Abstract
Acute fibrinous and organising pneumonia (AFOP) is a rare histological pattern of interstitial lung disease. The authors describe a 60-year-old woman admitted to the hospital for sustained fever, presenting with an alveolar opacity on chest X-ray, with the presumed diagnosis of community-acquired pneumonia and the onset of antibiotics. Since serological results suggested that Legionella pneumophila was the infectious agent, she was discharged on levofloxacin. A week later, she was again admitted with fever. CT scan showed opacities with crescentic morphology and a central ground-glass area suggestive of cryptogenic organising pneumonia. Microbiological, serological and autoimmunity tests were negative. She underwent surgical lung biopsy that revealed inflammatory infiltrate, macrophage desquamation, fibroblasts proliferation and fibrin deposition in the alveolar spaces, consistent with AFOP. She started corticotherapy with good response. Disease relapsed after prednisolone discontinuation, 10 months later. Currently, the patient is on prednisolone 5 mg/day without clinical and radiological recurrence.
Keywords: Pneumonia (infectious disease), Radiology, Interstitial lung disease
Background
Interstitial lung diseases (ILDs) cover a wide and heterogeneous range of disorders that are characterised by inflammation and fibrosis of the pulmonary interstitium. Acute fibrinous and organising pneumonia (AFOP), first described in 2002 by Beasley et al,1 belongs to this group. The diagnosis of ILD may be challenging, requiring the performance of invasive procedures and histological assessment to reach a definite diagnosis.
Case presentation
A 60-year-old Caucasian woman was admitted to the hospital with fever and night sweats, myalgias, anorexia and unquantified weight loss over the last 2 weeks. She also reported sporadic dry cough. Her personal history included peripheral vertiginous syndrome and hypercholesterolaemia, medicated for a long time with betahistine, cinnarizine and pitavistatin. On admission, she had an axillary temperature of 38°C, and inspiratory basal crackles could be heard on the right hemithorax.
Investigations
Analytically, C-reactive protein (CRP; 86 mg/L) and erythrocyte sedimentation rate (ESR; 96 mm/h) were elevated. Renal function and urinalyses were normal. Chest X-ray showed an alveolar opacity in the right lower lobe (figure 1), which prompted empirical antibiotic therapy with ceftriaxone and azithromycin. Clinical and radiological worsening (figure 2), with bilateral involvement, led to empirical antibiotic escalation to meropenem. Since serological results were compatible with acute Legionella pneumophila infection (equivocal IgM antibody titer), therapy was switched to levofloxacin, with clinical improvement (afebrile from the fourth day). The chest CT scan showed areas of parenchymal consolidation with air bronchogram at the base of both lung fields, surrounded by areas of ground-glass appearance and, scattered throughout lung parenchyma, predominantly in the lower lobes, nodular opacities with poorly defined limits. Considering the good clinical response, the patient was discharged, without fever, on the 10th day of levofloxacin, fulfilling 2 weeks of therapy at home. A week later, she was again admitted to the hospital for fever, dry cough and pleuritic chest pain. Auscultation revealed bibasal crackles consistent with de novo bilateral alveolar infiltrates seen at the chest X-ray. Inflammatory parameters remained high (ESR 107 mm/h and CRP 67 mg/L). A new CT scan was performed revealing multiple consolidation areas throughout the lung parenchyma, on both sides and resolution of some infiltrates present on the previous exam, consistent with migratory opacities. Some of these opacities showed crescentic morphology with a central ground-glass area (atoll sign) suggestive of cryptogenic organising pneumonia (figure 3). Microbiological (blood and sputum cultures), serological (no seroconversion for L. pneumophila was observed) and autoimmunity laboratory tests, (antinuclear antibodies, extractable nuclear antigens and anti-neutrophil cytoplasmic antibody) were negative. Transthoracic echocardiography revealed a thin layer of pericardial effusion but no signs of infective endocarditis. Lung function tests, with spirometry and lung volumes, showed restrictive ventilatory pattern with non-reversible obstructive component as well as impaired diffusing capacity for carbon monoxide (49% of predicted value). Arterial blood gas analysis featured hypoxaemia criteria at rest. The patient underwent flexible bronchofibroscopy, which revealed no endobronchial lesions, followed by bronchoalveolar lavage and transbronchial biopsies. The bronchoalveolar lavage specimen showed a predominance of macrophages (82%) and lymphocytes (17%; CD4/CD8 1.2), with no pathogenic micro-organisms and tumour cells. Transbronchial biopsy histology was inconclusive due to the small size of the samples. Finally, the patient underwent video-assisted thoracoscopic lung biopsy that revealed moderate enlargement of interalveolar septa by inflammatory infiltrate of mixed type, with lymphocytes, plasma cells and polymorphonuclear neutrophils associated with macrophage desquamation and fibrin deposition in the alveolar spaces. It further showed hyperplasia of type II pneumocytes and fibroblast proliferation, findings consistent with acute fibrinous and organising pneumonia (figure 4).
Figure 1.
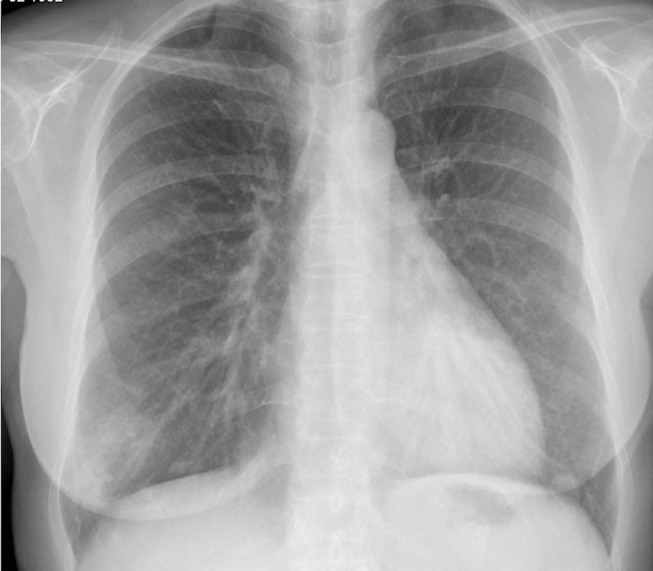
Chest X-ray revealing alveolar pattern infiltrate in the right lower lobe.
Figure 2.
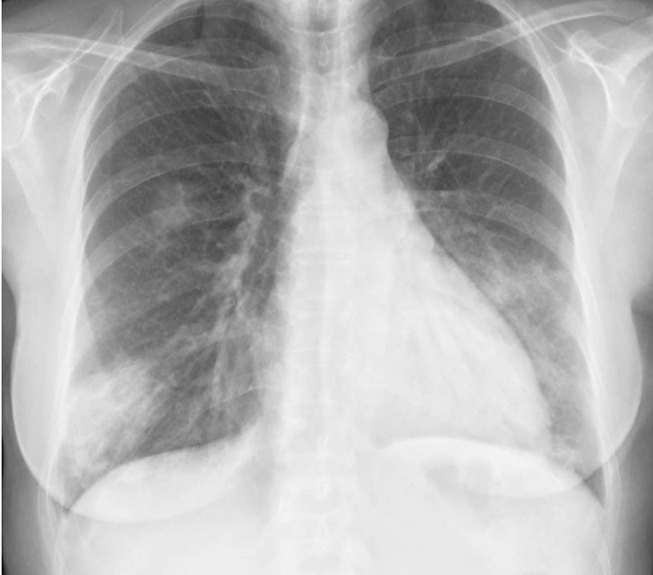
Chest X-ray (day 9) showing worsening of the alveolar opacity in the right lower lobe, with air bronchogram, as well as the appearance of new alveolar pattern opacities at the left lower lobe.
Figure 3.
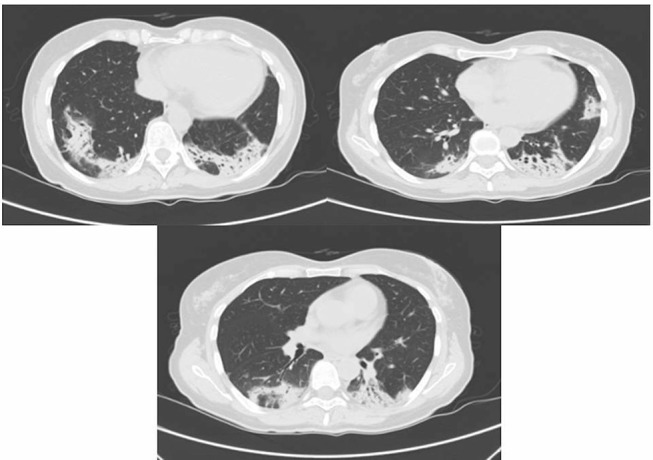
Contrast-enhanced CT scan showing areas of pulmonary consolidation with bilateral distribution and ground-glass opacities.
Figure 4.
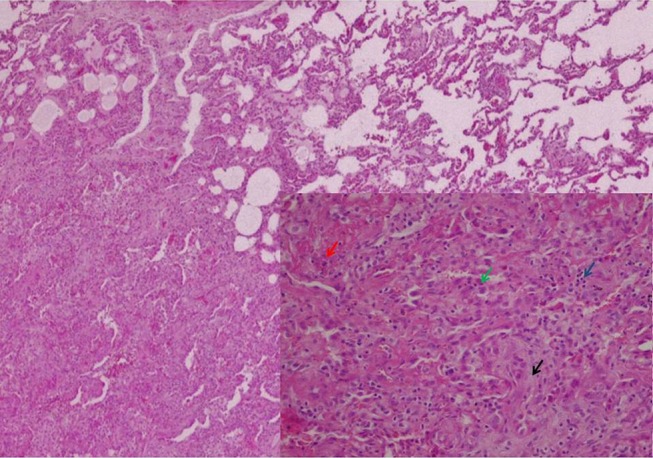
Surgical lung biopsy (H&E, original magnification 40×). In the lower right corner, in more detail, the characteristic changes of AFOP: alveolar spaces filled by fibrin (red arrow) and macrophages (blue arrow), hyperplasia of type II pneumocytes (green arrow) and foci of fibroblastic proliferation (black arrow) (H&E, original magnification 100×). AFOP, acute fibrinous and organising pneumonia.
Differential diagnosis
Commnity-acquired pneumonia has a broad differential diagnosis, including different forms of ILD. As seen in this patient, unfavourable response to standard empirical antibiotic therapy, without any serological or microbiological evidence of infective agent, in a previously healthy patient, should lead one to think of an alternative diagnosis.
Considering the possibility of ILD, a full workup including high resolution chest CT, pulmonary function tests and tissue sampling for histology, as well as laboratory exploration, should be performed and the case discussed within a multidisciplinary team with expertise on ILD in order to distinguish the different pathological entities.2
The hypothesis of pneumonia was first considered, further supported by an equivocal serology for Legionella and apparent good clinical response to antibiotics. However, the clinical and radiological course led to the suspicion of ILD.
Within the ILD, AFOP was assumed after the case discussion by a multidisciplinary team, considering the distinctive histological pattern, namely the presence of ‘fibrin balls’ that fill the alveolar spaces (the hallmark of this disease). The absence of hyaline membranes and eosinophils distinguishes this entity from diffuse alveolar damage and eosinophilic pneumonia, respectively, as seen in table 1.1–4
Table 1.
Histological and clinical features of AFOP, cryptogenic organising pneumonia, EP and diffuse alveolar damage
| AFOP | Cryptogenic organising pneumonia | EP | Diffuse alveolar damage | |
| Histological typical features | Organising pneumonia with prominent intra-alveolar fibrin balls and type II pneumocyte hyperplasia; the intervening lung parenchyma between the affected areas shows minimal changes | Excessive proliferation of granulation tissue within the bronchioles (bronchiolitis obliterans), alveolar ducts and alveoli, associated with chronic inflammation (lymphocytes and plasma cells). Extensive fibrosis not typically present |
Intra-alveolar and interstitial accumulation of eosinophils and, to a lesser extent, macrophages | Three phases: early exudative phase (capillary congestion, interstitial and alveolar oedema and eosinophilic hyaline membranes) followed by proliferative and fibrotic phases (proliferation of type II pneumocytes and progressive interstitial fibrosis) |
| Pulmonary involvement | Patchy | Patchy | Patchy | Diffuse |
| Hyaline membranes | Typically absent | Absent | Rarely present | Present |
| Fibrin | Major component, >50%,organised into balls | Lack of prominent airspace fibrin | May be present | Sometimes present |
| Aetiology | Possible association with infection, haematological disorder or drugs | Idiopathic | Idiopathic or secondary (fungal/parasitic infection, drug induced, immunological or systemic diseases) | More than 60 causes have been identified (shock, infection, trauma, drugs, pancreatitis) |
| Clinical course | Two forms: fulminant illness with rapid progression to death; subacute form, which may resolve after treatment with steroids | Clinical presentation mimics that of a community-acquired pneumonia; in many it is an acute illness of 1–2 weeks’ duration | Acute (1 week duration) and chronic (insidious) EP; peripheral blood eosinophilia is common | Fulminant clinical course, requiring management in an ICU with mechanical ventilation (ARDS); 50% mortality rate |
AFOP, acute fibrinous and organising pneumonia; ARDS, acute respiratory distress syndrome; ICU, intensive care unit.
Treatment
Once the association with infectious disease was excluded, the patient started systemic corticotherapy (prednisolone 1 mg/kg/day; 50 mg/day) with good clinical, functional and radiological response.
Outcome and follow-up
Disease relapsed, with fever, dyspnoea and pleuritic chest pain, after steroid tapering and withdrawal, 10 months later. Chest X-ray showed multiple nodular opacities, predominating in the upper lobes, some of them with a central cavity (figure 5). The patient restarted prednisolone at a dose of 40 mg/day with resolution of symptoms and radiological changes.
Figure 5.
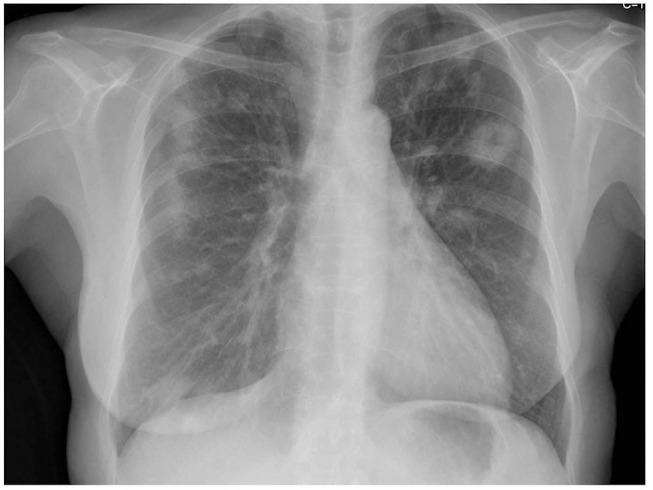
Chest X-ray showing multiple nodular opacities, predominating in the upper lobes, some of them with a central cavity; disease relapsed after prednisolone withdrawal, 10 months later.
Currently, at last follow-up, 2 years after initial presentation, the patient is on low-dose steroid therapy (prednisolone 5 mg/day) with no clinical nor radiological evidence of disease recurrence (figure 6).
Figure 6.
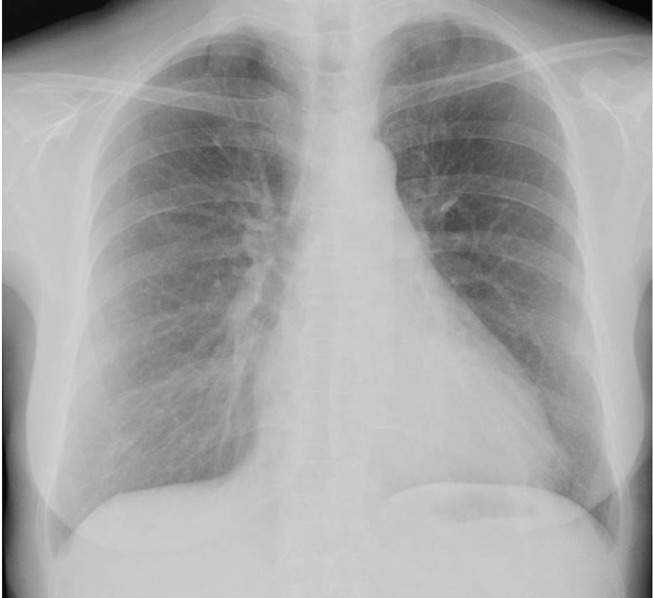
Chest X-ray, 2 years after the onset of prednisolone, with no changes.
Discussion
Although described in 2002 by Beasley et al1 there are as yet few cases of AFOP reported in the literature.
The clinical manifestations of AFOP are non-specific and include dyspnoea, fever and cough. The course can be indolent or it can progress swiftly, potentially with a fatal course.5 6 Its association with connective tissue disorders,6–8 haematopoietic stem cell and lung transplantation,9 infections10 11 and drug toxicity12 has been documented, as well as idiopathic cases,13 which seem to be the case in the reported patient. No previous conditions associated with AFOP were known nor any association with her medications’ history. In one recent review from our country, Gomes et al3 also described the presence of haematological disorders in 61.5% of the 13 cases, which was not seen prior to the diagnosis and along the follow-up of this case.
Chest X-ray findings are non-specific. Lung high-resolution CT scan is generally characterised by multifocal diffuse ground-glass infiltrates with basal predominance14 15 and, less frequently, as a lung lobe consolidation16 or a solitary nodule.17
The main differential diagnosis includes diffuse alveolar damage, eosinophilic pneumonia and organising pneumonia. The characterisation and distinction of this entities are resumed in table 1.
For a definite diagnosis of AFOP, it is necessary to biopsy affected lung tissue. The most commonly used methods to obtain a sample are surgical lung biopsy and CT guided biopsy; however, in some cases, transbronchial biopsy has also been used with success.
Histologically, the absence of hyaline membranes and noticeable eosinophils, the presence of intra-alveolar fibrin (fibrin balls) and a patchy distribution give AFOP a unique histological pattern. The presence of proliferating fibroblastic outbreaks (Masson bodies) and type II pneumocyte hyperplasia are also observed.18
Given the small number of cases reported in the literature, there are no further standard treatment regimens for AFOP. Response to therapy with corticosteroids and immunosuppressants (cyclophosphamide, mycophenolate mofetil and azathioprine)19 is the rule, especially in subacute forms of disease. The need for mechanical ventilation gives a worse prognosis. Relapse may occur during tapering of steroids.
Patient’s perspective.
This disease was a factor of stress for me. Its entrained evolution as well as the fact that it is a rare disease meant for me insecurity and discouragement. Now that I feel better, I thank the healthcare team for all the support and dedication to my case.
Learning points.
Acute fibrinous and organising pneumonia represents a rare and recently described form of interstitial lung disease (ILD).
The lack of response to standard antibiotic therapy for pneumonia should lead to consider alternative diagnosis as ILD.
The diagnostic evaluation of ILD invariably requires performing invasive tests; the definitive diagnosis is histological.
Footnotes
Contributors: The authors authorise the publication of the following article in the BMJ Case Reports, stating that it has not been published nor is it awaiting publication in another journal. The four authors declare that they have contributed in the elaboration of the article according to the ICMJE Recommendations. JRG and RM: conception and design, acquisition of data or analysis and interpretation of data. JRG, RM, PS and LC: drafting the article and revising it critically for important intellectual content. JRG, RM, PS and LC: final approval of the version published.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Beasley MB, Franks TJ, Galvin JR, et al. . Acute fibrinous and organizing pneumonia: a histological pattern of lung injury and possible variant of diffuse alveolar damage. Arch Pathol Lab Med 2002;126:1064–70. [DOI] [PubMed] [Google Scholar]
- 2.Travis WD, Costabel U, Hansell DM, et al. . An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013;188:733–48. 10.1164/rccm.201308-1483ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gomes R, Padrão E, Dabó H, et al. . Acute fibrinous and organizing pneumonia: a report of 13 cases in a tertiary university hospital. Medicine 2016;95:e4073 10.1097/MD.0000000000004073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feinstein MB, DeSouza SA, Moreira AL, et al. . A comparison of the pathological, clinical and radiographical, features of cryptogenic organising pneumonia, acute fibrinous and organising pneumonia and granulomatous organising pneumonia. J Clin Pathol 2015;68:441–7. 10.1136/jclinpath-2014-202626 [DOI] [PubMed] [Google Scholar]
- 5.Lee SM, Park JJ, Sung SH, et al. . Acute fibrinous and organizing pneumonia following hematopoietic stem cell transplantation. Korean J Intern Med 2009;24:156–9. 10.3904/kjim.2009.24.2.156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prahalad S, Bohnsack JF, Maloney CG, et al. . Fatal acute fibrinous and organizing pneumonia in a child with juvenile dermatomyositis. J Pediatr 2005;146:289–92. 10.1016/j.jpeds.2004.09.023 [DOI] [PubMed] [Google Scholar]
- 7.Hariri LP, Unizony S, Stone J, et al. . Acute fibrinous and organizing pneumonia in systemic lupus erythematosus: a case report and review of the literature. Pathol Int 2010;60:755–9. 10.1111/j.1440-1827.2010.02586.x [DOI] [PubMed] [Google Scholar]
- 8.Balduin R, Giacometti C, Saccarola L, et al. . Acute fibrinous and organizing pneumonia in a patient with collagen vascular disease "stigma". Sarcoidosis Vasc Diffuse Lung Dis 2007;24:78–80. [PubMed] [Google Scholar]
- 9.Renaud-Picard B, Dégot T, Biondini D, et al. . Successful lung retransplantation in a patient with acute fibrinous and organizing pneumonia: a case report. Transplant Proc 2015;47:182–5. 10.1016/j.transproceed.2014.08.039 [DOI] [PubMed] [Google Scholar]
- 10.Heo JY, Song JY, Noh JY, et al. . Acute fibrinous and organizing pneumonia in a patient with HIV infection and Pneumocystis jiroveci pneumonia. Respirology 2010;15:1259–61. 10.1111/j.1440-1843.2010.01845.x [DOI] [PubMed] [Google Scholar]
- 11.Canessa PA, Pratticò L, Sivori M, et al. . Acute fibrinous and organising pneumonia in Whipple's disease. Monaldi Arch Chest Dis 2008;69:186–8. 10.4081/monaldi.2008.382 [DOI] [PubMed] [Google Scholar]
- 12.Yokogawa N, Alcid DV. Acute fibrinous and organizing pneumonia as a rare presentation of abacavir hypersensitivity reaction. AIDS 2007;21:2116–7. 10.1097/QAD.0b013e3282f08c5a [DOI] [PubMed] [Google Scholar]
- 13.Tzouvelekis A, Koutsopoulos A, Oikonomou A, et al. . Acute fibrinous and organising pneumonia: a case report and review of the literature. J Med Case Rep 2009;3:74 10.1186/1752-1947-3-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Khouzaie TH, Dawamneh MF, Hazmi AM, et al. . Acute fibrinous and organizing pneumonia. Ann Saudi Med 2013;33:301–3. 10.5144/0256-4947.2013.301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Damas C, Morais A, Moura CS, et al. . Acute fibrinous and organizing pneumonia. Rev Port Pneumol 2006;12:615–20. [PubMed] [Google Scholar]
- 16.Akhtar A, Ul Abideen Z. Acute fibrinous and organizing pneumonia masquerading as a lower respiratory tract infection: a case report and review of the literature. BMC Res Notes 2015;8:38 10.1186/s13104-015-0984-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kobayashi H, Sugimoto C, Kanoh S, et al. . Acute fibrinous and organizing pneumonia: initial presentation as a solitary nodule. J Thorac Imaging 2005;20:289–92. [DOI] [PubMed] [Google Scholar]
- 18.Labarinas S, Gumy-Pause F, Rougemont AL, et al. . Is acute fibrinous and organizing pneumonia the expression of immune dysregulation? J Pediatr Hematol Oncol 2013;35:139–43. 10.1097/MPH.0b013e31827e5782 [DOI] [PubMed] [Google Scholar]
- 19.Bhatti S, Hakeem A, Torrealba J, et al. . Severe acute fibrinous and organizing pneumonia (AFOP) causing ventilatory failure: successful treatment with mycophenolate mofetil and corticosteroids. Respir Med 2009;103:1764–7. 10.1016/j.rmed.2009.07.009 [DOI] [PubMed] [Google Scholar]


