Abstract
Classical descriptions of the hypothalamus divide it into three rostro-caudal domains but little is known about their embryonic origins. To investigate this, we performed targeted fate-mapping, molecular characterisation and cell cycle analyses in the embryonic chick. Presumptive hypothalamic cells derive from the rostral diencephalic ventral midline, lie above the prechordal mesendoderm and express Fgf10. Fgf10+ progenitors undergo anisotropic growth: those displaced rostrally differentiate into anterior cells, then those displaced caudally differentiate into mammillary cells. A stable population of Fgf10+ progenitors is retained within the tuberal domain; a subset of these gives rise to the tuberal infundibulum – the precursor of the posterior pituitary. Pharmacological approaches reveal that Shh signalling promotes the growth and differentiation of anterior progenitors, and also orchestrates the development of the infundibulum and Rathke's pouch – the precursor of the anterior pituitary. Together, our studies identify a hypothalamic progenitor population defined by Fgf10 and highlight a role for Shh signalling in the integrated development of the hypothalamus and pituitary.
KEY WORDS: Fgf10, Shh, Chick, Hypothalamus, Prechordal mesendoderm
Summary: Anterior and then mammillary hypothalamic cells differentiate from Fgf10+ progenitors that are retained as central tuberal hypothalamic cells. Shh is required for anterior regionalisation and also for integrated hypothalamic/pituitary development.
INTRODUCTION
The hypothalamus is an evolutionarily ancient part of the brain that acts as a master homeostatic regulator. It controls the circadian cycle, endocrine function, energy and stress balance through the concerted activity of its resident neurons. These neurons are located in nuclei that are assigned to distinct rostro-caudal domains: anterior, tuberal and mammillary (Swanson, 1987). Particular subclasses of hypothalamic neurons, termed neuroendocrine neurons, project axons to the median eminence and posterior pituitary – the ventral-most regions of the tuberal hypothalamus. Here, they release neurohormones that act directly, or indirectly, via cells of the adjacent anterior pituitary, to govern endocrine function (Swanson, 1987). An understanding of hypothalamic development is therefore fundamental to our understanding of neuroendocrine axis formation and function in health and disease.
In the embryonic chick, as in all vertebrates examined, conserved molecular features define the developing hypothalamus and demarcate its antero-posterior (future rostro-caudal) domains. At neural plate stages, presumptive hypothalamic and telencephalic territories can be distinguished through homeodomain (HD) protein expression profiles: the presumptive telencephalon co-expresses Foxg1 and Six3, whereas the presumptive hypothalamus expresses only Six3 (Bovolenta et al., 1998; Bell et al., 2001; Chapman et al., 2002; Ahlgren et al., 2003; Sanchez-Arrones et al., 2012). At HH17, after neural tube formation, Shh, Fgf8, Fgf10, Fgf19 and Emx2 become detected in apparently non-overlapping progenitor domains along the antero-posterior axis of the developing hypothalamus (Schubert and Lumsden, 2005; García-Calero et al., 2006; Hurtado and Mikawa, 2006; Manning et al., 2006; Gimeno and Martinez, 2007; Parkinson et al., 2010). Previous studies have begun to reveal how these domains develop. In the anterior neural plate/neural tube, rostral diencephalic ventral midline (RDVM) cells are specified through signals that derive from underlying prechordal mesendoderm (PM) (Dale et al., 1997, 1999; reviewed by Placzek and Briscoe, 2005). In a subset of RDVM cells, Shh is then downregulated by BMPs that derive from the PM. Upregulation of BMP signalling in RDVM cells leads to expression of Tbx2, a step that promotes the development of RDVM cells to Fgf10+ and Emx2+ progenitors (Manning et al., 2006). Lineage-tracing studies show that Fgf10+ progenitors give rise to the infundibulum, an outgrowth of the tuberal hypothalamus (Pearson et al., 2011), whereas Emx2 appears to mark mammillary progenitors (Manning et al., 2006). As Shh is downregulated in RDVM cells, however, it becomes detected in adjacent anterior progenitors (Ohyama et al., 2005; Manning et al., 2006; Aglyamova and Agarwala, 2007).
Currently, the origins of anterior Shh+, tuberal Fgf10+ and mammillary Emx2+ progenitors, and their relationship with each other and with differentiated cells in the adult hypothalamus remain unresolved. Fate-mapping studies in the chick have provided inconsistent conclusions (Garcia-Lopez et al., 2004; Manning et al., 2006; Pearson et al., 2011) and lineage-tracing studies in the mouse have relied on promoters/enhancers that are not specific to hypothalamic progenitors (Alvarez-Bolado et al., 2012): as yet, no single early marker has been identified that delineates or defines the hypothalamic progenitor state. The controversy surrounding the position of hypothalamic progenitors fuels debate into which surrounding tissues may influence its development. Thus, although many studies highlight the importance of the PM (Shimamura and Rubenstein, 1997; Dale et al., 1997; Ohyama et al., 2005; Patten et al., 2003; Pera and Kessel, 1997; reviewed by Bedont et al., 2015; Burbridge et al., 2016), other studies suggest the PM is distant and thus less significant (Garcia-Calero et al., 2008; reviewed by Puelles and Rubenstein, 2015).
Here, we perform studies in the embryonic chick to address questions about the origin of the hypothalamus and the emergence of distinct rostro-caudal progenitor domains. We create an extensive fate-map of cells in the ventral midline/adjacent basal plate of the prosencephalon to show that hypothalamic progenitors lie above the PM at 9-10 somites. We reveal that these progenitors express Fgf10 and give rise to anterior and mammillary progenitor domains by bidirectional anisotropic growth and differentiation. Following this, the remaining Fgf10-positive progenitors give rise to cells within the tuberal domain. Finally, we uncover a crucial role for Shh signalling, both in growth and differentiation of anterior progenitors and in pituitary development (see Carreno et al., 2017). Together, our studies reveal how spatially distinct rostro-caudal domains of the hypothalamus are established.
RESULTS
Characterising the embryonic chick hypothalamus
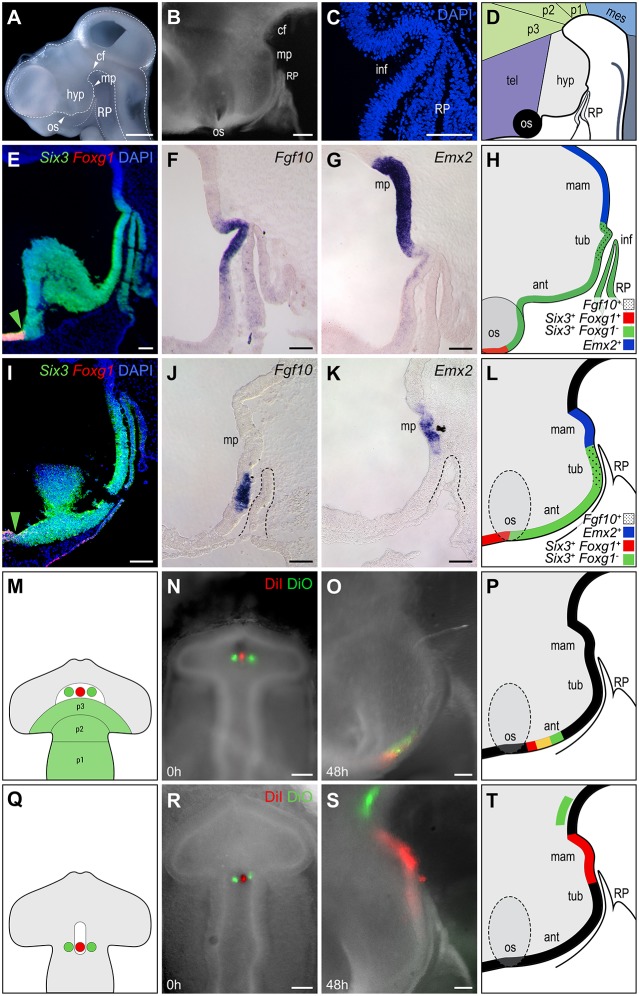
At embryonic days (E)3 (HH18-20) to E5 (HH26), the chick hypothalamus is clearly visible as a ventral protrusion with a rostral limit at the optic stalk and a caudal limit at the mammillary pouch (Fig. 1A; Fig. S1). A novel hemi-dissection technique preserves the association of Rathke's pouch (RP) with the hypothalamus (Fig. 1B). At E5, the tip of RP underlies the future infundibulum, a ventral outgrowth of the tuberal hypothalamus (Fig. 1C,D; Pearson et al., 2011). In situ hybridisation and analyses of sagittal sections at E5 reveals the telencephalic (Foxg1+Six3+)-hypothalamic (Foxg1−Six3+) boundary and shows that Six3 extends to the infundibulum, i.e. defines anterior and tuberal progenitor domains (Fig. 1E). Tuberal Fgf10+ progenitors lie above RP (Fig. 1F) and anterior to Emx2+ mammillary progenitors (Fig. 1G). Thus, at E5, anterior, tuberal and mammillary progenitor domains can be defined on the basis of gene expression profiles against known morphological landmarks: Six3+Foxg1−Fgf10− anterior progenitors extend from the optic stalk to RP; Six3+Foxg1−Fgf10+ tuberal progenitors lie above RP, and Emx2+Six3−Fgf10− mammillary progenitors extend from the infundibulum into the mammillary pouch (Fig. 1H).
Fig. 1.
Fate-mapping defines the extent of hypothalamic progenitors. (A-D) The position of the hypothalamus relative to adjacent structures, including Rathke's pouch (RP), shown: (A) in E5 whole-mount side view (dashed lines outline the neuroepithelium and RP); (B) after hemi-dissection at E3; (C) after sagittal sectioning (a high-power view of E5 chick to show the proximity of the infundibulum and RP); and (D) schematically (E5 chick). (E-L) Sagittal sections at E5 (E-H) or E3 (I-L) analysed by double fluorescent in situ hybridisation for Six3/Foxg1 (E,I) or by single in situ hybridisation for Fgf10 (F,J) or Emx2 (G,K), and illustrated schematically in H,L. Dashed lines in J,K outline RP. Green arrowheads in E,I indicate the telencephalic-hypothalamic boundary. (M-T) Fate-mapping of the medial prosencephalon (M-P) or prosencephalic neck (Q-T). (M,Q) Schematic representation of prosencephalon. White areas indicate the suggested position of hypothalamic progenitors from Garcia-Lopez et al. (2004) (M) or Manning et al. (2006) and Pearson et al. (2011) (Q). Circles show the position of focal DiI (pink) and DiO (green) injections. (N) Dorsal view of a 10-somite embryo after triple injection of DiI in the ventral midline of the medial prosencephalon and of DiO in adjacent basal plate (n=2). (O,P) Hemi-dissected side view of the same embryo incubated to HH20 (O), schematically represented in P. Labelled cells lie posterior to the optic stalk. (R) Dorsal view of a 9-somite embryo after triple injection of DiI in the ventral midline of the prosencephalic neck and of DiO in adjacent basal plate (n=2). (S,T) Hemi-dissected side view of the same embryo incubated to HH20 (S), schematically represented in T. DiI-labelled cells populate the ventral hypothalamus, extending from the mammillary pouch to the tuberal hypothalamus. DiO-labelled cells populate the basal plate of p2. Scale bars: 1 mm in A; 200 µm in B; 100 µm in C-S. ant, anterior hypothalamus; cf, cephalic flexure; hyp, hypothalamus; mam, mammillary hypothalamus; mes, mesencephalon; mp, mammillary pouch; os, optic stalk; p1-3, prosomeres 1-3; RP, Rathke's pouch; tel, telencephalon; tub, tuberal hypothalamus.
Anterior, tuberal and mammillary progenitor domains can similarly be identified at E3. Six3 is expressed in the Foxg1+ telencephalon, and in anterior and tuberal progenitors: its caudal limit aligns with the tip of RP (Fig. 1I). Fgf10 is expressed in tuberal progenitors that overlie RP (Fig. 1J) and Emx2 is expressed in mammillary progenitors (Fig. 1K). Thus, the E3 hypothalamus can be subdivided into a Foxg1−Six3+Fgf10− anterior progenitor domain rostral to RP, a Six3+Fgf10+Emx2− tuberal progenitor domain, which overlies RP, and a Emx2+Six3−Fgf10− mammillary progenitor domain (Fig. 1L). Furthermore, anterior, tuberal and mammillary progenitor domains can be conclusively identified in hemi-dissected E3 embryos, based on their position relative to RP.
We next determined the extent of hypothalamic progenitors in the ventral midline of 9-10 somite embryos. Previous studies have suggested very different origins for hypothalamic cells: chimaeric interspecies grafting studies have suggested that hypothalamic progenitors occupy the middle of the prosencephalon, anterior to prosomere 3 (Fig. 1M, white region; Garcia-Lopez et al., 2004), whereas focal-labelling studies have placed hypothalamic progenitors more posteriorly, at the prosencephalic neck (Fig. 1Q, white region; Manning et al., 2006; Pearson et al., 2011). We therefore targeted the extremes of each region (Fig. 1M,Q), injecting DiI (red) into the ventral midline and DiO (green) into adjacent basal plate cells, then analysing the position of descendants at E3. Ventral midline (DiI+) and basal plate cells (DiO+) in the middle of the prosencephalon contributed exclusively to the rostral-most part of the anterior hypothalamus, as determined in hemi-dissected view relative to the optic stalk and RP, and showed little growth (Fig. 1N-P). Transverse sections revealed that ventral midline and basal plate cells did not mix, but maintained their relative mediolateral position (Fig. S2). By contrast, ventral midline (DiI+) cells at the prosencephalic neck showed extensive expansion along the rostro-caudal axis and gave rise to mammillary and caudo-tuberal progenitor domains, as determined in hemi-dissected view relative to RP (Fig. 1R-T). Basal plate progenitors (DiO+) at the prosencephalic neck did not contribute to the hypothalamus but instead appeared to contribute to prosomere 2 (Fig. 1R-T). These results show that at 9-10 somites, hypothalamic progenitors extend from the prosencephalic neck to the middle of the prosencephalon. Their descendants populate the entire rostro-caudal extent of the hypothalamus, from the optic stalk to the mammillary pouch.
Hypothalamic progenitor domains originate in the RDVM above the PM
Many studies suggest that the PM induces the hypothalamus and then directs its subsequent development (reviewed by Bedont et al., 2015; Burbridge et al., 2016), but its exact position relative to hypothalamic progenitors remains unclear. Intriguingly, we noted that progenitors that give rise to the rostral-most anterior hypothalamus (Fig. 1M-P) appear to lie close to the anterior-most PM, the outline of which is apparent under bright-field view (Fig. 2A). We therefore asked whether progenitors that fate-map to the hypothalamus lie over the PM in the 9-10 somite embryo. To show that we could accurately target RDVM cells, we labelled them with DiI and immediately analysed embryos for Shh: DiI-labelled cells were observed above the Shh+ PM (Fig. 2B; and data not shown). To fully explore the extent to which PM cells underlie hypothalamic progenitors, DiI was targeted to the ventral midline overlying anterior (A), medial (B) and posterior (C) regions of the PM (Fig. 2C). Embryos were developed until E3 or E5, analysed in hemi-dissected view (Fig. 2D-L), then sectioned and analysed for marker expression to distinguish anterior, tuberal and mammillary progenitor domains (Figs S3 and S4).
Fig. 2.
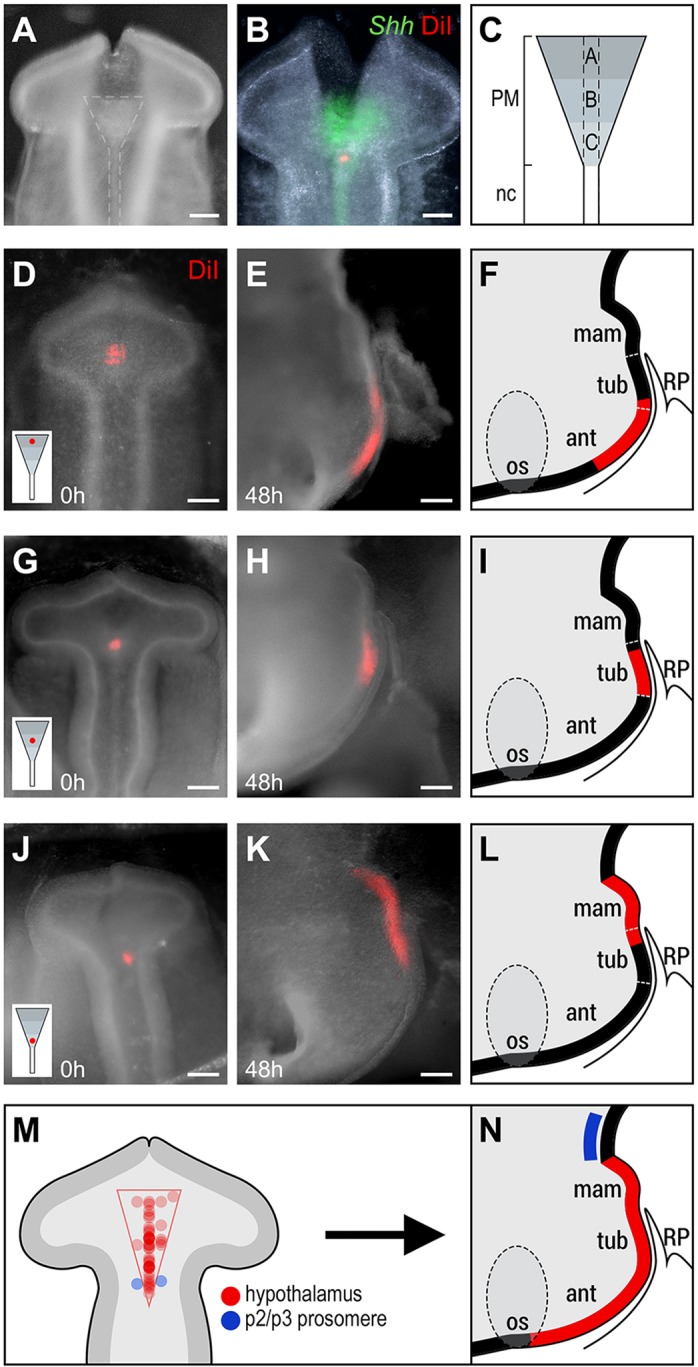
At 9-10 somites, hypothalamic progenitors lie above the PM. (A-C) Targeting neuroepithelium above the PM. (A) Whole-mount dorsal view of a 10-somite embryo after incision of the dorsal neural tube reveals the PM and notochord (dashed lines). (B) Double analysis of DiI and Shh. (C) Schematic showing subdivision of the PM/RDVM into three regions: A (anterior), B (middle) and C (posterior). Dashed lines indicate position of the ventral midline. (D-L) Representative chicks, showing the fate of neuroepithelial cells after focal DiI injections to regions A (n=4; D-F), B (n=4; G-I) and C (n=2; J-L). (D,G,J) Whole-mount dorsal view of 9-10 somite embryos showing DiI injection in regions A (D), B, (G) and C (J). Inset shows injection site relative to the PM. (E,F,H,I,K,L) Whole-mount side views (E,H,K) after development to HH20 and hemi-dissection to show position of DiI-labelled cells, schematically represented in F,I,L. (M,N) Summary. Schematics of 9-10 somite prosencephalon, showing injection sites of all fate-mapping experiments relative to the prosencephalic neck and PM (M; n=43 injections), and position of descendants at E3 (N). Circle intensity in M indicates multiple separate injections. Scale bars: 100 µm. ant, anterior hypothalamus; mam, mammillary hypothalamus; os, optic stalk; RP, Rathke's pouch; tub, tuberal hypothalamus.
Targeting relative to the PM produced consistent fate-maps. Neuroepithelial cells above the anterior PM (region A) gave rise to anterior and tuberal progenitors (n=4/4; Fig. 2D-F). Transverse sectioning, and labelling of alternate sections for Fgf10, confirmed that DiI was detected in the Fgf10− anterior progenitor domain and in the Fgf10+ rostro-tuberal progenitor domain (Fig. S3). No labelled cells were detected in the mammillary progenitor domain (not shown). Neuroepithelial cells above region B gave rise to tuberal progenitors (n=4/4; Fig. 2G-I), confirmed after transverse sectioning and labelling of alternate sections for Fgf10 (Fig. S3). Neuroepithelial cells above the posterior PM (region C) gave rise to Emx2− caudo-tuberal and Emx2+ mammillary progenitors (n=4/4; Fig. 2J-L; Fig. S4). Triple fate-mapping showed that, despite extensive growth along the rostro-caudal axis, cells maintain their relative position and show some growth/migration along the medio-lateral axis (Fig. S5A-I).
To begin to define the position of hypothalamic progenitors along the medio-lateral axis, we targeted ventral midline cells and adjacent basal plate cells (DiO) in regions overlying the PM. Basal plate progenitors contributed to basal hypothalamic regions. They showed growth along the rostro-caudal axis, but to a lesser extent than ventral midline progenitors (Fig. S5J-N). Together, these studies define the extent of hypothalamic progenitors in the ventral midline in the 9-10 somite embryo and begin to define their position in the medio-lateral axis. The position and shape of the PM suggests that it underlies and defines the limit of RDVM progenitors which will populate the forming anterior, tuberal and mammillary hypothalamus at E3 (Fig. 2M,N). Over this time, these progenitors expand in number; they grow extensively along the rostro-caudal axis, but maintain their relative position. Together, our studies show that hypothalamic progenitors become regionalised according to their position along the rostro-caudal axis, but that progenitors targeted above any region of the PM contribute some descendants to the tuberal progenitor domain.
Anterior and mammillary progenitors derive from Fgf10+ hypothalamic progenitors
We next analysed Six3, Fgf10 and Emx2 at 9-10 somites to address whether, at the time of fate-mapping, hypothalamic progenitors express these markers, and, if so, whether distinct progenitor domains are already apparent.
Whole-mount in situ hybridisation shows that Six3 is expressed throughout the ventral prosencephalon, including the telencephalon and hypothalamus. Expression is wide anteriorly and progressively narrower posteriorly (n=4; Fig. 3A). Fgf10 is similarly detected ventrally, but is confined to the middle and posterior prosencephalon (n=6; Fig. 3B), where its profile is similar to that of Six3 (i.e. tapering posteriorly). Expression of Emx2 is undetectable (Manning et al., 2006). Comparison with our fate-mapping data thus suggests that, in the 9-10 somite chick, hypothalamic progenitors express Six3 and Fgf10, but not Emx2.
Fig. 3.
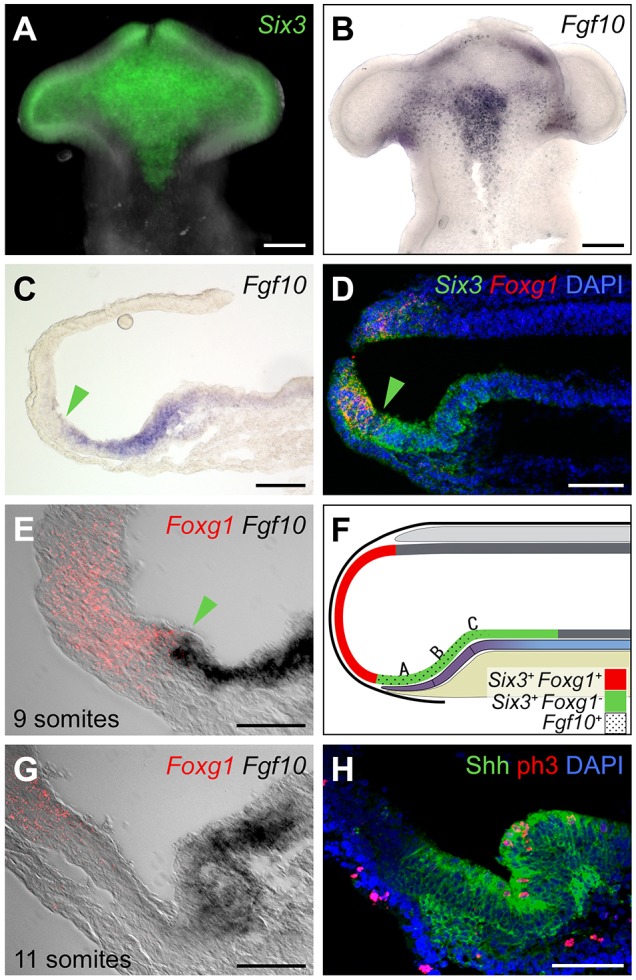
Hypothalamic progenitors at 9-10 somites express Fgf10. (A,B) Ventral flat-mount views of 10-somite isolated neuroepithelium after in situ hybridisation for Six3 (A; n=4) or Fgf10 (B; n=6). (C-F) Sagittal sections of 9-10 somite embryos. (C) In situ hybridisation for Fgf10 (n=5). (D-F) Double in situ hybridisation to detect Six3/Foxg1 (D) or Foxg1/Fgf10 (E) (n=3 each), shown schematically in F. Fgf10+ cells lie above the PM (purple indicates prechordal mesoderm; beige indicates endoderm) and abut the Foxg1− telencephalon. A,B,C refer to fate-mapped regions. Green arrowheads in C-E show the position of the tel-hyp boundary. (G,H) Sagittal sections of 11-somite embryos. (G) In situ hybridisation for Foxg1/Fgf10: anterior Fgf10− cells are apparent. (H) Immunohistochemical analysis for Shh/phospho-H3. A significantly higher density of phospho-H3+ cells is detected in tuberal compared with anterior domains (9.13±0.74 versus 3.13±0.72 phosphoH3+ cells/4.5×10−6 mm3; P<0.0001; n=8 sections; 4 embryos). Scale bars: 100 µm in A-D; 50 µm in E,G,H.
Analysis of Fgf10 in sagittal view suggests that Fgf10+ progenitors may extend as far as the telencephalic boundary (Fig. 3C). To confirm this, we performed double labelling of Six3/Foxg1 or Fgf10/Foxg1, followed by sagittal sectioning. This reveals that, at 9-10 somites, the anterior limit of Fgf10 immediately abuts the telencephalic boundary marked by Foxg1 (Fig. 3D-F). Therefore, at the time of fate-mapping, progenitors characteristic of tuberal (Six3+Fgf10+), but not anterior (Six3+Fgf10−) or mammillary (Fgf10−Emx2+), domains are present. Anterior progenitors can be detected only at 11 somites, approximately 1.5-2 h later than the fate-mapping studies (Fig. 3G), and appear concomitant with a characteristic folding of the hypothalamic floor and a distancing of the PM and the telencephalic/hypothalamic boundary (Fig. 3G,H). Mammillary progenitors appear still later, and are first detected at HH17 (Fig. S6). Together with our fate-mapping analysis, these results show that at 9-10 somites, the presumptive hypothalamus overlies the PM and is composed of Fgf10+ progenitors that give rise to anterior, then mammillary and tuberal domains.
Anterior and then mammillary domains arise by anisotropic growth
To further understand how anterior, mammillary and tuberal domains of the hypothalamus develop from Fgf10+ progenitors, we measured the rostro-caudal length of all three regions at HH10, HH15, HH17, HH20 and HH27 (Fig. 4). Despite the entire hypothalamic region expanding in size (∼8-fold expansion) over this period, the Fgf10+ progenitor domain undergoes remarkably little change in growth and remains a constant size (Fig. 4, red circles). By contrast, the anterior progenitor domain appears and increases dramatically in size between HH10+ and HH20 (Fig. 4, green circles). As growth of the anterior progenitor domain subsides, the mammillary progenitor domain forms and shows substantial growth from HH18 to HH27 (Fig. 4, blue circles; see also Fig. 1G,K). Fgf10+ progenitors are retained centrally and contribute exclusively to the tuberal domain. These data reveal the dynamic and bi-directional growth of the anterior and mammillary hypothalamus from Fgf10+ progenitors. A defining feature of the tuberal domain, the infundibulum, emerges over E4-E7 (Pearson et al., 2011), i.e. after the major growth phase of the anterior progenitor domain.
Fig. 4.
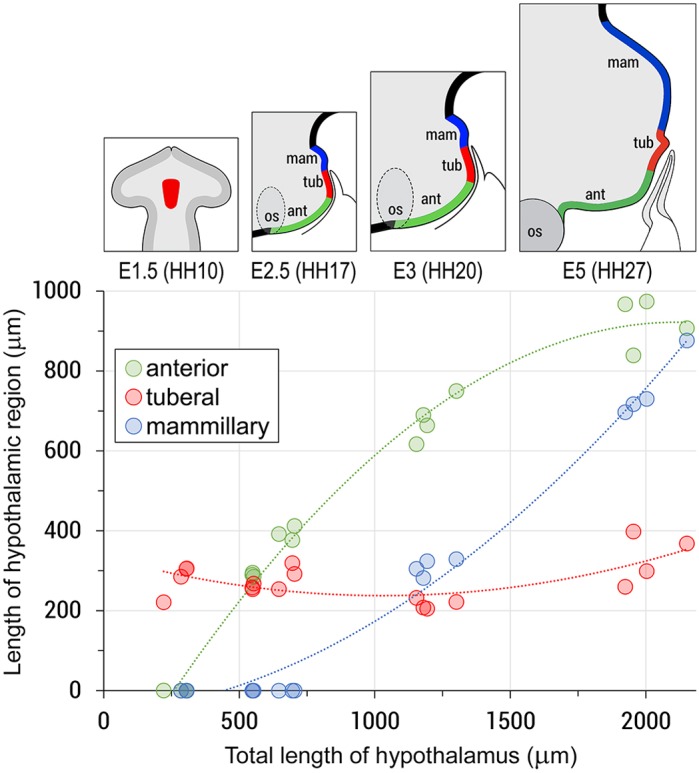
Growth of the hypothalamus is anisotropic. Scatter plot showing growth of anterior (green), tuberal (red) and mammillary (blue) progenitors from HH10 (E1.5) to HH27 (E5). Anterior and posterior domains emerge and grow by 922±63 µm and 910±85 µm; n=4 each; the lengths of the tuberal domains do not alter significantly (279±40 to 256±18, P>0.05; n=4 each). Circles represent analyses of individual chicks. Lines of best fit were generated using Microsoft Excel. Top schematics illustrate the growth of anterior (green) and posterior (blue) progenitors, and the relatively unchanged length of Fgf10+ hypothalamic (prospective tuberal) progenitors (red).
Anterior and mammillary progenitor domains arise from highly proliferative Fgf10+ progenitors
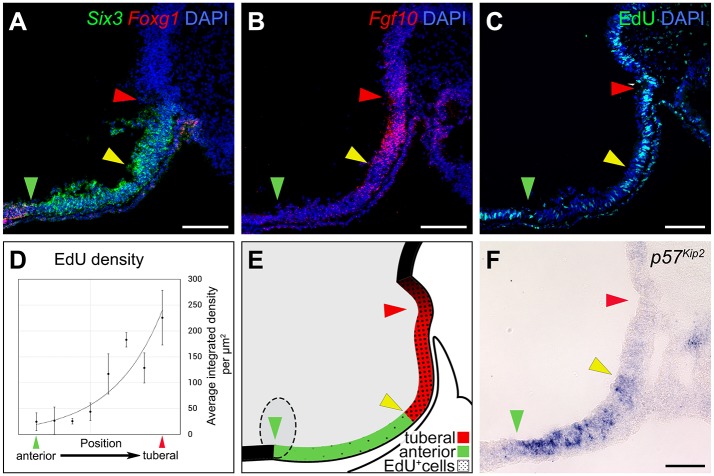
We next addressed whether localised proliferation could contribute to bi-directional growth of anterior and mammillary progenitor domains. Previous studies have shown that FGF signalling governs proliferation of hypothalamic progenitors (Pearson et al., 2011), implying that Fgf10+ progenitors self-propagate and are responsive to FGFs acting as autocrine signals. However, it remains possible that once initial pools of Fgf10− anterior and mammillary progenitors have formed, they then expand in response to FGFs acting as paracrine signals from presumptive tuberal regions. To distinguish these possibilities, we first analysed expression of Pea3, a marker of FGF-responding cells. From HH9 to HH26, Pea3 is expressed in Fgf10+ progenitors, but not in adjacent anterior and mammillary progenitors, suggesting that FGF signalling is autocrine (Fig. S7; data not shown). Next, we performed acute EdU labelling assays at HH15 and HH20, times when, respectively, anterior and mammillary progenitor domains expand rapidly. At HH15, labelling of serial adjacent sagittal sections shows that the Foxg1−Six3+Fgf10− anterior progenitor domain (Fig. 5A-C, green to yellow arrowheads) contains a lower number of EdU-labelled cells compared with the Foxg1−Six3+Fgf10+ tuberal progenitor domain (Fig. 5A-C, yellow to red arrowheads). Quantification of EdU fluorescence measured as density through the anterior/tuberal domains (Fig. 5C, green to red arrowheads) reveals a gradient of proliferation: EdU+ progenitors are detected at high density in the tuberal domain and at diminishing density through the anterior domain (Fig. 5D,E). Similarly, at HH20, the Emx2+ domain contains a lower density of EdU-labelled cells compared with the tuberal domain (Fig. S8). These results, together with our fate-maps, suggest that anterior and mammillary progenitor domains develop from highly proliferating Fgf10+ progenitors.
Fig. 5.
Anterior progenitors grow and differentiate from Fgf10+ hypothalamic progenitors. (A-C) Serial adjacent sagittal sections of HH15 embryos (n=3), analysed for Six3/Foxg1 (A), Fgf10 (B) or EdU (C). Green arrowheads show the tel-hyp boundary; the region between the yellow and red arrowhead indicates the Six3+ Fgf10+ progenitor domain. (D,E) Quantitative analysis of EdU labelling through anterior-tuberal progenitor domains (D; n=4), depicted schematically in E (arrowheads as in A-C). (F) Sagittal section of a HH15 embryo, analysed for p57kip2 expression (n=4). Expression is confined to anterior progenitors, and is higher rostrally (green arrowhead) and lower caudally (yellow arrowhead). Scale bars: 100 µm.
The decline in proliferation towards the rostral and caudal ends of both anterior and mammillary progenitor domains implies that cells begin to differentiate once displaced from the Fgf10+ domain. To explore a mechanism for this, we analysed expression of Cip/Kip family members, which encode cell cycle regulators of the G1-to-S-phase transition that promote cell cycle exit and differentiation (Galderisi et al., 2003). We detected p57Kip2, but not p27Kip1 or p21Cip1 (Fig. 5F, Fig. S9; and data not shown). At HH15, p57Kip2 is detected only in the anterior progenitor domain. Expression is graded, with highest levels detected in rostral-most and lowest levels in caudal-most anterior progenitors (Fig. 5F, green and yellow arrowheads, respectively). At HH18, p57Kip2 is also detected in caudal mammillary progenitors, but is, at best, only weakly detected in tuberal progenitors (Fig. S9A-C). By HH26, p57Kip2 shows graded expression through the mammillary domain, with highest levels detected most caudally (Fig. S9D-I). Thus, opposing gradients of proliferation and differentiation underlie the bi-directional growth of the developing anterior and mammillary hypothalamus.
Upregulation of Shh and p57Kip2 in anterior progenitors
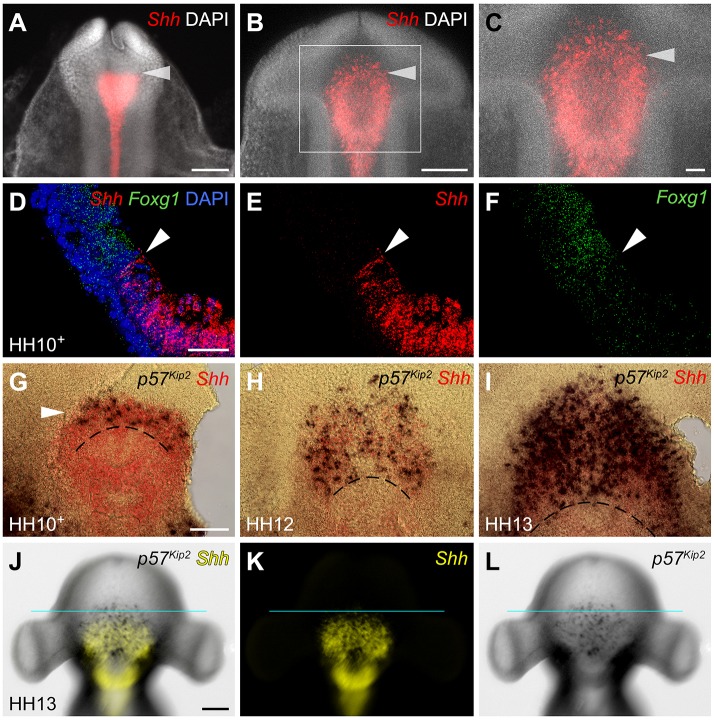
As the hypothalamus develops, Shh undergoes dynamic change, becoming downregulated in the presumptive tuberal hypothalamus and upregulated in the anterior hypothalamus (Manning et al., 2006). To address whether this change occurs as Fgf10+ progenitors develop into anterior progenitors, we examined Shh over 9-11 somites. At 9 somites, Shh expression in the prosencephalon (Fig. 6A) is similar to that of Fgf10 (Fig. 3B), suggesting a brief period of co-expression. However, from 11 somites, Shh becomes downregulated in Fgf10+ progenitors (Fig. 6B,C; Manning et al., 2006) and strong expression is now detected in cells at the periphery, including emerging anterior progenitors (Fig. 6B,C). Double in situ hybridisation and analysis of sagittal sections at 11 somites (HH10+) confirms that Shh+ anterior progenitors abut the Foxg1+ telencephalon (Fig. 6D-F, arrowhead). Analysis of phospho-H3 (a marker of M-phase) and of p57Kip2 suggests that at 11 somites, emerging anterior progenitors are already committing to a differentiation pathway: they upregulate p57Kip2 (Fig. 6G; expression of p57Kip2 is not detected in this region at 9-10 somites, data not shown) and show reduced phospho-H3 labelling, relative to neighbouring Fgf10+ progenitors (Fig. 3H). Shh+p57Kip2+ cells rapidly increase in number along the antero-posterior axis (Fig. 6H,I). Notably, however, at HH13, p57Kip2 expression is detected anterior to Shh (Fig. 6J-L). This, together with our cell cycle and fate-mapping analyses, suggests that emerging anterior progenitors downregulate Fgf10 and upregulate Shh and p57Kip2, then downregulate Shh as they differentiate.
Fig. 6.
Onset of Shh and p57Kip2 characterises anterior hypothalamic cells. (A-C) Ventral view of flat-mounted embryos at 8 (A) or 11 (B,C) somites after in situ hybridisation to detect Shh (n=5 each). Arrowhead indicates the position of forming ant-tub boundary. (D-F) Sagittal sections of 11-somite chick after double fluorescent in situ hybridisation to detect Shh/Foxg1, shown in double (D) or single (E,F) channel view (n=3). Arrowheads indicate the Shh-Foxg1 border. (G-I) Ventral views of flat-mounted isolated prosencephalon at HH10+ (11 somites, G) (n=2), HH12 (H) (n=3) and HH13 (I) (n=7), hybridised for p57Kip2/Shh. p57Kip2 expands over HH10+ to HH13. Dotted lines indicate approximate ant-tub boundary. (J-L) At HH13, p57Kip2 is detected anterior to Shh (blue line shows border; n=5). Scale bars: 100 µm in A,B,G-L; 50 µm in C-F.
Shh signalling over HH10-13 is required for establishment of the neuroendocrine axis
We next investigated the mechanism that governs the growth and differentiation of anterior progenitors. Conditional manipulation studies in mouse and zebrafish have suggested a role for neuroepithelial-derived Shh in these events (Shimogori et al., 2010; Blaess et al., 2015; Zhao et al., 2012; Muthu et al., 2016; Orquera et al., 2016), but no study has examined whether this reflects a role for Shh in the early differentiation of Fgf10+ progenitors.
To address this, we exposed HH9 (7-8 somite) embryos to cyclopamine, a pharmacological inhibitor of Shh signalling (Chen et al., 2002), then analysed embryos at progressive stages. Cyclopamine effectively (but transiently) inhibited Shh signalling, as judged by the downregulation of Ptch1 in anterior progenitors at HH10-13 (Fig. S10; Ptch1 is detected again by HH15). An additional consequence of inhibition of Shh signalling was the loss of detectable p57Kip2 (Fig. S11).
We then analysed anterior and tuberal progenitor domains at later times. All embryos in which Shh signalling was transiently eliminated showed consistent and predictable disruption to the anterior progenitor domain at E5. Although there was no significant difference in the length of the Fgf10+ progenitor domain, the length of the Six3+Foxg1− progenitor domain was significantly reduced (Fig. 7A-H). Six3 and Fgf10 maintained their common caudal boundary in cyclopamine-treated embryos (Fig. S12). Together, this means that transient blockade of Shh signalling leads to the specific loss of anterior progenitor territory.
Fig. 7.
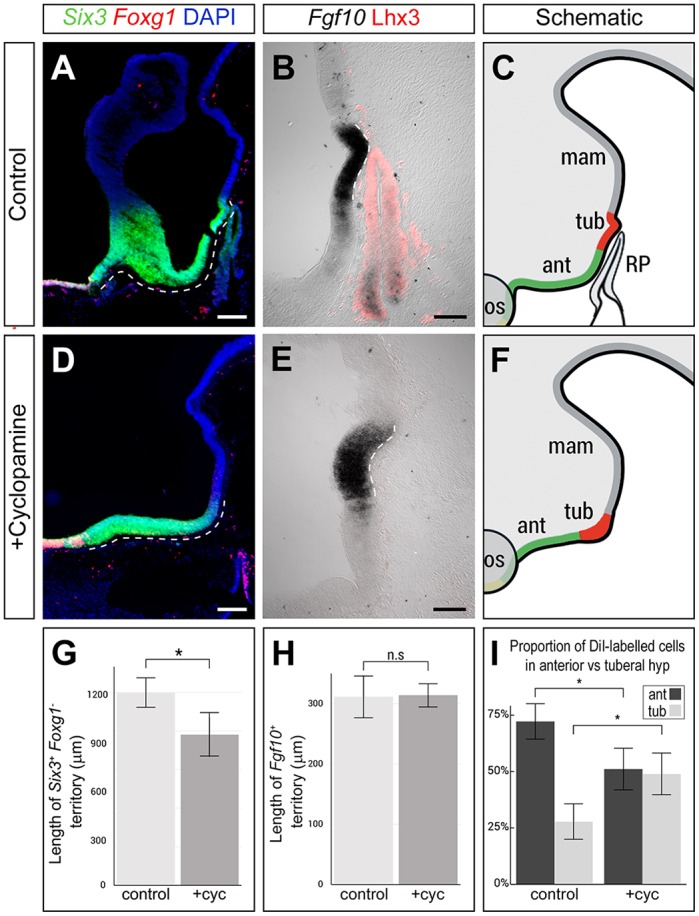
Shh signalling at HH10-HH15 is required for differentiation of the anterior hypothalamus and RP. (A-H) Analysis of Six3/Foxg1 and Fgf10/Lhx3 on E5 sagittal sections following PBS (control) or cyclopamine treatment at HH9. (A,D,G) Six3+Foxg1− territory (dashed lines in A,D) is significantly shorter in cyclopamine-treated embryos than in controls (*P<0.05; two-tailed unpaired t-test; n=5 each). (B,E,H) The length of the Fgf10+ tuberal territory (dashed lines in B,E) is not significantly affected in cyclopamine-treated embryos (P>0.05; two-tailed unpaired t-test; n=5 each). After cyclopamine exposure, the Fgf10+ tuberal progenitor domain is significantly wider and broader (total volume 3.2±0.37×106 µm3 compared with 1.45±0.05×106 µm3 in controls; P=0.0032; n=4 embryos). In addition, the infundibulum is absent and RP fails to develop (rudimentary pouch in this embryo shown in Fig. S13D). Sagittal views in A,B are serial adjacent; sagittal view in E is 45 µm from that in D; schematics in C,F summarise data from sections between A and B, D and E, respectively. (I) A significantly higher proportion of DiI-labelled cells are retained in Fgf10+ tuberal progenitors in cyclopamine-treated embryos compared with controls (*P<0.05; two-tailed unpaired t-test; n=4 each). Scale bars: 200 µm in A,D; 100 µm in B,E.
Notably, although the Fgf10+ progenitor domain was not expanded in length in cyclopamine-treated embryos, it appeared significantly wider and broader than in controls (Fig. 7E,F). This raises the possibility that although blockade of Shh signalling prevents growth and differentiation of anterior progenitors, it does not prevent continued proliferation of Fgf10+ progenitors, which then accumulate aberrantly. To test this, embryos were treated with cyclopamine or PBS at HH9, injected with DiI into region A at 9-10 somites, incubated until HH20, and then assayed for DiI labelling and Fgf10 expression to obtain a quantitative picture of the contribution of cells to anterior versus tuberal progenitor domains. In control embryos, significantly more cells contributed to the Fgf10− anterior than to the Fgf10+ tuberal progenitor domain. By contrast, in cyclopamine-treated embryos, there was a significant reduction to the contribution of cells to the anterior progenitor domain, and a significant increase to the contribution of cells to the Fgf10+ tuberal progenitor domain (Fig. 7I). Thus, a larger proportion of DiI-labelled cells remain in the Fgf10+ tuberal hypothalamus in cyclopamine-treated embryos. Together, this suggests a role for Shh signalling in the appropriate growth of anterior and tuberal hypothalamic domains.
To determine the consequences of the loss of anterior progenitor territory on neuronal differentiation, we examined the tract of the post-optic commissure (TPOC), a tract that develops from the earliest-differentiating neurons in the anterior hypothalamus (Ware and Schubert, 2011). We detected a significant reduction in the number of neurons in the TPOC, both 24 h and 48 h after the transient blockade of Shh signalling (Fig. 8). Finally, in cyclopamine-treated embryos, the infundibulum was completely absent (n=8/10; Fig. 7D,E) or present only as a rudimentary evagination (n=2/10; Fig. S13). Additionally, these embryos displayed a spectrum of disruptions to RP. In each, a rudimentary pouch formed that expressed Six3 (n=5/6) but did not express Lhx3 (n=4/4) (Fig. S13). In 7/10 embryos, the rudimentary pouch was not in close proximity to the ventral hypothalamus; instead, mesenchymal cells filled the intervening space (Fig. S13). Furthermore, the subset of cyclopamine-treated embryos that showed a rudimentary infundibulum exhibited a second ectopic rudimentary pouch-like structure (n=2/10; Fig. S13). Together, our studies reveal the importance of Shh signalling to the integrated development of the hypothalamo-pituitary axis.
Fig. 8.
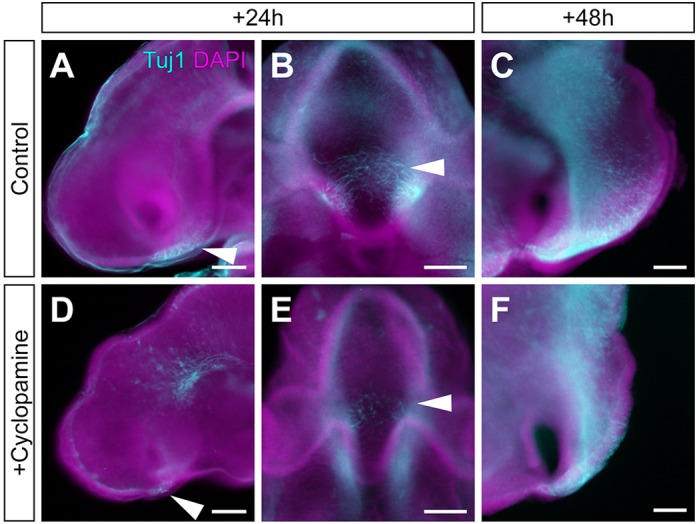
Reduction in tract of the post-optic commissure (TPOC) after transient blockade of Shh signalling. (A-F) TUJI immunolabelling of HH17 (A,B,D,E) or HH20 (C,F) embryos, in whole-mount lateral view (A,D), whole-mount ventral view (B,E) or isolated neuroepithelial view (C,F) after exposure to PBS (A-C, control) or cyclopamine (D,E). The TPOC (arrowheads) develops robustly in control embryos (A-C), but is significantly reduced in cyclopamine-exposed embryos after 24 h (88±52 µm, n=10) compared with controls (202±47 µm, n=5; P<0.01 two-tailed unpaired t-test) and 48 h (357±97 µm, n=8) compared with controls (623±78 µm, n=6; P<0.001; two-tailed unpaired t-test). Concomitantly, the anterior progenitor domain is reduced in length. Scale bars: 100 µm.
DISCUSSION
Here, we describe how rostro-caudal domains of the hypothalamus form in the embryonic chick. We show that the presumptive hypothalamus originates in neural tissue of the RDVM that lies directly above the PM and is composed of Fgf10+ progenitors. These give rise to anterior (Shh+Fgf10−) and mammillary (Emx2+Fgf10−) progenitors through bi-directional (rostral and caudal) expansion, whereas a stable population of Fgf10+ cells becomes restricted to the tuberal hypothalamus, including the infundibulum. Shh signalling is essential for the growth and differentiation of the anterior hypothalamus, including its resident neurons, and for development of the infundibulum and RP.
Fgf10 expression defines hypothalamic progenitors above the PM
Our fate-mapping studies define the location of hypothalamic progenitors at 9-10 somites. We pinpoint the position of anterior-most progenitors that will populate regions close to the optic stalk, posterior-most progenitors that will populate the caudal mammillary pouch and, along the medio-lateral axis, show that hypothalamic progenitors form a triangular territory that tapers posteriorly. This territory correlates with the expression profile of Fgf10, suggesting that Fgf10 is a defining marker for hypothalamic progenitors. In support of this, at 9-10 somites, Fgf10+ progenitors anteriorly abut Foxg1 progenitors to define the hypothalamic-telencephalic interface.
Our data lend weight to the idea that the hypothalamus arises from part of the neural tube characterised by proliferating progenitors (termed the acroterminal region; Puelles and Rubenstein, 2015), but show conclusively that, at 9-10 somites, the prospective hypothalamus is underlain by the PM. In fact, the position and shape of the PM anticipates the developing hypothalamus and thus could facilitate signalling between the two tissues as late as 9-10 somites. Our previous studies have shown that BMPs become active in the PM at the 7-somite stage (Ellis et al., 2015), and have shown that BMP signalling from the PM is required for Shh downregulation and Fgf10 upregulation in RDVM cells (Manning et al., 2006). These observations, and those here, suggest that BMP signalling from the PM induces Fgf10 in overlying neuroepithelial cells, to define the presumptive hypothalamus.
Development of anterior, tuberal and mammillary progenitor domains
We present numerous lines of evidence to show that Fgf10+ progenitors give rise to Fgf10− anterior and Fgf10− mammillary progenitors. First, fate-mapping shows that Fgf10+ progenitors contribute to the entire anterior and mammillary hypothalamus. Second, both phosphoH3 and EdU-labelling analyses reveal higher levels of proliferation in Fgf10+ progenitors than in emerging Shh+ anterior or Emx2+ mammillary progenitors, suggestive of rostral and caudal growth fronts. Third, although Fgf10+ progenitors proliferate rapidly, possibly in response to autocrine FGF signalling, the length of the Fgf10+ territory remains remarkably constant over E1.5 (HH10) to E5 (the latest stage examined), whereas anterior and mammillary domains become apparent and then lengthen throughout this period. This suggests that the rate at which cells are retained by, and displaced from, the Fgf10+ territory remains constant and ensures a stable population(s) of undifferentiated hypothalamic progenitors. We propose that displacement of cells away from the Fgf10+ territory marks an early step in the differentiation of cells to an anterior or mammillary fate. In this process, cells become refractory to paracrine FGF signals from the progenitor domain, which could promote loss of the undifferentiated hypothalamic progenitor state.
As yet, we have not explored the factors that determine whether Fgf10+ progenitors give rise to anterior versus mammillary progenitors. However, our studies suggest the position of Fgf10+ progenitors along the A-P axis at 9-10 somites may restrict their fate: region A progenitors give rise to anterior and rostro-tuberal, but not mammillary, progenitors, whereas region C progenitors give rise to caudo-tuberal and mammillary, but not anterior progenitors. This could reflect an exposure to different extrinsic factors: anterior hypothalamic progenitors lie above the pharyngeal endoderm/prechordal mesoderm, and in close proximity to the oral ectoderm/hypophyseal placode/nascent telencephalon, whereas posterior hypothalamic progenitors lie above the prechordal mesoderm and close to the notochord/nascent midbrain. Further studies are required to investigate this.
Shh signalling promotes the differentiation of Fgf10+ to Fgf10− anterior progenitors
Here, we have focused on the mechanism that promotes anterior development and describe an essential role for Shh signalling (Fig. 9). We demonstrate, using cyclopamine treatment, that Shh signalling is required for Fgf10+ progenitors to give rise to anterior progenitors. This, together with our cell cycle analyses, suggests that Shh acts on Fgf10+ progenitors to promote their differentiation (Fig. 9). In this model, Shh is downregulated in Fgf10+ progenitors but upregulated in emerging anterior progenitors (this study; Aglyamova and Agarwala, 2007), and Shh is then required to upregulate the differentiation marker p57Kip2. Such a mechanism suggests parallels to anterior hypothalamic development in zebrafish, where Shh signalling induces the transcription factor rx3 to select Shh+ anterior progenitors, then downregulates rx3, which allows progenitors to grow and differentiate appropriately (Muthu et al., 2016). Indeed, the proposed mechanism of action of Shh in growth and specification may be widely conserved: in mouse, conditional ablation of Shh from anterior hypothalamic cells prevents anterior hypothalamic differentiation (Shimogori et al., 2010; Zhao et al., 2012; reviewed by Blaess et al., 2015) and deletion of Shh from Hesx1+ cells leads to a phenotype that closely resembles the one we describe here, including the loss of anterior hypothalamic cells, and disruption to the tuberal hypothalamus (Carreno et al., 2017).
Fig. 9.
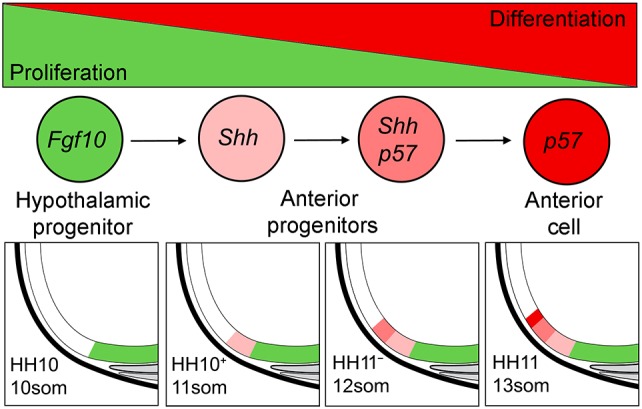
Model for anterior hypothalamic development from Fgf10+ progenitors. The hypothalamus forms from a stable population of highly proliferative Fgf10+ progenitors (green) that later contribute to tuberal domains (green). Shh signalling promotes the equal retention of Fgf10+ progenitors and displacement of Fgf10− progenitors (light pink). Over time, such progenitors become displaced more rostrally and express p57Kip2 (dark pink). These downregulate Shh and differentiate (red). Arrows indicate displacement of cells in caudal to rostral direction.
Our work shows that the decrease in anterior progenitors after cyclopamine exposure leads to a reduction in differentiated neurons of the TPOC. We have not investigated whether specific subsets of neurons are absent, but conditional deletion of Shh in zebrafish and mouse leads to a loss of both ‘anterior’ and ‘tuberal’ neurons (Shimogori et al., 2010; Zhao et al., 2012; Muthu et al., 2016; Orquera et al., 2016; Carreno et al., 2017). Thus, we suggest that anterior Shh+ progenitors will give rise to both differentiated anterior and tuberal neurons, a model supported through lineage-tracing studies in mouse (Alvarez-Bolado et al., 2012). Our studies are consistent with a model in which the earliest Fgf10+ progenitors to express Shh and p57Kip2 give rise to rostral-most hypothalamic neurons (e.g. those of the suprachiasmatic nucleus), then later ones differentiate to give rise to anterior neurons, and then the final ones give rise to tuberal neurons. In support of this, birthdating studies in mouse show that anterior neurons are generated prior to tuberal neurons (Shimada and Nakamura, 1973).
Coordinated development of hypothalamo-pituitary neuraxis
What is the fate of Fgf10+ progenitors that persist in the tuberal hypothalamus? Previous studies have shown that Fgf10 persists in non-proliferating infundibular cells and proliferating collar cells that are retained at the ventricular zone (Pearson et al., 2011). We detect p57Kip2, a regulator of cell-cycle exit, in a subset of Fgf10+ progenitors at HH18-26, and predict these are emerging infundibular cells. These require FGF signalling to form (Pearson et al., 2011), suggesting that infundibular cells can develop through prolonged exposure to autocrine FGF signalling. Collar cells, by contrast, do not immediately upregulate p57Kip2 (Fig. S9), suggesting that they persist as proliferating cells or as cells that may enter quiescence at a later stage. Studies in chick and mouse have described Fgf3/10+ stem-like cells within the tuberal hypothalamus that persist as quiescent cells in postnatal life (Hajihosseini et al., 2008; Pearson et al., 2011; Robins et al., 2013). Our studies raise the possibility that some descendants of embryonic Fgf10+ progenitors persist as quiescent stem-like cells in the postnatal hypothalamus.
Our data reveal that Shh signalling is necessary for both development of the infundibulum and of RP: transient blockade of Shh signalling prevents formation of the infundibulum and prevents the differentiation of the Lhx3+ RP. A rudimentary pouch is detected, but is separated from the hypothalamus by a loose meshwork of mesenchymal cells. Parallel studies in mouse show a highly similar phenotype after conditional deletion of Shh from Hesx1+ cells (Carreno et al., 2017). As yet, the mechanisms behind these phenotypes are not clear. Shh could act directly on each tissue – for example, in the tuberal hypothalamus, Shh could govern the transition of cycling Fgf10+ progenitors to non-cycling infundibular cells (Pearson et al., 2011). Alternatively, the effects of Shh could be indirect: blockade of Shh signalling leads to abnormal accumulation of Fgf10+ progenitors and abnormal broadening of the tuberal domain, which could in turn restrict infundibular development. Likewise, the failure of the infundibulum to form could, in turn, affect RP development (or vice-versa): many previous studies have suggested that reciprocal signalling events govern development of the infundibulum and RP, and that intimate contact between the two forming tissues is essential for their differentiation (reviewed by Davis et al., 2013; Rizzoti, 2015).
Nonetheless, severe disruptions to the neuronal hypothalamus, the infundibulum and RP reveal the importance of Shh signalling for the integrated development of the hypothalamo-pituitary neuraxis. Our work, together with that of Carreno et al. (2017), suggests that the action of Shh is evolutionarily conserved. Future studies using the chick embryo, an animal that is particularly well-suited to conditional interference, will allow us to determine whether Shh acts directly or indirectly to orchestrate development of the hypothalamo-pituitary axis and will enable us to probe the mechanisms that may direct Fgf10+ progenitors to populate the anterior, infundibular or mammillary domains.
MATERIALS AND METHODS
Chick embryos
Fertilised Bovan brown chicken eggs (Henry Stewart & Co., Norfolk, UK) were staged according to Hamburger and Hamilton (1951). All experiments conformed to relevant regulatory and ethical standards (University of Sheffield). Hemi-dissection of E3-E5 chick embryos was performed by removal of retina, mesenchyme and ectoderm surrounding one half of the embryo. Neuroepithelial tissue was isolated after dispase treatment (Pearson et al., 2011).
Fate mapping the ventral prosencephalon
Embryos (9-10 somites) were windowed and a dorsal incision made to access the ventral prosencephalon. Carbocyanine dyes, DiI (CM-DiI, Life Technologies) and DiO (Vybrant DiO, Life Technologies) were injected using a picospritzer II microinjection system (Parker Instrumentation). Injection sites were identified relative to the underlying PM and visualised through injection of blue food colouring (Dr Oetker).
Cell proliferation
HH15 or HH20 embryos were treated in ovo with 200 µl of 0.5 mM 5-ethynyl-2′-deoxyuridine (EdU, Life Technologies) for 90 min, fixed with 4% PFA and sagittally cryosectioned. Proliferating cells were detected using the Click-iT EdU kit (Life Technologies) as described by the supplier's protocol. To quantify EdU-labelling density, the antero-tuberal or tubero-mammillary hypothalamus were each partitioned into eight equal lengths and the integrated density of each measured in Adobe Photoshop.
Cyclopamine treatment
HH9 embryos were treated with 5 µl of 0.48 mM cyclopamine (Sigma) in PBS, pipetted over an incision in the vitelline membrane above the prosencephalon. After development, embryos were fixed in 4% paraformaldehyde, then processed by in situ hybridisation or immunohistochemistry. The optimal concentration of cyclopamine was determined through a concentration curve analysis.
In situ hybridisation and immunohistochemistry
Whole-mount and cryosectioned embryos were analysed by immunohistochemistry and in situ hybridisation according to standard techniques (Manning et al., 2006). Fluorescent in situ hybridisation was performed using the TSA Plus Cyanine 3/Fluorescein System (PerkinElmer, NEL753001KT) according to the supplier's protocol. Primary antibodies used were: anti-LHX3 (1:50; 67.4E12, DSHB) and anti-TUJ1 (1:1000; Covance). Secondary antibodies used were Alexa Fluor 488 and 594 (1:500; Molecular Probes).
Measurements of mRNA expression and morphological features
From HH10 to HH27, the tuberal progenitor domain was determined by measuring the length of Fgf10 expression domain; the anterior progenitor domain was determined by subtraction of this from the Six3+Foxg1− antero-tuberal domain, measured in serial adjacent sections; the mammillary progenitor domain was determined by measuring the Emx2+ territory. Measurements were carried out using ImageJ (v1.51).
Statistics
Statistics were performed and graphs were generated in Microsoft Excel.
Acknowledgements
We thank K. Storey (University of Dundee, UK) for p57Kip2 plasmid, anonymous reviewers for comments and Liz Manning for Fig. S6.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: T.F., M.T., M.A.P.; Methodology: T.F.; Formal analysis: T.F.; Investigation: T.F.; Resources: M.T., M.A.P.; Writing - original draft: M.A.P.; Writing - review & editing: T.F., M.T.; Visualization: T.F.; Supervision: M.T., M.A.P.; Funding acquisition: M.T., M.A.P.
Funding
This work was supported by the UK Medical Research Council (G0401310 to M.P.; G1100295 to M.T.) and the Wellcome Trust (202756/Z/16/Z to M.T.). Deposited in PMC for immediate release.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.153379.supplemental
References
- Aglyamova G. V. and Agarwala S. (2007). Gene expression analysis of the hedgehog signaling cascade in the chick midbrain and spinal cord. Dev. Dyn. 236, 1363-1373. 10.1002/dvdy.21146 [DOI] [PubMed] [Google Scholar]
- Ahlgren S., Vogt P. and Bronner-Fraser M. (2003). Excess FoxG1 causes overgrowth of the neural tube. J. Neurobiol. 57, 337-349. 10.1002/neu.10287 [DOI] [PubMed] [Google Scholar]
- Alvarez-Bolado G., Paul F. A. and Blaess S. (2012). Sonic hedgehog lineage in the mouse hypothalamus: from progenitor domains to hypothalamic regions. Neural Dev. 7, 4 10.1186/1749-8104-7-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedont J. L., Newman E. A. and Blackshaw S. (2015). Patterning, specification, and differentiation in the developing hypothalamus. Wiley Interdiscip. Rev. Dev. Biol. 4, 445-468. 10.1002/wdev.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell E., Ensini M., Gulisano M. and Lumsden A. (2001). Dynamic domains of gene expression in the early avian forebrain. Dev. Biol. 236, 76-88. 10.1006/dbio.2001.0301 [DOI] [PubMed] [Google Scholar]
- Blaess S., Szabó N., Haddad-Tóvolli R., Zhou X. and Álvarez-Bolado G. (2015). Sonic hedgehog signaling in the development of the mouse hypothalamus. Front. Neuroanat. 8, 156 10.3389/fnana.2014.00156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovolenta P., Mallamaci A., Puelles L. and Boncinelli E. (1998). Expression pattern of cSix3, a member of the Six/sine oculis family of transcription factors. Mech. Dev. 70, 201-203. 10.1016/S0925-4773(97)00183-4 [DOI] [PubMed] [Google Scholar]
- Burbridge S., Stewart I. and Placzek M. (2016). Development of the neuroendocrine hypothalamus. Compr. Physiol. 6, 623-643. 10.1002/cphy.c150023 [DOI] [PubMed] [Google Scholar]
- Carreno G., Apps J. R., Lodge E. J., Panousopoulos L., Haston S., Gonzalez-Meljem J. M., Hahn H., Andoniadou C. L. and Martinez-Barbera J. P. (2017). Hypothalamic sonic hedgehog is required for cell specification and proliferation of LHX3/LHX4 pituitary embryonic precursors. Development 144, 3289-3302. 10.1242/dev.153387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman S. C., Schubert F. R., Schoenwolf G. C. and Lumsden A. (2002). Analysis of spatial and temporal gene expression patterns in blastula and gastrula stage chick embryos. Dev. Biol. 245, 187-199. 10.1006/dbio.2002.0641 [DOI] [PubMed] [Google Scholar]
- Chen J. K., Taipale J., Cooper M. K. and Beachy P. A. (2002). Inhibition of Hedgehog signaling by direct binding of cyclopamine to Smoothened. Genes Dev. 16, 2743-2748. 10.1101/gad.1025302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale J. K., Vesque C., Lints T. J., Sampath T. K., Furley A., Dodd J. and Placzek M. (1997). Cooperation of BMP7 and SHH in the induction of forebrain ventral midline cells by prechordal mesoderm. Cell 90, 257-269. 10.1016/S0092-8674(00)80334-7 [DOI] [PubMed] [Google Scholar]
- Dale K., Sattar N., Heemskerk J., Clarke J. D., Placzek M. and Dodd J. (1999). Differential patterning of ventral midline cells by axial mesoderm is regulated by BMP7 and chordin. Development 126, 397-408. [DOI] [PubMed] [Google Scholar]
- Davis S. W., Ellsworth B. S., Peréz Millan M. I., Gergics P., Schade V., Foyouzi N., Brinkmeier M. L., Mortensen A. H. and Camper S. A. (2013). Pituitary gland development and disease: from stem cell to hormone production. Curr. Top. Dev. Biol. 106, 1-47. 10.1016/B978-0-12-416021-7.00001-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis P. S., Burbridge S., Soubes S., Ohyama K., Ben-Haim N., Chen C., Dale K., Shen M. M., Constam D. and Placzek M. (2015). ProNodal acts via FGFR3 to govern duration of Shh expression in the prechordal mesoderm. Development 142, 3821-3832. 10.1242/dev.119628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galderisi U., Jori F. P. and Giordano A. (2003). Cell cycle regulation and neural differentiation. Oncogene 22, 5208-5219. 10.1038/sj.onc.1206558 [DOI] [PubMed] [Google Scholar]
- García-Calero E., de Puelles E. and Puelles L. (2006). EphA7 receptor is expressed differentially at chicken prosomeric boundaries. Neuroscience 141, 1887-1897. 10.1016/j.neuroscience.2006.04.074 [DOI] [PubMed] [Google Scholar]
- García-Calero E., Fernández-Garre P., Martínez S. and Puelles L. (2008). Early mammillary pouch specification in the course of prechordal ventralization of the forebrain tegmentum. Dev. Biol. 320, 366-377. 10.1016/j.ydbio.2008.05.545 [DOI] [PubMed] [Google Scholar]
- Garcia-Lopez R., Vieira C., Echevarria D. and Martinez S. (2004). Fate map of the diencephalon and the zona limitans at the 10-somites stage in chick embryos. Dev. Biol. 268, 514-530. 10.1016/j.ydbio.2003.12.038 [DOI] [PubMed] [Google Scholar]
- Gimeno L. and Martinez S. (2007). Expression of chick Fgf19 and mouse Fgf15 orthologs is regulated in the developing brain by Fgf8 and Shh. Dev. Dyn. 236, 2285-2297. 10.1002/dvdy.21237 [DOI] [PubMed] [Google Scholar]
- Hajihosseini M. K., De Langhe S., Lana-Elola E., Morrison H., Sparshott N., Kelly R., Sharpe J., Rice D. and Bellusci S. (2008). Localization and fate of Fgf10-expressing cells in the adult mouse brain implicate Fgf10 in control of neurogenesis. Mol. Cell. Neurosci. 37, 857-868. 10.1016/j.mcn.2008.01.008 [DOI] [PubMed] [Google Scholar]
- Hamburger V. and Hamilton H. L. (1951). A series of normal stages in the development of the chick embryo. J. Morphol. 88, 49-92. 10.1002/jmor.1050880104 [DOI] [PubMed] [Google Scholar]
- Hurtado R. and Mikawa T. (2006). Enhanced sensitivity and stability in two-color in situ hybridization by means of a novel chromagenic substrate combination. Dev. Dyn. 235, 2811-2816. 10.1002/dvdy.20909 [DOI] [PubMed] [Google Scholar]
- Manning L., Ohyama K., Saeger B., Hatano O., Wilson S. A., Logan M. and Placzek M. (2006). Regional morphogenesis in the hypothalamus: a BMP-Tbx2 pathway coordinates fate and proliferation through Shh downregulation. Dev. Cell 11, 873-885. 10.1016/j.devcel.2006.09.021 [DOI] [PubMed] [Google Scholar]
- Muthu V., Eachus H., Ellis P., Brown S. and Placzek M. (2016). Rx3 and Shh direct anisotropic growth and specification in the zebrafish tuberal/anterior hypothalamus. Development 143, 2651-2663. 10.1242/dev.138305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama K., Ellis P., Kimura S. and Placzek M. (2005). Directed differentiation of neural cells to hypothalamic dopaminergic neurons. Development 132, 5185-5197. 10.1242/dev.02094 [DOI] [PubMed] [Google Scholar]
- Orquera D. P., Nasif S., Low M. J., Rubinstein M. and de Souza F. S. J. (2016). Essential function of the transcription factor Rax in the early patterning of the mammalian hypothalamus. Dev. Biol. 416, 212-224. 10.1016/j.ydbio.2016.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson N., Collins M. M., Dufresne L. and Ryan A. K. (2010). Expression patterns of hormones, signaling molecules, and transcription factors during adenohypophysis development in the chick embryo. Dev. Dyn. 239, 1197-1210. 10.1002/dvdy.22250 [DOI] [PubMed] [Google Scholar]
- Patten I., Kulesa P., Shen M. M., Fraser S. and Placzek M. (2003). Distinct modes of floor plate induction in the chick embryo. Development 130, 4809-4821. 10.1242/dev.00694 [DOI] [PubMed] [Google Scholar]
- Pearson C. A., Ohyama K., Manning L., Aghamohammadzadeh S., Sang H. and Placzek M. (2011). FGF-dependent midline-derived progenitor cells in hypothalamic infundibular development. Development 138, 2613-2624. 10.1242/dev.062794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pera E. M. and Kessel M. (1997). Patterning of the chick forebrain anlage by the prechordal plate. Development 124, 4153-4162. [DOI] [PubMed] [Google Scholar]
- Placzek M. and Briscoe J. (2005). The floor plate: multiple cells, multiple signals. Nat. Rev. Neurosci. 6, 230-240. 10.1038/nrn1628 [DOI] [PubMed] [Google Scholar]
- Puelles L. and Rubenstein J. L. R. (2015). A new scenario of hypothalamic organization: rationale of new hypotheses introduced in the updated prosomeric model. Front. Neuroanat. 9, 27 10.3389/fnana.2015.00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzoti K. (2015). Genetic regulation of murine pituitary development. J. Mol. Endocrinol. 54, R55-R73. 10.1530/JME-14-0237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins S. C., Stewart I., McNay D. E., Taylor V., Giachino C., Goetz M., Ninkovic J., Briancon N., Maratos-Flier E., Flier J. S. et al. (2013). α-Tanycytes of the adult hypothalamic third ventricle include distinct populations of FGF-responsive neural progenitors. Nat. Commun. 4, 2049 10.1038/ncomms3049 [DOI] [PubMed] [Google Scholar]
- Sanchez-Arrones L., Stern C. D., Bovolenta P. and Puelles L. (2012). Sharpening of the anterior neural border in the chick by rostral endoderm signalling. Development 139, 1034-1044. 10.1242/dev.067934 [DOI] [PubMed] [Google Scholar]
- Sanchez-Arrones L., Ferran J.,L., Hidalgo-Sanchez M. and Puelles L. (2015). Origin and early development of the chick adenohypophysis. Front. Neuroanat. 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert F. R. and Lumsden A. (2005). Transcriptional control of early tract formation in the embryonic chick midbrain. Development 132, 1785-1793. 10.1242/dev.01731 [DOI] [PubMed] [Google Scholar]
- Shimada M. and Nakamura T. (1973). Time of neuron origin in mouse hypothalamic nuclei. Exp. Neurol. 41, 163-173. 10.1016/0014-4886(73)90187-8 [DOI] [PubMed] [Google Scholar]
- Shimamura K. and Rubenstein J. L. (1997). Inductive interactions direct early regionalization of the mouse forebrain. Development 124, 2709-2718. [DOI] [PubMed] [Google Scholar]
- Shimogori T., Lee D. A., Miranda-Angulo A., Yang Y., Wang H., Jiang L., Yoshida A. C., Kataoka A., Mashiko H., Avetisyan M. et al. (2010). A genomic atlas of mouse hypothalamic development. Nat. Neurosci. 13, 767-775. 10.1038/nn.2545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson L. W. (1987). The hypothalamus. In Handbook of Chemical Neuroanatomy (ed. Björlund A., Hökfelt T., and Swanson L. W. ), pp. 1-124. Amsterdam: Elsevier. [Google Scholar]
- Ware M. and Schubert F. R. (2011). Development of the early axon scaffold in the rostral brain of the chick embryo. J. Anat. 219, 203-216. 10.1111/j.1469-7580.2011.01389.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Zevallos S. E., Rizzoti K., Jeong Y., Lovell-Badge R. and Epstein D. J. (2012). Disruption of SoxB1-dependent Sonic hedgehog expression in the hypothalamus causes septo-optic dysplasia. Dev. Cell 22, 585-596. 10.1016/j.devcel.2011.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]