Abstract
While single-center and cross-sectional studies have suggested modest impact of liver donation on donor psychological well-being, few studies have assessed these outcomes prospectively among a large cohort. We conducted one of the largest, prospective, multi-center studies of psychological outcomes in living liver donors within the Adult-to-Adult Living Donor Liver Transplantation Cohort Study (A2ALL-2) Consortium. 271 (91%) of 297 eligible donors were interviewed at least once at pre-donation, 3-, 6-, 12- and 24-months post-donation using validated measures. We found that living liver donors reported low rates of major depressive (0–3%), alcohol abuse (2–5%), and anxiety syndromes (2–3%) at any given assessment in their first two years after donation. Between 4.7–9.6% of donors reported impaired mental well-being at various time points. We identified significant predictors for donors’ perceptions of being better people and experiencing psychological growth following donation, including age, gender, relationship to recipient, ambivalence and motivation regarding donation, and feeling that donation will make life more worthwhile. Our results highlight the need for close psychosocial monitoring for those donors whose recipients died (n=27), some of whom experienced guilt and concerns of responsibility. Careful screening and targeted, data-driven follow-up holds promise for optimizing psychological outcomes following this procedure for potentially vulnerable donors.
INTRODUCTION
The use of living liver donation has been a critical strategy in response to the shortage of deceased donor liver grafts for patients needing the life-saving intervention. However, living liver donors (LLD) undergo a major surgical operation with no medical benefit for themselves. The procedure is not only physically demanding on LLD (1, 2) but can also involve psychological burden (3, 4). For this reason, LLD are typically chosen to be healthy adults, both physically and emotionally (5). Given these burdens, it is imperative to have a comprehensive understanding of the psychological effects of LLD and to ensure that long-term harm is not being caused by donation.
The longitudinal effects of donation on LLD psychological well-being have not been well characterized beyond several single-center studies (6–8). Six to twelve months after donation, most LLD report their overall psychological well-being to be equivalent to or better than a normative general population or a control population of healthy adults (6), but prior studies have not systematically assessed pre-donation psychological status. Despite the overall stability in donor well-being after donation, it is not the case that all donors fare equally well (6, 9–11).
From small, single-center studies, we know that LLD who donate to recipients with hepatocellular carcinoma (HCC) and those who urgently donated to recipients with acute liver failure have significantly worse mental well-being prior to donation than normative populations (6). However, three months after donation, the mental well-being of these LLD are not significantly different from normative populations (12). Some reports have suggested worse psychological outcomes among donors whose recipients suffered complications post-transplant (3, 9), whereas other reports have not found this association (12). In a larger cross-sectional study, past or present psychiatric history, holding a graduate degree, and concerns about the donor’s own well-being prior to donation were all associated with poorer psychological outcomes, compared to population norms (13).
In a single-institution Japanese study, the rate of new onset psychiatric complications was less than 5% among LLD (14). Furthermore, in a longer-term cross-sectional analysis of LLD in the Adult-to-Adult Living Donor Liver Transplantation Cohort Study (A2ALL), most donors maintained above average health-related quality of life (HRQOL) up to 11 years post-donation (10, 15). In a recent study,(16) almost all (97%) of LLD indicated they would donate again, regardless of complications, and similar results were found in donors 3–9 years post-donation in A2ALL (15). Although the existing literature provides a snapshot of the typical trajectory of psychological outcomes for LLD, current prospective studies of donor psychological outcomes have not rigorously addressed potential predictors of psychological outcomes (6, 11, 17).
Understanding the impact of liver donation surgery not only on clinical outcomes, but also on donors’ psychological well-being, is critical for several reasons. Having a data-driven understanding of psychological outcomes is critical for donor informed consent and helps set expectations for post-donation recovery. An improved understanding of psychological outcomes may include identification of potential psychological benefits of donation. In addition, identification of donors at higher risk for poor psychological outcomes would allow transplant centers to monitor and treat potentially vulnerable donors during their recovery and aid in the development of targeted interventions. The purpose of the present study is to evaluate trends in psychological outcomes over time and potential predictors of these outcomes in a prospective, multi-center study of LLD up to two years after donation.
METHODS
Patients and study design
The A2ALL-2 consortium consists of eight US transplant centers and one transplant center in Toronto, Canada. Centers began enrolling LLDs and their recipients between February and July of 2011, and all centers ended enrollment on January 31, 2014. All centers followed the medical and psychosocial evaluation and exclusion criteria for selecting LLD now included in the current US national policy (18). As our study was observational, screening protocols were not standardized across centers. Donors in the current prospective study of HRQOL were enrolled on or before their scheduled donation date. Study participants were also required to be English-speaking to participate in telephone interviews. The study was approved by the institutional review boards and privacy boards of the University of Michigan Data Coordinating Center and each of the nine participating transplant centers. All donors provided written informed consent.
Procedure
The A2ALL Health-Related Quality of Life (HRQOL) study survey was implemented using computer-assisted telephone interview methods, which ensure consistent wording and reduce missing data by requiring a response (or reason for no response) before moving on to subsequent questions (19–21). Interviewers were trained in computer-assisted telephone interview methods. Pre-donation interviews were conducted within one month prior to the donation, and post-donation interviews were conducted at 3, 6, 12, and 24 months after donation. Participants were interviewed for 35–45 minutes each time and were compensated $20 for each completed interview. Data collection ended on July 15, 2014, after which donors who did not complete all post-donation interviews were administratively censored (n=29 at one year and 66 additionally at two years post-donation). Clinical information, including donor hospitalizations, complications, and recipient indication for transplantation were abstracted from medical records.
Measures
Psychological Outcomes
The major depressive, anxiety, and alcohol abuse modules of the Primary Care Evaluation of Mental Health Disorders (PRIME-MD) were assessed. Alcohol abuse was defined as any endorsement of the following items more than once in the preceding 6 months: drinking alcohol despite health problems, drinking alcohol during responsibilities, missed obligations due to drinking, problems getting along with other people due to drinking, or driving after drinking. The PRIME-MD is a validated tool designed to identify clinically significant mental health problems in primary care, but has also been successfully implemented in other patient populations (22–24). The modules are useful for identifying syndromes likely to meet diagnostic criteria (25–27).
The Mental Component Summary (MCS) of the Short-Form-36 (SF-36), version 2, summarizes the mental well-being of respondents. General population, norm-based scoring of the MCS was used to allow comparison to the US population, which is calibrated to have a mean score of 50 and a standard deviation of 10. The SF-36 is one of the most widely used HRQOL outcome measures in the biomedical literature (28, 29).
The Posttraumatic Growth Inventory-Short Form (PTGI-SF) (Cronbach’s alpha=0.93 in the present sample) is a 10-item measure used to assess positive outcomes reported by individuals who have experienced traumatic events. In the present study, it was asked with reference to the donation experience. Higher scores indicate a greater degree of perceived positive change following donation. Prior research suggests that it is a useful scale in determining how well patients are able to reconstruct or strengthen their perceptions of self, others, and the meaning of events (30). The PTGI-SF was only administered at one and two years post-donation.
The Simmons Better Person Scale (Cronbach’s alpha=0.78 in the present sample) is a 10-item scale that assesses whether a respondent perceives themselves to be a better person for having donated. An example item is, “Since the donation, I think more highly of myself.” Ratings range from 1=not at all true to 10=very true. Items are averaged, with higher scores indicating greater perceptions of being a better person (31).
LLD were also asked a single question of whether they would make the same decision to donate again. If their recipients died, LLD were asked a single question as to whether they felt guilty about the death and whether they felt responsible for the death (both on 1–10 scales with 1=not at all guilt/responsible and 10=very guilty/responsible) (31). Guilt and responsibility were defined as scores of 6 or more on the 10 point scale.
Potential Predictors of Psychological Outcomes
We examined donor demographics (age, sex, race/ethnicity, education, and marital status), clinical characteristics (length of donation hospital stay, post-donation re-hospitalizations within the first month, and post-operative complications within the first month), donor-recipient relationship (first degree relatives, spouse/partner, other biological or non-biological relatives, and unrelated people including friends and others), whether the donor knew of recipient death prior to the survey time point, and pre-donation survey items representing donors’ physical and mental health, and perceptions about donation.
Several pre-donation survey items included in the current study were based on instruments developed to assess donor experiences during the pre-donation process (31). These instruments included items that asked about: (a) other donation behavior (e.g. blood donation); (b) decision-making items including whether there were other possible donors for the transplant candidate; (c) a seven-item scale that assessed ambivalence about donating (Cronbach’s alpha = 0.57 in this sample); (d) whether someone encouraged or discouraged the donor to donate; (e) anticipated long-term health effects of donation; (f) feeling life would be more worthwhile if the donor donated; and (g) a two-item measure that assessed whether donors had a history of family disapproval of their behavior in the past (“black sheep donors”). Simmons’ (31) 11 items pertaining to motivations to donate were averaged to summarize the motivation to donate (Cronbach’s alpha=0.77 in the present sample). The scale ranged from 1 (weak motivation to donate) to 7 (strong motivation to donate). Other potential pre-donation predictors included the Campbell global life satisfaction item (32), which captures how donors feel about life as a whole, the MCS and Physical Component Summary (PCS) scores from the SF-36 (29), and the Patient Health Questionnaire-9 (PHQ-9) (33) depression score (Cronbach’s alpha = 0.73 in the present sample).
Statistical Analysis
Descriptive statistics were used to summarize demographic characteristics of LLD. We compared those who responded to the A2ALL HRQOL survey to those who did not respond (did not consent or were not interviewed) using t tests for continuous variables and Pearson’s chi-squared or Fisher’s exact tests for categorical variables.
Among LLD who responded to the survey, we also examined their psychological characteristics over time. At each assessment time point, we calculated means and standard deviations for continuous variables and percentages for dichotomous variables. For dichotomous outcomes, we also estimated endorsement cumulatively by calculating the percent who endorsed the outcome at any time post-donation. PRIME-MD factors were evaluated as three separate outcomes (major depressive, alcohol abuse, and non-panic, generalized anxiety syndromes) and as a group of syndromes at each time point. Lasagna plots were used to illustrate subject-specific changes over time in PRIME-MD syndromes and willingness to donate again for those donors who had each outcome at any time point (34). We hypothesized that willingness to donate again could differ based on whether the recipient died, the length of donation hospital stay, and whether the donor had post-donation complications. Because only 30 donors ever reported an unwillingness to donate again during the study period, we were not adequately powered to do multivariable modeling. Instead, we performed unadjusted repeated measures logistic regression models using generalized estimating equation (GEE) to test for these associations.
We were interested in identifying pre-donation predictors of donation-related outcomes. However, because several outcomes had low endorsement, we made an a priori decision to only model binary outcomes with endorsement >10% at a given time to help ensure reliability and generalizability of model results. To identify pre-donation predictors of two continuous donation-related outcomes measures, the Simmons Better Person Scale and PTGI-SF, GEE models with sandwich standard error estimators were fit among donors who completed the pre-donation survey and at least one post-donation survey. Predictor variable selection was guided by the method of best subsets (35), adjusted for time. Predictors were retained in models if p-values from overall tests (over all levels for categorical variables) were less than 0.05, or if Bonferroni-corrected pairwise tests against the reference category were significant for categorical variables.
Recipient indications for liver transplant were missing for some donors. Therefore, to evaluate the impact of recipient diagnosis on donors’ psychological outcomes, a subgroup analysis was conducted among donors with such information using similar modeling methods as in the main analysis. Recipient indications tested in this cohort included hepatitis C virus cirrhosis, HCC and other primary hepatic malignancy, alcohol-related cirrhosis, cryptogenic cirrhosis, primary biliary cirrhosis, primary sclerosing cholangitis, and other liver disease/cirrhosis.
Because there may be differences in screening protocols and other factors across centers, we assessed both the magnitude of center effects and the effect of center adjustment on other covariate coefficients (reflecting possible confounding). To do so, we conducted a sensitivity analysis including center indicators in final models. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Inc, Cary, NC). Lasagna plots were generated using R version 3.2.3.
RESULTS
Among 297 donors who consented to the study, 271 (91.2%) were interviewed at least once, with 245 interviewed at both pre- and post-donation, eight at only pre-donation, and 18 at only post-donation time points (Figure 1).
Figure 1.
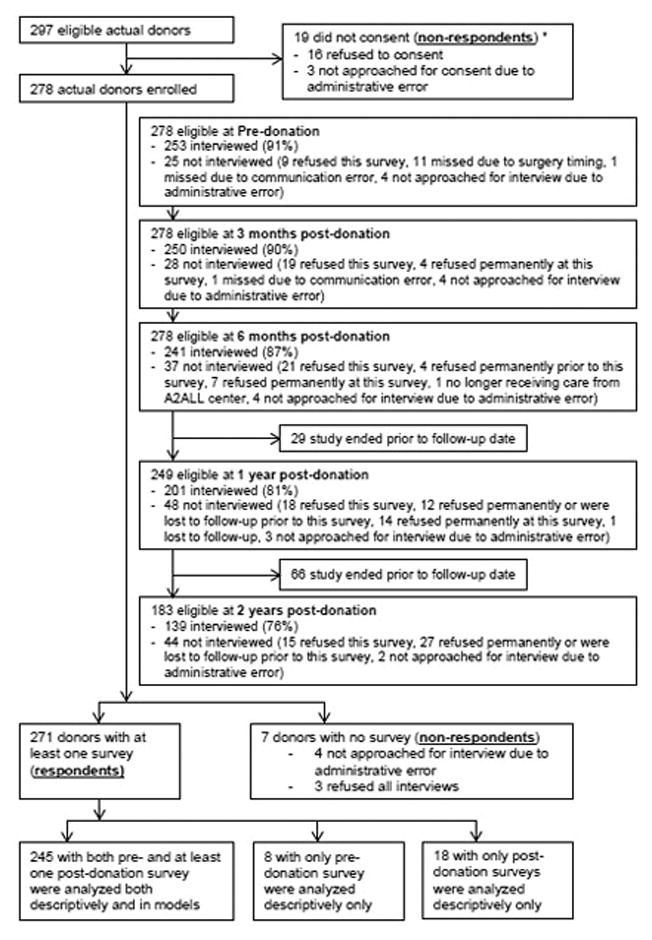
Subject flow diagram. This diagram shows the number of eligible actual donor who consented to the study, were interviewed by the survey center, and were included in descriptive analyses and models. Donors were eligible at each time point if they had reached that time point before being administratively censored at the end of study on July 15, 2014.
Note: The above chart appears in DiMartini et al (in press) AJT 2016. There were 30 potential donors consented to the study but did not donate. These 30 subjects were not included in this flow chart.
* The donation statuses for these 19 donor candidates were unknown as they didn’t consent to this study.
We compared demographic characteristics of respondents (n = 271) and non-respondents (n = 26 including 19 potential donors who did not consent and seven actual donors who were not interviewed), and no significant differences were found for sex, age or race/ethnicity (p = 0.74, 0.36 and 0.11, respectively). Respondents were predominantly female (57%), white (80%), married (63%), and employed full time (61%) (Table 1). Most had education beyond the high school level (83%) and over half donated to a first degree relative (53%). Only 10% (n=27) of respondents learned about their recipient’s death during the study follow-up period.
Table 1.
Demographic and donation-related characteristics of respondents (n=271).
| Characteristic | % (n) or Mean (SD) |
|---|---|
| Female | 57.2% (155) |
| Age at donation | 36.79 (10.51) |
| Race/Ethnicity a | |
| Non-Hispanic White | 80.4% (218) |
| Hispanic | 9.2% (25) |
| Native American or Alaskan Native | 1.8% (5) |
| Asian | 3.0% (8) |
| Black or African American | 2.6% (7) |
| Native Hawaiian or other Pacific Islander | 2.6% (7) |
| Other | 0.4% (1) |
| Education at survey | |
| High school or less | 17.3% (47) |
| Vocational or some college | 29.2% (79) |
| College graduate | 28.8% (78) |
| Postgraduate | 18.1% (49) |
| Unknown | 6.6% (18) |
| Married or had long-term partner | 63.1% (171) |
| Relation to transplant recipient | |
| First degree relative | 53.1% (144) |
| Parent | 2.2% (6) |
| Child | 36.2% (98) |
| Sibling | 14.8% (40) |
| Spouse/partner | 6.3% (17) |
| Other biological or non-biological relative | 19.2% (52) |
| Unrelated | 21.4% (58) |
| Post-donation length of hospital stay (days), Mean (SD) | 5.50 (1.99) |
| Range | 1–24 |
| Number of post-operative complications during the first month post-donation b | |
| 0 | 80.4% (218) |
| ≤ 1 | 19.2% (52) |
| Number of hospitalizations during the first month post-donation b | |
| 0 | 91.5% (248) |
| ≤ 1 | 7.7% (21) |
|
| |
| Post-donation recipient vital status from donor reported survey data (n=263) | |
|
| |
| Donor ever aware of recipient death c | 10.3% (27) |
| Weeks post-donation that recipient death occurred (n=27) | 16.11 (18.22) |
|
| |
| Pre-donation predictors from survey data (n=253) | |
|
| |
| History of other donation behavior (e.g. blood donation) | 71.5% (181) |
| There were other possible donors for the transplant candidate | 41.9% (106) |
| Ambivalence to donate (scale of 0=no ambivalence to 7= highest ambivalence) | 1.97 (1.58) |
| Someone encouraged the donor to donate | 13.4% (34) |
| Someone discouraged the donor to donate | 46.6% (118) |
| Anticipated long-term health effects of donation | 51.0% (129) |
| Feeling life would be more worthwhile if the donor donated (scale of 1=very unlikely to 10=very likely) b | 6.80 (2.79) |
| History of family disapproval of donor’s behavior, % yes | 28.5% (72) |
| Average of motivations to donate (scale of 1 to 7 with higher score indicating more or stronger motivation) | 4.97 (0.94) |
| Feeling about life as a whole (scale of 1= complete dissatisfaction to 7= complete satisfaction) | 6.11 (0.90) |
| SF-36 Mental Component Summary | 58.37 (7.19) |
| SF-36 Physical Component Summary | 56.20 (3.88) |
| PHQ-9 depression score (scale of 0 to 27), Mean (SD) | 1.45 (2.30) |
| Range | 0–16 |
Race/ethnicity: Native American or Alaskan Native, Asian, Black or African American, Native Hawaiian or other Pacific Islander, and Other were collapsed into one category in the modeling and in the comparison of respondents vs. non-respondents
Missing < 1%.
n=5 reported that they did not know recipient vital status at at least one time point.
Patient Health Questionnaire-9 =PHQ-9; Short-Form-36 = SF-36
Psychological characteristics
Table 2 shows the psychological characteristics by pre- and post-donation time points. On average, donors’ responses on the Better Person Scale and PTGI-SF were both at about the midpoint of their scales across all time points in which they were administered. On the Better Person scale, ranging from one (low) to 10 (high), donors’ three-month and six-month post-donation scores were about half a point and a third of a point higher relative to two-years post-donation, respectively. Although decreasing, this magnitude of change is unlikely to be clinically meaningful, but does suggest that feelings of self-worth persist over time (33).
Table 2.
Psychological Outcome Characteristics over Time.
| Outcome | Pre-donation (n=253) % (n) or Mean (SD) |
3 Months Post-donation (n=250) % (n) or Mean (SD) |
6 Months Post-donation (n=241) % (n) or Mean (SD) |
1 Year Post-donation (n=201) % (n) or Mean (SD) |
2 Years Post-donation (n=139) % (n) or Mean (SD) |
Endorsement at any post-donation time point(n=263) % (n) |
|---|---|---|---|---|---|---|
| Better Person Scale (1=low, 10=high) | - | 5.02 (2.46) | 4.71 (2.54) | 4.62 (2.75) | 4.57 (2.57) | - |
| Post Traumatic Growth Inventory-Short Form (0=low, 50=high) a | - | - | - | 25.23 (13.13) | 24.92 (13.69) | - |
| Would not make the same decision to donate again b | - | 8.0% (20) | 4.2% (10) | 5.0% (10) | 5.8% (8) | 11.4% (30) |
| Any PRIME-MD Syndrome c | 5.5% (14) | 4.0% (10) | 5.9% (14) | 9.5% (19) | 5.8% (8) | 14.1% (37) |
| Major Depressive Syndrome (12-mo prevalence = 6.6%) d | 0.4% (1) | 0.4% (1) | 0.0% (0) | 2.5% (5) | 0.0% (0) | 2.3% (6) |
| Alcohol Abuse Syndrome (12-mo prevalence = 5.9%) e | 4.0% (10) | 2.4% (6) | 4.2% (10) | 5.5% (11) | 3.6% (5) | 8.4% (22) |
| Non-Panic General Anxiety Syndrome (12-mo prevalence = 3.1%) f | 2.0% (5) | 1.6% (4) | 1.7% (4) | 3.5% (7) | 2.2% (3) | 5.3% (14) |
| Donors whose recipient are no longer alive g | - | n=13 | n=18 | n=14 | n=14 | n=27 |
| Feel guilty about death (6 or higher on scale of 1 – not at all guilty to 10 – very guilty) | - | 7.7% (1) | 33.3% (6) | 21.4% (3) | 0.0% (0) | 33.3% (9) |
| Feel responsible for death (6 or higher on scale of 1 – not at all responsible to 10 – very responsible) | - | 0.0% (0) | 22.2% (4) | 21.4% (3) | 7.1% (1) | 22.2% (6) |
|
| ||||||
| General HRQOL | ||||||
| SF-36 MCS (US mean = 50, SD = 10, higher is better) g | 58.37 (7.19) | 58.16 (9.46) | 58.67 (8.32) | 57.95 (10.92) | 59.52 (7.53) | - |
| SF-36 MCS Impaired (Below 0.5 SD of the mean) g | 4.7% (12) | 9.6% (24) | 7.9% (19) | 9.5% (19) | 5.0% (7) | 18.6% (49) |
Missing n=1 at 1 year.
Missing n=5 at 6 months.
Missing n=2 at 3 months, n=3 at 6 months, and n=1 at 1 year; prevalence figures taken from Center for Behavioral Health Statistics and Quality. (2015). Behavioral health trends in the United States: Results from the 2014 National Survey on Drug Use and Health (HHS Publication No. SMA 15-4927, NSDUH Series H-50). Retrieved from http://www.samhsa.gov/data/
Missing n=1 at 3 months, n=2 at 6 months, and n=1 at 1 year.
Missing n=3 at 6 months.
Missing n=1 at 3 months and n=3 at 6 months.
Missing n=1 at 6 months.
Health-related quality of life=HRQOL; Mental Component Summary=MCS; Patient Health Questionnaire-9 =PHQ-9; Physical Component Summary=PCS; Primary Care Evaluation of Mental Health Disorders (PRIME-MD); Short-Form-36 = SF-36
Only 8% of donors reported they would not make the same decision to donate again at three months post-donation; this decreased to around 5% at subsequent follow-up assessments. Overall, 11% (n=30) reported they would not donate again at some point during the study follow-up. Although a few of these donors indicated they would not donate again consistently across all post-donation time points, most only indicated once or twice during follow-up that they would not donate again (Figure 2). Based on unadjusted repeated measures regression models, donors whose recipients died were 8.0 times more likely to report unwillingness to donate again than donors whose recipients did not die (95% CI 2.9–22.3, p=0.047); post-donation complications and length of hospital stay were not associated with donor’s unwillingness to donate again (p=0.95 and p=0.90, respectively).
Figure 2.
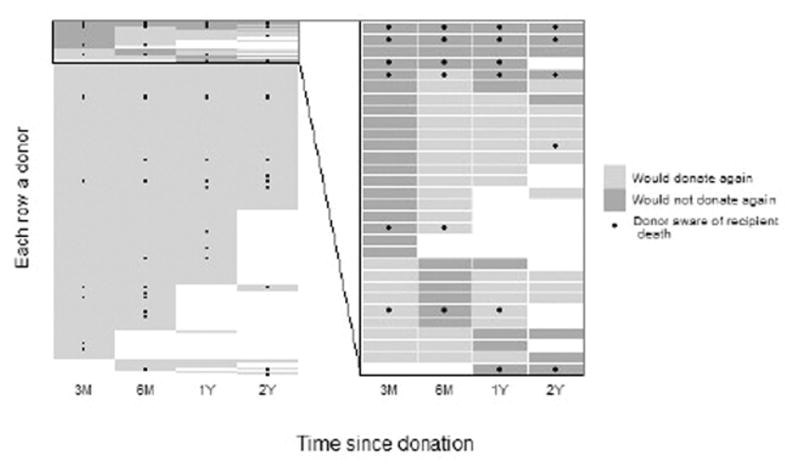
Donor-specific willingness to donate again (light grey) or not (dark grey) are shown for each survey: 3 months (3M), 6 months (6M), 1 year (1Y) and 2 years (2Y). Each row in the graph represents a donor and white boxes indicate missing surveys. Recipient deaths that were known to the donor are shown with black dots, with the first dot in each row representing the time point when the donor first reported awareness of recipient death. The left side includes all donors with post-donation surveys (n=263) and the right side shows only donors who reported they would not donate again at one or more time points (n=30).
With respect to PRIME-MD syndromes, 4% to 9.5% of donors had at least one syndrome at any given time point, with little change over time observed (Table 2). The most common syndrome was alcohol abuse (2% – 5%), followed by anxiety syndrome (2% – 3%) and major depressive syndrome (0% – 3%). Among donors with any type of syndrome at any time point (n=43, Figure 3 right panel), 30 had a syndrome at only one time point; eight at two time points; two at three time points; two at four time points; and one at all five time points. Regarding the individual syndromes, 37 only had one syndrome (yellow, red and blue in Figure 3) at any time, six had two syndromes (orange and purple) during at least one time point, and no one had all three syndromes at the same time. In addition, 26 out of 43 had no syndromes at pre-donation but developed them during post-donation follow-up.
Figure 3.
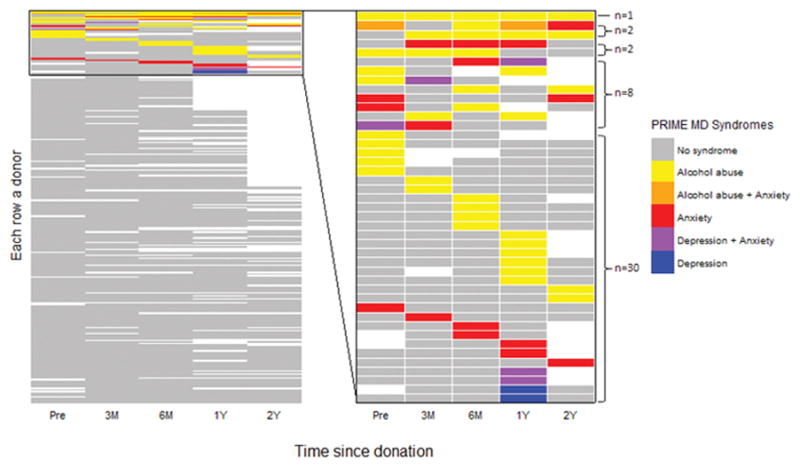
Donor-specific PRIME-MD syndromes by time point: pre-donation (Pre), 3 months (3M), 6 months (6M), 1 year (1Y) and 2 years (2Y). In the graph, each row represents a donor and white boxes indicate missing surveys. The left side includes all donors (n=271) and the right side shows only donors who had any syndrome at one or more time points (n=43).
On average, donors’ MCS scores were similar across all pre- and post-donation time points and were about 8 to 9.5 points higher than the US general population (Table 2). At pre-donation, 4.7% of donors were considered impaired on the MCS (defined as 0.5 SD below the US normative mean); it increased to 9.6% at three months post-donation, but then decreased back to 5% at two years post-donation.
Of donors reporting recipient death during the study follow-up (n=27), nine (33%) had ever felt guilty and six (22%) had ever felt responsible for the recipient death at some point after their recipient died.
Predictors of Psychological Outcomes
No binary outcomes were modeled because no outcomes had endorsement >10% at at least one time point to help ensure reliability of model results.
Significant predictors of the Simmons Better Person Scale included time since donation, relationship to recipient, sex, recipient death, and several pre-donation psychological factors (Table 3). Scores on the Better Person scale were decreasing over time until one year post-donation. Donors donating to a first degree relative had higher scores compared to those donating to unrelated recipients (β=0.84, 95% CI 0.19–1.49), on average, while female donors (β= −0.70, 95% CI −1.21 to −0.18) and donors whose recipient died (β= −1.24, 95% CI −1.89 to −0.59) had lower scores. Higher pre-donation ambivalence, anticipation that life will be more worthwhile after donation, higher average of donation motivations, and history of other donation behavior (actual or intended) were all associated with higher scores on the Simmons Better Person Scale.
Table 3.
Predictors of Simmons Better Person Scale (0=low, 10=high) from Repeated Measures Linear Regression Models (n=245).
| Regression coefficient | 95% CI | P-value | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Post-donation time point | <.001 | |||
| 3M vs. 2Y | 0.58 | 0.30 | 0.86 | <.001 |
| 6M vs. 2Y | 0.34 | 0.07 | 0.61 | .01 |
| 1Y vs. 2Y | 0.12 | −0.14 | 0.37 | .36 |
| Donor recipient relationship | .054 | |||
| First degree relative vs. Unrelated | 0.84 | 0.19 | 1.49 | .012 |
| Spouse/partner vs. Unrelated | −0.15 | −1.49 | 1.19 | .82 |
| Other biological or non-biological relative vs. Unrelated | 0.46 | −0.32 | 1.24 | .25 |
| Female vs. Male | −0.70 | −1.21 | −0.18 | .008 |
| Recipient death (time dependent) | −1.24 | −1.89 | −0.59 | <.001 |
| Pre-donation predictors | ||||
| Ambivalence scale (0-no ambivalence to 7-ambivalence) | 0.24 | 0.07 | 0.40 | .005 |
| If donated, I will feel my life is more worthwhile (1-very unlikely to 10-very likely) | 0.27 | 0.17 | 0.37 | <.001 |
| Average of motivations to donate (scale of 1 to 7 with higher score meaning stronger motivation) | 0.61 | 0.31 | 0.91 | <.001 |
| History of other donation behavior | 0.79 | 0.21 | 1.37 | .008 |
Variables tested for inclusion but not significant: donor demographics (age at donation, race/ethnicity, education, marital status), clinical characteristics (length of hospital stay, whether donor was re-hospitalized or had complication during the first month post-donation), whether there were other possible donors for the transplant candidate, whether someone encouraged or discouraged the donor to donate, whether donor anticipated long-term health effects of donation, black sheep donor, how donor felt about life as a whole, pre-donation SF-36 MCS and PCS, and PHQ-9 depression score.
Mental Component Summary=MCS; Patient Health Questionnaire-9 =PHQ-9; Physical Component Summary=PCS; Short-Form-36 = SF-36
The PTGI-SF average scores were not significantly different between one year and two years post-donation (Table 4). Older donors experienced less growth (β= −1.58 per 10-year increase in age, 95% CI −2.98 to −0.17), on average, as did those who were discouraged to donate (β= −3.56, 95% CI −6.68 to −0.45). In contrast, donors who anticipated pre-donation that their life would be more worthwhile after donation had significantly more growth as measured by the PTGI-SF.
Table 4.
Predictors of Posttraumatic Growth Inventory (PTGI) (0=low, 50=high) from Repeated Measures Linear Regression Models (n=192).
| Regression coefficient | 95% CI | P-value | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Post-donation time point | ||||
| 1Y vs. 2Y | 0.22 | −1.57 | 2.02 | .81 |
| Age at donation (per 10 year increase) | −1.58 | −2.98 | −0.17 | .03 |
| If donated, I will feel my life is more worthwhile (1-very unlikely to 10-very likely) | 2.06 | 1.51 | 2.61 | <.001 |
| Anyone discouraged to donate | −3.56 | −6.68 | −0.45 | .03 |
Variables tested for inclusion but not significant: donor demographics (sex, race/ethnicity, education, marital status), clinical characteristics (length of hospital stay, whether donor was re-hospitalized or had complication during the first month post-donation), donor recipient relationship, recipient death, history of other donation behavior, whether there were other possible donors for the transplant candidate, ambivalence to donate, whether someone encouraged the donor to donate, whether donor anticipated long-term health effects of donation, black sheep donor, average of motivations to donate, how donor felt about life as a whole, SF-36 MCS and PCS, and PHQ-9 depression score.
Mental Component Summary=MCS; Patient Health Questionnaire-9 =PHQ-9; Physical Component Summary=PCS; Short-Form-36 = SF-36
For both modelled outcomes, sensitivity analyses including center indicators in models showed similar results for the predictors identified above. Center was significant in predicting the Simmons Better Person Scale (overall p-value=0.03), but not significant in predicting PTGI-SF (p=0.09). For the Simmons Better Person Scale, using the center with the largest number of donors (n=90) as the reference category, the differences from the other eight centers ranged from −0.24 (p=0.61) to 1.41 points (p=0.0008). Only the center with the 1.41 point difference was found to be significantly different from the reference center.
The subgroup analysis (n=226) examining the effects of recipient indications for liver transplant on donors’ psychological outcomes did not reveal any recipient indication/diagnosis significantly associated with the Better Person Scale or PTGI-SF, except for cryptogenic cirrhosis. Donors whose recipients had an indication for transplant of cryptogenic cirrhosis had an average of 7.5 points (95% CI 0.67–14.4) more post traumatic growth compared to donors whose recipients’ indication for transplant did not include cryptogenic cirrhosis.
DISCUSSION
We conducted one of the largest, multi-center prospective studies of living liver donors’ psychological well-being to date. At two years post-donation, nearly 95% of donors interviewed reported they would make the decision to donate again if they could. However, it is useful to note that up to 11% of our sample indicated at some point during the post-donation study period that they would not donate again, and this rate is somewhat higher than reported in prior studies of living liver donors (9, 36). The slight discrepancy may be due to the fact that our participants were reporting their experience to a survey center that was not directly associated with the donation team.
In our cohort, we found that LLDs report low rates of major depressive, alcohol abuse, and anxiety syndromes at any given time point in their first two years following donation (generally < 5% for any individual syndrome at any given time point). This finding is compatible with earlier A2ALL cohort research (1) and other prospective studies (12) that have investigated rates of LLD psychiatric symptoms. Furthermore, our donors reported mental well-being that is consistent with or better than that of the general population and other LLD populations, on average (6, 10, 11, 16). That said, there exists a minority of patients that describe impairment in this domain, even before donation (10).
While it is generally good news that few donors experience these psychiatric syndromes or impaired mental well-being, the fact that alcohol abuse was endorsed at all among liver donors is worrisome. To explore a post-hoc hypothesis, we examined whether PRIME-MD alcohol abuse syndrome in donors was associated with recipient alcohol cirrhosis diagnosis -- a “birds of a feather flock together” hypothesis -- but found no association across all time points (p=0.26). We are aware of no prior research that has looked at drinking behavior among LLDs. However, at the pre-donation survey, 4% of our sample endorsed alcohol abuse syndrome in the previous 6 months. Some donors also endorsed symptoms of alcohol abuse syndrome at 3- and 6-months post-donation surveys. Especially given the timeframe for liver regeneration in donors, it would be prudent for LLDs to be more closely monitored for their alcohol use both pre- and post-donation (37).
Donors whose recipients had died were more likely to report unwillingness to donate again, compared to donors whose recipients did not die. Furthermore, a third of those donors whose recipients died felt guilty and 22% felt responsible at some point for their recipient’s death. Our findings highlight these donors may benefit from additional monitoring to ensure that they receive adequate psychosocial support and treatment, if necessary (10, 38, 39).
Our study has several strengths, including the large, multi-center, prospective design and the use of standardized patient-reported outcomes to describe the sample over time. A recent review has highlighted the need for exactly this type of prospective living donor outcomes study, as well as stronger, evidence-based psychosocial screening criteria (17). Consistent with prior research, we found that many donors experienced positive psychological outcomes as a result of their donation, including feelings of self-worth, and personal growth. Although low levels of endorsement for many of the outcomes did not allow for statistical modeling, our observational findings are worth highlighting in and of themselves. Future research on longer-term psychological outcomes is warranted, as some key psychological sequelae to donation may not become apparent until much later after the donation experience (15).
Acknowledgments
This study was presented in part at the annual meeting of the American Transplant Congress, Boston, MA, June 15, 2016.
This is publication number #39 of the Adult-to-Adult Living Donor Liver Transplantation Cohort Study.
This study was supported by the National Institute of Diabetes & Digestive & Kidney Diseases through cooperative agreements (grants U01-DK62444, U01-DK62467, U01-DK62483, U01-DK62484, U01-DK62494, U01-DK62496, U01-DK62498, U01-DK62505, U01-DK62531, U01-DK62536, U01-DK85515, U01-DK85563, and U01-DK85587). Additional support was provided by Health Resources and Services Administration (HRSA), and the American Society of Transplant Surgeons (ASTS).
The following individuals were instrumental in the planning and conduct of this study at each of the participating institutions:
Columbia University Medical Center, New York, NY (DK62483): PI: Jean C. Emond, MD; Co-Is: Robert S. Brown, Jr., MD, MPH, James Guarrera, MD, FACS, Martin R. Prince, MD, PhD, Benjamin Samstein, MD, Elizabeth Verna, MD, MS; Study Coordinators: Taruna Chawla, MD, Scott Heese, MPH, Connie Kim, BS, Theresa Lukose, PharmD, Tarek Mansour, MB BCH, Joseph Pisa, BA, Rudina Odeh-Ramadan, PharmD, Jonah Zaretsky, BS.
Lahey Hospital & Medical Center, Burlington, MA (DK85515): PI: Elizabeth A. Pomfret, MD, PhD, FACS; Co-Is: Christiane Ferran, MD, PhD, Fredric Gordon, MD, James J. Pomposelli, MD, PhD, FACS, Mary Ann Simpson, PhD; Study Coordinators: Erick Marangos, Agnes Trabucco, BS, MTASCP.
Northwestern University, Chicago, IL (DK62467): PI: Michael M.I. Abecassis, MD, MBA; Co-Is: Talia B. Baker, MD, Zeeshan Butt, PhD, Laura M. Kulik, MD, Daniela P. Ladner, MD, Donna M. Woods, PhD; Study Coordinator: Patrice Al-Saden, RN, CCRC, Tija Berzins, Amna Daud, MD, MPH, Elizabeth Rauch, BS, Teri Strenski, PhD, Jessica Thurk, BA, MA, Erin Wymore, BA, MS, CHES.
University of California Los Angeles, Los Angeles, CA (DK62496): PI: Johnny C. Hong, MD; Co-I: Ronald W. Busuttil, MD, PhD; Study Coordinator: Janet Mooney, RN, BSN.
University of California San Francisco, San Francisco, CA (DK62444): PI: Chris E. Freise, MD, FACS; Co-I: Norah A. Terrault, MD, MPH; Study Coordinators: Alexandra Birch, BS, Dulce MacLeod, RN.
University of Colorado, Aurora, CO (DK62536): PI: James R. Burton, Jr., MD; Co-Is: Gregory T. Everson, MD, FACP, Igal Kam, MD, James Trotter, MD, Michael A. Zimmerman, MD; Study Coordinators: Jessica Fontenot, BS, Carlos Garcia, RN, BS, Anastasia Krajec, RN.
University of Michigan Health System, Ann Arbor, MI (DK62498): PI: Robert M. Merion, MD, FACS; DCC Staff: Yevgeniya Abramovich, BA, Mary Akagi, MS, CCRP, Douglas R. Armstrong, BSN, MS, Charlotte A. Beil, MPH, Carl L. Berg, MD, Abby Brithinee, BA, Tania C. Ghani, MS, MSI, Brenda W. Gillespie, PhD, Beth Golden, BScN, Margaret Hill-Callahan, BS, LSW, Lisa Holloway, BS, CCRC, Terese A. Howell, BS, CCRC, Anna S.F. Lok, MD, Monique Lowe, MSI, Anna Nattie, BA, Akinlolu O. Ojo, MD, PhD, Samia Shaw, AAIT, Abigail Smith, MS, Robert A. Wolfe, PhD, Gary Xia, BA.
University of North Carolina, Chapel Hill, NC (DK62505): PI: Paul H. Hayashi, MD, MPH; Study Coordinator: Tracy Russell, MA.
University of Pennsylvania, Philadelphia, PA (DK62494): PI: Abraham Shaked, MD, PhD, Kim Olthoff MD; Co-Is: David S. Goldberg, MD, Karen L. Krok, MD, K. Rajender Reddy, MD, Mark A. Rosen, MD, PhD, Robert M. Weinrieb, MD; Study Coordinators: Brian Conboy, PA, MBA, Mary Kaminski, PA-C, Debra McCorriston, RN, Mary Shaw, RN, BBA.
University of Pittsburgh Medical Center, Pittsburgh, PA (DK85587): PI: Abhinav Humar, MD; Co-Is: Andrea F. DiMartini, MD, Mary Amanda Dew, PhD, Mark Sturdevent, MD; Study Coordinators: Megan Basch, RN, Sheila Fedorek, RN, CCRC, Leslie Mitrik, BS, Mary L. McNulty, MLS.
University of Toronto, Toronto, ON, CA (DK85563): PI: David Grant, MD, FRCSC; Co-Is: Oyedele Adeyi, MD, FCAP, FRCPC, Susan Abbey, MD, FRCPC, Hance Clarke, MSc, MD, FRCPC, Susan Holtzman, PhD, Joel Katz, CRC, PhD, Gary Levy, BSc, FRCPC, MD, Nazia Selzner, MD, PhD; Study Coordinators: Kimberly Castellano, BSc, Andrea Morillo, BM, BCh, Erin Winter, BSc.
University of Virginia, Charlottesville, VA (DK62484): PI: Carl L. Berg, MD; Co-I: Timothy L. Pruett, MD; Study Coordinator: Jaye Davis, RN.
Virginia Commonwealth University - Medical College of Virginia Campus, Richmond, VA (DK62531): PIs: Adrian H. Cotterell, MD, FACS, Robert A. Fisher, MD, FACS; Co-Is: Martha K. Behnke, PhD, Adrian H. Cotterell, MD, FACS, Ann S. Fulcher, MD, Pamela M. Kimball, PhD, HCLD, Mary E. Olbrisch, PhD, ABPP, Marc P. Posner, MD, FACS, Mark A. Reimers, PhD, Amit Sharma, MD, R. Todd Stravitz, MD, FACP; Study Coordinators: April Ashworth, RN, BSN, Joanne Davis, RN, Sarah Hubbard, Andrea Lassiter, BS, Luke Wolfe, MS.
National Institute of Diabetes and Digestive and Kidney Diseases, Division of Digestive Diseases and Nutrition, Bethesda, MD: Edward Doo, MD, James E. Everhart, MD, MPH, Jay H. Hoofnagle, MD, Stephen James, MD, Patricia R. Robuck, PhD, Leonard B. Seeff, MD, Averell H. Sherker, MD, FRCPC, Rebecca J. Torrance, RN, MS.
Abbreviations
- A2ALL
Adult-to-Adult Living Donor Liver Transplantation Cohort Study (A2ALL)
- GEE
generalized estimating equation
- HCC
hepatocellular carcinoma
- HRQOL
health-related quality of life
- LLD
living liver donors
- MCS
Mental Component Summary
- PCS
Physical Component Summary
- PHQ-9
Patient Health Questionnaire-9
- PRIME-MD
Primary Care Evaluation of Mental Health Disorders
- PTGI-SF
Posttraumatic Growth Inventory-Short Form
- SF-36
Short-Form-36
Footnotes
DISCLOSURES
The authors have nothing to declare.
References
- 1.Abecassis MM, Fisher RA, Olthoff KM, Freise CE, Rodrigo DR, Samstein B, et al. Complications of living donor hepatic lobectomy--a comprehensive report. Am J Transplant. 2012 May;12(5):1208–17. doi: 10.1111/j.1600-6143.2011.03972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghobrial RM, Freise CE, Trotter JF, Tong L, Ojo AO, Fair JH, et al. Donor morbidity after living donation for liver transplantation. Gastroenterology. 2008 Aug;135(2):468–76. doi: 10.1053/j.gastro.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Erim Y, Beckmann M, Kroencke S, Valentin-Gamazo C, Malago M, Broering D, et al. Psychological strain in urgent indications for living donor liver transplantation. Liver Transpl. 2007 Jun;13(6):886–95. doi: 10.1002/lt.21168. [DOI] [PubMed] [Google Scholar]
- 4.Trotter JF, Hill-Callahan MM, Gillespie BW, Nielsen CA, Saab S, Shrestha R, et al. Severe psychiatric problems in right hepatic lobe donors for living donor liver transplantation. Transplantation. 2007 Jun 15;83(11):1506–8. doi: 10.1097/01.tp.0000263343.21714.3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pascher A, Sauer IM, Walter M, Lopez-Haeninnen E, Theruvath T, Spinelli A, et al. Donor evaluation, donor risks, donor outcome, and donor quality of life in adult-to-adult living donor liver transplantation. Liver Transpl. 2002 Sep;8(9):829–37. doi: 10.1053/jlts.2002.34896. [DOI] [PubMed] [Google Scholar]
- 6.Parikh ND, Ladner D, Abecassis M, Butt Z. Quality of life for donors after living donor liver transplantation: a review of the literature. Liver Transpl. 2010 Dec;16(12):1352–8. doi: 10.1002/lt.22181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toyoki Y, Ishido K, Kudo D, Umehara M, Kimura N, Narumi S, et al. Donor quality of life after living donor liver transplantation: single-institute experience. Transplant Proc. 2012 Mar;44(2):341–3. doi: 10.1016/j.transproceed.2012.01.034. [DOI] [PubMed] [Google Scholar]
- 8.Ishizaki M, Kaibori M, Matsui K, Kwon AH. Change in donor quality of life after living donor liver transplantation surgery: a single-institution experience. Transplant Proc. 2012 Mar;44(2):344–6. doi: 10.1016/j.transproceed.2012.01.026. [DOI] [PubMed] [Google Scholar]
- 9.Kim-Schluger L, Florman SS, Schiano T, O’Rourke M, Gagliardi R, Drooker M, et al. Quality of life after lobectomy for adult liver transplantation. Transplantation. 2002 May 27;73(10):1593–7. doi: 10.1097/00007890-200205270-00012. [DOI] [PubMed] [Google Scholar]
- 10.Ladner DP, Dew MA, Forney S, Gillespie BW, Brown RS, Jr, Merion RM, et al. Long-term quality of life after liver donation in the adult to adult living donor liver transplantation cohort study (A2ALL) J Hepatol. 2015 Feb;62(2):346–53. doi: 10.1016/j.jhep.2014.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kroencke S, Nashan B, Fischer L, Erim Y, Schulz KH. Donor quality of life up to two years after living donor liver transplantation: a prospective study. Transplantation. 2014 Mar 15;97(5):582–9. doi: 10.1097/01.TP.0000438206.04348.b2. [DOI] [PubMed] [Google Scholar]
- 12.Erim Y, Beckmann M, Valentin-Gamazo C, Malago M, Frilling A, Schlaak JF, et al. Quality of life and psychiatric complications after adult living donor liver transplantation. Liver Transpl. 2006 Dec;12(12):1782–90. doi: 10.1002/lt.20907. [DOI] [PubMed] [Google Scholar]
- 13.DuBay DA, Holtzman S, Adcock L, Abbey S, Greenwood S, Macleod C, et al. Adult right-lobe living liver donors: quality of life, attitudes and predictors of donor outcomes. Am J Transplant. 2009 May;9(5):1169–78. doi: 10.1111/j.1600-6143.2009.02614.x. [DOI] [PubMed] [Google Scholar]
- 14.Kimura H, Onishi Y, Sunada S, Kishi S, Suzuki N, Tsuboi C, et al. Postoperative Psychiatric Complications in Living Liver Donors. Transplant Proc. 2015 Jul-Aug;47(6):1860–5. doi: 10.1016/j.transproceed.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 15.Dew MA, DiMartini AF, Ladner DP, Simpson MA, Pomfret EA, Gillespie BW, et al. Psychosocial Outcomes 3 to 10 Years After Donation in the Adult to Adult Living Donor Liver Transplantation Cohort Study. Transplantation. 2016 Jun;100(6):1257–69. doi: 10.1097/TP.0000000000001144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Humphreville VR, Radosevich DM, Humar A, Payne WD, Kandaswamy R, Lake JR, et al. Longterm health-related quality of life after living liver donation. Liver Transpl. 2016 Jan;22(1):53–62. doi: 10.1002/lt.24304. [DOI] [PubMed] [Google Scholar]
- 17.Duerinckx N, Timmerman L, Van Gogh J, van Busschbach J, Ismail SY, Massey EK, et al. Predonation psychosocial evaluation of living kidney and liver donor candidates: a systematic literature review. Transpl Int. 2014 Jan;27(1):2–18. doi: 10.1111/tri.12154. [DOI] [PubMed] [Google Scholar]
- 18.Organ Procurement and Transplantation Network/United Network for Organ Sharing (OPTN/UNOS) [Last accessed 2/9/16];OPTN Policies, Policy 14: Living donation. Updated 12/1/15. http://optn.transplant.hrsa.gov/governance/policies/
- 19.Li C, Ford ES, Zhao G, Tsai J, Balluz LS. A comparison of depression prevalence estimates measured by the Patient Health Questionnaire with two administration modes: computer-assisted telephone interviewing versus computer-assisted personal interviewing. Int J Public Health. 2012 Feb;57(1):225–33. doi: 10.1007/s00038-011-0253-9. [DOI] [PubMed] [Google Scholar]
- 20.Harlow BL, Rosenthal JF, Ziegler RG. A comparison of computer-assisted and hard copy telephone interviewing. Am J Epidemiol. 1985 Aug;122(2):335–40. doi: 10.1093/oxfordjournals.aje.a114105. [DOI] [PubMed] [Google Scholar]
- 21.Groves RM, Mathiowetz NA. Computer assisted telephone interviewing: effects on interviewers and respondents. Public Opin Q. 1984 Spring;48(1B):356–69. doi: 10.1093/poq/48.1b.356. [DOI] [PubMed] [Google Scholar]
- 22.Karp JF, Dew MA, Wahed AS, Fitzgerald K, Bolon CA, Weiner DK, et al. Challenges and Solutions for Depression Prevention Research: Methodology for a Depression Prevention Trial for Older Adults with Knee Arthritis and Emotional Distress. Am J Geriatr Psychiatry. 2015 Nov 17; doi: 10.1016/j.jagp.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boyko EJ, Seelig AD, Jacobson IG, Hooper TI, Smith B, Smith TC, et al. Sleep characteristics, mental health, and diabetes risk: a prospective study of U.S. military service members in the Millennium Cohort Study. Diabetes Care. 2013 Oct;36(10):3154–61. doi: 10.2337/DC13-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colenda CC, Legault C, Rapp SR, DeBon MW, Hogan P, Wallace R, et al. Psychiatric disorders and cognitive dysfunction among older, postmenopausal women: results from the Women’s Health Initiative Memory Study. Am J Geriatr Psychiatry. 2010 Feb;18(2):177–86. doi: 10.1097/JGP.0b013e3181c65864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999 Nov 10;282(18):1737–44. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 26.Spitzer RL, Kroenke K, Linzer M, Hahn SR, Williams JB, deGruy FV, 3rd, et al. Health-related quality of life in primary care patients with mental disorders. Results from the PRIME-MD 1000 Study. JAMA. 1995 Nov 15;274(19):1511–7. [PubMed] [Google Scholar]
- 27.Spitzer RL, Williams JB, Kroenke K, Linzer M, deGruy FV, 3rd, Hahn SR, et al. Utility of a new procedure for diagnosing mental disorders in primary care. The PRIME-MD 1000 study. JAMA. 1994 Dec 14;272(22):1749–56. [PubMed] [Google Scholar]
- 28.Jay CL, Butt Z, Ladner DP, Skaro AI, Abecassis MM. A review of quality of life instruments used in liver transplantation. J Hepatol. 2009 Nov;51(5):949–59. doi: 10.1016/j.jhep.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992 Jun;30(6):473–83. [PubMed] [Google Scholar]
- 30.Tedeschi RG, Calhoun LG. The Posttraumatic Growth Inventory: measuring the positive legacy of trauma. J Trauma Stress. 1996 Jul;9(3):455–71. doi: 10.1007/BF02103658. [DOI] [PubMed] [Google Scholar]
- 31.Simmons R, Simmons R, Marine S. Gift of life: The effect of organ transplantation on individual, family, and societal dynamics. New Brunswick, NJ: Transaction Books; 1987. [Google Scholar]
- 32.Campbell A, Converse PE, Rodgers WL. The quality of American life: Perceptions evaluations, and satisfactions. Russell Sage Foundation; 1976. [Google Scholar]
- 33.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001 Sep;16(9):606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003 May;41(5):582–92. doi: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]
- 35.Harrell FE. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. New York, NY: Springer; 2001. [Google Scholar]
- 36.Miyagi S, Kawagishi N, Fujimori K, Sekiguchi S, Fukumori T, Akamatsu Y, et al. Risks of donation and quality of donors’ life after living donor liver transplantation. Transpl Int. 2005 Jan;18(1):47–51. doi: 10.1111/j.1432-2277.2004.00028.x. [DOI] [PubMed] [Google Scholar]
- 37.Bramstedt KA, Stowe J, Lemberg B. The dilemma of alcohol use by potential living liver donors. Prog Transplant. 2006 Mar;16(1):24–7. doi: 10.1177/152692480601600106. [DOI] [PubMed] [Google Scholar]
- 38.Yucetin L, Bozoklar CA, Yanik O, Tekin S, Tuncer M, Demirbas A. An Investigation of Post-Traumatic Growth Experiences Among Living Kidney Donors. Transplant Proc. 2015 Jun;47(5):1287–90. doi: 10.1016/j.transproceed.2015.04.027. [DOI] [PubMed] [Google Scholar]
- 39.DiMartini AF, Dew MA, Butt Z, Simpson MA, Ladner DP, Smith AR, et al. Patterns and predictors of sexual function after liver donation: The Adult-to-Adult Living Donor Liver Transplantation Cohort study. Liver Transpl. 2015 May;21(5):670–82. doi: 10.1002/lt.24108. [DOI] [PMC free article] [PubMed] [Google Scholar]


