 6.4% in each group experienced an upper respiratory tract infection, which appeared to be the most common AE.47 Only 1 patient in the TCZ-SC group developed grade 1 hepatic steatosis.47
It is critically important that all healthcare professionals remain responsible for ensuring appropriate pharmacovigilance of adverse drug reactions (ADRs). The appropriate national regulators should always be informed of ADRs at the earliest available opportunity and healthcare professionals should be responsible for including as much detail as possible in their reports to aid in their overall assessment and classification. Because of the likely structural batch to batch variation with biologic medicinal products it is critically important to include details of the brand name and batch number of the medication. The results of one Dutch study have suggested despite this being an EU policy in reality this information is rarely recorded.56 To facilitate this, appropriate systems must be in place to record both the brand names and batch numbers of all biologics administered to all patients.
Product availability and dosing schedule
TCZ is available both as an IV infusion and as a subcutaneous injection.2
As an intravenous infusion it is formulated as a clear to opalescent, colorless to pale yellow 20mg/ml solution for injection of which there are 3 vials available, 400mg/20mL, 200mg/10 mL and 80mg/4mL1. In moderate to severe RA, IV TCZ is indicated once every 4 weeks at a dose of 8mg/kg in UK and 4mg/kg increasing to up to 8mg/kg based on patient response in the USA.5,57 For individuals whose body weight is more than 100 kg, doses exceeding 800 mg per infusion are not recommended.5 In the pediatric population, the recommended dose in sJIA and pJIA, in patients above 2 y of age, is 8mg/kg IV once every 2 weeks when their weight is greater than or equal to 30kg. In patients weighting less than 30kg, 12mg/kg IV and 10mg/kg IV once every 2 weeks is recommended in sJIA and pJIA, patient's respectively.5 Prior to reconstitution the vials must be stored in a refrigerator at 2°C–8°C and should be kept in the carton to protect the biologic drug from exposure to light.5 The summary of product characteristics states that in adults with RA and children with sJIA or pJIA with a weight greater than or equal to 30 kg the requisite dose of TCZ must be diluted to 100 mL with sodium chloride 0.9%.3 For children with sJIA or pJIA who weigh less than 30 kg the requisite dose should be diluted in 50 mL sodium chloride 0.9%.5 In all cases of infusion preparation, the bag should be gently inverted to mix the solution while avoiding foaming.5 The shelf life of the unopened vial of TCZ is 30 months however after dilution the SmPC states that the prepared solution is physically and chemically stable at 30°C for 24 hours.5 Despite this the SmPC advises that from a microbiological point of view the infusion should be administered as soon as possible after preparation and the bag should not be kept for longer than 24 hours at 2°C–8°C.5
The subcutaneous formulation of TCZ is ready prepared as a clear to yellow liquid formulated as a 162 mg dose in 0.9 mL and is delivered via a prefilled syringe.58 The recommended posology of SC administration is 162 mg once every week.58
There is limited information available regarding switching from one formulation to another. Oqata et al evaluated the efficacy and safety of switching from intravenous to subcutaneous TCZ monotherapy in RA.59 In this study disease control of RA was maintained without serious safety concerns, however it did note that the efficacy may be reduced in patients with heavier body weights.59 These concerns were reflected in a similar study conducted by Iwamoto et al.60 On the other hand, Burmester et al, following the SUMMACTA study demonstrated comparable long-term efficacy and safety of TCZ SC compared with IV TCZ.46
Advantages and disadvantages of tocilizumab relative to other products available to treat the same disease
A number of biologic monoclonal antibodies targeting different inflammatory cytokines are available to treat RA. These include the TNFα inhibitors infliximab (INX), adalimumab (ADA), etanercept (ETA), golimumab (GOL) and certolizumab pegol (CTL), the B cell depleting agent rituximab (RTX), IL-1 inhibitor anakinra (ANK) and finally the T cell inhibitor abatacept (ABC). Currently the EMA have approved all of the above agents to be used to treat RA in adults however only ETA, TCZ, ABC, ADA and canakinumab are licensed for use in JIA.61-69 Based on this selection of options available it is important to consider which biologic is the most appropriate for a patient based on comparisons of the relative efficacy profiles, safety profiles, costs and also individual patient factors.
Structural differences and immunogenicity
The relative structural differences in biologics available to treat RA and the relative differences in the genetic technology used to produce them gives rise to different pharmacokinetic, pharmacodynamic and immunogenic profiles. Over the last 40 y recombinant DNA technology has progressed significantly from the development of murine monoclonal antibodies using hybridomas through to the chimeric, humanized and finally fully human therapeutic monoclonal antibodies we see today.
Initially developed murine based antibodies were shown to be very poorly tolerated in humans due to the increased likelihood of immune reactions post treatment and a poor efficacy profile.70 One study looking at the relative immunogenicity of monoclonal antibodies developed between 1984 and 2003 acknowledged the difficulties in drawing specific conclusions from direct comparisons of monoclonal antibodies. It was reported that broadly speaking, chimeric monoclonal antibodies such as INX or RTX with an antibody structure composed of a human constant region and a murine variable region demonstrated significant reduction in immunogenicity when compared with their murine counterparts.71 It was also suggested that further humanization of the antibody variable regions leads to a subsequent further decrease in immunogenicity, however they were unable to comment on the immunogenicity profiles of fully human monoclonal antibodies.71 As TCZ is a humanized immunoglobulin it is also much less immunogenic than the chimeric alternatives. Results from the ADACTA study did not show any statistical differences in hypersensitivity reactions when comparing subcutaneous TCZ with SC ADA and no anaphylaxis was reported in either group.72
While structure has a significant role in the immunogenicity profile of a monoclonal antibody it can also lead to other changes in the pharmacokinetic profile. The best example of this is CTL which has a somewhat unique structural difference when compared with the other monoclonal antibodies including TCZ. CTL is a humanized FAb fragment conjugated to polyethylene glycol.65 In this molecule the conjugation of polyethylene glycol to the antibody fragment increases the size and aqueous solubility of the molecule leading to increases in plasma half-life and a subsequent reduction in the frequency of dosing required.73 The absence of an fc binding region in CTL reduces antibody and compliment dependant cellular toxicity and also prevents the drug from crossing the placenta.74 This structural difference and several small scale studies and case reports have led the British Society of Rheumatology to advise that CTL is the only TNFα inhibitor that can be used in all 3 trimesters of pregnancy.75
Patient acceptability
Biological monoclonal antibodies are available as either IV infusions or as SC injections via a syringe or a pre filled pen device. INX and RTX are only available as IV infusions whereas ADA, ETA, GOL and CTZ are only available as SC injections.61-66 TCZ and ABA are available as both IV infusions and SC injections.5,68,76,77
In the UK, IV biologics are generally administered on hospital infusion units and SC biologics are normally delivered to patients direct to their homes via a homecare provider. Homecare delivery in the most cases is very convenient for patients as their medication is delivered direct to their home. Homecare is also generally a cheaper alternative to IV infusions as the patient does not have to make use of an infusion unit with the associated costs of the staff running it. Infusion units also have a finite number of patients they can infuse in a week. Because of this, clinicians often view SC injections as a more favorable option if the patient is eligible. However, using the subcutaneous injections does mean that adequate training and support must be provided to the patient to ensure the correct injection technique.
Certain patients may not be eligible for SC injections. Patients who are considered to be either intentionally or un-intentionally non-compliant to their treatment regimens may be considered for IV infusion options over SC injections. From an efficacy view point, IV infusions administered on a hospital infusion unit ensure that the biologic is administered at a dose and frequency in line with the clinician directions. This subsequently ensures the maximum possibility that the biologic treatment will be effective and that no biologic medication is wasted. Wastage can occur both intentionally or unintentionally. One way is by either an intentional or unintentionally non-compliant patient stockpiling medication which they haven't injected without informing their clinician. The reasons for doing this can include fear of reprisal, not understanding the economic and health impact of missing doses, not being able to remember when to inject, not having the manual dexterity to inject themselves, lacking the understanding of how the biologic should be stored or forgetting where it was stored when the time comes to inject.
Of the subcutaneous formulations that exist there is also a variation in how often they must be injected. Patients who are needle phobic or are already having SC injection with other drugs may prefer a biologic that is injected less frequently. The most frequent maintenance injections are required in patients using ETA (either twice weekly or weekly),63 TCZ and ABArequire weekly injections,76,77 ADA and CTZ require fortnightly injections,62,65 GOL requires monthly injections.64
Work life also will heavily impact a patient's selection about which biologics to use as many patients will not be able to routinely attend clinic for infusions but also may struggle to be present at home to accept routine deliveries of subcutaneous injections from a homecare company. A study conducted by Huynh et al looked into patient treatment preferences and highlighted the perceived benefits patients saw in having either SC injections of biologics or IV infusions. Patients receiving IV infusions preferred this mainly because it was perceived as being more safe and easier to manage whereas patients receiving SC injections cited time constraints and the fact that it was easy to manage. The majority of patients not already treated on a biologic preferred the SC route.78
This highlights the requirement of a clinician to fully assess all the needs of a patient before choosing which biologic will be the most appropriate. Because TCZ exists as both IV and SC preparations it offers the patient choice and flexibility in the event that TCZ is the right clinical choice for the condition.
Economic and commercial considerations
Biologic drugs are expensive. One US based study estimated that the average direct cost to treat a patient with RA increases approximately threefold when patients were treated with an anti TNF monoclonal antibody compared with those treated with DMARDs alone.79 For many years, patient access to these monoclonal antibodies has been prohibited by their high cost. This high cost has led to strict criteria being adopted nationally to ensure that only patients who have the worst disease activity initiate biologics and that continuation is dependent on an adequate response. More and more biologics are being approved to treat inflammatory conditions. These include new biologics with novel molecular targets and biosimilar biologics based on the innovator biologic. Because of this it is crucial that clinicians choose biologics that are most likely to be cost effective; however the cost of a biologic can be complex to accurately assess due to a variety of patient access schemes and potential dosing schedules available.
The introduction of biosimilar monoclonal antibodies has led to increased competition in the biologics market which in time is expected to lead to significant cost savings.80 However, despite the fact that these cheaper alternatives now exist in the market, cost effectiveness should always be the prime consideration when choosing a biologic rather than just cost alone. Cost effectiveness is a complicated issue that encompasses a variety of factors including the cost of a drug, expected efficacy / side effects based on evidence from clinical trials but also factoring in expected compliance issues. Because of this complex multifactorial picture regarding which biologic to choose physicians should engage their patients in the decision making process as much as possible to adopt a treatment strategy which is most likely to be appropriately adhered to and thus successfully treat the patient.78
Comparative safety and tolerability
Some biologics registries have reported the drug survival of a limited number of biologics including TCZ and indicated that TCZ is as well if not better tolerated than TNFα inhibitors.81-83 One large meta-analysis conducted by the Cochrane group demonstrated that patients treated with TCZ had similar rates of serious infections to patients treated with TNF inhibitors excluding INX.84 Tuberculosis reactivation appears to be less common in patients treated with TCZ when compared with patients treated with TNF inhibitors. This is evidenced by the incidences seen in clinical trials of TNF inhibitors compared with the extremely low incidence seen in a worldwide TCZ trials database.85,86 Results from the United Health Care database reported that gastrointestinal perforation is more common in patients treated with TCZ than in patients treated with TNF inhibitors.87 Patients treated with TCZ have also demonstrated a greater incidence of increased fasting levels of plasma lipids including HDL, LDL and total cholesterol however, these increases in HDL and TC have also been seen in patients treated with TNF inhibitors.88,89 This risk factor does not specifically warrant choosing one biologic over another however it does reinforce the need for lipid monitoring before and during biologic treatment.
Efficacy considerations
The efficacy of TCZ when administered with methotrexate has been well established in the pivotal phase 3 clinical trials. However these were placebo or DMARD active comparator controlled studies which prevented the direct comparison of TCZ with other biologic agents. The following summarizes the evidence of direct and indirect studies involving TCZ and other drugs used in the treatment of RA.
Indirect comparisons of the efficacy of TCZ compared with other biologic monoclonal antibodies in treating moderate to severe RA has been evaluated in several systematic reviews. One review of double blind controlled studies comparing the efficacy of ADA, INX, ETA and TCZ in RA patients with an inadequate response to DMARDs noted that TCZ had a similar ACR20 and ACR50 but a superior ACR70 response at 24 to 30 weeks when compared with ADA, INX and ETA.90 These findings are backed up by a further review conducted by Gallego-Galisteo et al which concluded that patients treated with either TCZ or a TNF inhibitor plus methotrexate (MTX) had similar ACR50 responses at week 24 to 30 post initiation of the biologic monoclonal antibody.91 One review which analyzed the response of patients with an inadequate response to TNF inhibitors switched to either TCZ, RTX, ABA or GOL noted no difference in ACR50 response.92 In terms of radiographic damage seen in patients with RA both TCZ and the TNF inhibitors when taken with methotrexate were effective at slowing X-ray progression of joint damage when compared with methotrexate taken alone. However, when taken alone without methotrexate TCZ, ADA and ETA were still found to be significantly better than MTX with TCZ demonstrating the least X-ray progression of joint damage.93
TCZ has been shown to be highly effective when used as monotherapy in RA. TCZ monotherapy was more efficacious than non-biologic DMARDs at slowing joint damage in the Study of Active Controlled Monotherapy Used for RA, an IL-6 inhibitor (SAMURAI) study, even in patients at high risk for structural damage.94Contrary to findings with TNF agents, add-on (TCZ plus MTX) therapy was not superior to TCZ monotherapy in inadequate responders to MTX in the ACT-RAY study; ACR responses, swollen and tender joint counts, DAS28 change from baseline, DAS28 ≤ 3.2 and Genant-modified total Sharp Score were not significantly different between TCZ plus MTX and TCZ monotherapy (p>0.05), though proportions of patients achieving DAS28 <2.6 and patients without radiographic progression were significantly higher with TCZ plus MTX (p<0.05).95These differences in efficacy are unlikely to be due to immunogenicity because the proportions of patients with neutralising antidrug antibodies were similar between monotherapy (4.4%) and combination therapy (3.7%).95
In the ACT-SUREand ACT-STARstudies, which were real-world-type safety studies in patients with active RA despite receiving biologics or DMARDs, comparable improvements in clinical signs and symptoms were observed in patients receiving TCZ monotherapy or TCZ plus DMARDs, although precise reasons for not receiving DMARDs are unknown.96,97 Long-term data from the Safety and Efficacy of TCZ monotherapy, in patients with RA (STREAM study) demonstrated that TCZ monotherapy is not associated with clinically relevant decline in efficacy over time and that ACR response rates and improvements in DAS28 were sustained over 5 y of TCZ monotherapy.50
The efficacy of TCZ compared with anti TNF was reported in the ADACTA study. In the ADACTA phase 4 trial 325 patients were blinded and randomized to receive either TCZ IV (8mg/kg) monotherapy monthly or ADA 40 mg SC monotherapy every 2 weeks. The results demonstrated that at week 24 patients treated with TCZ observed a greater decrease in their DAS28 score than patients treated with ADA alone (−3.3 vs. −1.8; P<0.0001), patients treated with TCZ were more likely to achieve DAS28 remission than patients treated with ADA (39.9% vs. 10.5% respectively) and TCZ also demonstrated favorable ACR20, ACR50 and ACR70 response rates when compared with ADA. These findings demonstrated that TCZ was statistically superior to ADA as monotherapy for reduction of signs and symptoms in RA where MTX was inappropriate.72
Future applications of tocilizumab: Use in giant cell arteritis, polymyalgia rheumatica and large vessel vasculitis
Long-term treatment with high dose glucocorticoids (GC) has been the standard of care for management of GCA, PMR and vasculitis in general. There is however a shift in our approach with increasing knowledge of adverse effects with chronic GC requirement. Inflammatory joint conditions, as described above, have been the forerunner of innovative GC-free therapies in disease management using biologic agents such as TCZ.
Dasgupta and Panayiwere the first to demonstrate elevated serum levels of patients with GCA and PMR, including in some patients with normal acute phase reactants.98 IL-6 protein and RNA have been since demonstrated in tissues and circulation of patients with these conditions and has been shown to mirror disease activity.99 Recent reports of Interleukin-6 blockade with TCZ promise to deliver better outcomes in these diseases.
A single center, Phase 2 randomized control trial by Villiger et al. with 30 newly diagnosed or relapsing GCA patients showed efficacy of TCZ for induction and maintenance of remission. Patients were randomized (2:1) to receive TCZ 8 mg/kg IV every 4 weeks for 52 weeks; or placebo IV every 4 weeks for 52 weeks; all given in combination with prednisone starting at 1 mg/kg per day and tapered to 0 mg according to a standard reduction scheme defined in the study protocol. The primary outcome was the proportion of patients who achieved complete remission of disease at a prednisolone dose of 0·1 mg/kg per day at week 12. At 12 weeks 17 from 20 patients on TCZ and 4 from 10 patients in the placebo group achieved complete remission (85% v 40%; risk difference 45%, 95% CI = 11–79; p = 0·0301). At 52 weeks, 17 in the TCZ group and 2 in placebo group were relapse-free (85% v 20%, risk difference 65%, 95% CI 36–94; p = 0·0010).100
Evans et al. reported on a case series of 8 patients with 18FFDG-PET CT positive refractory LVV, all the patients had a complete response with 6 to 12 infusions of TCZ 8 mg/Kg/month. Five patients had relapse after stopping TCZ with one patient showing revascularization on ultrasound (US) scan after TCZ infusion.101 A retrospective study of 44 patients with refractory TA showed efficacy of TCZ with clinical, biologic and radiological response, as well as steroid-sparing efficacy the parameters for response.8 The TENOR study in 20 patients with GC treatment naïve PMR showed that 3 IV infusions of TCZ as 8 mg/Kg monotherapy was efficacious in inducing remission and reducing GC requirement.102
GiACTA, a large randomized clinical trial (RCT) has assessed SC TCZ 162 mg either weekly or every second week with a 6-month and 12-month prednisolone taper arms n 251 patients with newly diagnosed or relapsed/refractory GCA.9The trial has completed first 12 months follow up and results were presented in form of an abstract at the ACR in Washington 2016F.9 In the primary comparison, 53% and 56% of patients in the 2 TCZ groups achieved sustained GC free remission at 12 months (defined as the absence of flare, normalization of C-reactive protein after week 12 and adherence to the protocol-defined prednisone taper) compared with only 14% in the prednisone group (p < 0.0001). The median cumulative GC dose was 1.8 g in both TCZ groups compared with 3.3 g and 3.8 g in the 2 placebo groups (one with a short and one with a long GC tapering course).9 The incidence of adverse events was similar among all treatment arms.9 Another RCT (SIRRESTA) evaluating the efficacy of Sirukumab (a human monoclonal antibody against the IL-6), in patients with new or refractory/relapsed GCA is underway with the objective of recruiting 150 patients. The Phase 2 results with Sirukumab are encouraging.103
Conclusion
Biologic therapy is now a mainstay of treatment of many inflammatory conditions. IL-6 participates in the host defense against environmental pathogens,whereas dysregulation of IL-6 production has been implicated in the pathogenesis of various autoimmune and chronic inflammatory diseases. TCZ is currently the only drug targeting IL-6 receptors, and more information from trials and real clinical experience is becoming available for further use not only in RA, sJIA, and pJIA. Based on a review for quality, safety and efficacy, TCZ is approved for SC or IV use for RA, sJIA, pJIA, having an overall positive benefit-risk profile for the indications mentioned above. TCZ has the largest database on monotherapy and has already demonstrated greater efficacy than MTX or DMARDs in lowering disease activity and reducing radiographic progression. The strongest clinical and economic standpoint is its effectiveness as monotherapy. It is becoming increasingly important to establish treatment strategies for individual patients and establish the strong therapeutic potential of TCZ. TCZ promises to be an exciting new therapy in GCA, PMR and LVV.
Abbreviations
- ABC
Abatacept
- ACR
American College of Rheumatology
- ADA
Adalimumab
- AE
Adverse Event
- ANC
Absolute neutrophil count
- ANK
Anakinra
- AUC
Area under the curve
- CHO
Chinese Hamster Ovary
- CHMP
Committee for Medicinal Products for Human Use
- Cmax
Maximum concentration of TCZ in the plasma
- CRP
C Reactive Protein
- CTL
Certolizumab Pegol
- CV
Cardiovascular
- CYP450
Cytochrome P450 Enzyme
- DAS
Disease Activity Score
- DMARDs
Disease-Modifying Anti-Rheumatic Drugs
- EMA
European Medicines Agency
- EPAR
European Public Assessment Report
- ETA
Etanercept
- EU
European Union
- FAB
Fragment Antigen Binding
- GC
Glucocorticoids
- GCA
Giant Cell Arteritis
- GOL
Golimumab
- HDL
High Density Lipoprotein
- IL-6
Interleukin 6
- IL-6R
Interleukin 6 receptor
- IL-17
Interleukin 17
- INX
Infliximab
- IV
Intravenous
- JAKs
Janus kinases
- LDL
Low Density Lipoprotein
- LVV
Large Vessel Vasculitis
- MAPK
Mitogen-Activated Protein Kinases
- MMP
Matrix Metalloproteinase
- MTX
Methotrexate
- NSAID
Non-Steroidal Anti Inflammatory Drug
- pJIA
Polyarticular Juvenile Idiopathic Arthritis
- PMR
Polymyalgia Rheumatica
- RA
Rheumatoid Arthritis
- RANKL
Nuclear Factor Kappa B Ligand
- RTX
Rituximab
- SC
Subcutaneous
- sJIA
Systemic Juvenile Idiopathic Arthritis
- SmPC
Summary of Product Characteristics
- TA
Takayasu Arteritis
- TCZ
Tocilizumab
- TC
Total Cholesterol
- Th17
T helper 17 cells
- TGF-β
Transforming Growth Factor Beta
- TNF-α
Tumour necrosis factor alpha
- Treg
Regulatory T-cells
- USA
United States of America
- US
Ultrasound.
- VEGF
Vascular Endothelial Growth Factor
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Assessment Report For RoActemra [Internet] 1st ed. London: European Medicines Agency; 2009. [accessed 2017January3]. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/000955/WC500054888.pdf [Google Scholar]
- [2].RoACTEMRA [Internet] F Hoffmann-La Roche Ltd; c2017 [accessed 2017January2]. http://www.roche.com/products/product-details.htm?productId=30d444d8-7658-469e-9fce-f4de549c00c4 [Google Scholar]
- [3].ACTEMRA [Internet] Genentech USA; c2016 [accessed 2017January2]. http://www. actemra.com/ [Google Scholar]
- [4].Higuchi T, Nakanishi T, Takada K, Matsumoto M, Okada M, Horikoshi H, Suzuki K. A case of multicentric castleman's disease having lung lesion successfully treated with humanized anti-interleukin-6 receptor antibody, Tocilizumab. J Korean Med Sci 2010; 25(9):1364-7; PMID:20808682; https://doi.org/ 10.3346/jkms.2010.25.9.1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].RoActemra 20mg/ml Concentrate for Solution for Infusion Electronic Medicines Compendium; 29/07/16 [Updated 9/August/16; accessed 4/January/17]. Available from: https://www.medicines.org.uk/emc/medicine/22311/SPC/RoActemra+20mg+ml+Concentrate+for+Solution+for+Infusion/ [Google Scholar]
- [6].Ito H, Takazoe M, Fukuda Y, Hibi T, Kusugami K, Andoh A, Matsumoto T, Yamamura T, Azuma J, Nishimoto N, et al.. A pilot randomized trial of a human anti-interleukin-6 receptor monoclonal antibody in active Crohn's disease. Gastroenterology 2004; 126(4):989-96; PMID:15057738; https://doi.org/ 10.1053/j.gastro.2004.01.012 [DOI] [PubMed] [Google Scholar]
- [7].Illei GG, Shirota Y, Yarboro CH, Daruwalla J, Tackey E, Takada K, Fleisher T, Balow JE, Lipsky PE. Tocilizumab in Systemic Lupus Erythematosus: Data on safety, preliminary efficacy, and Impact on circulating plasma cells from an open-label phase I dosage-escalation study. Arthritis Rheum 2010; 62(2):542-52; PMID:20112381; https://doi.org/ 10.1002/art.27221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Abisror N, Mekinian A, Lavigne C, Vandenhende MA, Soussan M, Fain O, Club Rhumatismes et Inflammation, and SNFMI . Tocilizumab in refractory Takayasu arteritis: a case series and updated literature review. Autoimmun Rev 2013; 12:1143-9; PMID:23820042; https://doi.org/ 10.1016/j.autrev.2013.06.019 [DOI] [PubMed] [Google Scholar]
- [9].Stone J, Tuckwell K, Dimonaco S, Klearman M, Aringer M, Blockmans D, et al.. Efficacy and Safety of Tocilizumab in Patients with Giant Cell Arteritis: Primary and Secondary Outcomes from a Phase 3, Randomized, Double-Blind, Placebo-Controlled Trial [abstract]. Arthritis Rheumatol (Hoboken, NJ) 2016; 68(suppl 10) [Google Scholar]
- [10].Hagihara K, Kawase I, Tanaka T, Kishimoto T. Tocilizumab ameliorates clinical symptoms in polymyalgia rheumatica. J Rheumatol 2010; 37(5):1075-6; PMID:20439532; https://doi.org/ 10.3899/jrheum.091185 [DOI] [PubMed] [Google Scholar]
- [11].Study of Tocilizumab to Treat Polymyalgia Rheumatica - Full Text View - ClinicalTrials.gov [Internet]. [cited 2016July10]. Available from: https://clinicaltrials.gov/ct2/show/NCT01396317 [Google Scholar]
- [12].Thonhofer R, Hiller M, Just H, Trummer M, Siegel C, Dejaco C. Treatment of refractory adult-onset still's disease with tocilizumab: report of two cases and review of the literature. Rheumatol Int 2011; 31(12):1653-6; PMID:21240503; https://doi.org/ 10.1007/s00296-010-1631-y [DOI] [PubMed] [Google Scholar]
- [13].Iwamoto M, Nara H, Hirata D, Minota S, Nishimoto N, Yoshizaki K. Humanized monoclonal anti-interleukin-6 receptor antibody for treatment of intractable adult-onset Still's disease. Arthritis Rheum 2002; 46(12):3388-9; PMID:12483747; https://doi.org/ 10.1002/art.10620 [DOI] [PubMed] [Google Scholar]
- [14].Matsumoto K, Nagashima T, Takatori S, Kawahara Y, Yagi M, Iwamoto M, Okazaki H, Minota S. Glucocorticoid and cyclosporine refractory adult onset Still's disease successfully treated with tocilizumab. Clin Rheumatol 2009; 28(4):485-7; PMID:19184270; https://doi.org/ 10.1007/s10067-009-1097-z [DOI] [PubMed] [Google Scholar]
- [15].Tocilizumab (RoActemra (EU), Actemra (US)) UKMi New Drugs Online Database [Internet]. [cited 2016June19]. Available from: http://www.ukmi.nhs.uk/applications/ndo/record_view_open.asp?newDrugID = 4214 [Google Scholar]
- [16].Calabrese LH, Rose-John S. “IL-6 biology: implications for clinical targeting in rheumatic disease. Nat Rev Rheumatol 2014; 10(12): 720-727; PMID:25136784; https://doi.org/ 10.1038/nrrheum.2014.127 [DOI] [PubMed] [Google Scholar]
- [17].Srirangan S, Choy EH. The role of interleukin 6 in the pathophysiology of rheumatoid arthritis. Ther Adv Musculoskelet Dis 2010; 2(5):247-56; PMID:22870451; https://doi.org/ 10.1177/1759720X10378372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Simpson RJ, Hammacher A, Smith DK, Matthews JM, Ward LD. Interleukin-6: Structure-function relationships. Protein Sci 1997; 6(5):929-55; PMID:9144766; https://doi.org/ 10.1002/pro.5560060501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kishimoto T. Interleukin-6: from basic science to medicine-40 years in immunology. Annu Rev Immunol 2005; 23:1-21; PMID:15771564; https://doi.org/ 10.1146/annurev.immunol.23.021704.115806 [DOI] [PubMed] [Google Scholar]
- [20].Mihara M, Hashizume M, Yoshida H, Suzuki M, Shiina M. IL-6/IL-6 receptor system and its role in physiological and pathological conditions. Clin Sci 2012; 122(4):143-59; PMID:22029668; https://doi.org/ 10.1042/CS20110340 [DOI] [PubMed] [Google Scholar]
- [21].Luchtefeld M, Preuss C, Rühle F, Bogalle EP, Sietmann A, Figura S, Müller W, Grote K, Schieffer B, Stoll M. Gp130-dependent release of acute phase proteins is linked to the activation of innate immune signaling pathways. PloS One 2011; 6(5):19427; https://doi.org/ 10.1371/journal.pone.0019427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Garbers C, Thaiss W, Jones GW, Waetzig GH, Lorenzen I, Guilhot F, Lissilaa R, Ferlin WG, Grötzinger J, Jones SA, et al.. Inhibition of classic signaling is a novel function of soluble glycoprotein 130 (sgp130), which is controlled by the ratio of interleukin 6 and soluble interleukin 6 receptor. J Biol Chem 2011; 286(50):42959-70; PMID:21990364; https://doi.org/ 10.1074/jbc.M111.295758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Rose-John S. IL-6 trans-signaling via the soluble IL-6 receptor: importance for the pro-inflammatory activities of IL-6. Int J Biol Sci 2012; 8(9):1237-47; PMID:23136552; https://doi.org/ 10.7150/ijbs.4989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Honjo T, Reth M, Radbruch A, Alt F. Molecular Biology of B Cells. Elsevier; 2014 [Google Scholar]
- [25].Bondeson J, Wainwright SD, Lauder S, Amos N, Hughes CE. The role of synovial macrophages and macrophage-produced cytokines in driving aggrecanases, matrix metalloproteinases, and other destructive and inflammatory responses in osteoarthritis. Arthritis Res Ther 2006; 8(6):1; https://doi.org/ 10.1186/ar2099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kimura A, Kishimoto T. IL‐6: Regulator of Treg/Th17 balance. Euro J Immunol 2010; 40(7):1830-5; PMID:20583029; https://doi.org/ 10.1002/eji.201040391 [DOI] [PubMed] [Google Scholar]
- [27].Yoshitake F, Itoh S, Narita H, Ishihara K, Ebisu S. Interleukin-6 directly inhibits osteoclast differentiation by suppressing receptor activator of NF-κB signaling pathways. J Biol Chem 2008; 283(17):11535-40; PMID:18296709; https://doi.org/ 10.1074/jbc.M607999200 [DOI] [PubMed] [Google Scholar]
- [28].Cohen T, Nahari D, Cerem LW, Neufeld G, Levi BZ. Interleukin 6 induces the expression of vascular endothelial growth factor. J Biol Chem 1996; 271(2):736-41; PMID:8557680; https://doi.org/ 10.1074/jbc.271.2.736 [DOI] [PubMed] [Google Scholar]
- [29].Higuchi T, Nakanishi T, Takada K, Matsumoto M, Okada M, Horikoshi H, Suzuki K. A case of multicentric Castleman's disease having lung lesion successfully treated with humanized anti-interleukin-6 receptor antibody, tocilizumab. J Korean Med Sci 2010; 25(9):1364-7; PMID:20808682; https://doi.org/ 10.3346/jkms.2010.25.9.1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Guideline for the use of intravenous tocilizumab in the treatment of adult patients with rheumatoid arthritis.pdf [Internet]. [cited 2016June19]. Available from: http://www.rheumatology.org.uk/includes/documents/cm_docs/2014/b/bsr_bhpr_guideline_for_the_use_of_intravenous_tocilizumab_in_the_treatment_of_adult_patients_with_rheumatoid_arthritis.pdf [Google Scholar]
- [31].Lally F, Smith E, Filer A, Stone MA, Shaw JS, Nash GB, Buckley CD, Ed Rainger G. A novel mechanism of neutrophil recruitment in a coculture model of the rheumatoid synovium. Arthritis Rheum 2005; 52(11):3460-9; https://doi.org/ 10.1002/art.21394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Choy E. Understanding the dynamics: pathways involved in the pathogenesis of rheumatoid arthritis. Rheumatology 2012; 51(4 Suppl):v3-11; PMID:22718924; https://doi.org/ 10.1093/rheumatology/kes113 [DOI] [PubMed] [Google Scholar]
- [33].Reiff A. Treatment of Systemic Juvenile Idiopathic Arthritis with Tocilizumab-the Role of Anti-Interleukin-6 Therapy After a Decade of Treatment. Biol Ther 2012; 2(1):1-2; PMID:24392296; https://doi.org/ 10.1007/s13554-012-0001-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Caparbo VF, Prada F, Silva CA, Regio PL, Pereira RM. Serum from children with polyarticular juvenile idiopathic arthritis (pJIA) inhibits differentiation, mineralization and may increase apoptosis of human osteoblasts “in vitro.” Clin Rheumatol 2009; 28(1):71-7; PMID:18685881; https://doi.org/ 10.1007/s10067-008-0985-y [DOI] [PubMed] [Google Scholar]
- [35].GUIDELINE ON DEVELOPMENT, PRODUCTION, CHARACTERISATION AND SPECIFICATIONS FOR MONOCLONAL ANTIBODIES AND RELATED PRODUCTS [Internet]. 1st ed. London: European Medical Agency; 2009. [cited 18August2016]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003074.pdf [Google Scholar]
- [36].ICH Topic Q 6 B Specifications: Test Procedures and Acceptance Criteria for Biotechnological/Biological Products [Internet]. 1st ed. London: European Medical Agency; 1999. [cited 18August2016]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002824.pdf [Google Scholar]
- [37].ICH Topic Q5E Comparability of Biotechnological/Biological products [Internet]. London: European Medicines Agency; ࣮EMEA 2006 [accessed 2017January3]. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002805.pdf [Google Scholar]
- [38].Vezér B, Buzás Z, Sebeszta M, Zrubka Z. Authorized manufacturing changes for therapeutic monoclonal antibodies (mAbs) in European Public Assessment Report (EPAR) documents. Curr Med Res Opin 2016; 32(5):829-34; https://doi.org/ 10.1185/03007995.2016.1145579 [DOI] [PubMed] [Google Scholar]
- [39].Nishimoto N, Yoshizaki K, Miyasaka N, Yamamoto K, Kawai S, Takeuchi T, Hashimoto J, Azuma J, Kishimoto T. Treatment of rheumatoid arthritis with humanized anti-interleukin-6 receptor antibody: a multicenter, double-blind, placebo-controlled trial. Arthritis Rheum 2004; 50(6):1761-9; https://doi.org/ 10.1002/art.20303 [DOI] [PubMed] [Google Scholar]
- [40].Sebba A. Tocilizumab: the first interleukin-6-receptor inhibitor. Am J Health Syst Pharm 2008; 65:1413-18; PMID:18653811; https://doi.org/ 10.2146/ajhp070449 [DOI] [PubMed] [Google Scholar]
- [41].Emery P, Keystone E, Tony HP, Cantagrel A, Van Vollenhoven R, Sanchez A, Alecock E, Lee J, Kremer J. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Ann Rheum Dis 2008; 67(11):1516-23; PMID:18625622; https://doi.org/ 10.1136/ard.2008.092932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Smolen JS, Beaulieu A, Rubbert-Roth A, Ramos-Remus C, Rovensky J, Alecock E, Woodworth T, Alten R, OPTION Investigators . Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet 2008; 371(9617):987-97; https://doi.org/ 10.1016/S0140-6736(08)60453-5 [DOI] [PubMed] [Google Scholar]
- [43].Jones G, Sebba A, Gu J, Lowenstein MB, Calvo A, Gomez-Reino JJ, Siri DA, Tomšič M, Alecock E, Woodworth T, et al.. Comparison of tocilizumab monotherapy versus methotrexate monotherapy in patients with moderate to severe rheumatoid arthritis: the AMBITION study. Ann Rheum Dis 2010; 69(01):88-96; PMID:19297346; https://doi.org/ 10.1136/ard.2008.105197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kremer JM, Blanco R, Brzosko M, Burgos-Vargas R, Halland AM, Vernon E, Ambs P, Fleischmann R. Tocilizumab inhibits structural joint damage in rheumatoid arthritis patients with inadequate responses to methotrexate: Results from the double-blind treatment phase of a randomized placebo-controlled trial of tocilizumab safety and prevention of structural joint damage at one year. Arthritis Rheum 2011; 63(3):609-21; https://doi.org/ 10.1002/art.30158 [DOI] [PubMed] [Google Scholar]
- [45].Genovese MC, McKay JD, Nasonov EL, Mysler EF, da Silva NA, Alecock E, Woodworth T, Gomez-Reino JJ. Interleukin-6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to disease-modifying antirheumatic drugs: The tocilizumab in combination with traditional disease-modifying antirheumatic drug therapy study. Arthritis Rheumat 2008; 58(10):2968-80; https://doi.org/ 10.1002/art.23940 [DOI] [PubMed] [Google Scholar]
- [46].Burmester GR, Rubbert-Roth A, Cantagrel A, Hall S, Leszczynski P, Feldman D, Rangaraj MJ, Roane G, Ludivico C, Lu P, et al.. A randomised, double-blind, parallel-group study of the safety and efficacy of subcutaneous tocilizumab versus intravenous tocilizumab in combination with traditional disease-modifying antirheumatic drugs in patients with moderate to severe rheumatoid arthritis (SUMMACTA study). Ann Rheum Dis 2014; 73(1):69-74; PMID:23904473; https://doi.org/ 10.1136/annrheumdis-2013-203523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kivitz A, Olech E, Borofsky MA, Zazueta BM, Navarro-Sarabia F, Radominski SC, Merrill JT, Pei J, Rowell L, Nasmyth-Miller C, et al.. The efficacy and safety of tocilizumab subcutaneous Q2w and following escalation from Q2w to Qw therapy in combination with traditional dmards in patients with moderate to severe rheumatoid arthritis at 96 weeks. Arthritis Rheumatol 2014; 66:S1076-7 [Google Scholar]
- [48].Chaitow J, De Benedetti F, Brunner H, Ruperto N, Allen R, Murray K, Schneider R, Woo P, Wright S, Kenwright A, et al.. Tocilizumab in patients with systemic juvenile idiopathic arthritis: Efficacy data from the placebo-controlled 12-week part of the phase III tender trial. Intern Med J 2011; 41:32-; https://doi.org/ 10.1111/j.1445-5994.2011.02489.x [DOI] [Google Scholar]
- [49].Brunner HI, Ruperto N, Zuber Z, Keane C, Harari O, Kenwright A, Lu P, Cuttica R, Keltsev V, Xavier RM, et al.. Efficacy and safety of tocilizumab in patients with polyarticular-course juvenile idiopathic arthritis: results from a phase 3, randomised, double-blind withdrawal trial. Ann Rheum Dis 2014; 74(6):1110-7; PMID:24834925; https://doi.org/ 10.1136/annrheumdis-2014-205351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Nishimoto N, Miyasaka N, Yamamoto K, Kawai S, Takeuchi T, Azuma J. Long-term safety and efficacy of tocilizumab, an anti-IL-6 receptor monoclonal antibody, in monotherapy, in patients with rheumatoid arthritis (the STREAM study): evidence of safety and efficacy in a 5-year extension study. Ann Rheum Dis 2009; 68(10):1580-4; PMID:19019888; https://doi.org/ 10.1136/ard.2008.092866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Farah Z, Ali S, Price-Kuehne F, Mackworth-Young CG. Tocilizumab in refractory rheumatoid arthritis: long-term efficacy, safety, and tolerability beyond 2 years. Biologics 2016; 10:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].De Benedetti F, Brunner H, Ruperto N, Cuttica R, Malattia C, Schneider R, Woo P, Eleftheriou D, Baildam E, Burgos-Vargas R, et al.. Efficacy and safety of tocilizumab (TCZ) in patients with systemic juvenile idiopathic arthritis (SJIA): tender 52-week data. Pediatric Rheumatol 2012; 10(1):1; PMID:22222048; https://doi.org/ 10.1186/1546-0096-10-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Yamamoto K, Goto H, Hirao K, Nakajima A, Origasa H, Tanaka K, Tomobe M, Totsuka K. Longterm safety of tocilizumab: results from 3 years of followup postmarketing surveillance of 5573 patients with rheumatoid arthritis in Japan. J Rheumatol 2015; 42(8):1368-75; PMID:26034149; https://doi.org/ 10.3899/jrheum.141210 [DOI] [PubMed] [Google Scholar]
- [54].Genovese MC, Rubbert-Roth A, Smolen JS, Kremer J, Khraishi M, Gómez-Reino J, Sebba A, Pilson R, Williams S, et al.. Longterm safety and efficacy of tocilizumab in patients with rheumatoid arthritis: a cumulative analysis of up to 4.6 years of exposure. J Rheumatol 2013; 40(6):768-80; PMID:23457383; https://doi.org/ 10.3899/jrheum.120687 [DOI] [PubMed] [Google Scholar]
- [55].Nishimoto N, Ito K, Takagi N. Safety and efficacy profiles of tocilizumab monotherapy in Japanese patients with rheumatoid arthritis: meta-analysis of six initial trials and five long-term extensions. Modern Rheumatol 2010; 20(3):222-32; PMID:20221663; https://doi.org/ 10.3109/s10165-010-0279-5 [DOI] [PubMed] [Google Scholar]
- [56].Klein K, Scholl JH, Vermeer NS, Broekmans AW, Van Puijenbroek EP, De Bruin ML, Stolk P. Traceability of Biologics in The Netherlands: An Analysis of Information-Recording Systems in Clinical Practice and Spontaneous ADR Reports. Drug Saf 2016; 39(2): 185-92; PMID:26719190; https://doi.org/ 10.1007/s40264-015-0383-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Actemra, Highlights of prescribing Information [Internet] Genentech; c2013 [accessed 2017January3]. https://www.gene.com/download/pdf/actemra_prescribing.pdf [Google Scholar]
- [58].RoActemra 162 mg Solution for Injection in Pre-Filled Syringe Electronic Medicines Compendium; 29/07/16 [Updated 9/8/16; accessed 4/January/17]. Available from: http://www.medicines.org.uk/emc/medicine/28809/ [Google Scholar]
- [59].Ogata A, Tanimura K, Sugimoto T, Inoue H, Urata Y, Matsubara T, Kondo M, Ueki Y, Iwahashi M, Tohma S, et al.. Phase III study of the efficacy and safety of subcutaneous versus intravenous tocilizumab monotherapy in patients with rheumatoid arthritis. Arthritis Care Res (Hoboken) 2014; 66(3):344-54; PMID:23983039; https://doi.org/ 10.1002/acr.22110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Iwamoto N, Fukui S, Umeda M, Nishino A, Nakashima Y, Suzuki T, Horai Y, Nonaka F, Okada A, Koga T, et al.. Evaluation of switching from intravenous to subcutaneous formulation of tocilizumab in patients with rheumatoid arthritis. Modern Rheumatol 2016; 26(5):662-666; PMID:26708444; https://doi.org/ 10.3109/14397595.2015.1129692 [DOI] [PubMed] [Google Scholar]
- [61].Remicade 100 mg powder for concentrate for solution for infusion Electronic Medicines Compendium; 9/06/16 [Updated 21/July/16; accessed 4/January/17]. Available from: http://www.medicines.org.uk/emc/medicine/3236 [Google Scholar]
- [62].Humira 40 mg/0.4 ml Pre-filled Syringe and Pre-filled Pen Electronic Medicines Compendium; 12/12/16 [Updated 30/December/16; accessed 4/January/17]. Available from: http://www.medicines.org.uk/emc/medicine/31860 [Google Scholar]
- [63].Enbrel 50 mg solution for injection in pre-filled pen Electronic Medicines Compendium; 1/04/16 [Updated 6/May/16; accessed 4/January/17]. Available from: http://www.medicines.org.uk/emc/medicine/22143 [Google Scholar]
- [64].Simponi 100 mg solution for injection Electronic Medicines Compendium; 13/10/16 [Updated 11/November/16; accessed 4/January/17]. Available from: http://www.medicines.org.uk/emc/medicine/28316 [Google Scholar]
- [65].Cimzia 200 mg solution for injection in pre-filled syringe Electronic Medicines Compendium; 01/12/16 [Updated 20/December/16; accessed 4/January/17]. Available from: http://www.medicines.org.uk/emc/medicine/22323 [Google Scholar]
- [66].Mabthera 100 mg and 500 mg Concentrate for Solution for Infusion Electronic Medicines Compendium; 26/05/16 [Updated 8/June/16; accessed 4/January/17]. Available from: http://www.medicines.org.uk/emc/medicine/2570 [Google Scholar]
- [67].Kineret 100 mg solution for injection in a pre-filled syringe Electronic Medicines Compendium; 28/01/16 [Updated 31/March/16; accessed 4/January/17]. Available from: http://www.medicines.org.uk/emc/medicine/23104 [Google Scholar]
- [68].ORENCIA 250 mg powder for concentrate for solution for infusion Electronic Medicines Compendium; 01/08/16 [Updated 1/September/16; accessed 4/January/17]. Available from: http://www.medicines.org.uk/emc/medicine/19714 [Google Scholar]
- [69].Ilaris 150 mg powder for solution for injection Electronic Medicines Compendium; 1/08/16 [Updated 10/August/16; accessed 4/January/17]. Available from: http://www.medicines.org.uk/emc/medicine/22718 [Google Scholar]
- [70].James K, Bell GT. Human monoclonal antibody production. Current status and future prospects. J Immunol Methods 1987; 100(1-2):5-40; PMID:3298441; https://doi.org/ 10.1016/0022-1759(87)90170-0 [DOI] [PubMed] [Google Scholar]
- [71].Hwang WY, Foote J. Immunogenicity of engineered antibodies. Methods 2005; 36(1):3-10; PMID:15848070; https://doi.org/ 10.1016/j.ymeth.2005.01.001 [DOI] [PubMed] [Google Scholar]
- [72].Gabay C, Emery P, Van Vollenhoven R, Dikranian A, Alten R, Pavelka K, Klearman M, Musselman D, Agarwal S, Green J, et al.. Tocilizumab monotherapy versus adalimumab monotherapy for treatment of rheumatoid arthritis (ADACTA): a randomised, double-blind, controlled phase 4 trial. Lancet 2013; 381(9877):1541-50; https://doi.org/ 10.1016/S0140-6736(13)60250-0 [DOI] [PubMed] [Google Scholar]
- [73].Veronese FM, Mero A. The Impact of PEGylation on Biological Therapies. Biodrugs 2008; 22(5):315-29; PMID:18778113; https://doi.org/ 10.2165/00063030-200822050-00004 [DOI] [PubMed] [Google Scholar]
- [74].Goel N, Stephens S. Certolizumab Pegol. MAbs 2010; 2(2):137-47; PMID:20190560; https://doi.org/ 10.4161/mabs.2.2.11271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Flint J, Panchal S, Hurrell A, Van de Venne M, Gayed M, Schreiber K, Arthanari S, Cunningham J, Flanders L, Moore L, et al.. BSR and BHPR guideline on prescribing drugs in pregnancy and breastfeeding—Part I: standard and biologic disease modifying anti-rheumatic drugs and corticosteroids. Rheumatology 2016; 55(9):1693-7; PMID:26750124; https://doi.org/ 10.1093/rheumatology/kev404 [DOI] [PubMed] [Google Scholar]
- [76].RoActemra 162 mg Solution for Injection in Pre-Filled Syringe Electronic Medicines Compendium; 29/07/16 [Updated 9/August/16; accessed 5/January/17]. Available from: https://www.medicines.org.uk/emc/medicine/28809 [Google Scholar]
- [77].ORENCIA 125 mg solution for injection (pre-filled syringe) Electronic Medicines Compendium; 01/08/16 [Updated 1/September/16; accessed 5/January/17]. Available from: https://www.medicines.org.uk/emc/medicine/27216 [Google Scholar]
- [78].Huynh TK, Østergaard A, Egsmose C, Ole Rintek M. Preferences of patients and health professionals for route and frequency of administration of biologic agents in the treatment of rheumatoid arthritis. Patient Prefer Adherence 2014; 8:93-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Michaud K, Messer J, Choi HK, Wolfe F. Direct medical costs and their predictors in patients with rheumatoid arthritis: a three-year study of 7527 patients. Arthritis Rheum 2003; 48:2750-62; PMID:14558079; https://doi.org/ 10.1002/art.11439 [DOI] [PubMed] [Google Scholar]
- [80].Simoens S. Biosimilar medicines and cost-effectiveness. Clinicoecon Outcomes Res 2011; 3:29-36; PMID:21935330; https://doi.org/ 10.2147/CEOR.S12494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Leffers HCB, Østergaard M, Glintborg B, Krogh NS, Tarp U, Lorenzen T, Hansen A, Dreyer L, Hetland ML. Three-year drug survival and effectiveness of abatacept and tocilizumab in patients with rheumatoid arthritis treated in routine care. Results from the Nationwide Danish Danbio Registry. Arthritis Rheum 2013; 65(Suppl 10) [Google Scholar]
- [82].Hetland ML, Christensen IJ, Tarp U, Dreyer L, Hansen A, Hansen IB, Kollerup G, Linde L, Lindegaard HM, Poulsen UE, et al.. Direct comparison of treatment responses, remission rates, and drug adherence in patients with rheumatoid arthritis treated with adalimumab, etanercept, or infliximab: results from eight years of surveillance of clinical practice in the nationwide Danish DANBIO registry. Arthritis Rheum 2010; 62(1):22-32; PMID:20039405; https://doi.org/ 10.1002/art.27227 [DOI] [PubMed] [Google Scholar]
- [83].Hishitani Y, Ogata A, Shima Y, Hirano T, Ebina K, Kunugiza Y, Shi K, Narazaki M, Hagihara K, Tomita T, et al.. Retention of tocilizumab and anti-tumour necrosis factor drugs in the treatment of rheumatoid arthritis. Scand J Rheumatol 2013; 42(4):253-9; PMID:23470089; https://doi.org/ 10.3109/03009742.2012.762037 [DOI] [PubMed] [Google Scholar]
- [84].Singh JA, Wells GA, Christensen R, Ghogomu E, Maxwell L, Macdonald JK, Filippini G, Skoetz N, Francis D, Lopes LC, et al.. Adverse effects of biologics: a network meta-analysis and Cochrane overview. Cochrane Database Syst Rev 2011; 16(2):CD008794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Gómez-Reino JJ, Carmona L, Valverde VR, Mola EM, Montero MD. Treatment of rheumatoid arthritis with tumor necrosis factor inhibitors may predispose to significant increase in tuberculosis risk: a multicenter active-surveillance report. Arthritis Rheum 2003; 48(8):2122-7; PMID:12905464; https://doi.org/ 10.1002/art.11137 [DOI] [PubMed] [Google Scholar]
- [86].van Vollenhoven RF, Nishimoto N, Yamanaka H. Experience with Mycobacterium tuberculosis infection reported in the tocilizumab worldwide RA safety database. Ann Rheum Dis 2009; 68(Suppl 3):567 [Google Scholar]
- [87].Gout T, Ostör AJ, Nisar MK. Lower gastrointestinal perforation in rheumatoid arthritis patients treated with conventional DMARDs or tocilizumab: a systematic literature review. Clin Rheumatol 2011; 30(11):1471-4; PMID:21833686; https://doi.org/ 10.1007/s10067-011-1827-x [DOI] [PubMed] [Google Scholar]
- [88].Koike T, Harigai M, Inokuma S, Ishiguro N, Ryu J, Takeuchi T, Takei S, Tanaka Y, Sano Y, Yaguramaki H, et al.. Effectiveness and safety of tocilizumab: postmarketing surveillance of 7901 patients with rheumatoid arthritis in Japan. J Rheumatol 2014; 41(1):15-23; PMID:24187110; https://doi.org/ 10.3899/jrheum.130466 [DOI] [PubMed] [Google Scholar]
- [89].Daïen CI, Duny Y, Barnetche T, Daurès J, Combe B, Morel J. Effect of TNF inhibitors on lipid profile in rheumatoid arthritis: a systematic review with meta-analysis. Ann Rheum Dis 2012; 71:862-8; PMID:22267329; https://doi.org/ 10.1136/annrheumdis-2011-201148 [DOI] [PubMed] [Google Scholar]
- [90].Bergman GJ, Hochberg MC, Boers M, Wintfeld N, Kielhorn A, Jansen JP. Indirect comparison of tocilizumab and other biologic agents in patients with rheumatoid arthritis and inadequate response to disease-modifying antirheumatic drugs. Semin Arthritis Rheum 2010; 39(6):425-41; PMID:20223500; https://doi.org/ 10.1016/j.semarthrit.2009.12.002 [DOI] [PubMed] [Google Scholar]
- [91].Gallego-Galisteo M, Villa-Rubio A, Alegre-del Rey E, Márquez-Fernández E, Ramos-Báez JJ. Indirect comparison of biological treatments in refractory rheumatoid arthritis. J Clin Pharm Ther 2012; 37(3):301-7; PMID:21831256; https://doi.org/ 10.1111/j.1365-2710.2011.01292.x [DOI] [PubMed] [Google Scholar]
- [92].Salliot C, Finckh A, Katchamart W, Lu Y, Sun Y, Bombardier C, Keystone E. Indirect comparisons of the efficacy of biological antirheumatic agents in rheumatoid arthritis in patients with an inadequate response to conventional disease-modifying antirheumatic drugs or to an anti-tumour necrosis factor agent: a meta-analysis. Ann Rheum Dis 2011; 70(2):266-71; PMID:21097801; https://doi.org/ 10.1136/ard.2010.132134 [DOI] [PubMed] [Google Scholar]
- [93].Jones G, Darian-Smith E, Kwok M, Winzenberg T. Effect of biologic therapy on radiological progression in rheumatoid arthritis: what does it add to methotrexate? Biologics 2012; 6:155-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Nishimoto N, Hashimoto J, Miyasaka N, Yamamoto K, Kawai S, Takeuchi T, Murata N, van der Heijde D, Kishimoto T. Study of active controlled monotherapy used for rheumatoid arthritis, an IL-6 inhibitor (SAMURAI): evidence of clinical and radiographic benefit from an x ray reader-blinded randomised controlled trial of tocilizumab. Ann Rheum Dis 2007; 66(9):1162-7; PMID:17485422; https://doi.org/ 10.1136/ard.2006.068064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Dougados M, Kissel K, Sheeran T, Tak PP, Conaghan PG, Mola EM, Schett G, Amital H, Navarro-Sarabia F, Hou A, et al.. Adding tocilizumab or switching to tocilizumab monotherapy in methotrexate inadequate responders: 24-week symptomatic and structural results of a 2-year randomised controlled strategy trial in rheumatoid arthritis (ACT-RAY). Ann Rheum Dis 2013; 72(1):43-50; PMID:22562983; https://doi.org/ 10.1136/annrheumdis-2011-201282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Sibilia J, Graninger W, Ostor A, et al.. Comparison of tocilizumab as monotherapy or with add-on DMARDS in patients with rheumatoid arthritis and an inadequate response to previous treatments: ACT-SURE results. Ann Rheum Dis 2011; 70:466 [Google Scholar]
- [97].`Weinblatt ME, Kremer J, Cush J, Rigby W, Teng LL, Devenport J, Singh N, Lepley D, Genovese MC. Tocilizumab as monotherapy or in combination with nonbiologic DMARDs: 24-week results of an open-label, clinical practice study (ACT-STAR). Arthritis Care Res 2013; 65(3):362-71; https://doi.org/ 10.1002/acr.21847 [DOI] [PubMed] [Google Scholar]
- [98].Dasgupta B, Panayi GS. Interleukin-6 in the serum of patients with polymyalgia rheumatica and giant cell arteritis. Br J Rheumatol 1990; 29:456-8; PMID:2124160; https://doi.org/ 10.1093/rheumatology/29.6.456 [DOI] [PubMed] [Google Scholar]
- [99].Weyand CM, Fulbright JW, Hunder GG, Evans JM, Goronzy JJ. Treatment of giant cell arteritis: interleukin-6 as a biologic marker of disease activity. Arthritis Rheum 2000; 43:1041-8; PMID:10817557; https://doi.org/ 10.1002/1529-0131(200005)43:5%3c1041::AID-ANR12%3e3.0.CO;2-7 [DOI] [PubMed] [Google Scholar]
- [100].Villiger PM, Adler S, Kuchen S, Wermelinger F, Dan D, Fiege V, Bütikofer L, Seitz M, Reichenbach S. Tocilizumab for induction and maintenance of remission in giant cell arteritis: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet 2016; 387:1921-27; PMID:26952547; https://doi.org/ 10.1016/S0140-6736(16)00560-2 [DOI] [PubMed] [Google Scholar]
- [101].Evans J, Steel L, Borg F, Dasgupta B. Long-term efficacy and safety of tocilizumab in giant cell arteritis and large vessel vasculitis. RMD Open 2016; 2:e000137; PMID:26819753; https://doi.org/ 10.1136/rmdopen-2015-000137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Devauchelle V, Berthelot JM, Cornec D. Efficacy and safety of tocilizumab as first line therapy in patients with recent Polymyalgia Rheumatica (PMR): results of the first longitudinal prospective study (Tenor). Ann Rheum Dis 2015; 74:526; PMID:24347569; https://doi.org/ 10.1136/annrheumdis-2015-eular.272724347569 [DOI] [Google Scholar]
- [103].SIRRESTA study ClinicalTrials.gov Identifier: NCT02531633 (available from https://clinicaltrials.gov/ct2/show/NCT02531633?term=sirukumab&rank=1) [Google Scholar]


