Abstract
CMAH (cytidine monophosphate-N-acetylneuraminic acid hydroxylase) is responsible for the oxidation of cytidine monophosphate-N-acetylneuraminic acids in mammals. However, humans cannot oxidize cytidine monophosphate-N-acetylneuraminic acid to cytidine monophosphate-N-glycolylneuraminic acid due to a primary exon deletion of the CMAH gene. To understand the effects and implications of the lack of CMAH activity in more detail, a Cmah knock-out model in mice is of keen interest in basic and applied research. The analysis method to determine the phenotype of this mouse model is herein described in detail, and is based on the detection of both N-acetylneuraminic acid and N-glycolylenuraminic acid in the liver and milk of wild-type and Cmah knock-out mice. Endogenous sialic acids are released and derivatized with o-phenylenediamine to generate fluorogenic derivatives, which can be subsequently analyzed by HPLC. The presented protocol can be also applied for the analysis of milk and tissue samples from various other origins, and may be of use to investigate the nutritional and health effects of N-glycolylneuraminic acid.
Keywords: Biochemistry, Issue 125, CMAH, Sialic acid, Neu5Gc, N-glycolylneuraminic acid, Neu5Ac, HPLC-FLD, mouse milk
Introduction
N-acetylneuraminic acid (Neu5Ac) and N-glycolylneuraminic acid (Neu5Gc) are the most common sialic acids in most mammals1. Although able of synthesizing Neu5Ac endogenously, humans are not capable of producing Neu5Gc due to a primary exon deletion on the CMAH gene encoding for a CMP-Neu5Ac hydroxylase2,3. However, animal-based food products can be dietary sources of Neu5Gc4,5,6, leading to the production of anti-Neu5Gc antibodies and therefore trigger an immune response towards Neu5Gc7. This dietary effect of Neu5Gc is suspected to contribute to chronic inflammation and various other diseases8,9,10. In order to comprehensively understand the effects of Neu5Gc in humans, an animal model for the systematic study of the effects of foodborne sialic acids is highly desirable. Although protocols based on polymerase chain reaction (PCR) for analyzing of knock-out mice are well established and a convenient way for the genotypical assessment, the functional analysis of phenotype on the metabolic level requires more specific analysis methods. The phenotype of a Cmah knock-out mouse model can be assessed by isolating and analyzing the composition of sialic acids in liver or milk samples. Several methods for the detection of sialic acids in animal tissues have been reported previously: reacting sialic acids with resorcinol11 or thiobarbituric acid12 result in the formation of a chromophoric product and can be simply analyzed using a platereader based setup, but only the total sialic acid content may be determined. Alternatively, the analysis of sialic acids was also described using gas chromatography13, MALDI-ToF mass spectrometry14 or amperometric methods15. However, the most commonly applied sialic acid analysis methods are based on hydrolytic release, followed by fluorescence derivatization and subsequent high performance liquid chromatography16,17,18.
Protocol
Procedures involving animal subjects have been approved by the Ethical Committee of the Experimental Animal Center of Nanjing Agricultural University in accordance to the National Guidelines for Experimental Animal Welfare (Ministry of Science and Technology, PR of China, 2006) with the animals housed in a SPF facility (Permission ID: SYXK-J-2011-0037).
1. Cmah Knock-out Mouse Model
Use wild-type C57Bl/6 mice from the Comparative Medicine Centre of Yangzhou University (China). NOTE: Cmah knock-out mice were generated based on the information provided from previous Cmah knock-out studies17,19 and obtained commercially using a CRISPR/Cas920 strategy by removing 92 base pairs from exon 6 of the Cmah gene, which is also deleted in the human CMAH gene19. Positive F0-mice (2 individuals) were crossed with wild type mice to obtain heterozygous F1-mice. After crossing heterozygous F1-mice (5 individuals), homozygous Cmah knock-out F2-mice could be successfully obtained (3 individuals).
- Perform genotyping of mice using genomic DNA according to the method shown by Zangala21 using a commercial DNA purification kit.
- Amplify a corresponding DNA fragment of the Cmah gene by using commercial DNA polymerase and the primer pair 5'gaaagggctcggctctgtatgaa3' and 5'tttaaaatgtcccgggtgagaagc3'. Perform the gene amplification using 34 PCR cycles consisting of denaturation at 94 °C for 40 s, annealing at 63 °C for 40 s, and elongation at 72 °C for 1 min.
- Visualize PCR products on an 1% agarose gel. One should observe a single band (0.5 kb) for the wild-type individual, a double band (0.4 and 0.5 kb) for the heterozygous knock-out individual, and a single band (0.4 kb) for the homozygous knock-out individual.
2. Sample Collection
3. Isolation of Sialic Acids from Milk
Prepare 100 mL of a 2 M acetic acid solution by adding 12 mL of glacial acetic acid into 88 mL of distilled H2O.
Transfer 50 µL of milk into a 1.5 mL centrifuge tube and add 1.2 mL of the prepared aqueous acetic acid solution.
Incubate the suspension for 4 h at 80 °C and centrifuge the sample at 14,000 x g for 10 min.
Transfer the top 1,000 µL of the supernatant into a fresh 1.5 mL centrifuge tube and remove the solvent by centrifugal evaporation at room temperature to complete dryness (depending on the attached vacuum pump this will take 4 - 20 h). Re-dissolve the sample in 500 µL of distilled H2O.
- Prepare a micro-anion exchange column. This step will specifically enrich anionic compounds and remove cationic and neutral compounds from the milk and liver samples, and therefore increases the selectivity of the fluorogenic OPD labeling agent for sialic acid derivatization.
- Transfer for each sample 200 mg of the anion exchange resin (Dowex 1X8) into empty 3 mL column tubes with an attached stopcock.
- Add 2 mL of the aqueous acetic acid solution prepared in step 3.1 to replace the chloride ions in the resin with acetate ions. Close the stopcock as soon as the solvent reaches the top of the resin.
- Gently add 2 mL of distilled H2O, open the stopcock and stop the flow as soon as most of the solvent reaches the top of the resin. Make sure that the resin is always covered with solvent.
- Wash the resin column two more times with 2 mL of distilled H2O for removing the excess acetate.
Transfer the resulting 500 µL of sample solvent from step 3.4. onto the resin column and discard the flow-through. Gently wash the resin with 2 mL of distilled H2O. Elute the sample anions from the resin using 1 mL of a 50 mM ammonium acetate solution (386 mg of ammonium acetate dissolved in 100 mL of distilled H2O) into a 1.5 mL centrifuge tube. Remove the solvent by centrifugal evaporation at room temperature to dryness. NOTE: Dry samples can be stored at 4-6 °C for up to 12 months.
4. Isolation of Sialic Acids from Liver Tissue
Gently thaw 20 - 50 mg of mouse liver and transfer it into a Dounce tissue grinder (1 mL or 2 mL of volume). Add 1.2 mL of the aqueous acetic acid solution prepared in step 3.1 and homogenize the tissue by gentle shearing for 10 s. Transfer the resulting suspension into a 1.5 mL centrifuge tube.
Follow steps 3.3. to 3.6. NOTE: Dry samples can be stored at 4-6 °C for up to 12 months.
5. Preparation of a Mixed Sialic Acid Standard
Weigh in between 5 - 8 mg of N-acetylneuraminic acid on an analytical balance into a 1.5 mL centrifuge tube. Note the exact weight and add 164 µL of distilled H2O for every mg (i.e. if the weight of Neu5Ac is 7.2 mg then add 7.2 x 164 = 1, 181 µL of distilled H2O). This results in a 20 mM Neu5Ac stock solution which can be stored at -20 °C for up to 12 months.
Obtain N-glycolylneuraminic acid at smaller quantities at a moderate price such as in 1 mg aliquots. Add 154 µL of distilled H2O directly to the compound in order to obtain a ~20 mM Neu5Gc stock solution. Transfer the solution into a 1.5 mL centrifuge tube. This solution can be stored at -20 °C for up to 12 months.
Combine 5 µL from each stock solution from step 5.1 and 5.2 into a fresh 1.5 mL centrifuge tube and remove the solvent by centrifugal evaporation at room temperature to dryness. NOTE: The mixed sialic acid standard can be stored at 4 - 6 °C for up to 12 months.
6. Fluorescence Derivatization of Sialic Acids
Prepare 10 mL of OPD-solution, consisting of 100 mg of o-phenylenediamine and 208 mg of sodium hydrogen sulfite in 10 mL of distilled H2O.
Add 20 µL of OPD-solution to the sialic acid samples derived from mouse milk (step 3), mouse liver (step 4), or the mixed sialic acid standard (step 5). Vortex vigorously for 30 s and incubate samples for 4 h at 80 °C in the dark (i.e. wrap the microtubes in aluminum foil).
Let the samples cool down for 5 min, add 80 µL of distilled H2O and centrifuge the tubes at 14,000 x g for 1 min.
Transfer 80 µL from the supernatant into a 300 µL high-recovery HPLC vial. The derivatized sialic acid samples can be stored at 4 - 6 °C for up to one week.
7. HPLC Analysis of Sialic Acid Derivatives
Analyze the samples using a standard HPLC system connected to an online fluorescence detector.
Use a reversed phase C18 column with the standard dimensions of 250 mm length and 4.6 mm diameter for the analysis.
- Prepare solvent A by diluting 200 mL of stock solution with 800 mL of LCMS-grade water (LCMS - liquid chromatography mass-spectrometry). The stock solution itself can be prepared as follows:
- Add 46 g of formic acid to 800 mL of LCMS-grade H2O.
- Adjust the pH to 4.5 by dropwise adding ammonium hydroxide solution (puriss. p.a.).
- Transfer the solvent to a measuring cylinder and fill up to 1,000 mL with LCMS-grade H2O. This stock solution can be stored at 4 - 6 °C for up to 3 months.
For solvent B, use LCMS-grade acetonitrile.
- Separate the sialic acids derivatives at a 1 mL/min flow rate with the following gradient elution:
- Start by adding 10% of solvent B mixed to solvent A.
- From 0 min to 15 min, gradually increase the proportion of solvent B with a linear gradient to 60%.
- From 15 min to 16 min, rapidly increase the proportion of solvent B with a linear gradient from 60% to 90%. This initiates the washing of the HPLC column.
- To further wash the HPLC column, keep the level of solvent B at 90% between 16 min and 18 min before gradually reducing the level of solvent B again to 10% between 18 min and 19 min.
- Re-equilibrate HPLC column to the starting conditions of 10% B between 19 and 24 min.
Inject 50 µL of sample into the HPLC system.
Monitor eluents using the fluorescence detector excitation/emission wavelengths of 373/448 nm, and the derivatized sialic acids can be expected at the approximate retention times of 9 min (for Neu5Gc-OPD) and 10 min (for Neu5Ac-OPD).
Calculate the relative amount of Neu5Gc (FNeu5Gc) from the fluorescence peak areas of the Neu5Ac (ANeu5Ac) and Neu5Gc (ANeu5Gc) as follows: FNeu5Gc [%] = 100×ANeu5Gc/(ANeu5Ac+ANeu5Gc). In case of the milk and liver samples from the homozygous Cmah knock-out mouse FNeu5Gc should be 0%, whereas the values for FNeu5Gc of heterozygous and wild-type mice may vary widely depending on mouse age and tissue type (between 2% and >90%) with expected error margins of ±12%.
Representative Results
A schematic overview of the described analysis method is shown in Figure 1 and includes the isolation of sialic acids from milk and liver samples of wild-type and Cmah knock-out mutant mice, and the fluorescence derivatization and HPLC analysis of these components. Figure 2 and Figure 3 show representative HPLC chromatograms of derivatized sialic acids of milk and liver samples from homo- and heterozygous knock-out Cmah mice (-/- and +/-) and wild type mice. Figure 4 shows the obtained relative amounts of N-glycolylneuraminic acid (Neu5Gc) of the analyzed mouse samples calculated from the HPLC peak areas.
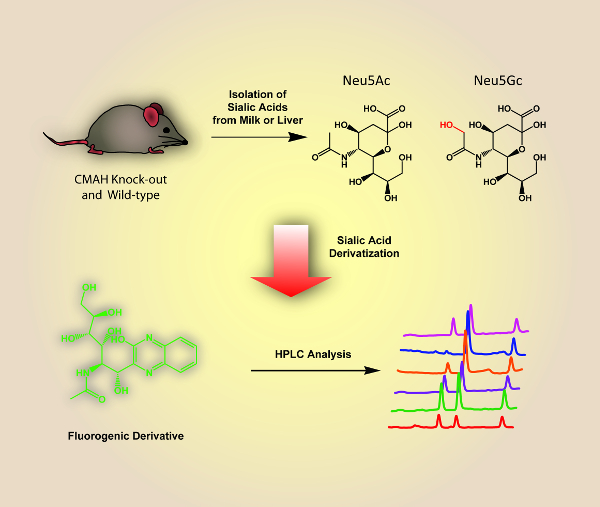
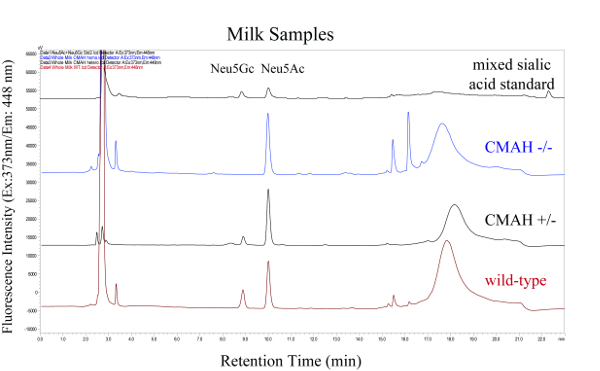
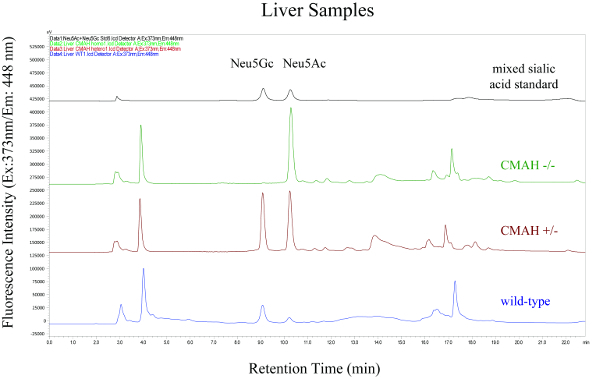
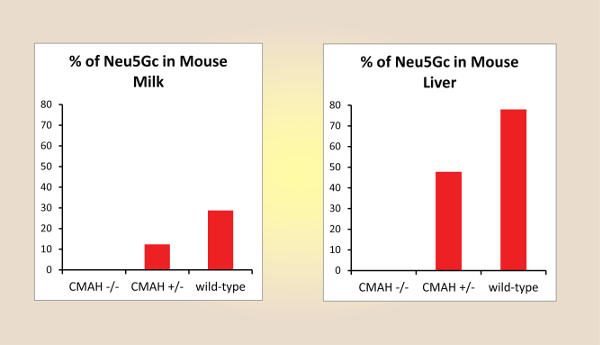
Figure 1 : Schematic Overview of the Described Analysis Method for Sialic Acids from Mouse-derived Milk and Liver Samples. Please click here to view a larger version of this figure.
Figure 2: HPLC Chromatograms of Fluorescence Labeled Sialic Acids from Milk Samples Derived from Homo- and Heterozygous Knock-out Cmah Mice (-/- and +/-) and Wild-type Mice. The top chromatogram is the mixed sialic acid standard. Please click here to view a larger version of this figure.
Figure 3: HPLC Chromatograms of Fluorescence Labeled Sialic Acids from Liver Samples Derived from Homo- and Heterozygous Knock-out Cmah Mice (-/- and +/-) and Wild-type Mice. The top chromatogram is the mixed sialic acid standard. Please click here to view a larger version of this figure.
Figure 4: Relative Amounts of N-glycolylneuraminic Acid (Neu5Gc) of the Analyzed Mouse Samples Calculated from the HPLC Peak Areas.Please click here to view a larger version of this figure.
Discussion
The herein presented protocol allows the phenotypical assessment of homozygous Cmah knock-out mice by analyzing and quantifying the relative amounts of Neu5Gc of milk and liver samples. The analysis was performed using a standard HPLC setup with fluorescence detection. The most critical step of this procedure is the preparation of the anion exchange columns and performing the anion exchange chromatography; to settle the resin properly and to collect the right washing and elution fractions takes a bit of practice.
Alternatively, the herein used derivatization agent OPD can be easily replaced with the more expensive derivatization agent DMB (1,2-diamino-4,5-methylenedioxybenzene). Furthermore, the analysis of the obtained sialic acid derivatives from step 6.5. can be also analyzed using mass-spectrometric detection instead of the fluorescence detection (i.e. Shimadzu Nexera UPLC system coupled with MS-2020 detector). After applying steps 7.2 - 7.6, the sialic acid derivatives are directly detected using positive ionization mode scanning for the m/z ratios of 382.0 and 398.0 (these are the H+-adducts of Neu5Ac-OPD and Neu5Gc-OPD, respectively).
One limitation of this method is that the Neu5Gc content can only be quantified relative to the Neu5Ac amount, but not in an absolute manner. To do so, the addition of a distinct and quantifiable sialic acid as an internal standard would be needed at the initial stage of the sample preparation. Another shortcoming is that only homozygous but not heterozygous Cmah knock-out mice can be unambiguously identified, as the relative amount of Neu5Gc heterozygous knock-out mice may vary strongly depending on sample type (i.e. tissue) and age of the individual mice. Future developments of this method may include the addition of internal standards and the absolute quantification of sialic acids in mice.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This work was supported in part by the Natural Science Foundation of China (grant numbers 31471703, A0201300537 and 31671854 to J.V. and L.L.), and the 100 Foreign Talents Plan (grant number JSB2014012 to J.V.).
References
- Lamari FN, Karamanos NK. Separation methods for sialic acids and critical evaluation of their biologic relevance. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;781(1-2):3–19. doi: 10.1016/s1570-0232(02)00432-4. [DOI] [PubMed] [Google Scholar]
- Irie A, Koyama S, Kozutsumi Y, Kawasaki T, Suzuki A. The molecular basis for the absence of N-glycolylneuraminic acid in humans. J Biol Chem. 1998;273(25):15866–15871. doi: 10.1074/jbc.273.25.15866. [DOI] [PubMed] [Google Scholar]
- Chou HH, et al. A mutation in human CMP-sialic acid hydroxylase occurred after the Homo-Pan divergence. Proc Natl Acad Sci U S A. 1998;95(20):11751–11756. doi: 10.1073/pnas.95.20.11751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehan IF, et al. Large-Scale Glycomics of Livestock: Discovery of Highly Sensitive Serum Biomarkers Indicating an Environmental Stress Affecting Immune Responses and Productivity of Holstein Dairy Cows. J Agric Food Chem. 2015;63(48):10578–10590. doi: 10.1021/acs.jafc.5b04304. [DOI] [PubMed] [Google Scholar]
- Wang B, McVeagh P, Petocz P, Brand-Miller J. Brain ganglioside and glycoprotein sialic acid in breastfed compared with formula-fed infants. Am J Clin Nutr. 2003;78(5):1024–1029. doi: 10.1093/ajcn/78.5.1024. [DOI] [PubMed] [Google Scholar]
- Yao HL, et al. Quantification of sialic acids in red meat by UPLC-FLD using indoxylsialosides as internal standards. Glycoconj J. 2016;33(2):219–226. doi: 10.1007/s10719-016-9659-1. [DOI] [PubMed] [Google Scholar]
- Nguyen DH, Tangvoranuntakul P, Varki A. Effects of natural human antibodies against a nonhuman sialic acid that metabolically incorporates into activated and malignant immune cells. J Immunol. 2005;175(1):228–236. doi: 10.4049/jimmunol.175.1.228. [DOI] [PubMed] [Google Scholar]
- Byres E, et al. Incorporation of a non-human glycan mediates human susceptibility to a bacterial toxin. Nature. 2008;456(7222):648–652. doi: 10.1038/nature07428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedlund M, Padler-Karavani V, Varki NM, Varki A. Evidence for a human-specific mechanism for diet and antibody-mediated inflammation in carcinoma progression. Proc Natl Acad Sci U S A. 2008;105(48):18936–18941. doi: 10.1073/pnas.0803943105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangvoranuntakul P, et al. Human uptake and incorporation of an immunogenic nonhuman dietary sialic acid. Proc Natl Acad Sci U S A. 2003;100(21):12045–12050. doi: 10.1073/pnas.2131556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svennerholm L. Quantitative estimation of sialic acids. II. A colorimetric resorcinol-hydrochloric acid method. Biochim Biophys Acta. 1957;24(3):604–611. doi: 10.1016/0006-3002(57)90254-8. [DOI] [PubMed] [Google Scholar]
- Warren L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959;234(8):1971–1975. [PubMed] [Google Scholar]
- Kakehi K, Maeda K, Teramae M, Honda S, Takai T. Analysis of sialic acids by gas chromatography of the mannosamine derivatives released by the action of N-acetylneuraminate lyase. J Chromatogr. 1983;272(1):1–8. doi: 10.1016/s0378-4347(00)86097-1. [DOI] [PubMed] [Google Scholar]
- Wheeler SF, Domann P, Harvey DJ. Derivatization of sialic acids for stabilization in matrix-assisted laser desorption/ionization mass spectrometry and concomitant differentiation of alpha(2 --> 3)- and alpha(2 --> 6)-isomers. Rapid Commun Mass Spectrom. 2009;23(2):303–312. doi: 10.1002/rcm.3867. [DOI] [PubMed] [Google Scholar]
- Hurum DC, Rohrer JS. Determination of sialic acids in infant formula by chromatographic methods: a comparison of high-performance anion-exchange chromatography with pulsed amperometric detection and ultra-high-performance liquid chromatography methods. J Dairy Sci. 2012;95(3):1152–1161. doi: 10.3168/jds.2011-4988. [DOI] [PubMed] [Google Scholar]
- Ito M, et al. An improved fluorometric high-performance liquid chromatography method for sialic acid determination: an internal standard method and its application to sialic acid analysis of human apolipoprotein. E. Anal Biochem. 2002;300(2):260–266. doi: 10.1006/abio.2001.5470. [DOI] [PubMed] [Google Scholar]
- Naito Y, et al. Germinal Center Marker GL7 Probes Activation-Dependent Repression of N-Glycolylneuraminic Acid, a Sialic Acid Species Involved in the Negative Modulation of B-Cell Activation. Mol Cell Biol. 2007;27(8):3008–3022. doi: 10.1128/MCB.02047-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzi AE, et al. High-pressure liquid chromatography of sialic acids on a pellicular resin anion-exchange column with pulsed amperometric detection: a comparison with six other systems. Anal Biochem. 1990;188(1):20–32. doi: 10.1016/0003-2697(90)90523-c. [DOI] [PubMed] [Google Scholar]
- Hedlund M, et al. N-glycolylneuraminic acid deficiency in mice: implications for human biology and evolution. Mol Cell Biol. 2007;27(12):4340–4346. doi: 10.1128/MCB.00379-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt RJ, et al. CRISPR-Cas9 knockin mice for genome editing and cancer modeling. Cell. 2014;159(2):440–455. doi: 10.1016/j.cell.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zangala T. Isolation of genomic DNA from mouse tails. J Vis Exp. 2007. p. e246. [DOI] [PMC free article] [PubMed]
- Willingham K, et al. Milk collection methods for mice and Reeves' muntjac deer. J Vis Exp. 2014. [DOI] [PMC free article] [PubMed]
- Goncalves LA, Vigario AM, Penha-Goncalves C. Improved isolation of murine hepatocytes for in vitro malaria liver stage studies. Malar J. 2007;6:169. doi: 10.1186/1475-2875-6-169. [DOI] [PMC free article] [PubMed] [Google Scholar]


