ABSTRACT
The success of immunotherapeutics over the past decade has fundamentally altered the therapeutic landscape in melanoma and non-small cell lung (NSCLC) cancer care. Multiple clinical trials have confirmed significant improvements in survival with a variety of immunotherapeutic strategies. The careful and appropriate selection of standard of care (SOC) therapies is key to the successful design and interpretation of these trials. To date immunotherapeutic trials have used best supportive care, matched placebo, chemotherapy, targeted therapy or, more recently, established immunotherapeutics in melanoma clinical trials as SOCs. Each of these SOC choices has a fundamental impact on the selection and validity of response assessment criteria and clinical endpoints. As yet there is no established approach, thus new data must be interpreted with an understanding of the limitations of the current paradigm. Additionally, the pace of development has mandated the use of novel clinical trial designs, answering multiple therapeutic questions simultaneously and designed to expedite regulatory approval. This review addresses the most important challenges in the selection of SOC in immunotherapeutic trials and the current and future challenges in trial design.
KEYWORDS: clinical trial design, immune-related response, immunotherapy trials, immunotherapy, standard of care
Introduction
The past decade has seen unprecedented achievements in the field of cancer immunotherapy, which has led to several novel therapeutic strategies being approved. These recent successes have led to many new challenges in immuno-oncology clinical trial design, in particular the parameters for measuring benefit and ethical considerations.
The premise of cancer immunotherapy is to utilize innate and adaptive immunological pathways to induce antineoplastic activity while limiting toxicities traditionally expected from cytotoxic therapies. Building on the initial work of William Coley, cancer vaccines were initially investigated as a mechanism for engaging the adaptive immune system, with modest success. Early approved agents were often non-specific immunologic inducers, such as BCG in bladder cancer and high-dose IL-2 in melanoma, and their therapeutic impact could not be generalized to a broader population with metastatic disease. Monoclonal antibodies, such as trastuzumab, rituximab and cetuximab, offered a new, more targeted strategy that act through both immunologic, such as antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC), and non-immunologic mechanisms, including direct receptor blockade or local delivery of cytotoxic chemotherapy.1-3 However it was not until 2010, with the success of sipuleucel-T in prostate cancer, that an immunological therapeutic option designed to induce tumor-targeted T-lymphocytes as the prime mechanism of anti- tumor activity was approved by the Food and Drug Administration (FDA).4 The checkpoint inhibitors then expanded on this success, with the cytotoxic T-lymphocyte antigen-4 (CTLA-4) antagonist, ipilimumab, shown to improve survival in melanoma and programmed cell death-1 (PD-1/PD-L1) directed therapies later expanding the range of tumors that would benefit from immunotherapy, including non-small cell lung cancer (NSCLC), renal cell cancer (RCC), urothelial cancer and many others.5-7
These clinical benefits have been hard won after years of failed T-cell activating immunotherapeutic trials in the preceding 2 decades. The experience of these trials, particularly in NSCLC and melanoma, have shown the importance of trial design with appropriate standard of care (SOC) comparators that generate reliable results or signals of true activity.,8,9 The SOC comparator is an important factor in the success of immunotherapeutic trials because it impacts the effective measurement of trial endpoints, innovations in trial design and statistical analysis, ethics of trial participation, the combination novel therapeutics with existing standards of care, and requirements for regulatory approval.
New trial designs that more efficiently bring a drug to clinical practice are altering the drug development and regulatory approval paradigm. Phase 1 trials with expansion cohorts or those testing multiple hypotheses simultaneously in early phase may more rapidly deliver promising new therapies to the clinic, through adaptable hypothesis testing, biomarker refinement and appropriate patient selection.10 In this review we examine the challenges and considerations inherent in comparing SOC treatments against new immunotherapies entering large phase III clinical trials, with particular emphasis on recent developments in melanoma and NSCLC. For clarity, we focus primarily on clinical trials involving checkpoint inhibitors as they encompass the important challenges currently presented by SOC selection, while acknowledging that emerging immunotherapeutics, such as chimeric antigen receptor (CAR) T-cells or dendritic cell/tumor cell fusions, may present unique challenges as they become more widely used.
Clinical trial experience
As new immunotherapies approach regulatory approval their efficacy is examined in large phase II or III clinical trials against recognized SOCs. The choice of SOC depends on both the tumor subtype and stage of disease examined. The evolution of these SOCs is informative as many have changed significantly over the past 10 years, and as immunotherapies have been applied in a wider range of clinical settings. Furthermore, while early trials of immunotherapeutics sought to establish signals of activity against placebo, and as such were less concerned with comparative efficacy against a therapeutic SOC, as the impact of immunotherapeutics has become more significant so the choice of SOC has become a vital consideration in the design, undertaking and interpretation of clinical trials.
Immunotherapies have usually been initially tested in advanced disease where few effective therapeutic options exist or in the adjuvant setting where there is no effective adjuvant therapy, or in addition to an effective adjuvant therapy. In both these situations, selection of best supportive care (BSC) and/or placebo administration as SOC is an ethical and widely accepted approach. Placebo was used as the SOC in 2 seminal trials of advanced disease. The first, by Kantoff et al., of sipuleucel-T (an active cellular immunotherapy consisting of peripheral blood mononuclear cells activated ex vivo in the presence of the PA2024 recombinant fusion protein) in castrate-resistant prostate cancer,4 and the second by Hodi et al. of ipilimumab in metastatic or unresectable melanoma.11 Both demonstrated improved OS compared with placebo, which then led to the respective drug's approval.5 Prior to these trials vaccines had also been tested against placebo in the adjuvant setting, such as the recombinant MAGE-A3 vaccine with AS15 immunostimulant and tecemotide in NSCLC without success,8,9 although in both of these examples the vaccine was given after the accepted SOC (chemotherapy or chemoradiotherapy). In the case of vaccines, as opposed to T-cell activating therapies, the inclusion of placebo is critical where the vaccine adjuvant can cause an immunological response on its own. There are now several trials exploring checkpoint inhibitors as adjuvant therapy.12 Recently, data using ipilimumab versus placebo in resected Stage III melanoma demonstrated both a relapse free (HR 0.76; 95%CI: 0.64 – 0.89; P = <0.001) and overall survival benefit (HR 0.72; 95% CI: 0.58 – 0.88; P = 0.001) in a double-blinded study.13 Other studies being conducted include pembrolizumab (an anti-PD-1 antibody) vs. placebo in the adjuvant setting in resected stage III melanoma, KEYNOTE-054 (NCT02362594) and resected early-stage NSCLC, KEYNOTE-091 (NCT02504372), and atezolizumab (an anti-PD-L1 antibody) being compared against BSC in resected early-stage NSCLC (NCT02486718).
More recently though, as the efficacy of immunotherapy has been proven in the initial clinical trials,14,15 new medications have been compared in advanced disease against a well-established chemotherapeutic SOC. The trials that led to the approval of anti-PD-1 therapy in NSCLC compared pembrolizumab or nivolumab with docetaxel, the standard second-line chemotherapeutic option,16-18 and first-line trials have used platinum-based chemotherapy as the comparator, the widely accepted first-line SOC (as shown in Fig. 1). While the design of second-line trials in NSCLC is reasonably straightforward as docetaxel therapy is a widely accepted therapy and treatment is usually limited, first-line trials are made more complex by the nature of the SOC. There are several potential regimens with equivalent efficacy and the use of maintenance chemo- or targeted therapy has also been shown to improve survival.19-22 Therefore, the SOC in this setting depends on tumor histology, performance status, response to initial therapy and physician preference. In addition, treatment duration is an important SOC consideration when comparing therapies that are given until disease progression, such as the PD-1/PD-L1 inhibitors and maintenance chemotherapy. If maintenance chemotherapy were omitted in a SOC arm, any PFS difference may unduly favor the experimental immunotherapy.
Figure 1.
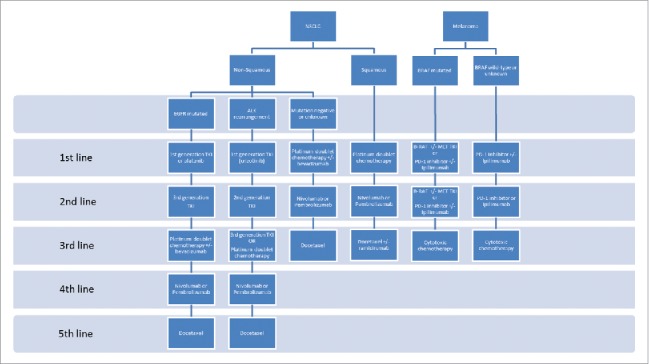
Current standard of care therapy in advanced NSCLC and Melanoma Notes: The current standards of care systemic therapy for metastatic non-small cell lung carcinoma (NSCLC) and melanoma. EGFR = epidermal growth factor receptor; ALK = anaplastic lymphoma kinase rearrangement; TKI = tyrosine kinase inhibitor; PD-1 = programmed cell death protein-1.
The first reported phase III trial of NSCLC in the first-line setting compared ipilimumab with carboplatin-paclitaxel, without maintenance therapy or bevacizumab,23 while first-line trials of PD-directed checkpoint inhibitors have used a variety of chemotherapy combinations as SOC. Trials such as the recently reported KEYNOTE-024 with pembrolizumab that demonstrated a significant improvement in PFS (HR 0.50; 95%CI: 0.37 – 0.68, P<0.001),24 and NEPTUNE with durvalumab and tremelimumab (NCT02542293), used investigator's choice chemotherapy with maintenance therapy as SOC, whereas other trials have excluded maintenance therapy, such as CheckMate 026 comparing nivolumab with platinum doublet chemotherapy (NCT02041533), and still others restricted chemotherapy to carboplatin-paclitaxel-bevacizumab with maintenance bevacizumab, such as IMpower 150 with atezolizumab. The CheckMate 026 trial, which included only PD-L1 positive tumors, is particularly interesting as its primary end point was PFS, while also prohibiting maintenance chemotherapy. This alone could have skewed the PFS results in favor of nivolumab therapy, but an early press release has indicated that the trial did not meet its primary end point.25 Whether this failure is due to overperformance of the SOC comparator, or intrinsic to the efficacy of nivolumab in this setting should be elucidated in the publication of the full results. These differences reflect the great challenge in choosing an appropriate SOC for NSCLC, which is in fact a complex set of diseases with a rapidly developing therapeutic environment. However, the choice of SOC is crucially important to ensure the validity of trial endpoints and applicability of results.
The evolution of SOC selection in melanoma offers an important contrast, largely due to the relative paucity of well-established and effective chemotherapeutic options. Dacarbazine, or its oral analog temozolomide, carboplatin-paclitaxel, and nab-paclitaxel have remained the only chemotherapeutic options in metastatic melanoma for many years despite response rates of only 10–15%.26-29 Early trials of ipilimumab, pembrolizumab and nivolumab in advanced melanoma all used either dacarbazine alone or investigator's choice chemotherapy as SOC comparators, to establish their efficacy in both previously treated and untreated patients.30-33 However, the clear and rapid demonstration of superior efficacy for these agents in the treatment of advanced melanoma, has meant immune checkpoint inhibitors are now the SOC in both the first- and second-line settings in BRAF wild-type tumors, or sequenced with BRAF inhibitors in those patients with an activating BRAF mutation. In this situation, SOC choices are more directly comparable to experimental immunotherapeutics, but the pace of therapeutic innovation and continual gains in survival of patients with advanced melanoma has meant SOC changes have outstripped the ability of trials to incorporate them accommodated in a standard phased drug development program. This has led to more innovative trial designs aimed at addressing multiple therapeutic questions simultaneously.
Innovative trial design
Among the key aspects of recent immunotherapeutic advances has been the acceleration of the standard drug development and approval pathway. The accelerated FDA approvals for pembrolizumab and nivolumab in melanoma were on the basis of a phase Ib trial and subgroup analysis of a phase III trial respectively,32,34 and bypassed the standard progression from phase I “safety” to phase III “comparative efficacy” trials. Although, accelerated approval has been granted to “breakthrough” therapies in the past, such as ALK inhibitors in NSCLC, checkpoint inhibitor trials with innovative new designs that challenge the position of a traditional SOC show this unconventional approach will continue beyond regulatory approval. These trials seek to optimise the dosing of individual immunotherapies or novel combinations in a phase III trial setting, bring an SOC into an earlier phase trial with the explicit aim of regulatory approval, or use new trial design to investigate a new medication across multiple indications, rather than merely a “head-to-head” comparison with an established SOC. Three phase II/III trials of pembrolizumab demonstrated the dose finding approach while simultaneously establishing efficacy. KEYNOTE-002 in metastatic melanoma and KEYNOTE-010 in advanced lung cancer both investigated pembrolizumab at 2 mg/kg and 10 mg/kg doses compared with SOC chemotherapy,16,31 while KEYNOTE-006 in metastatic melanoma compared 2 different dosing schedules of pembrolizumab (2-weekly vs. 3-weekly dosing at 10 mg/kg) against ipilimumab as the SOC.35
Optimal dosing of immunotherapy combinations is also being investigated in the phase III setting, with a current phase III trial comparing nivolumab 3 mg/kg and ipilimumab 1 mg/kg against nivolumab 1 mg/kg and ipilimumab 3 mg/kg in melanoma (NCT02714218). This trial is a good example of adaptation in trial design challenging the established paradigm in the face of a rapidly changing therapeutic environment. Prior to public reporting of the initial results from trials of the combination of ipilimumab and nivolumab, showing superior response rates and survival outcomes with the combination, compared with either agent alone, but at the cost of significantly increased toxicity, so this trial aimed at optimising dosing and minimising toxicity was initiated without including a traditional SOC comparator. As companies aim to stay ahead of a dynamic and competitive market, trials that challenge familiar design or incorporate new practice-changing findings before reporting or approval by regulatory bodies are likely to more frequent.
The paradigm established with the approval of the PD-1 inhibitors has also led to the design of new early phase trials aimed at drug registration. The LUNG-MAP study, a biomarker-driven, second-line, phase II/III multi-sub-study “Master Protocol” trial, evaluating multiple biomarker-targeted therapies in squamous cell carcinoma (SqCC) NSCLC aimed specifically at drug registration (NCT02154490) is an example of using multiple arms targeted at subpopulations within a broader group. The trial includes experimental arms with nivolumab, durvalumab and ipilimumab in those patients without a known biomarker for targeted therapy, and all arms are compared against docetaxel as SOC. These trials challenge the position of an established SOC as the only acceptable comparator in the phase III trial setting. If a new drug can convincingly demonstrate improved efficacy in an early phase setting, and receive regulatory approval, then phase III trials will increasingly be used to optimise delivery of medications rather than demonstrate definitive efficacy. This, in turn, will further challenge those regulatory bodies when evaluating the comparative efficacy of new medications, and determining which new therapies offer good value for money without concrete survival data. These represent significant challenges in the introduction of immunotherapeutics in routine clinical practice in a timely and accessible fashion.
Response assessment
Novel trial designs offer exciting new mechanisms to explore drug efficacy, but of particular importance is establishing a reliable method for evaluating efficacy between immunotherapies and SOC in late phase clinical trials. To this end, it is critical to understand the differences in the response kinetics between immunotherapies and chemo- or molecularly targeted therapies. Chemotherapy and targeted therapies have well-defined patterns of response, with susceptible tumors demonstrating a predictable decrease in tumor size over a period of weeks. Radiological techniques serve as reliable assessment tools of drug efficacy with consistency between individual patients, trial locations and therapeutic agents. However, the well-documented phenomena of delayed immune response, prolonged stable disease and “pseudoprogression” have meant that traditional response assessments may underestimate the benefit of immunotherapeutics when compared with a traditional SOC.36,37 As yet there has been no consensus approach adopted in immunotherapeutic clinical trials, although investigators have insisted on survival endpoints being the major endpoints rather than response for most studies.
The Response Evaluation Criteria in Solid Tumors (RECIST), based on earlier WHO criteria, was developed to standardise response assessment and in chemotherapy trials of advanced malignancy.38,39 However, atypical patterns of response seen in immunotherapeutic trials led to the adaptation of these guidelines to recognize that response or prolonged disease stabilization can occur after an initial increase in tumor size or the appearance of new lesions.40 The key differences between these immune-related response criteria (irRC) and RECIST 1.1 are the definition of progressive disease (PD) and the use of bidimensional, rather than unidimensional, target lesions measurements. PD is defined by RECIST 1.1 as an increase of ≥ 20% in tumor burden from baseline or nadir, or the appearance of new lesions. By contrast, irRC defines PD as an increase of ≥ 25% in bidimensional tumor burden from baseline, nadir or a reset baseline (where tumor burden has increased at the first scheduled assessment). irRC also requires confirmation of PD at 2 consecutive time points at least 4 weeks apart, whereas RECIST 1.1 does not, and new lesions are incorporated in the assessment of total tumor burden and do not define PD as with RECIST 1.1.
The altered patterns of response are particularly important when considering the outcomes with immunotherapeutics compared with chemotherapy or targeted-therapy SOCs. While RECIST 1.1 is well-established and clinically validated across thousands of clinical trials, is likely to underestimate the benefit of immunotherapies, particularly in the assessment of progression-free survival. Prior to recent immunotherapy success, vaccine trials had demonstrated the phenomenon of late response to immunotherapy,41 but most trials have continued to use RECIST or modified WHO criteria to define response.8,42 In addition, regulatory agencies have not accepted irRC as a validated end-point for drug approval, but recent trials of checkpoint inhibitors have used varying approaches to this dilemma.
The initial trials of ipilimumab in melanoma used modified WHO criteria with bidimensional measurement of tumor burden but without the additional criteria included in the irRC. Many trials have used RECIST 1.1 without adaptation, including the landmark KEYNOTE-010, CheckMate 057, and CheckMate 017 trials comparing PD-1 inhibition with docetaxel in previously treated NSCLC.16-18 While other trials have reported responses based on RECIST 1.1, but included irRC assessment as a sensitivity analysis, such as KEYNOTE-002 (comparing pembrolizumab with investigator's choice chemotherapy in ipilimumab-refractory melanoma),31 or as a secondary end point, such as POPLAR 2 (comparing atezolizumab with docetaxel in previously treated NSCLC).43 Later trials such as KEYNOTE-006 comparing pembrolizumab with ipilimumab in advanced melanoma, used RECIST 1.1 to evaluate response endpoints, but irRC to manage therapeutic decisions.35 Similarly, Lynch et al. used irRC to guide clinical care, but assessed response with both modified WHO criteria and irRC.23
The exact extent to which immune-related responses, as distinct from RECIST-assessed responses, occur is unclear as many trials have not undertaken irRC assessment. However, some trials offer an insight into their frequency. In the dose finding trial of ipilimumab in advanced melanoma, 22 of 227 (9.7%) patients treated with ipilimumab at 10 mg/kg who were characterized as having PD went on to have responses consistent with response on irRC (5 with irPR and 17 with irSD).40 In the phase I trial of pembrolizumab in melanoma (KEYNOTE-001), 24 of 327 (7.3%) patients with sufficient follow-up imaging, demonstrated atypical responses (15 with early pseudoprogression at <12 weeks, and 9 patients with delayed pseudoprogression at >12 weeks). Furthermore, those patients with PD by RECIST criteria but not irRC demonstrated improved survival compared with patients who have PD assessed by both RECIST and irRC. In this larger analysis, 262 of 584 demonstrated PD by RECIST 1.1, but a smaller subset 84 (14.4%) had PD by RECIST 1.1 but not irRC. Median OS for patients with PD by RECIST 1.1 but not irRC was 22.5 months (95% CI: 16.5 months - not yet reached), compared with 8.4 months (95%CI: 6.6 – 9.9 months).
Atypical immune responses are uncommon, but they appear to impact survival in meaningful ways when immunotherapies are compared with chemotherapeutic or targeted SOCs. The effective assessment of these responses and further delineation of their impact on outcomes across other tumor types are important challenges to overcome, particularly in the regulatory assessment of immunotherapeutics by drug approval bodies.
Statistical design
Statistical design of trials and interim analysis of outcomes are also impacted by differing patterns of response, and are particularly important in the regulatory approval of immunotherapies. The delayed responses and long-term survival seen with immunotherapies, not seen with traditional SOC, particularly in melanoma and NSCLC, may result in loss of statistical power and underestimation of study duration.44 Statistical design of clinical studies are usually based on the assumption that the risk of any event (for example death or recurrence) is proportional between therapies at all points during the study. The altered kinetics of response and potential for long-term survival or cure with immunotherapies, compared with traditional SOC, mean this critical assumption may be violated. This may lead to a loss of statistical power to demonstrate a difference between therapies.
Interim analyses are most often planned on a time-to-event basis, such as the interim analysis after 380 deaths in the CheckMate 057 trial, with the assumption that all participants will be risk of the specified event for the duration of the trial. However, if patients are cured, “functionally cured” or are long-term survivors they will no longer be at risk of the pre-specified event, thereby reducing the at-risk population remaining in the study and increasing the trial duration required to achieve the desired number of events and level of statistical power. An example was the phase III registration trial of ipilimumab in melanoma where the final analysis of survival took place 2 y later than initially estimated, with only 414 of a planned 416 events having occurred.11,45 The significant rate of long-term survival meant though 80% of deaths were observed in the first 3 y of the study it took a further 2 y to accumulate the final 20%, thereby delaying result accumulation and reporting.
The importance of interim analyses is also demonstrated in the approval of both pembrolizumab and nivolumab in the treatment of NSCLC.46,47 While pembrolizumab was granted accelerated approval on the basis of ORR of significant magnitude and duration in the phase 1 (KEYNOTE-001) trial, interim results from the phase III KEYNOTE-010 trial were included in the final risk-benefit analysis, and confirmed benefit for pembrolizumab over docetaxel.46 In contrast, nivolumab was approved by the FDA in the second-line treatment of NSCLC solely on the basis on the interim analysis of OS in the CheckMate 057.47 In this trial there was a 6 month delay before the benefit of nivolumab became apparent in Kaplan-Meier analysis. This is likely to be driven by the delayed response pattern of checkpoint inhibiting MAbs and the large group of non-responders skewing the early part of the curve when compared against the response pattern seen with chemotherapeutic SOC. This means early analyses would not necessarily demonstrate superior efficacy against pre-specified stopping criteria.
In addition late or long-term responders are underestimated in interim analyses. The phase III trial of tremelimumab, an IgG2 CTLA-4-blocking MAb, compared with chemotherapy in metastatic melanoma, was stopped early after the OS results crossed the prespecified futility boundary at an interim analysis.48 However, the trial did demonstrate non-significantly improved OS with tremelimumab over chemotherapy 12.6 vs 10.7 months (HR 0.88; P = 0.127) and a significantly improved duration of response 35.8 vs 13.4 months (P = 0.0011), despite a comparably low ORR 10.7% vs 9.8%. This differing pattern and duration of response may not have been effectively recognized in the original statistical plan, and may have affected the interim analysis and failed to adequately recognize the minority of patients receiving significant benefit from the medication. The importance of the study design cannot be underestimated as ipilimumab, which was compared with placebo, was granted FDA approval and tremelimumab was not.
These challenges will likely need to be met with new statistical approaches that account for differing response patterns, in particular taking into account the effect of the comparator arm where an SOC's response kinetics differ from newer immunotherapies.
Endpoint selection
One of the most important considerations in the selection of SOC in clinical trials of immunotherapeutics is their impact on the selection of appropriate clinical trial endpoints. As discussed above, the delayed response kinetics and the atypical responses seen with new immunotherapeutics may underestimate their clinical benefit if using standard response measurements and/or timelines. This impact is particularly important when considering PFS as an end point, as although it is an attractive marker for efficacy that requires a smaller sample size and shorter follow-up, it is has been shown in many immunotherapeutic trials that clinically meaningful and statistically significant improvements in OS can be found despite no significant difference in PFS.5,18 This is in stark contrast to standard trials of chemotherapy, in which differences in PFS are more readily demonstrated and confirmation of changes in OS requires a greater number of subjects, longer timelines and balancing of subsequent therapies.
For this reason most published clinical trials of immunotherapy to date in melanoma and NSCLC have used OS alone as the primary end point,5,17,18,30 or a combination of OS and PFS as co-primary endpoints.16 While many studies showed an improvement in both PFS and OS, particularly in trials of melanoma, reported hazard ratios (HRs) were most often better for OS than PFS, and some trials demonstrated improvements in OS only. However, OS as a primary end point is limited too as subsequent or crossover therapy can influence the likelihood of a positive trial result. This phenomenon was observed in an exploratory analysis of the IMPACT trial of sipuleucel-T in prostate cancer, where subsequent infusion of an autologous cellular therapy in a subset of the control group improved this group's survival over the remainder of the control group, and affected the OS benefit seen in the population as a whole.49 After adjusting for this subsequent therapy the OS benefit of sipuleucel-T would have improved from the reported 4.1 months to 7.8 months. While OS remains the preferred primary end point in trials of immunotherapy against a traditional SOC, in situations where immunotherapy has become established as the SOC, or where immunotherapy is added to a traditional SOC backbone, this limitation has been partially overcome. PFS then becomes a reliable and valid marker of efficacy as both the experimental and comparator arms will have similar response kinetics. This can be seen in the design of current clinical trials where many use PFS as the sole primary end point or in combination with OS (see Table 2).
Table 2.
Selected ongoing clinical trials of immunotherapeutics in melanoma and NSCLC.
| Immunotherapy | Trial Name | Phase | Population | Start Date | N | SOC | 2nd arm | 3rd arm | 4th arm | Primary Endpoint | Clinicaltrials.gov Identifier |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Melanoma | |||||||||||
| Pembrolizumab | |||||||||||
| KEYNOTE-054 | III | Resected stage III melanoma | 07/2015 | 900 | Placebo | Pembrolizumab 200 mg | RFS | NCT02362594 | |||
| KEYNOTE-022 | I/II | Previously treated MM | 05/2014 | 177 | Placebo + Dabrafenib + Trametinib | Pembrolizumab + Dabrafenib + Trametinib | Pembrolizumab + Dabrafenib | Pembrolizumab + Trametinib | DLTs | NCT02130466 | |
| PFS | |||||||||||
| MASTERKY-265 | Ib/III | Previously untreated MM | 12/2014 | 660 | Pembrolizumab + placebo | Pembrolizumab + T-VEC | PFS | NCT02263508 | |||
| OS | |||||||||||
| NCI/SWOG trial | III | Fully resected stage III/IV melanoma | 10/2015 | 1378 | Interferon or Ipilimumab | Pembrolizumab 200 mg | OS | NCT02506153 | |||
| RFS | |||||||||||
| PD-L1 status | |||||||||||
| ECHO-301 | III | MM | 06/2016 | 600 | Pembrolizumab | Pembrolizumab + epacadostat (IDO-1 inhibitor) | PFS | NCT02752074 | |||
| OS | |||||||||||
| Nivolumab | |||||||||||
| CheckMate 064 | II | MM | 04/2013 | 177 | Nivolumab 1 mg/kg + Ipilimumab 3 mg/kg | Ipilimumab 3 mg/kg + Nivolumab 1 mg/kg | AEs | NCT01783938 | |||
| CheckMate 238 | III | Fully resected stage III/IV melanoma | 03/2015 | 800 | Ipilimumab | Nivolumab | RFS | NCT02388906 | |||
| Combination | |||||||||||
| IIIb/IV | Previously untreated MM | 03/2016 | 340 | Nivolumab 1 mg/kg + Ipilimumab 3 mg/kg | Nivolumab 3 mg/kg + Ipilimumab 1 mg/kg | AEs | NCT02714218 | ||||
| NCI sponsored | III | Previously untreated MM | 07/2015 | 300 | Dabrafenib + Trametinib followed by Nivolumab + ipilimumab | Nivolumab + ipilimumab followed by Dabrafenib + Trametinib | OS | NCT02224781 | |||
| NSCLC | |||||||||||
| Ipilimumab | |||||||||||
| III | Previously untreated SqCC mNSCLC | 10/2014 | 867 | Carboplatin-Paclitaxel-placebo | Carboplatin-Paclitaxel-Ipilimumab | OS | NCT02279732 | ||||
| Pembrolizumab | |||||||||||
| KEYNOTE-042 | III | Previously untreated and PD-L1 positive mNSCLC without activating mutation | 10/2014 | 1240 | Platinum-based chemotherapy | Pembrolizumab 200 mg (up to 35 doses) | OS | NCT02220894 | |||
| KEYNOTE-091 | III | Stage IB/II-IIIA NSCLC post-resection | 11/2015 | 1380 | Placebo | Pembrolizumab 200 mg (18 doses) | DFS | NCT02504372 | |||
| KEYNOTE-189 | III | Previously untreated non-SqCC mNSCLC | 10/2015 | 570 | Platinum-Pemetrexed | Pembrolizumab 200 mg + Platinum-Pemetrexed | PFS | NCT02775435 | |||
| OS | |||||||||||
| KEYNOTE-407 | III | Previously untreated SqCC mNSCLC | 06/2016 | 560 | Carboplatin-Paclitaxel/nab-paclitaxel | Pembrolizumab 200 mg + Carboplatin-Paclitaxel/nab-paclitaxel | PFS | NCT02775435 | |||
| OS | |||||||||||
| Nivolumab | |||||||||||
| CheckMate 026 | III | Previously untreated PD-L1 + mNSCLC | 03/2014 | 535 | Invetsigator's choice chemotherapy | Nivolumab 3 mg/kg | PFS in strong PD-L1 positive | NCT02041533 | |||
| CheckMate 078 | III | Previously treated mNSCLC | 12/2015 | 500 | Docetaxel | Nivolumab 3 mg/kg | OS | NCT02613507 | |||
| CheckMate 227 | III | Previously untreated mNSCLC | 08/2015 | 1980 | Platinum doublet chemotherapy | Nivolumab | Nivolumab + Ipilimumab | Nivolumab + Platinum doublet chemotherapy | PFS | NCT02477826 | |
| OS | |||||||||||
| CheckMate 722 | III | Previously treated EGFR mutant/ T790m negative mNSCLC | 10/2016 | 429 | Platinum doublet chemotherapy | Nivolumab + Platinum doublet chemotherapy | Nivolumab + Ipilimumab | PFS | NCT02864251 | ||
| Atezolizumab | |||||||||||
| OAK | III | Previously treated mNSCLC | 03/2014 | 1225 | Docetaxel | Atezolizumab 1200 mg | OS | NCT02008227 | |||
| IMpower150 | III | Previously untreated Non-SqCC mNSCLC | 03/2015 | 1200 | Carboplatin-Paclitaxel-Bevacizumab | Atezolizumab 1200 mg -Carboplatin-Paclitaxel | Atezolizumab 1200 mg -Carboplatin-Paclitaxel-Bevacizumab | PFS | NCT02366143 | ||
| IMpower130 | III | Previously untreated Non-SqCC mNSCLC | 04/2015 | 550 | Carboplatin + Nab-Paclitaxel | Atezolizumab 1200 mg -Carboplatin-Paclitaxel | PFS | NCT02367781 | |||
| IMpower010 | III | Resected IB – IIIA NSCLC | 10/2015 | 1127 | BSC | Atezolizumab 1200 mg | DFS | NCT02486718 | |||
| IMpower110 | III | Previously untreated mNSCLC | 07/2015 | 570 | Platinum + Pemetrexed/Gemcitabine | Atezolizumab 1200 mg | PFS | NCT02409342 | |||
| OS | |||||||||||
| IMpower131 | III | Previously untreated SqCC mNSCLC | 06/2015 | 1025 | Carboplatin-Paclitaxel/nab-paclitaxel | Atezolizumab 1200 mg +Carboplatin-Paclitaxel/nab-paclitaxel | PFS | NCT02367794 | |||
| OS | |||||||||||
| IMpower132 | III | Previously untreated non-SqCC mNSCLC | 04/2016 | 568 | Platinum-Pemetrexed | Atezolizumab 1200 mg + Platinum-Pemetrexed | PFS | NCT02657434 | |||
| IMpower210 | III | Previously treated mNSCLC | 07/2016 | 563 | Docetaxel | Atezolizumab 1200 mg | OS | NCT02813785 | |||
| Durvalumab | |||||||||||
| ARCTIC | III | Previously treated mNSCLC | 01/2015 | 730 | Vinorelbine | Durvalumab | OS | NCT02352948 | |||
| Sub-study 1 | Gemcitabine | PFS | |||||||||
| Erlotinib | |||||||||||
| ARCTIC | III | Previously treated mNSCLC | 01/2015 | 730 | Vinorelbine | Durvalumab | Durvalumab + Tremelimumab | Tremelimumab | NCT02352948 | ||
| Sub-study 2 | Gemcitabine | ||||||||||
| Erlotinib | |||||||||||
| PACIFIC | III | Stage III NSCLC following chemoradiation | 05/2014 | 702 | Placebo | Durvalumab | NCT02125461 | ||||
| MYSTIC | III | Previously untreated mNSCLC | 07/2015 | 1092 | Platinum based chemo | Durvalumab | Durvalumab + Tremelimumab | PFS | NCT02453282 | ||
| OS | |||||||||||
| NEPTUNE | III | Previously untreated mNSCLC | 11/2015 | 800 | Platinum based chemo | Durvalumab + Tremelimumab | OS | NCT02542293 | |||
| abound2L+ | II | Previously treated mNSCLC | 01/2015 | 240 | nab-Paclitaxel | nab-Paclitaxel + Durvalumab | nab-Paclitaxel + CC-486 | PFS | NCT02250326 | ||
| Combination | |||||||||||
| Lung-MAP sub-study | III | Previously treated mNSCLC (without actionable biomarkers) | 12–2015 | 250 | Nivolumab | Nivolumab + Ipilimumab | PFS | NCT02785952 | |||
| OS | |||||||||||
Notes: SOC = standard of care; MM = metastatic melanoma; mNSCLC = metastatic NSCLC; BRAF WT = BRAF wild-type; OS = overall survival; PFS = progression-free survival; ORR = overall response rate; SqCC = squamous cell carcinoma; non-SqCC = non squamous cell carcinoma
These findings highlight a fundamental challenge in the design of immunotherapeutic trials where OS is the most reliable indicator of efficacy. As the FDA guidance on clinical trial endpoints points out, despite being a universally accepted outcome measure, OS is significantly affected by subsequent therapies, crossover to the experimental immunotherapy following progression on the SOC is particularly difficult. Ideally, from an ethical perspective, all trial participants would have access to an active experimental therapy at some point in their treatment course, while still enabling the trial to demonstrate sufficient and valid benefit. However, as some authors have argued, cross over may not be ethical if it undermines the trial's ability to answer the question for which it was designed.50 However, even if cross over is prohibited, as in KEYNOTE-010 in NSCLC, there may be unintended consequences that potentially confound trial results. Patients may withdraw from the trial if assigned the SOC arm, or will seek alternative access to similar medications, either with off-label prescription or in clinical trials of other agents. In KEYNOTE-010, 34/343(9.9%) assigned to the docetaxel arm withdrew consent, and 45/343 (13.1%) went on to receive off-trial immunotherapy following progression on docetaxel.16 The potential for confounding will need to be actively addressed in future trials of immunotherapy against traditional SOC, either through pre-planned modeling and statistical analysis, or by the development of validated immune-specific endpoints as surrogates for OS benefit. New technologies, such as those measuring circulating tumor DNA (ctDNA), have been shown to correlate with temporal changes in disease activity,51 and correlate strongly with the likelihood of an objective response to TIL immunotherapy.52
An alternative approach is the use of novel endpoints, as demonstrated in the phase III trial of the “first-in-class” injected oncolytic virus talimogene laherparepvec (T-VEC) against a GM-CSF control arm. T-VEC is a herpes simplex virus type 1-derived oncolytic virus designed to enhance an individual's anti-tumor response by replicating solely within tumors and producing granulocyte macrophage colony-stimulating factor (GM-CSF).53 In this trial, the primary end point was durable response rate (DRR) defined as the rate of CR and PR lasting more than 6 months but beginning within 12 months of T-VEC administration. ORR and OS were secondary endpoints. T-VEC administration resulted in a statistically significantly improved DRR and ORR, and an improved OS, though not statistically so (p = 0.051). Nevertheless, on the basis of these results the FDA approved T-VEC for use in October 2015 and it was subsequently also approved by the EMA in December 2015. This trial demonstrated how the atypical kinetics of response can be effectively taken into account in the design and analysis of immunotherapeutic trials. Importantly, regulatory agencies have also demonstrated that they are willing to adapt to this changing environment and consider novel outcomes that do not necessarily fit the standard outcome framework.
Future considerations for combination therapies
As immunotherapeutics have shown improved clinical outcomes inevitably combination trials have been initiated where immunotherapies are combined either with each other, chemotherapeutic agents, or targeted therapies - as can be seen from the multiple ongoing combination trials in Table 2. Combined immune checkpoint blockade has already shown impressive success in melanoma, at the cost of significantly increased toxicity, in the CheckMate 067 and 069 trials (see Table 1.) with significant improvements in ORR and PFS.54,55 There are also early signs in NSCLC that the combination of chemotherapy and pembrolizumab may have an additive effect. The initial report from the KEYNOTE-021 trial showed a significantly improved ORR for the combination therapy over chemotherapy alone (55% vs 29%, P = 0.002).56 Results are still awaited for targeted and immunotherapy combinations in melanoma and NSCLC, but the combination of a MEK inhibitor (cobimetinib) and an anti-PD-L1 mAb (atezolizumab) showed a response rate of 17% in the phase 1 trial of microsatellite stable colorectal cancer, in which PD-axis directed therapy is usually ineffective.57 This provides early evidence for the potential for success of novel therapeutic combinations.
Table 1.
Pivotal published clinical trials of immunotherapeutics in melanoma and NSCLC.
| Immunotherapy | Trial | Phase | Intervention | Population | Recruitment Period | N | SOC | 1st Comparator | 2nd Comparator | Primary Endpoint | Results | p value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Melanoma | ||||||||||||
| Ipilimumab | ||||||||||||
| Hodi et al.11 | III | Ipilimumab v placebo | Previously treated metastatic melanoma (MM) | 09/2004 – 08/2008 | 676 | gp100 | Ipilimumab + gp100 | Ipilimumab 3 mg/kg | OS | Median OS: | p<0.001 | |
| Ipilimumab + gp100: 10.0 mo | ||||||||||||
| Ipilimumab: 10.1 mo gp100: 6.4 mo | ||||||||||||
| Ribas et al. | II | Tremelimumab vs Dacarbazine (DTIC or Temozolomide | Previously untreated MM | 03/2006 – 07/2007 | 655 | Dacarbazine or Temozolomide | Tremelimumab | N/A | OS | Median OS: | p = 0.127 | |
| Tremelimumab: 12.7 mo | ||||||||||||
| Chemotherapy: 10.8 mo | ||||||||||||
| Robert et al.30 | III | Ipilimumab vs Dacarbazine (DTIC) | Previously untreated MM | 08/2006 – 01/2008 | 502 | Dacarbazine | Ipilimumab + Dacarbazine | N/A | OS | Median OS: | p<0.001 | |
| Ipilimumab + DTIC: 11.2 mo | ||||||||||||
| DTIC: 9.1 mo | ||||||||||||
| Pembrolizumab | ||||||||||||
| Ribas et al.31 (KEYNOTE-002) | II | Chemotherapy vs Pembrolizumab | MM after Ipilimumab or BRAF/MEK inhibitor | 11/2012 – 11/2013 | 540 | Investigator's choice chemotherapy | Pembrolizumab 2 mg/kg | Pembrolizumab 10 mg/kg | OS (interim analysis PFS) | 6 month PFS: | p<0.001 | |
| Pembrolizumab (2 mg/kg) – 34% | ||||||||||||
| Pembrolizumab (10 mg/kg) – 38% | ||||||||||||
| Chemotherapy – 16% | ||||||||||||
| Robert et al.35 (KEYNOTE-006) | III | Ipilimumab vs Pembrolizumab | Previously treated MM (2nd line) | 09/2013 – 03/2014 | 834 | Ipilimumab 3 mg/kg | Pembrolizumab 10 mg/kg Q2W | Pembrolizumab 10 mg/kg Q3W | OS and PFS | 6 month PFS: | ||
| Pembrolizumab Q2W – 47.3% | p<0.001 | |||||||||||
| Pembrolizumab Q3W– 46.4% | p<0.001 | |||||||||||
| Ipilimumab – 26.5% | ||||||||||||
| 1 y OS: | ||||||||||||
| Pembrolizumab Q2W – 74.1% | p<0.001 | |||||||||||
| Pembrolizumab Q3W– 68.4% | p = 0.004 | |||||||||||
| Ipilimumab – 58.2% | ||||||||||||
| Nivolumab | ||||||||||||
| Weber et al.32 (CheckMate 037) | III | Nivolumab vs Dacarbazine (DTIC) or Carboplatin-Paclitaxel | Previously treated MM | 12/2012 – 01/2014 | 405 | Dacarbazine or Carboplatin/Paclitaxel | Nivolumab 3 mg/kg | N/A | ORR | ORR: | ||
| OS | Nivolumab: 31.7% | |||||||||||
| Chemotherapy: 10.6% | ||||||||||||
| OS not yet reported | ||||||||||||
| Robert et al.33 (CheckMate 066) | III | Nivolumab vs Dacarbazine (DTIC) | Previously untreated MM (BRAF WT) | 01/2013 – 02/2014 | 518 | Dacarbazine | Nivolumab 3 mg/kg | N/A | OS | 1 y OS: | p<0.001 | |
| Nivolumab: 72.9% | ||||||||||||
| Dacarbazine: 42.1% | ||||||||||||
| Combination | ||||||||||||
| Postow et al.58 | I | Ipilimumab vs Nivolumab + Ipilimumab | Previously untreated MM | 09/2013 – 02/2014 | 175 (142 BRAF WT) | Ipilimumab 3 mg/kg | Ipilimumab 3 mg/kg + Nivolumab 1 mg/kg Q3W; then Nivolumab 3 mg/kg Q2W | N/A | ORR in BRAF WT only | ORR: | p<0.001 | |
| Hodi et al.59(CheckMate 069) | Ipilimumab + nivolumab: 61% | |||||||||||
| Ipilimumab: 11% | ||||||||||||
| 1 y OS: | p = 0.26 | |||||||||||
| Ipilimumab + nivolumab: 73% | ||||||||||||
| Ipilimumab: 65% | ||||||||||||
| Larkin et al.54 | III | Ipilimumab vs Nivolumab + Ipilimumab OR Nivolumab alone | Previously untreated MM | 07/2013 – 03/2014 | 945 | Ipilimumab 3 mg/kg | Nivolumab 3 mg/kg Q2W | Ipilimumab 3 mg/kg + Nivolumab 1 mg/kg Q3W | PFS and OS | Median PFS: | p<0.001 | |
| Wolchok et al.60(CheckMate 067) | Ipilimumab: 2.9 mo | |||||||||||
| Ipilimumab + nivolumab: 11.5 mo | ||||||||||||
| Nivolumab: 6.9 mo | ||||||||||||
| OS not yet reported | ||||||||||||
| Talimogene Laherparepvec (T-VEC) | ||||||||||||
| Antbacka et al.53 | III | T-VEC vs GM-CSF | Previously untreated MM | 05/2009 – 07/2011 | 436 | Intralesional T-VEC | Subcutaneous GM-CSF | N/A | DRR | DRR: | p<0.001 | |
| T-VEC: 16.3% | ||||||||||||
| GM-CSF: 2.1% | ||||||||||||
| NSCLC | ||||||||||||
| Ipilimumab | ||||||||||||
| Lynch et al.23 | II | Ipilimumab +/− Carboplatin-Paclitaxel | Previously untreated unresectable or metastatic NSCLC (mNSCLC) | 02/2008 – 02/2009 | 204 | Carboplatin-Paclitaxel | Concurrent regimen: | Phased regimen: | irPFS | Median irPFS: | ||
| Ipilimumab 10 mg/kg + Carboplatin/Paclitaxel Q3W for 4 doses then 2 doses of chemotherapy | 2 doses of Carboplatin/Paclitaxel Q3W then 4 doses of chemotherapy + Ipilimumab 10 mg/kg | Chemotherapy: 4.6 mo | p = 0.13 | |||||||||
| Concurrent Rx: 5.5 mo | p = 0.05 | |||||||||||
| Phased Rx: 5.7 mo | ||||||||||||
| Median OS: | ||||||||||||
| Chemotherapy: 8.3 mo | p = 0.48 | |||||||||||
| Concurrent Rx: 9.7 mo | p = 0.23 | |||||||||||
| Phased Rx: 12.2 mo | ||||||||||||
| Pembrolizumab | ||||||||||||
| Herbst et al.16 (KEYNOTE-010) | II/III | Pembrolizumab vs Docetaxel | Previously treated mNSCLC PD-L1 >1% | 08/2013 – 02/2015 | 1034 | Docetaxel | Pembrolizumab 2 mg/kg | Pembrolizumab 10 mg/kg | PFS and OS | Median PFS | ||
| Chemotherapy: 4.0 mo | ||||||||||||
| Pembrolizumab (2 mg/kg): 3.9 mo | p = 0.07 | |||||||||||
| Pembrolizumab (10 mg/kg): 4.0 mo | p = 0.004 | |||||||||||
| Median OS: | ||||||||||||
| Chemotherapy: 8.5 mo | p<0.001 | |||||||||||
| Pembrolizumab (2 mg/kg): 10.4 mo | p<0.001 | |||||||||||
| Pembrolizumab (10 mg/kg): 12.7 mo | ||||||||||||
| Reck et al.24 (KEYNOTE-024) | III | Chemotherapy vs Pembrolizumab | Previously untreated mNSCLC without activating mutation | 08/2014 | 305 | Platinum-based chemotherapy | Pembrolizumab 200 mg | N/A | PFS | Median PFS | p<0.001 | |
| Chemotherapy: 6.0 mo | ||||||||||||
| Pembrolizumab: 10.3 mo | ||||||||||||
| Langer et al.56 (KEYNOTE-021) | II | Chemotherapy +/− pembrolizumab | Previously untreated mNSCLC | 11/2014 – 01/2016 | 123 | Carboplatin-Pemetrexed | Pembrolizumab 200 mg + Carboplatin-Pemetrexed | N/A | ORR | ORR: | p = 0.002 | |
| Chemotherapy: 29% | ||||||||||||
| Chemotherapy + pembrolizumab: 55% | ||||||||||||
| Nivolumab | ||||||||||||
| Brahmer et al.17 (CheckMate 017) | III | Nivolumab vs Docetaxel | Previously treated SqCC mNSCLC | 10/2012 – 12/2013 | 272 | Docetaxel | Nivolumab 3 mg/kg Q2W | N/A | OS | Median OS | p<0.001 | |
| Docetaxel: 6.0 mo | ||||||||||||
| Nivolumab: 9.2mo | ||||||||||||
| Borghaei et al.18 (CheckMate 057) | III | Nivolumab vs Docetaxel | Previously treated Non-SqCC mNSCLC | 11/2012 – 12/2013 | 582 | Docetaxel | Nivolumab 3 mg/kg Q2W | N/A | OS | Median OS | p = 0.002 | |
| Docetaxel: 9.4mo | ||||||||||||
| Nivolumab: 12.2mo | ||||||||||||
| Atezolizumab | ||||||||||||
| Fehrenbacher et al.43 (POPLAR II) | II | Atezolizumab vs Docetaxel | Previously treated mNSCLC | 08/2013 – 03/2014 | 287 | Docetaxel | Atezolizumab 1200 mg | N/A | OS | Median OS | p = 0.04 | |
| Docetaxel: 9.7 mo | ||||||||||||
| Atezolizumab: 12.6 mo | ||||||||||||
Notes: SOC = standard of care; MM = metastatic melanoma; mNSCLC = metastatic NSCLC; BRAF WT = BRAF wild-type; OS = overall survival; PFS = progression-free survival; ORR = overall response rate; SqCC = squamous cell carcinoma; non-SqCC = non squamous cell carcinoma
If the early promise of these immunotherapies is fulfilled then it is likely that immunotherapy alone or in combination will supplant the existing SOC in melanoma and NSCLC. This suggests that new immunotherapies will either need to be added to the existing backbone or compared directly against the immunotherapeutic SOC, bringing with it concerns about patient safety, the need for agreed response criteria and the informed design of trial statistics. Indeed studies combining chemotherapy with both nivolumab and ipilimumab have already launched and it remains to be seen whether such combinations are tolerable and efficacious. However, the initial trials of combination therapy indicate that immunotherapy may become the therapeutic standard in the very near future for many different cancer types.
Conclusion
The field of cancer immunotherapy is rapidly expanding, and as numerous clinical trials with a dizzying array of agents, including cancer vaccines, checkpoint inhibitors and novel treatment strategies, are undertaken, so the broader cancer community must adjust to the unique challenges presented by these developments. The current clinical trial and drug development framework is, at times, ill-suited to the direct comparison of therapies with distinct mechanisms of action, response kinetics and impact on commonly accepted clinical outcomes. The SOC in each trial fundamentally affects the design, management and interpretation of these trials, and only effective communication between drug developers, researchers, clinicians and regulatory bodies will enable the development of a modern, ethical and responsive approach to clinical trial execution. Novel approaches to trial end point selection, definition and measurement will be required if we are to be able to rise to these challenges. We owe it to our patients to do just that.
Disclosure of potential conflicts of interest
Dr. Rivalland has received honoraria from AstraZeneca.Dr. Scott has nothing to disclose.Dr. John has received honoraria from Pfizer, Novartis, BMS, AstraZeneca and Roche
References
- [1].Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nat Rev Cancer 2012; 12(4):278-87; PMID:22437872; http://dx.doi.org/ 10.1038/nrc3236 [DOI] [PubMed] [Google Scholar]
- [2].Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, Pegram M, Oh DY, Dieras V, Guardino E, et al.. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Eng J Med 2012; 367(19):1783-91;PMID:23020162;http://dx.doi.org/ 10.1056/NEJMoa1209124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Weiner GJ. Rituximab: mechanism of action. Semin Hematol 2010; 47(2):115-23;PMID:20350658;http://dx.doi.org/ 10.1053/j.seminhematol.2010.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, et al.. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Eng J Med 2010; 363(5):411-22;PMID:20818862;http://dx.doi.org/ 10.1056/NEJMoa1001294 [DOI] [PubMed] [Google Scholar]
- [5].Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al.. Improved survival with ipilimumab in patients with metastatic melanoma. N Eng J Med 2010; 363(8):711-23; PMID:20525992; http://dx.doi.org/ 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al.. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Eng J Med 2012; 366(26):2443-54; PMID:22658127; http://dx.doi.org/ 10.1056/NEJMoa1200690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, Dawson N, O'Donnell PH, Balmanoukian A, Loriot Y, et al.. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet (London, England) 2016; 387(10031):1909-20; PMID:26952546; http://dx.doi.org/ 10.1016/S0140-6736(16)00561-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Butts C, Socinski MA, Mitchell PL, Thatcher N, Havel L, Krzakowski M, Nawrocki S, Ciuleanu T-E, Bosquée L, Trigo JM, et al.. Tecemotide (L-BLP25) versus placebo after chemoradiotherapy for stage III non-small-cell lung cancer (START): a randomised, double-blind, phase 3 trial. Lancet Oncol 2014; 15(1):59-68; PMID:24331154; http://dx.doi.org/ 10.1016/S1470-2045(13)70510-2 [DOI] [PubMed] [Google Scholar]
- [9].Vansteenkiste JF, Cho BC, Vanakesa T, De Pas T, Zielinski M, Kim MS, Jassem J, Yoshimura M, Dahabreh J, Nakayama H, et al.. Efficacy of the MAGE-A3 cancer immunotherapeutic as adjuvant therapy in patients with resected MAGE-A3-positive non-small-cell lung cancer (MAGRIT): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2016; 17(6):822-35; PMID:27132212; http://dx.doi.org/ 10.1016/S1470-2045(16)00099-1 [DOI] [PubMed] [Google Scholar]
- [10].Bates SE, Berry DA, Balasubramaniam S, Bailey S, LoRusso PM, Rubin EH. Advancing clinical trials to streamline drug development. Clin Cancer Res 2015; 21(20):4527-35; PMID:26473188; http://dx.doi.org/ 10.1158/1078-0432.CCR-15-0039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA. Improved survival with ipilimumab in patients with metastatic melanoma. N Eng J Med 2010; 363:711-23; http://dx.doi.org/ 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Franklin C, Livingstone E, Roesch A, Schilling B, Schadendorf D. Immunotherapy in melanoma: recent advances and future directions. Eur J Surg Oncol 2016; [Epub ahead of print]; PMID:27769635; http://dx.doi.org/ 10.1016/j.ejso.2016.07.145 [DOI] [PubMed] [Google Scholar]
- [13].Eggermont AMM, Chiarion-Sileni V, Grob J-J, Dummer R, Wolchok JD, Schmidt H, Hamid O, Robert C, Ascierto PA, Richards JM, et al.. Prolonged Survival in stage III Melanoma with Ipilimumab adjuvant therapy. N Eng J Med 2016; 375(19): 1845–1855; PMID:27717298; http://dx.doi.org/ 10.1056/NEJMoa1611299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, Stankevich E, Pons A, Salay TM, McMiller TL, et al.. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 2010; 28(19):3167-75; PMID:20516446; http://dx.doi.org/ 10.1200/JCO.2009.26.7609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, et al.. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Eng J Med 2013; 369(2):134-44;PMID:23724846; http://dx.doi.org/ 10.1056/NEJMoa1305133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, et al.. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet (London, England) 2016; 387(10027): 1540–1550; PMID:26712084; http://dx.doi.org/ 10.1016/s0140-6736(15)01281-7 [DOI] [PubMed] [Google Scholar]
- [17].Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, et al.. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Eng J Med 2015; 373(2):123-35; PMID:26028407; http://dx.doi.org/ 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al.. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Eng J Med 2015; 373(17):1627-39; PMID:26412456; http://dx.doi.org/ 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Paz-Ares L, de Marinis F, Dediu M, Thomas M, Pujol JL, Bidoli P, Molinier O, Sahoo TP, Laack E, Reck M, et al.. Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (PARAMOUNT): a double-blind, phase 3, randomised controlled trial. Lancet Oncol 2012; 13(3):247-55; PMID:22341744; http://dx.doi.org/ 10.1016/S1470-2045(12)70063-3 [DOI] [PubMed] [Google Scholar]
- [20].Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Eng J Med 2006; 355(24):2542-50;PMID:17167137;http://dx.doi.org/ 10.1056/NEJMoa061884 [DOI] [PubMed] [Google Scholar]
- [21].Cappuzzo F, Ciuleanu T, Stelmakh L, Cicenas S, Szczesna A, Juhasz E, Esteban E, Molinier O, Brugger W, Melezinek I, et al.. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol 2010; 11(6):521-9; PMID:20493771; http://dx.doi.org/ 10.1016/S1470-2045(10)70112-1 [DOI] [PubMed] [Google Scholar]
- [22].Perol M, Chouaid C, Perol D, Barlesi F, Gervais R, Westeel V, Crequit J, Lena H, Vergnenegre A, Zalcman G, et al.. Randomized, phase III study of gemcitabine or erlotinib maintenance therapy versus observation, with predefined second-line treatment, after cisplatin-gemcitabine induction chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol 2012; 30(28):3516-24; PMID:22949150; http://dx.doi.org/ 10.1200/JCO.2011.39.9782 [DOI] [PubMed] [Google Scholar]
- [23].Lynch TJ, Bondarenko I, Luft A, Serwatowski P, Barlesi F, Chacko R, Sebastian M, Neal J, Lu H, Cuillerot JM, et al.. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J Clin Oncol 2012; 30(17):2046-54;PMID:22547592;http://dx.doi.org/ 10.1200/JCO.2011.38.4032 [DOI] [PubMed] [Google Scholar]
- [24].Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Cső szi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, et al.. Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. N Eng J Med 2016; 375(19): 1823–1833; PMID:27718847; http://dx.doi.org/ 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- [25].Bristol-Myers Squibb Bristol-myers squibb statement on top-line results from checkmate-026 5 August 2016. [accessed 15September2016]. http://news.bms.com/press-release/bristol-myers-squibb-announces-top-line-results-checkmate-026-phase-3-study-opdivo-niv
- [26].Serrone L, Zeuli M, Sega FM, Cognetti F. Dacarbazine-based chemotherapy for metastatic melanoma: thirty-year experience overview. J Exp Clin Cancer Res 2000; 19(1):21-34 [PubMed] [Google Scholar]
- [27].Patel PM, Suciu S, Mortier L, Kruit WH, Robert C, Schadendorf D, Trefzer U, Punt CJ, Dummer R, Davidson N, et al.. Extended schedule, escalated dose temozolomide versus dacarbazine in stage IV melanoma: final results of a randomised phase III study (EORTC 18032). Eur J Cancer (Oxford, England: 1990) 2011; 47(10):1476-83; PMID:21600759; http://dx.doi.org/ 10.1016/j.ejca.2011.04.030 [DOI] [PubMed] [Google Scholar]
- [28].Rao RD, Holtan SG, Ingle JN, Croghan GA, Kottschade LA, Creagan ET, Kaur JS, Pitot HC, Markovic SN. Combination of paclitaxel and carboplatin as second-line therapy for patients with metastatic melanoma. Cancer 2006; 106(2):375-82; PMID:16342250; http://dx.doi.org/ 10.1002/cncr.21611 [DOI] [PubMed] [Google Scholar]
- [29].Hersh EM, Del Vecchio M, Brown MP, Kefford R, Loquai C, Testori A, Bhatia S, Gutzmer R, Conry R, Haydon A, et al.. A randomized, controlled phase III trial of nab-Paclitaxel versus dacarbazine in chemotherapy-naive patients with metastatic melanoma. Annal Oncol 2015; 26(11):2267-74; http://dx.doi.org/ 10.1093/annonc/mdv324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Robert C, Thomas L, Bondarenko I, O'Day S, Weber J, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ, et al.. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Eng J Med 2011; 364(26):2517-26; PMID:21639810; http://dx.doi.org/ 10.1056/NEJMoa1104621 [DOI] [PubMed] [Google Scholar]
- [31].Ribas A, Puzanov I, Dummer R, Schadendorf D, Hamid O, Robert C, Hodi FS, Schachter J, Pavlick AC, Lewis KD, et al.. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol 2015; 16(8):908-18; PMID:26115796; http://dx.doi.org/ 10.1016/S1470-2045(15)00083-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Weber JS, D'Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, Hoeller C, Khushalani NI, Miller WH Jr., Lao CD, et al.. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2015; 16(4):375-84; PMID:25795410; http://dx.doi.org/ 10.1016/S1470-2045(15)70076-8 [DOI] [PubMed] [Google Scholar]
- [33].Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E, et al.. Nivolumab in previously untreated melanoma without BRAF mutation. N Eng J Med 2015; 372(4):320-30; PMID:25399552; http://dx.doi.org/ 10.1056/NEJMoa1412082 [DOI] [PubMed] [Google Scholar]
- [34].Robert C, Ribas A, Wolchok JD, Hodi FS, Hamid O, Kefford R, Weber JS, Joshua AM, Hwu WJ, Gangadhar TC, et al.. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet (London, England) 2014; 384(9948):1109-17; PMID:25034862;http://dx.doi.org/ 10.1016/S0140-6736(14)60958-2 [DOI] [PubMed] [Google Scholar]
- [35].Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, et al.. Pembrolizumab versus Ipilimumab in advanced melanoma. N Eng J Med 2015; 372(26):2521-32; PMID:25891173; http://dx.doi.org/ 10.1056/NEJMoa1503093 [DOI] [PubMed] [Google Scholar]
- [36].Nishino M, Jagannathan JP, Krajewski KM, O'Regan K, Hatabu H, Shapiro G, Ramaiya NH. Personalized tumor response assessment in the era of molecular medicine: cancer-specific and therapy-specific response criteria to complement pitfalls of RECIST. AJR Am J Roentgenol 2012; 198(4):737-45;PMID:22451534;http://dx.doi.org/ 10.2214/AJR.11.7483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Chiou VL, Burotto M. Pseudoprogression and immune-related response in solid tumors. J Clin Oncol 2015; 33(31):3541-3; PMID:26261262; http://dx.doi.org/ 10.1200/JCO.2015.61.6870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, et al.. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000; 92(3):205-16; PMID:10655437; http://dx.doi.org/ 10.1093/jnci/92.3.205 [DOI] [PubMed] [Google Scholar]
- [39].Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al.. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer (Oxford, England: 1990) 2009; 45(2):228-47; PMID:19097774; http://dx.doi.org/ 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- [40].Wolchok JD, Hoos A, O'Day S, Weber JS, Hamid O, Lebbe C, Maio M, Binder M, Bohnsack O, Nichol G, et al.. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 2009; 15(23):7412-20; PMID:19934295; http://dx.doi.org/ 10.1158/1078-0432.CCR-09-1624 [DOI] [PubMed] [Google Scholar]
- [41].Mitchell PL, Quinn MA, Grant PT, Allen DG, Jobling TW, White SC, Zhao A, Karanikas V, Vaughan H, Pietersz G, et al.. A phase 2, single-arm study of an autologous dendritic cell treatment against mucin 1 in patients with advanced epithelial ovarian cancer. J Immunother Cancer 2014; 2(16; PMID:24995129; http://dx.doi.org/ 10.1186/2051-1426-2-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Schwartzentruber DJ, Lawson DH, Richards JM, Conry RM, Miller DM, Treisman J, Gailani F, Riley L, Conlon K, Pockaj B, et al.. gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N Eng J Med 2011; 364(22):2119-27; PMID:21631324; http://dx.doi.org/ 10.1056/NEJMoa1012863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, Park K, Smith D, Artal-Cortes A, Lewanski C, et al.. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet (London, England) 2016; 387(10030): 1837–1846; http://dx.doi.org/ 10.1016/s0140-6736(16)00587-0 [DOI] [PubMed] [Google Scholar]
- [44].Chen TT. Statistical issues and challenges in immuno-oncology. J Immunother Cancer 2013; 1:18; PMID:24829754; http://dx.doi.org/ 10.1186/2051-1426-1-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Chen TT. Predicting analysis times in randomized clinical trials with cancer immunotherapy. BMC Med Res Methodol 2016; 16(12; PMID:26830911; http://dx.doi.org/ 10.1186/s12874-016-0117-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Sul J, Blumenthal GM, Jiang X, He K, Keegan P, Pazdur R. FDA approval summary: pembrolizumab for the treatment of patients with metastatic non-small cell lung cancer whose tumors express programmed death-ligand 1. Oncologist 2016; 21(5):643-50; PMID:27026676; http://dx.doi.org/ 10.1634/theoncologist.2015-0498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kazandjian D, Suzman DL, Blumenthal G, Mushti S, He K, Libeg M, Keegan P, Pazdur R. FDA approval summary: nivolumab for the treatment of metastatic non-small cell lung cancer with progression on or after platinum-based chemotherapy. Oncologist 2016; 21(5):634-42;PMID:26984449;http://dx.doi.org/ 10.1634/theoncologist.2015-0507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ribas A, Kefford R, Marshall MA, Punt CJA, Haanen JB. Phase, III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J Clin Oncol 2013; 31:616-22; http://dx.doi.org/ 10.1200/jco.2012.44.6112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Gomella L, Nabhan C, DeVries T, Whitmore J, Frohlich M, George D. 683 estimating the overall survival benefit of sipuleucel-t in the impact trial accounting for crossover treatment in control subjects with autologous immunotherapy generated from cyropreserved cells. J Urol; 187(4):e278-e9; http://dx.doi.org/ 10.1016/j.juro.2012.02.765 [DOI] [Google Scholar]
- [50].Prasad V, Grady C. The misguided ethics of crossover trials. Contemp Clin Trials 2014; 37(2):167-9; PMID:24365533; http://dx.doi.org/ 10.1016/j.cct.2013.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Chia PL, Do H, Morey A, Mitchell P, Dobrovic A, John T. Temporal changes of EGFR mutations and T790M levels in tumour and plasma DNA following AZD9291 treatment. Lung cancer (Amsterdam, Netherlands) 2016; 98:29-32; PMID:27393503 [DOI] [PubMed] [Google Scholar]
- [52].Xi L, Pham T, Payabyab EC, Sherry RM, Rosenberg SA, Raffeld M. Circulating Tumor DNA as an Early Indicator of Response to T-Cell Transfer Immunotherapy in Metastatic Melanoma. Clin Cancer Res 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Andtbacka RH, Kaufman HL, Collichio F, Amatruda T, Senzer N, Chesney J, Delman KA, Spitler LE, Puzanov I, Agarwala SS, et al.. Talimogene Laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol 2015; 33(25):2780-8; PMID:26014293; http://dx.doi.org/ 10.1200/JCO.2014.58.3377 [DOI] [PubMed] [Google Scholar]
- [54].Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, et al.. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Eng J Med 2015; 373(1):23-34; PMID:26027431; http://dx.doi.org/ 10.1056/NEJMoa1504030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Eng J Med 2015; 372; doi: 10.1056/NEJMoa1414428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Langer CJ, Gadgeel SM, Borghaei H, Papadimitrakopoulou VA, Patnaik A, Powell SF, Gentzler RD, Martins RG, Stevenson JP, Jalal SI, et al.. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol 2016; 17(11): 1497–1508; http://dx.doi.org/ 10.1016/s1470-2045(16)30498-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Bendell J, Tae Won K, Cheng Ean C, Yung-Jue B, Lee C, Desai J, Lewin J, Wallin J, Das Thakur M, Mwawasi G, et al.. LBA-01Safety and efficacy of cobimetinib (cobi) and atezolizumab (atezo) in a Phase 1b study of metastatic colorectal cancer (mCRC). Annal Oncol 2016; 27(suppl 2):ii140 [Google Scholar]
- [58].Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, Linette GP, Meyer N, Giguere JK, Agarwala SS, et al.. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Eng J Med 2015; 372(21):2006-17; PMID:25891304; http://dx.doi.org/ 10.1056/NEJMoa1414428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Hodi FS, Chesney J, Pavlick AC, Robert C, Grossmann KF, McDermott DF, Linette GP, Meyer N, Giguere JK, Agarwala SS, et al.. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol 2016; 17(11): 1558–1568; http://dx.doi.org/ 10.1016/s1470-2045(16)30366-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, Lao C, Schadendorf D, Ferrucci PF, Smylie M, et al.. Updated results from a phase III trial of nivolumab (NIVO) combined with ipilimumab (IPI) in treatment-naive patients (pts) with advanced melanoma (MEL) (CheckMate 067). ASCO Meeting Abstracts 2016; 34(15_suppl):9505 [Google Scholar]


