Abstract
A 16-year-old young man presented to the emergency room with new-onset generalised tonic-clonic seizures. Examination showed a Glasgow score of 13 and predominantly crural left hemiparesis. Imaging demonstrated a right frontoparietal haemorrhage of non-vascular origin with perilesional oedema. Surgical drainage was carried out, but rebleeding occurred within 24 hours following surgery, and again 1 week after discharge. On reinterrogation and examination, Ehrlichia canis infection was suspected and empirical management with doxycycline was begun. Improvement was evident 72 hours after antibiotic initiation, and PCR confirmed the diagnosis; thus, doxycycline was continued for 6 months. After 2 years, seizures recurred and treatment was reinstated with good clinical response. However, seizures reappeared whenever treatment discontinuation was attempted. Lacking alternatives, doxycycline was maintained up to the third year following the initial episode. Subsequently, the patient showed complete resolution without neurological sequelae up to his last follow-up visit, 12 months following treatment cessation.
Keywords: infection (neurology), emergency medicine, travel medicine
Background
Previously unrecognised pathogens have emerged as humans are increasingly exposed to microbes that evolve within mammalian-tick enzootic cycles.1 2 These pathogens include obligate intracellular bacteriae of the family Anaplasmataceae, which among other characteristics share the ability to modify surface immunogenic proteins.1 This latter feature probably contributes to persistent infectivity of reservoir hosts and facilitates infection of new alternative niches such as humans.1
Within this family, there is a genus of small gram-negative bacteriae, measuring 0.5 to 1.5 µm, known as ehrlichiae. They are obligate intracellular pleomorphic coccobacilli with special tropism for macrophages or granulocytes and are found within the cytosol forming mulberry-like inclusions, which represent replication within endosomal vacuoles.1 2 Ehrlichiae were recognised as distinct from other obligate intracellular bacteriae of medical importance in 1945, by the German bacteriologist Paul Ehrlich.2 They are the causative agents of monocytic ehrlichiosis and granulocytic ehrlichiosis, prolonged febrile pathologies characteristic of dogs, which in humans have clinical and geographical similarities to Rocky Mountain Spotted Fever.1–3
Ehrlichia canis was described in 1963. It is the primary aetiological agent of canine monocytic ehrlichiosis, a globally distributed potentially fatal disease representing the most severe form of ehrlichiae infections,4 5 as well as of tropical canine pancytopaenia, a haemorrhagic disease.2–4 E. canis is transmitted by the brown dog tick Rhipicephalus sanguineus and infects monocytes and macrophages.4 5 In humans, the first case of human ehrlichiosis assumed to be due to E. canis was reported in the USA in 1986, in a 51-year-old man.2 6 It has since been recognised to be associated with various non-specific clinical manifestations developing after a tick bite, such as fever, encephalopathy, mild hepatitis, acute tubular necrosis, anaemia and thrombocytopaenia.2 6 However, the following report depicts the first case of primary cerebral infection by E. canis, manifesting as intraparenchymal haemorrhage.
Case presentation
A right-handed 16-year-old young man presented to the emergency department, 2 hours after having had two episodes of new-onset generalised tonic–clonic seizures with partial recovery. Background history was negative for recent febrile episodes, trauma, anticoagulation usage, exposure to toxic substances and use of recreational drugs. The patient did not have a significant medical history, and family history was negative. On physical examination, vital signs were normal and stable, and ophthalmoscopy did not reveal abnormalities. However, Glasgow score was 13, given by a decreased palpebral opening and verbal response. Furthermore, he showed predominantly crural left haemiparesis. There was no nuchal rigidity or further findings during examination.
Investigations
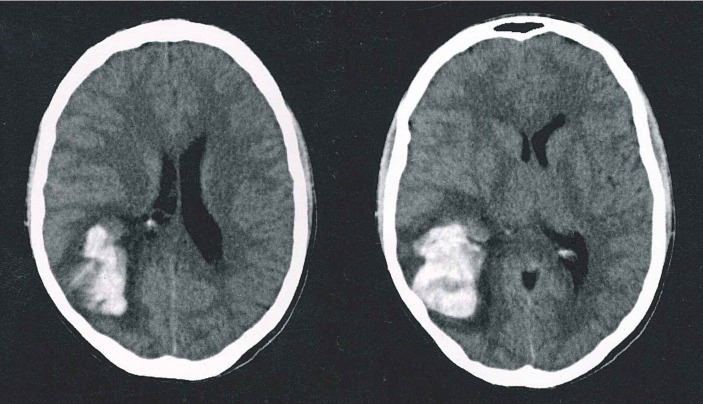
Simple cerebral CT was performed, given the acute onset of symptoms and the physical examination findings previously described. It showed a right frontoparietal haemorrhage, with perilesional oedema leading to mild ventricular compression, without ventricular drainage or midline shift (see figure 1).
Figure 1.
Simple axial CT showing right frontoparietal cerebral haemorrhage with important perilesional oedema and mild ventricular compression.
Given these findings, tomographic angiography was performed so as to further investigate the case and differential diagnoses. It did not demonstrate vascular malformations or other abnormalities explicative of the haemorrhage.
Complete blood count was not suggestive of infection; platelet count was 250 x 109/L, and mean platelet volume was 12 fL. Furthermore, partial thromboplastin time was 25.8 (normal control of 27.1), prothrombin time was 10.7 (normal control of 10.7) with an international normalised ratio of 1. Light transmission aggregometry showed an 85% maximal aggregation in response to ADP, epinephrine, collagen, ristocetin and arachidonic acid, and evaluation by the haematology group ruled out the presence of blood dyscrasia as a possible cause of the haemorrhage.
Lumbar puncture opening pressure was 18 cm of H2O. Cerebrospinal fluid was crystal clear with glucose of 54 mg/dL and total protein of 37 mg/dL. Microscopic examination showed no leucocytes or red blood cells, while Gram and Wright staining were negative for bacteriae and polymorphonuclear cells, respectively.
About 2 weeks later, E. canis infection was suspected based on reinterrogation of the patient's mother. She revealed that the patient's dog had been diagnosed with the infection. On new physical examination, lesions compatible with tick bites were identified in-between toes. Thus, we performed PCR tests in cerebrospinal fluid (CSF) and blood, which effectively confirmed the diagnosis.
Differential diagnosis
Our initial consideration, given the patient's age, was that the haemorrhage was most probably due to a vascular abnormality, but angiographic results showed this was not the case.
Even though differential diagnosis approach to a non-traumatic intracerebral haemorrhage in a young patient must take into account an infectious aetiology, we were not initially inclined towards this possibility because the patient did not show physical signs suggestive of acute infection, complete blood count was normal, and we had no microbiological evidence of common pathogens of the central nervous system (CNS). Both CSF and blood cultures were negative for infection. Furthermore, antibody detection tests for Rickettsia conorii, R. rickettsii, Chlamydia trachomatis and Mycoplasma pneumoniae were negative, while Mycobacterium tuberculosis DNA was not detected in CSF.
In addition, in developing countries, a ruptured cyst in neurocysticercosis must be considered as a diagnostic option. However, this was a less likely diagnosis, as CT did not reveal the typical cysts or calcifications seen in the disease.
Even though a rapidly progressive tumour was also a possible diagnosis, it was not considered at first, given that imaging was not suggestive of the presence of a mass. Furthermore, histopathological evaluation of tissue extracted during surgery did not confirm the presence of malignancy.
Other possible explanations were hypertensive stroke and cocaine use, which we were able to rule out with careful clinical history, physical examination and laboratory studies.
Ehrlichia infection was only suspected on symptom worsening and a thorough history taking process 1 week after the patient was discharged.
Treatment
Initially, appropriate anticonvulsive management with oral phenytoin 600 mg daily as a single dose was begun and continued after discharge for a total of 6 months. After angiography was obtained, surgical drainage was carried out. Reoperation was performed the following day, because Glasgow deteriorated and haemiparesis worsened. Friable tissue macroscopically suggestive of malignancy was observed during the second intervention. However, histopathological evaluation did not confirm the presence of tumour tissue.
Once the diagnosis of E. canis infection was suspected on worsening haemiparesis, empirical management with oral doxycycline 200 mg per day as a single dose was initiated. It led to clinical improvement within 72 hours. PCR results in blood samples confirmed the diagnosis; consequently, doxycycline 200 mg per day was continued. Because we were unable to find reports of specific treatment options for intraparenchymal haemorrhage due to this pathogen, long-term antibiotic was instated until complete recovery was attained, with periodical assessment of hepatic and renal function. Doxycycline was discontinued following 6 months of initial presentation, after paresis was completely reversed and as seizure recurrence or neurological deterioration was not evidenced.
Outcome and follow-up
Clinical improvement was apparent 24 hours after having completed the second surgical drainage, and the patient was discharged within 1 week of observation, during which no additional convulsive episodes were observed. Haemiparesis had almost completely receded and Glasgow score was 15/15 by the time he was discharged. Physical examination showed residual left-sided diminished strength, ++/++++ symmetrical reflexes and preserved sensation. Appropriate oral anticonvulsive prophylaxis, as mentioned above, was given on discharge.
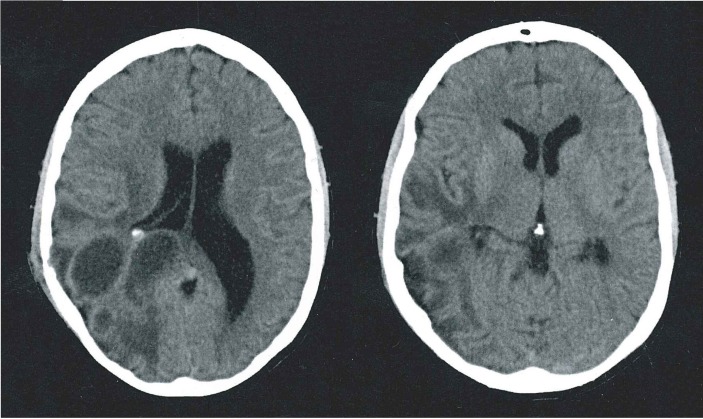
One week ensuing, haemiparesis worsened. Tomographic imaging on this occasion was suggestive of rebleed, associated with extensive oedema (figure 2). On reinterrogation, the patient's mother referred that her son used to sleep with his dog, which had been recently diagnosed with E. canis infection. Physical examination was positive for petechiae-like lesions in soles and in-between toes, suggestive of tick bites. Given these findings and background history, CSF and blood analyses for E. canis infection were performed extrainstitutionally at a reference laboratory and empirical antibiotic initiated as mentioned above. By the third month, paresis showed complete resolution and as seizure or neurological deterioration were not seen, antibiotic therapy together with anticonvulsive treatment was discontinued following 6 months of initial presentation.
Figure 2.
Simple axial CT suggestive of right frontoparietal cerebral rebleeding with significant associated oedema.
A recurrence of tonic–clonic seizures with the same characteristics was seen 2 years after the first episode. Contrast MRI showed a circumferential gadolinium-enhancing frontoparietal lesion with extensive perilesional oedema, located at the same site previously described. Doxycycline 200 mg/day was reinstated given our previous experience and continued until there was no clinical or radiological evidence of haemorrhage a few months after.
On various ensuing occasions in which antibiotic therapy was suspended, seizures recurred. Thus, in view of the lack of alternatives, antibiotic therapy was continued until the third year following the initial presentation, with hepatic, renal and blood analysis controls, which did not show abnormalities. Subsequently, the antibiotic was effectively stopped and the patient showed complete resolution, with no neurological deficit whatsoever and normal imaging controls (see figure 3) up to his last follow-up visit, which was carried out 1 year after medication was discontinued.
Figure 3.
Normal simple T1-weighted axial MRI, performed subsequent to doxycycline discontinuation 3 years after presentation.
Discussion
Human infection with E. canis is rare, but it has been reported in a group of patients identified in Venezuela, who presented with fever of over 39°C, rash, headache, myalgia and cytopaenia.7 These are recognised as the classical presenting symptoms in E. canis infection. Nonetheless, they were not present in our patient; rather, diagnosis was achieved through a thorough clinical interview, during which a clear epizootic association could be ascertained. The present clinical case highlights the complexity and variability of newly emerging human infections1 2 by establishing E. canis as a causative agent of intraparenchymal haemorrhage, not associated with systemic findings. Furthermore, it emphasises the importance of the clinical interview in the process of establishing an appropriate clinical diagnosis, as the only clue we had that was suggestive of E. canis infection was the patient's interaction with his pet and the detailed physical examination performed. In their natural hosts, ehrlichiae avoid clearance by the immune system, establishing a persistent infection.8 Therefore, chronicity and reactivation with episodes of reduced immunity are characteristic of ehrlichioses,2 just as observed in this case report.
In humans, CSF examination in patients diagnosed with ehrlichiosis reveals predominantly lymphocytic pleocytosis found in 73% of patients and an elevated protein concentration in up to 76%.8 9 There are also reports of perivascular infiltration by lymphocytes, plasma cells and macrophages (some containing ehrlichiae inclusions) in the brain and leptomeninges of patients with E. chaffeensis infection.8 Laboratory data therefore supports CNS involvement in ehrlichial infection.
A case of frequent complex partial seizures secondary to dual infection with Anaplasma phagocytophilum and E. chaffeensis was reported in 200610; in contrast to the case presented here, encephalopathy was diagnosed through medial temporal lobe and multifocal cortical signal increase in fluid-attenuated inversion recovery MRI.10 Further cases of CNS involvement have been described in up to 20% to 70% of patients with human monocytic ehrlichiosis.4 8 9 11 In children, CNS manifestations comprise hyper-reflexia, meningismus, confusion, broad-based gait, cranial neuropathy, ataxia and seizures.11 Long-term language and motor neurological sequelae have been occasionally observed.11 12 In contrast, meningitis and meningoencephalitis are rare complications in human granulocytic ehrlichiosis. Few patients develop focal neurological signs and symptoms.13 About 1% shows CNS involvement.8 However, a number of different peripheral nervous system manifestations have been described.1 11 13
The association of granulocytic ehrlichiosis with meningitis has been reported in dogs,14 although some studies utilising broadly reactive PCR to evaluate brain tissue and CSF suggest that Ehrlichia is unlikely to be related with the development of canine meningoencephalitis.15 Although neurological signs attributable to ehrlichiosis are rare, CNS lesions are commonly seen in postmortem examination of infected veterinary specimens.16
On the other hand, canine monocytic ehrlichiosis is recognised as a haemorrhagic disease in dogs.3 The pathology is characterised by thrombocytopaenia,3 which is the most common haematological finding in dogs with E. canis infection.17 Thrombocytopaenia is hypothesised to be caused by peripheral sequestration secondary to vasculitis, destruction in the reticuloendothelial system, inhibition of platelet migration and suppressed production during the chronic phase of the disease.2 8 17 18 It has also been proposed that an overproduction of antiplatelet antibodies observed as early as day seven postinfection, could lead to platelet destruction.19 However, a bleeding tendency seen in dogs with normal platelet counts suggests that haemorrhage is likely attributable to a qualitative platelet defect, as opposed to thrombocytopaenia.20 Animal studies have demonstrated that compared with controls, dogs infected with E. canis have reduced platelet aggregation in response to ADP, epinephrine and collagen.20–25
Mechanisms that are thought to contribute to reduced platelet function include the presence of exhausted platelets that continue to circulate,26 impairment of platelet factor 3 release by an unidentified agent27 and coating of the platelet membrane with light-chain proteins.20 23 Also, it has been suggested that specific antibodies directed against platelet membrane glycoproteins may interfere with platelet aggregation.28
Another possible explicative mechanism for the occurrence of haemorrhage is the formation of microorganism clusters within the vacuole in platelets.8 Thus, it has been suggested that bleeding in ehrlichiosis is of multifactorial origin.25 In consequence, there are tangible explanations and support as to why our patient developed cerebral haemorrhage without thrombocytopaenia. Since platelet function tests were normal in our patient, we believe that haemorrhage was possibly due to localised vasculitis within cerebral vessels, secondary to an inflammatory process caused by the infection.
In summary, evidence provides a sufficient basis to establish a clinical association between ehrlichiosis and CNS infection and clinical manifestations. However, to our knowledge, apart from a case of seizures and anaemia in a dog diagnosed with E. canis infection,16 there are no further reports of neurological focal deficits secondary to this specific pathogen, needless to say, of intraparenchymal haemorrhage secondary to E. canis infection in a human. The only report of intracranial haemorrhage in a human is a recently published case of E. chaffeensis infection. However, apart from it being a different causative agent, the case differed from ours in that it was a subarachnoid haemorrhage presenting with thunderclap-type headache.18
Learning points.
Infection by ehrlichial species commonly causing disease in humans is associated with non-specific central nervous system manifestations.
Although rare, human ehrlichiosis caused by Ehrlichia canis may manifest exclusively in the brain, in this case as an intraparenchymal haemorrhage, without associated systemic findings.
Central nervous system haemorrhage in ehrlichiosis may course without thrombocytopaenia and is possibly linked to altered platelet function due to the presence of microorganism clusters within the platelets.
The history of a zoonotic link can be a major factor orienting the diagnosis in an otherwise unexplained a traumatic central nervous system haemorrhage.
Footnotes
Contributors: JFR provided initial care for the patient and conceived the idea of writing the case. JFR, CGB and MFC were part of the clinical team providing follow-up care, and the three contributed to extract, summarise and interpret the data from clinical records. CGB and MFC performed the literature review. CGB drafted the first version of the manuscript and selected the most illustrative images. MFC obtained informed consent from the patient. JFR, CGB and MFC proof-read and edited the article and approved the final version of it.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Dumler JS. Ehrlichioses and Anaplasmosis : Guerrant RL, Walker DH, Weller PF, Tropical infectious diseases: principles, pathogens and practice. New York: Elsevier, 2011:339–43. [Google Scholar]
- 2.Mounzer KC, Dinubile MJ. Ehrlichial infections. Clin Dermatol 1996;14:289–93. 10.1016/0738-081X(96)00014-4 [DOI] [PubMed] [Google Scholar]
- 3.Rar V, Golovljova I. Anaplasma, Ehrlichia, and 'Candidatus Neoehrlichia' bacteria: pathogenicity, biodiversity, and molecular genetic characteristics, a review. Infect Genet Evol 2011;11:1842–61. 10.1016/j.meegid.2011.09.019 [DOI] [PubMed] [Google Scholar]
- 4.McBride JW. Ehrlichia : Barrett AD, Stanberry LR, Vaccines for Biodefense and Emerging and Neglected Diseases. Oxford: Elsevier, 2009:919–37. [Google Scholar]
- 5.Walker DH, Paddock CD, Dumler JS. Emerging and re-emerging tick-transmitted rickettsial and ehrlichial infections. Med Clin North Am 2008;92:1345–61. 10.1016/j.mcna.2008.06.002 [DOI] [PubMed] [Google Scholar]
- 6.Flicek BF. Rickettsial and other tick-borne infections. Crit Care Nurs Clin North Am 2007;19:27–38. 10.1016/j.ccell.2006.11.001 [DOI] [PubMed] [Google Scholar]
- 7.Perez M, Bodor M, Zhang C, et al. Human infection with Ehrlichia canis accompanied by clinical signs in Venezuela. Ann N Y Acad Sci 2006;1078:110–7. 10.1196/annals.1374.016 [DOI] [PubMed] [Google Scholar]
- 8.Dumler JS, Walker DH. Tick-borne ehrlichioses. Lancet Infect Dis 2001;1:21–8. 10.1016/S1473-3099(09)70296-811871406 [DOI] [Google Scholar]
- 9.Ratnasamy N, Everett ED, Roland WE, et al. Central nervous system manifestations of human ehrlichiosis. Clin Infect Dis 1996;23:314–9. 10.1093/clinids/23.2.314 [DOI] [PubMed] [Google Scholar]
- 10.Young NP, Klein CJ. Encephalopathy with seizures having PCR-positive Anaplasma phagocytophilum and Ehrlichia chaffeensis. Eur J Neurol 2007;14:e3–4. 10.1111/j.1468-1331.2006.01582.x [DOI] [PubMed] [Google Scholar]
- 11.Glaser C, Christie L, Bloch KC. Rickettsial and ehrlichial infections. Handb Clin Neurol 2010;96:143–58. 10.1016/S0072-9752(09)96010-9 [DOI] [PubMed] [Google Scholar]
- 12.Jacobs RF, Schutze GE. Ehrlichiosis in children. J Pediatr 1997;131:184–92. 10.1016/S0022-3476(97)70152-5 [DOI] [PubMed] [Google Scholar]
- 13.Carter N, Miller NR. Fourth nerve palsy caused by Ehrlichia chaffeensis. J Neuroophthalmol 1997;17:47–50. 10.1097/00041327-199703000-00010 [DOI] [PubMed] [Google Scholar]
- 14.Maretzki CH, Fisher DJ, Greene CE. Granulocytic ehrlichiosis and meningitis in a dog. J Am Vet Med Assoc 1994;205:1554–6. [PubMed] [Google Scholar]
- 15.Barber RM, Li Q, Diniz PP, et al. Evaluation of brain tissue or cerebrospinal fluid with broadly reactive polymerase chain reaction for Ehrlichia, Anaplasma, spotted fever group Rickettsia, Bartonella, and Borrelia species in canine neurological diseases (109 cases). J Vet Intern Med 2010;24:372–8. 10.1111/j.1939-1676.2009.0466.x [DOI] [PubMed] [Google Scholar]
- 16.Meinkoth JH, Hoover JP, Cowell RL, et al. Ehrlichiosis in a dog with seizures and nonregenerative Anemia. J Am Vet Med Assoc 1989;195:1754–5. [PubMed] [Google Scholar]
- 17.Harrus S, Bark H, Waner T. Canine monocytic ehrlichiosis: an update. Compend Contin Educ Pract Vet 1997;19:431. [Google Scholar]
- 18.Harrus S, Waner T, Neer TM. Ehrlichia canis infection Greene C, Infectious diseases of the dog and cat. Missouri: Elsevier, 2011:227–37. [Google Scholar]
- 19.Harrus S, Waner T, Weiss DJ, et al. Kinetics of serum antiplatelet antibodies in experimental acute canine ehrlichiosis. Vet Immunol Immunopathol 1996;51:13–20. 10.1016/0165-2427(95)05516-9 [DOI] [PubMed] [Google Scholar]
- 20.Kuehn NF, Gaunt SD. Clinical and hematologic findings in canine ehrlichiosis. J Am Vet Med Assoc 1985;186:355–8. [PubMed] [Google Scholar]
- 21.Harrus S, Waner T, Eldor A, et al. Platelet dysfunction associated with experimental acute canine ehrlichiosis. Vet Rec 1996;139:290–3. 10.1136/vr.139.12.290 [DOI] [PubMed] [Google Scholar]
- 22.Harrus S, Waner T, Bark H, et al. Recent advances in determining the pathogenesis of canine monocytic ehrlichiosis. J Clin Microbiol 1999;37:2745–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varela F, Font X, Valladares JE, et al. Thrombocytopathia and light-chain proteinuria in a dog naturally infected with Ehrlichia canis. J Vet Intern Med 1997;11:309–11. 10.1111/j.1939-1676.1997.tb00471.x [DOI] [PubMed] [Google Scholar]
- 24.Cortese L, Pelagalli A, Piantedosi D, et al. Platelet aggregation and haemostatic response in dogs naturally co-infected by Leishmania infantum and Ehrlichia canis. J Vet Med A Physiol Pathol Clin Med 2006;53:546–8. 10.1111/j.1439-0442.2006.00883.x [DOI] [PubMed] [Google Scholar]
- 25.Brandão LP, Hasegawa MY, Hagiwara MK, et al. Platelet aggregation studies in acute experimental canine ehrlichiosis. Vet Clin Pathol 2006;35:78–81. 10.1111/j.1939-165X.2006.tb00091.x [DOI] [PubMed] [Google Scholar]
- 26.O'Brien JR. 'Exhausted' platelets continue to circulate. Lancet 1978;2:1316–7. 10.1016/S0140-6736(78)92087-1 [DOI] [PubMed] [Google Scholar]
- 27.Lovering SL, Pierce KR, Adams LG. Serum complement and blood platelet adhesiveness in acute canine ehrlichiosis. Am J Vet Res 1980;41:1266–71. [PubMed] [Google Scholar]
- 28.Waner T, Leykin I, Shinitsky M, et al. Detection of platelet-bound antibodies in beagle dogs after artificial infection with Ehrlichia canis. Vet Immunol Immunopathol 2000;77:145–50. 10.1016/S0165-2427(00)00225-7 [DOI] [PubMed] [Google Scholar]