Abstract
Sleepiness is commonly seen in adolescents and can negatively impact school performance. Little research has investigated the impact of sleepiness in juvenile animals on spatial learning. Sprague-Dawley juvenile (<30 days) and young adult (>60 days) rats were sleep deprived for 24 hours and tested, along with controls, in a water maze task. Sleep deprived juveniles were slower to learn the location of the hidden platform than controls; however, adult performance was not impaired. Sleep deprivation did not impair recall during a probe trial for either age group. Sleep deprivation prior to testing slowed spatial learning in juveniles but not adults.
Keywords: Young Rats, Development, Morris Water Maze, Total Sleep Deprivation
Sleepiness is a major factor that could contribute to poor academic performance and behavioral problems for school age adolescents (Shochat, Cohen-Zion, & Tzischinsky, 2014; Taras & Potts-Datema, 2005; Wolfson & Carskadon, 2003). A reduction in sleep time is correlated with an increase in teacher reports of inattention and cognitive problems (Gruber et al., 2012). There are many factors that can contribute to sleep loss including social pressures, school activities, and changes in biology (Carskadon, Acebo, & Jenni, 2004; Jenni & O’Connor, 2005). Additionally, sleep disorders such as sleep disordered breathing in children can have a large impact on a child’s functioning (de Carvalho et al., 2013).
Sleep appears to play a critical role in the consolidation of certain types of memories (Diekelmann, Wilhelm, & Born, 2009; Maski, 2015), even though the mechanisms by which this occurs are not fully understood (Frank & Benington, 2006; Huber & Born, 2014). Hippocampal-dependent memory processes are especially susceptible to disruptions of sleep (Kreutzmann, Havekes, Abel, & Meerlo, 2015; Walker, 2008). Sleep deprivation has been shown to impair the learning of a hippocampal-dependent contextual fear task, but not an amygdala-dependent cued fear task (McDermott et al., 2003). Deprivation of sleep impaired rodent performance in the hippocampal-dependent reference water maze task (Chang, Wu, & Lan, 2009; Guan, Peng, & Fang, 2004; Tartar et al., 2006; Ward, McCarley, & Strecker, 2009), but not spatial working memory (Ward et al., 2009). Sleep enhanced performance in a hippocampal-dependent object place recognition task (Binder et al., 2012), while sleep deprivation impaired performance on this same task (Prince et al., 2014). Sleep deprivation also impaired the recall of the hippocampal-dependent social transmission of food preference task (Wooden et al., 2014). Additionally, deficits in hippocampal plasticity have been noted following the disruption of sleep (Davis, Harding, & Wright, 2003; McDermott et al., 2003; Prince et al., 2014; Tartar et al., 2006) and have been extensively reviewed by others (Kreutzmann et al., 2015; Prince & Abel, 2013).
To the author’s knowledge, no studies have compared the effects of sleepiness on learning a hippocampal-dependent task in juvenile and adult rats. In the present experiment, juvenile (<30 days) and young adult (>60 days) rats were sleep deprived prior to learning the hippocampal-dependent reference memory water maze task. It was hypothesized that younger rats would be more sensitive to the effects of sleep deprivation on learning than young adult rats.
Method
Subjects
Juvenile male Sprague-Dawley rats (n=16, Age 23–27 days) and young adult male rats (n=15, Age 70–125 days; Harlan, Indianapolis, IN, USA) were used in the experiment. Rats were group housed in standard cages. Housing was in a climate controlled facility (room temperature 21 °C ± 1) with a 12:12 hr light-dark cycle (lights on at 10:00). All behavioral testing was conducted at the beginning of the lights on period. Food and water were available ad libitum. All animals were treated in accordance with the Guide for the Care and Use of Laboratory Animals, 8th ed. (2011). All procedures were approved by the university Institutional Animal Care and Use Committee (IACUC).
Sleep Deprivation
Rats were sleep deprived with an automated device (Pinnacle Technology, Lawrence, KS, USA). The effectiveness of this device for producing sleep deprivation has been previously validated in our laboratory (Wooden et al., 2014) and in others as well (Hines, Schmitt, Hines, Moss, & Haydon, 2013; Naylor et al., 2012; Sims, Wu, & Dale, 2013; Wisor, Clegern, & Schmidt, 2011). Briefly, the device consists of a cylindrical acrylic cage (30.5 cm in diameter) with a slowly rotating bar (approximately 5 RPM) attached to the bottom. The movement of the bar is controlled by a connected computer. The rotating bar “nudges” the rat to prevent sleep. Food and water were available ad libitum in the device. Sleep deprivation was achieved by individually placing rats in the device and programming the bar to spin for 4 s and stop spinning for 12 s, repeated for the 24 hour period. Previously, we found that this protocol results in approximately 95% wakefulness over 24 hours and a near-complete elimination of REM sleep (less than 1%) (Wooden et al., 2014). Rats in the control group were individually placed in a similar container with the same bedding, food, and water access for 24 hours. Juvenile and young adult rats were randomly assigned to either control group (juvenile n=8, young adult n=7) or sleep deprivation group (juvenile n=8, young adult n=8).
Water Maze
In the water maze task, each rat underwent three blocks of four trials separated by a 30-min period. This version of the water maze task allows rats to be fully trained in approximately 2 h (Frick, Stillner, & Berger-Sweeney, 2000), which is necessary for manipulations that cannot be given on multiple days, such as following 24-h of sleep deprivation. Rats were tested in the water maze (diameter = 1.5 m) during the first two hours of the 12 h lights-on period immediately following sleep deprivation or control conditions. On each trial, rats were placed in the water maze facing the wall in one of three quadrants that did not contain the hidden platform. The starting position was in a semi-random order so that no starting point was repeated and no point was used more than three times. The location of the hidden platform remained constant. After finding the hidden platform, the rat was allowed to remain on the platform for approximately 10 s before being dried and placed in a holding cage. If the rat did not find the hidden platform within 60 s, the rat was placed on the platform by the experimenter for approximately 10 s before being placed in a holding cage for an additional 60 s. Between trial blocks, rats were group housed in a dry cage. Sleep deprived rats were observed during these 30 min intervals in order to prevent sleep with gentle handling and sensory stimulation; however, the sleep deprived rats engaged primarily in grooming and social interaction during this period.
Thirty minutes after the last learning trial, rats were given a single 30 s probe trial, during which the platform was completely removed from the water maze. The starting point for this trial was the same for all rats, and was in a quadrant that that the platform had not been in. A video tracking system (Videomex, Columbus Instruments, Columbus, OH, USA) was utilized to record rodent behavior in the water maze.
Analysis
The main dependent variables collected in both tests were latency and path distance rats took to find the platform in the water maze. In probe trial data, the main dependent variables were percent time and swim distance spent in the quadrant of the pool that formerly contained the hidden platform. Water maze testing was analyzed by factorial repeated measures analysis of variance (ANOVA). Probe trial data were analyzed by independent samples t-test. All data analysis was conducted utilizing SPSS (version 20.0, IBM Corp, Armonk, NY, USA) with an alpha level of 0.05.
Results
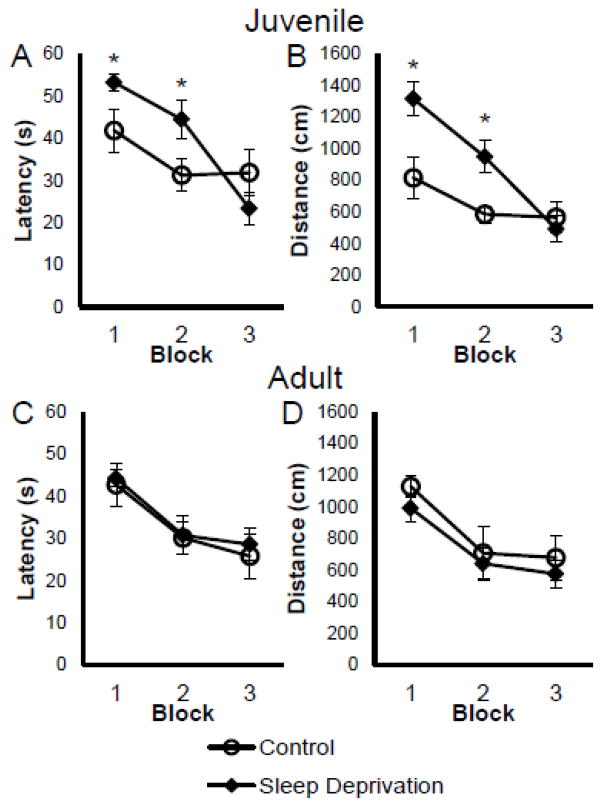
Sleep deprived juvenile rats took longer to learn the water maze task than non-sleep deprived rats. There was a significant trial x group interaction in latency to find the hidden platform (F(11,154)=2.516, p=.006) and distance (F(11,154)=2.095, p=.024). As seen in Figure 1A and 1B, control juvenile rats indicated a significant decrease in latency to find the platform during the second block of trials (p=.044) and a significant decrease in distance to the platform during the first block (p=.011) and second block of trials (p=.007). In the third block of trials, control and sleep deprived juvenile rats performance was similar in latency (p=.214) and distance to the platform (p=.576).
Figure 1.
Twenty-four hours of sleep deprivation prior to testing delayed the learning of the hidden platform location in juvenile, but not adult rats. Figures depicts mean (±SEM) latency (A) and path length (B) to reach the hidden platform over 3 blocks (average of 4 trials each) for sleep deprived (n=8; black diamond) and cage control (n=8, open circle) juvenile rats. Bottom figures depict mean (±SEM) latency (C) and path length (D) to reach the platform for sleep deprived (n=8) and cage control (n=7) adult rats.
* p < .05
The sleep deprivation protocol did not affect young adult learning in the water maze task (see Figures 1C and 1D). There was no significant trial x group interaction in latency to find the hidden platform (F(11,143)=0.312, p=.982) or distance (F(11,143)=.354, p=.971).
Overall, juvenile rats indicated learning the location of the platform by a decrease in both latency (F(11,154)=7.024, p<.001) and distance (F(11,154)=7.326, p<.001) over the 12 trials. Young adult rats also showed the same indication of learning by a decrease in latency (F(11,143)=4.856, p<.001) and distance (F(11,143)=5.344, p<.001).
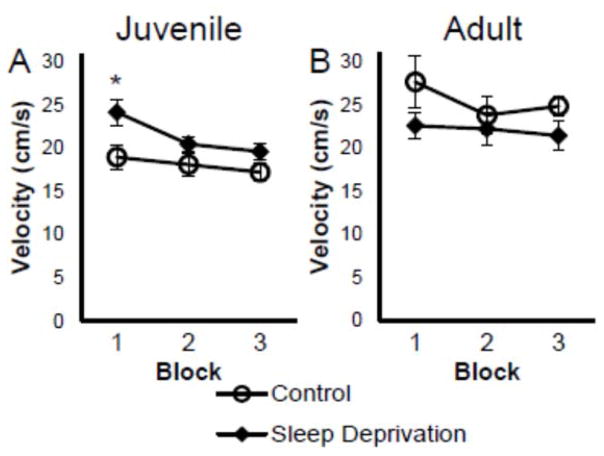
Interestingly, in juvenile rats, there was a significant main effect in swim velocity (F(1,14)=7.369, p=.017) where sleep deprived juvenile rats were significantly faster (M=21.43 cm/s, SEM±.86) than control rats (M=18.12 cm/s, SEM±.86; see Figure 2A), especially in the first block of trials (p=.026). In young adult rats, there was no significant difference between sleep deprived (M=22.08 cm/s, SEM±1.53) and control rats (M=25.44 cm/s, SEM±1.64; F(1,13)=2.249, p=.158; see Figure 2B).
Figure 2.
Twenty-four hours of sleep deprivation prior to testing altered swim velocity in the first block of trials for juvenile, but not adult rats. Figures depicts mean (±SEM) velocity in juvenile (A) and adult rats (B) over 3 blocks (average of 4 trials each) for sleep deprived (black diamond) and cage control (open circle) rats.
* p < .05
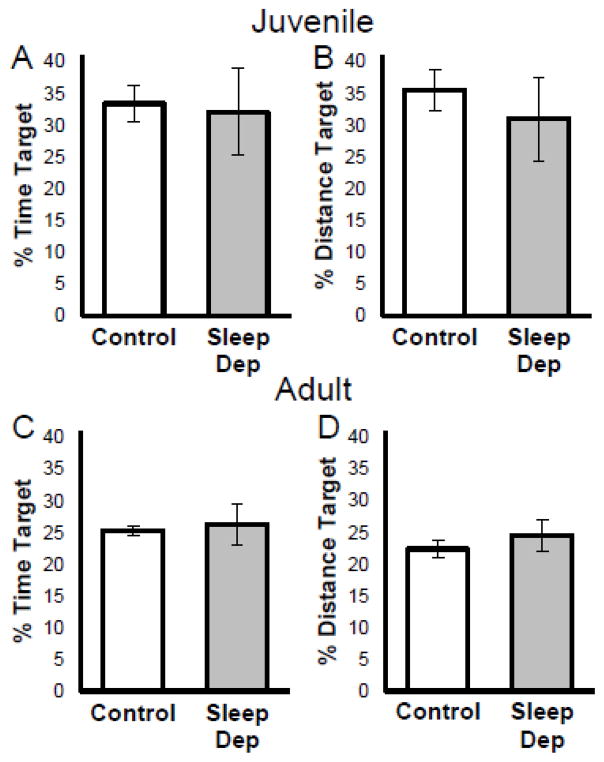
As seen in Figures 3A & 3B, no differences were observed between control and sleep deprived juvenile rats in a probe trial following learning trials. There was no significant difference as measured by percent time spent in the target quadrant (t(14)=0.175, p=.863) or percent distance traveled in the target quadrant (t(14)=0.608, p=.553)
Figure 3.
Twenty-four hours of sleep deprivation prior to testing did not impair recall testing of the platform location during a probe trial. Figures depicts mean (±SEM) percent time (A) and percent distance (B) during the probe trial that rats spent searching in the target quadrant that formerly contained the hidden platform for sleep deprived (gray bars) and cage control (white bars) juvenile rats. Bottom figures depict mean (±SEM) percent time (C) and percent distance (D) during the probe trial spent in the target quadrant for adult rats.
* p < .05
No differences were observed between control and sleep deprived young adult rats in a probe trial following learning trials (see Figures 3C & 3D). There was no significant difference as measured by percent time spent in the target quadrant (t(13)=0.302, p=.768) or percent distance traveled in the target quadrant (t(13)=0.743, p=.470)
Discussion
In this study, juvenile and young adult rats were sleep deprived for 24 h prior to performing an abbreviated water maze task. During the learning trials, sleep deprived juvenile rats had a slower learning curve than cage control rats; however, by the end of learning trials, both groups performed similarly. The sleep deprivation protocol used in this experiment, however, did not seem to hinder learning in young adult rats. Probe trial results reflected the similar performance between groups by the end of learning trials. Therefore, juvenile rats seemed to be more sensitive to the effects of sleep deprivation than young adults in learning the water maze task. All rats, though, were ultimately able to recall the platform location following learning.
To the authors’ knowledge, this is the first study to look at the effects of total sleep deprivation on water maze learning in juvenile rats. Rapid eye movement (REM) sleep deprivation has previously been shown to interfere with spatial memory consolidation in three week old rats, but not adult rats (Li et al., 2009). This study utilized a platform over water technique to produce REM deprivation. Additionally, this study specifically looked at the role of sleep in the recall of a task in which learning occurred before sleep deprivation (Li et al., 2009). In the present experiment, rats were sleep deprived before training. Training rats while sleepy is meant to replicate real-world scenarios where students are asked to learn in school while sleepy (Gruber et al., 2012; Shochat et al., 2014). Acute sleep deprivation is less common than sleep restriction or sleep fragmentation as a means for increasing sleepiness in adolescents. However, acute sleep deprivation can provide an experimental model for inducing similar sleepiness to sleep restriction and fragmentation as measured behavioral and chemically (Córdova et al., 2006; Deurveilher, Bush, Rusak, Eskes, & Semba, 2015; Kim et al., 2012; McKenna et al., 2007). It should also be noted that while both sleep deprived and control juvenile rats eventually reached similar performance and recall in the water maze, the platform location did remain constant over the course of many learning trials. This suggests that while it may be possible for sleepy adolescents to adequately learn new information, learning may only occur if the same information is presented many times (which is often not the case in more naturalistic learning environments).
A device control condition was not used in the present experiment. Previous testing has shown that device controls that produced the non-specific stress of movement without sleep deprivation did not impact water maze performance (Tartar et al., 2006) or social transmission of food preference testing (Wooden et al., 2014). Since young adult rats were not impacted by sleep deprivation produced by bar rotation, it is unlikely that similar tactile stimulation without sleep deprivation would have produced any effect. Research from other laboratories using the same sleep deprivation device has shown a modest increase in corticosterone levels following 6 hours of sleep deprivation (in mice). However, this corticosterone increase was far less than the increases seen in other stress models (Naidoo et al., 2014). Additionally, negative performance in the water maze due to sleep deprivation is not dependent on the adrenal stress response (Ruskin, Dunn, Billiot, Bazan, & LaHoste, 2006).
Previous research has shown sleep deprivation prior to learning can impair rodent performance in a water maze task (Chang et al., 2009; Guan et al., 2004; Tartar et al., 2006). In our experience, however, rats perform differently depending on the nature of the strain of rat used, sleep deprivation method, and water maze protocol utilized (Tartar et al., 2006; Ward et al., 2009). In the present study, young adult rats did not indicate a performance deficit in the water maze task. It is possible that the water maze task was “simpler” for the adult rats due to some type of cognitive reserve because of the smaller size of the water maze tank (over 40% less surface area) than used in previous research (Ward et al., 2009), however still challenging enough for the loss of sleep to impact learning in juveniles. It is also possible that sleep deprivation selectively impacted motivation. Juvenile rats demonstrated slower swim speed velocities, primarily in the first block of training, while adult rat swim velocities remained consistent among trials. This could be an indication of decreased motivation to escape the water in juvenile rats. Future studies should use a separate measure of motivation. Sleep loss has been shown to alter motivation of a rodent for a food reward (Hanlon, Andrzejewski, Harder, Kelley, & Benca, 2005) but not water reward in adult and aged rats (Christie, McCarley, & Strecker, 2010). Disturbances of sleep have not been shown to impact performance in visible platform tasks in the water maze which test for impairments of motivation or physical ability (Guan et al., 2004; Smith & Rose, 1996; Tartar et al., 2006), however, to the authors’ knowledge, no tests comparing young and adult rats have been reported. This protocol should be added to future research studies.
Another possible explanation for the present results is that juvenile rats may have had impaired attention which slowed their ability to learn the task. Sleep loss does negatively impact measures of attention in rodents (Christie, McKenna, Connolly, McCarley, & Strecker, 2008; Córdova et al., 2006). However, as recently reviewed by Kreutzmann and colleagues (Kreutzmann et al., 2015), there is ample evidence that the disruption of sleep impacts neural plasticity within the hippocampus by changing molecular functions.
It should be noted that a limitation to the present experiment is that only male rats were tested in the present experiment. In humans, poor sleep quality has been associated with deficits executive functioning more in adolescent boys than in adolescent girls (Kuula et al., 2015). However, future research should confirm whether findings generalize to female rats as well, especially since female rat performance in the water maze may show a larger deficit due to sleep deprivation than male rats (Hajali, Sheibani, Esmaeili-Mahani, & Shabani, 2012).
In conclusion, 24 h of sleep deprivation prior to learning impaired the speed in which juvenile rats learned the reference memory water maze task. The same sleep deprivation protocol did not impair performance in young adults. This work demonstrates the importance of prior sleep in learning a new task. Adolescents with increased somnolence during the school day are likely to demonstrate difficulties in learning new information. While these data suggest new information can still be learned in this state, it will be learned at a slower pace than in those who are not sleepy.
Acknowledgments
This work was supported by the National Institute of Mental Health (R15MH087934) and a Faculty Research Support Fund (UHCL) awarded to CPW. We thank Jesus Bautista, Emmanuel Aguero, Kimberly Hood, Jennifer Taylor, and Jennifer Pido for their excellent technical assistance.
Contributor Information
Christopher P. Ward, University of Houston-Clear Lake and Baylor College of Medicine
Jessica I. Wooden, University of Houston-Clear Lake
Ryan Kieltyka, University of Houston-Clear Lake.
References
- Binder S, Baier PC, Mölle M, Inostroza M, Born J, Marshall L. Sleep enhances memory consolidation in the hippocampus-dependent object-place recognition task in rats. Neurobiology of Learning and Memory. 2012;97(2):213–9. doi: 10.1016/j.nlm.2011.12.004. http://doi.org/10.1016/j.nlm.2011.12.004. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Acebo C, Jenni OG. Regulation of adolescent sleep: implications for behavior. Annals of the New York Academy of Sciences. 2004;1021:276–91. doi: 10.1196/annals.1308.032. http://doi.org/10.1196/annals.1308.032. [DOI] [PubMed] [Google Scholar]
- Chang HM, Wu UI, Lan CT. Melatonin preserves longevity protein (sirtuin 1) expression in the hippocampus of total sleep-deprived rats. Journal of Pineal Research. 2009;47(3):211–20. doi: 10.1111/j.1600-079X.2009.00704.x. http://doi.org/10.1111/j.1600-079X.2009.00704.x. [DOI] [PubMed] [Google Scholar]
- Christie MA, McCarley RW, Strecker RE. Twenty-four hours, or five days, of continuous sleep deprivation or experimental sleep fragmentation do not alter thirst or motivation for water reward in rats. Behavioural Brain Research. 2010;214(2):180–6. doi: 10.1016/j.bbr.2010.05.020. http://doi.org/10.1016/j.bbr.2010.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie MA, McKenna JT, Connolly NP, McCarley RW, Strecker RE. 24 hours of sleep deprivation in the rat increases sleepiness and decreases vigilance: introduction of the rat-psychomotor vigilance task. Journal of Sleep Research. 2008;17(4):376–84. doi: 10.1111/j.1365-2869.2008.00698.x. http://doi.org/10.1111/j.1365-2869.2008.00698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Córdova CA, Said BO, McCarley RW, Baxter MG, Chiba AA, Strecker RE. Sleep deprivation in rats produces attentional impairments on a 5-choice serial reaction time task. Sleep. 2006;29(1):69–76. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3628810&tool=pmcentrez&rendertype=abstract. [PMC free article] [PubMed] [Google Scholar]
- Davis CJ, Harding JW, Wright JW. REM sleep deprivation-induced deficits in the latency-to-peak induction and maintenance of long-term potentiation within the CA1 region of the hippocampus. Brain Research. 2003;973(2):293–7. doi: 10.1016/s0006-8993(03)02508-3. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12738073. [DOI] [PubMed] [Google Scholar]
- De Carvalho LBC, do Prado LBF, Ferrreira VR, da Rocha Figueiredo MB, Jung A, de Morais JF, do Prado GF. Symptoms of sleep disorders and objective academic performance. Sleep Medicine. 2013;14(9):872–6. doi: 10.1016/j.sleep.2013.05.011. http://doi.org/10.1016/j.sleep.2013.05.011. [DOI] [PubMed] [Google Scholar]
- Deurveilher S, Bush JE, Rusak B, Eskes GA, Semba K. Psychomotor vigilance task performance during and following chronic sleep restriction in rats. Sleep. 2015;38(4):515–28. doi: 10.5665/sleep.4562. http://doi.org/10.5665/sleep.4562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekelmann S, Wilhelm I, Born J. The whats and whens of sleep-dependent memory consolidation. Sleep Medicine Reviews. 2009;13(5):309–21. doi: 10.1016/j.smrv.2008.08.002. http://doi.org/10.1016/j.smrv.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Frank MG, Benington JH. The role of sleep in memory consolidation and brain plasticity: dream or reality? The Neuroscientist: A Review Journal Bringing Neurobiology, Neurology and Psychiatry. 2006;12(6):477–88. doi: 10.1177/1073858406293552. http://doi.org/10.1177/1073858406293552. [DOI] [PubMed] [Google Scholar]
- Frick KM, Stillner ET, Berger-Sweeney J. Mice are not little rats: species differences in a one-day water maze task. Neuroreport. 2000;11(16):3461–5. doi: 10.1097/00001756-200011090-00013. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11095500. [DOI] [PubMed] [Google Scholar]
- Gruber R, Michaelsen S, Bergmame L, Frenette S, Bruni O, Fontil L, Carrier J. Short sleep duration is associated with teacher-reported inattention and cognitive problems in healthy school-aged children. Nature and Science of Sleep. 2012;4:33–40. doi: 10.2147/NSS.S24607. http://doi.org/10.2147/NSS.S24607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Z, Peng X, Fang J. Sleep deprivation impairs spatial memory and decreases extracellular signal-regulated kinase phosphorylation in the hippocampus. Brain Research. 2004;1018(1):38–47. doi: 10.1016/j.brainres.2004.05.032. http://doi.org/10.1016/j.brainres.2004.05.032. [DOI] [PubMed] [Google Scholar]
- Hajali V, Sheibani V, Esmaeili-Mahani S, Shabani M. Female rats are more susceptible to the deleterious effects of paradoxical sleep deprivation on cognitive performance. Behavioural Brain Research. 2012;228(2):311–8. doi: 10.1016/j.bbr.2011.12.008. http://doi.org/10.1016/j.bbr.2011.12.008. [DOI] [PubMed] [Google Scholar]
- Hanlon EC, Andrzejewski ME, Harder BK, Kelley AE, Benca RM. The effect of REM sleep deprivation on motivation for food reward. Behavioural Brain Research. 2005;163(1):58–69. doi: 10.1016/j.bbr.2005.04.017. http://doi.org/10.1016/j.bbr.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Hines DJ, Schmitt LI, Hines RM, Moss SJ, Haydon PG. Antidepressant effects of sleep deprivation require astrocyte-dependent adenosine mediated signaling. Translational Psychiatry. 2013;3:e212. doi: 10.1038/tp.2012.136. http://doi.org/10.1038/tp.2012.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber R, Born J. Sleep, synaptic connectivity, and hippocampal memory during early development. Trends in Cognitive Sciences. 2014;18(3):141–152. doi: 10.1016/j.tics.2013.12.005. http://doi.org/10.1016/j.tics.2013.12.005. [DOI] [PubMed] [Google Scholar]
- Jenni OG, O’Connor BB. Children’s sleep: an interplay between culture and biology. Pediatrics. 2005;115(1 Suppl):204–16. doi: 10.1542/peds.2004-0815B. http://doi.org/10.1542/peds.2004-0815B. [DOI] [PubMed] [Google Scholar]
- Kim Y, Bolortuya Y, Chen L, Basheer R, McCarley RW, Strecker RE. Decoupling of sleepiness from sleep time and intensity during chronic sleep restriction: evidence for a role of the adenosine system. Sleep. 2012;35(6):861–9. doi: 10.5665/sleep.1890. http://doi.org/10.5665/sleep.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzmann J, Havekes R, Abel T, Meerlo P. Sleep deprivation and hippocampal vulnerability: changes in neuronal plasticity, neurogenesis and cognitive function. Neuroscience. 2015 doi: 10.1016/j.neuroscience.2015.04.053. http://doi.org/10.1016/j.neuroscience.2015.04.053. [DOI] [PubMed]
- Kuula L, Pesonen AK, Martikainen S, Kajantie E, Lahti J, Strandberg T, Räikkönen K. Poor sleep and neurocognitive function in early adolescence. Sleep Medicine. 2015;16(10):1207–12. doi: 10.1016/j.sleep.2015.06.017. http://doi.org/10.1016/j.sleep.2015.06.017. [DOI] [PubMed] [Google Scholar]
- Li S, Tian Y, Ding Y, Jin X, Yan C, Shen X. The effects of rapid eye movement sleep deprivation and recovery on spatial reference memory of young rats. Learning & Behavior. 2009;37(3):246–53. doi: 10.3758/LB.37.3.246. http://doi.org/10.3758/LB.37.3.246. [DOI] [PubMed] [Google Scholar]
- Maski KP. Sleep-Dependent Memory Consolidation in Children. Seminars in Pediatric Neurology. 2015;22(2):130–134. doi: 10.1016/j.spen.2015.03.008. http://doi.org/10.1016/j.spen.2015.03.008. [DOI] [PubMed] [Google Scholar]
- McDermott CM, LaHoste GJ, Chen C, Musto A, Bazan NG, Magee JC. Sleep Deprivation Causes Behavioral, Synaptic, and Membrane Excitability Alterations in Hippocampal Neurons. J Neurosci. 2003;23(29):9687–9695. doi: 10.1523/JNEUROSCI.23-29-09687.2003. Retrieved from http://www.jneurosci.org/content/23/29/9687.long. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna JT, Tartar JL, Ward CP, Thakkar MM, Cordeira JW, McCarley RW, Strecker RE. Sleep fragmentation elevates behavioral, electrographic and neurochemical measures of sleepiness. Neuroscience. 2007;146(4):1462–73. doi: 10.1016/j.neuroscience.2007.03.009. http://doi.org/10.1016/j.neuroscience.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidoo N, Davis JG, Zhu J, Yabumoto M, Singletary K, Brown M, … Baur JA. Aging and sleep deprivation induce the unfolded protein response in the pancreas: implications for metabolism. Aging Cell. 2014;13(1):131–41. doi: 10.1111/acel.12158. http://doi.org/10.1111/acel.12158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor E, Aillon DV, Barrett BS, Wilson GS, Johnson DA, Johnson DA, … Petillo PA. Lactate as a biomarker for sleep. Sleep. 2012;35(9):1209–22. doi: 10.5665/sleep.2072. http://doi.org/10.5665/sleep.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince T-M, Abel T. The impact of sleep loss on hippocampal function. Learning & Memory (Cold Spring Harbor, NY) 2013;20(10):558–69. doi: 10.1101/lm.031674.113. http://doi.org/10.1101/lm.031674.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince TM, Wimmer M, Choi J, Havekes R, Aton S, Abel T. Sleep deprivation during a specific 3-hour time window post-training impairs hippocampal synaptic plasticity and memory. Neurobiology of Learning and Memory. 2014;109:122–30. doi: 10.1016/j.nlm.2013.11.021. http://doi.org/10.1016/j.nlm.2013.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruskin DN, Dunn KE, Billiot I, Bazan NG, LaHoste GJ. Eliminating the adrenal stress response does not affect sleep deprivation-induced acquisition deficits in the water maze. Life Sciences. 2006;78(24):2833–8. doi: 10.1016/j.lfs.2005.11.003. http://doi.org/10.1016/j.lfs.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Shochat T, Cohen-Zion M, Tzischinsky O. Functional consequences of inadequate sleep in adolescents: a systematic review. Sleep Medicine Reviews. 2014;18(1):75–87. doi: 10.1016/j.smrv.2013.03.005. http://doi.org/10.1016/j.smrv.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Sims RE, Wu HHT, Dale N. Sleep-wake sensitive mechanisms of adenosine release in the basal forebrain of rodents: an in vitro study. PloS One. 2013;8(1):e53814. doi: 10.1371/journal.pone.0053814. http://doi.org/10.1371/journal.pone.0053814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C, Rose GM. Evidence for a paradoxical sleep window for place learning in the Morris water maze. Physiology & Behavior. 1996;59(1):93–7. doi: 10.1016/0031-9384(95)02054-3. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8848497. [DOI] [PubMed] [Google Scholar]
- Taras H, Potts-Datema W. Sleep and student performance at school. The Journal of School Health. 2005;75(7):248–54. doi: 10.1111/j.1746-1561.2005.00033.x. http://doi.org/10.1111/j.1746-1561.2005.00033.x. [DOI] [PubMed] [Google Scholar]
- Tartar JL, Ward CP, McKenna JT, Thakkar M, Arrigoni E, McCarley RW, … Strecker RE. Hippocampal synaptic plasticity and spatial learning are impaired in a rat model of sleep fragmentation. The European Journal of Neuroscience. 2006;23(10):2739–48. doi: 10.1111/j.1460-9568.2006.04808.x. http://doi.org/10.1111/j.1460-9568.2006.04808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MP. Cognitive consequences of sleep and sleep loss. Sleep Medicine. 2008;9(Suppl 1):S29–34. doi: 10.1016/S1389-9457(08)70014-5. http://doi.org/10.1016/S1389-9457(08)70014-5. [DOI] [PubMed] [Google Scholar]
- Ward CP, McCarley RW, Strecker RE. Experimental sleep fragmentation impairs spatial reference but not working memory in Fischer/Brown Norway rats. Journal of Sleep Research. 2009;18(2):238–44. doi: 10.1111/j.1365-2869.2008.00714.x. http://doi.org/10.1111/j.1365-2869.2008.00714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisor JP, Clegern WC, Schmidt MA. Toll-like receptor 4 is a regulator of monocyte and electroencephalographic responses to sleep loss. Sleep. 2011;34(10):1335–45. doi: 10.5665/SLEEP.1274. http://doi.org/10.5665/SLEEP.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson AR, Carskadon MA. Understanding adolescents’ sleep patterns and school performance: a critical appraisal. Sleep Medicine Reviews. 2003;7(6):491–506. doi: 10.1016/s1087-0792(03)90003-7. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/15018092. [DOI] [PubMed] [Google Scholar]
- Wooden JI, Pido J, Mathews H, Kieltyka R, Montemayor BA, Ward CP. Sleep deprivation impairs recall of social transmission of food preference in rats. Nature and Science of Sleep. 2014;6:129–35. doi: 10.2147/NSS.S68611. http://doi.org/10.2147/NSS.S68611. [DOI] [PMC free article] [PubMed] [Google Scholar]