Abstract
The role of dendritic cells (DCs) and their targeted manipulation in the body’s response to implanted materials is an important and developing area of investigation, and a large component of the emerging field of biomaterials-based immune engineering. The key position of DCs in the immune system, serving to bridge innate and adaptive immunity, is facilitated by rich diversity in type and function and places DCs as a critical mediator to biomaterials of both synthetic and natural origins. This review presents current views regarding DC biology and summarizes recent findings in DC responses to implanted biomaterials. Based on these findings, there is promise that the directed programming of application-specific DC responses to biomaterials can become a reality, enabling and enhancing applications almost as diverse as the larger field of biomaterials itself.
Keywords: biomaterials, dendritic cells, immunology, scaffolds, microparticles
Introduction
Biomaterials are used as tools for regenerative therapies aimed at replacing lost or dysfunctional tissues [1]. Emerging tissue engineering approaches typically employ some combination of materials, cells and biomolecules (e.g., proteins). It has long been recognized that the cellular component of these combination products, depending on the source, could trigger severe immunological reactions similar to that seen in transplantation of allogeneic or xenogeneic tissue [2]. More recently, researchers have reported that the biomaterial component may also evoke significant immunological barriers to integration and tissue regeneration [3]. This inflammatory response against the biomaterial component of tissue-engineered constructs has been very well characterized and is collectively known as the foreign body response (FBR). The known primary cellular mediators of this inflammatory response are macrophages, along with neutrophils. Briefly, following implantation, protein adsorption on the surface of the biomaterial results in initiation of the coagulation cascade, complement system (which can polarize immune cells towards an inflammatory response) and the formation of a provisional matrix. These phenomena have been extensively investigated on different biomaterial surfaces and it is thought that they are correlated to the physico-chemical surface properties of the biomaterial, thereby linking biomaterial properties with host immune cell responses [4]. Following matrix formation, antigen presenting cells, including macrophages and dendritic cells (DCs), can be recruited to the implant site by chemokines released by the matrix as well as surrounding cells. Macrophages, in particular, persist at the implantation site, adhering to the implant surface and coalescing with neighboring macrophages to form a giant cell body, which attempts to engulf the material. Within this encapsulation, macrophages secrete a number of inflammatory mediators, including reactive oxygen species and degradative enzymes that can be detrimental to the structure and functionality of the implanted biomaterial [4]. The presence of exogenous biologics only exacerbates this immune response, with foreign cell-associated antigen release prompting chronic inflammation, typically mediated by T-cells. Dendritic cells play a critical role as enablers of this chronic adaptive response against tissue engineered constructs which typically deliver immunogenic cells, proteins and other biologics [3]. Interestingly, they may also contribute to immune response against the material component of these combination constructs. Herein, we discuss the current knowledge on DC responses to implanted biomaterials, with particular relevance to tissue engineering scaffolds.
Dendritic Cells in the Immune Response
The mammalian immune system is composed of two sets of mechanisms that collude to shield the host from would-be invaders, the innate and the adaptive immune systems. The innate immune system has evolved to recognize certain non-self-entities to which we, as a species are continually exposed (e.g., pathogen-associated molecular patterns; PAMPs), whereas adaptive immunity educates the body to never-before seen invaders. Notably, one cell type is distinctly efficient at bridging the innate immune system to adaptive immunity – the dendritic cell (DC) [5].
Dendritic cells are the ‘sentinel’ of the immune system for their role in patrolling, scavenging and recognizing non-self-components throughout the host [5]. These cells are phagocytic, and the most efficient antigen presenting cells (APCs) with the capacity to instigate either inflammatory or anti-inflammatory adaptive immunity. Following tissue damage, in situ immature DCs capture released antigen and subsequently migrate back to lymphoid organs via chemokine gradients, where they initiate clonal selection and expansion of specific, rare T cells. These expanded T cell clones have receptors specific for antigens that are processed and present on the surfaces of DCs during the migration process. Moreover, antigen-specific T cells and subsequently mobilized B cells, macrophages, natural killer (NK) cells and eosinophils home to the site of insult where a combination of broad and specific assault is unleashed to abolish an invading threat. Critically, DCs also activate suppressive immune networks for induction of tolerance towards self-antigens. The direction and magnitude of immune responses are influenced by DC activation level and phenotype — either an activated phenotype providing an inflammatory reaction, or conversely, a tolerogenic phenotype for regulatory measures [6]. The versatility of DC responses is in part owing to the diversity of receptors on the surfaces of DCs, as well as, the heterogeneity of DC subsets.
Heterogeneity of Dendritic Cells
Dendritic cells were first discovered in the laboratory of Ralph Steinman in 1973. While controversial at the time, Steinman later shared the 2011 Nobel Prize in Physiology or Medicine for his discovery of the DC and its role in adaptive immunity. Steinman et al., described these cells as being large (~10 μm) mononuclear cells with elongated, stellate processes (or dendrites) extending in multiple directions from the cell body [7]. The subsets of this cell type varies between different mammals, so for the sake of brevity, here we only discuss DC heterogeneity in humans. Currently, cells are designated as DCs based on specific cell surface markers or clusters of differentiation (CD) and high expression levels of MHC class I and class II. Moreover, DCs are leukocytes distinguished based on their lack of markers found on other cells: CD3 (T cells), CD19 (B cells), CD56 (NK cells), CD14 (monocytes), CD15 (granulocytes), and CD34 (stem cells). Accordingly, DCs have been classically termed lineage-negative (lin-) DR+ cells [8].
Dendritic cells can be grouped on four different levels: i) precursor population (i.e. lineage), ii) function, iii) final polarity of immune response and, iv) anatomical localization. According to current understanding, DCs are identified as either ‘myeloid’ or ‘plasmacytoid’. Myeloid DCs (mDCs) are characterized by the expression of CD11c, CD13, CD33 and CD11b, and its lack of expression of CD14 and CD16. Myeloid CD11c+ DCs can be further split into CD1c+, CD14+ and CD141+ fractions. On the other hand, plasmacytoid DCs (pDCs) typically do not express myeloid markers and are recognized via the surface markers CD123, CD303 and CD304 [6].
These two major subsets of DCs differentiate in an array of cells with differential functional capabilities and primary loci in mammalian hosts. In humans, there are 5 major classes that have been characterized, namely: (1) Peripheral Blood DC (PBDC), (2) Epithelial and Interstitial DC, (3) Thymic DC (TDC), (4) Lymphoid DC (LDC) and (5) Bone marrow DC (BM-DC) [9].
Peripheral blood DCs represents about 0.5 – 1.5% of the total peripheral blood mononuclear cells (PBMCs), and consists of both myeloid and plasmacytoid DCs. The mDCs in this compartment express CD13, CD33, CD45RO, and have impressive antigen uptake and T-cell stimulatory capacities. Exposure of this DC subtype to bacterial components (e.g., lipopolysachharide [LPS]) results in secretion of pro-inflammatory cytokines, particularly interleukin (IL)-12. Myeloid PBDCs have high expression of toll-like receptor2 (TLR2) and TLR4 [10], but levels of CD16, blood dendritic cell antigen-1 (BDCA-1) and BDCA-3 vary depending the subclass. Human blood also contains immature forms of pDCs. These pDCs, expressing high levels of IL-3α, BDCA-2, BDCA-4, CD4, CD62L and immunoglobulin-λ-like transcript (ILT) [11], are noted for their production of IFN-α in response to CpG, certain viruses and CD40L. Toll-like receptor7 (TLR7) and TLR9 as well as C-type lectins – CD205 and CD209 are highly conserved on this distinct DC subset [11].
Epithelial and Interstitial DCs are found in peripheral tissue and are known for their antigen-capturing and migratory capacities. This subset of DCs include Langerhans cells and can contain CD1c+ mDCs, C141+ mDc, CD14+ DCs, as well as a few pDCs. Moreover, they are excellent stimulators of naïve T cells, and also release high quantities of inflammatory cytokines (e.g., IL-12) upon maturation [6].
Thymic DCs As their name suggest, they are found in the thymus but can be comprised of pDCs, immature CD11c+ mDCs and mature CD11c+ mDCs. Thymic pDCs are similar in immunophenotype to the aforementioned peripheral blood pDCs. Mature CD11c+ mDCs make up about 7% of thymic DCs and are marked by their expression of DC-LAMP, CCR7 and IL-12 production [12].
Lymphoid tissues (e.g., spleen, lymph nodes) are also resident to significant numbers of DCs. Human lymphoid tissues can contain CD1c+ mDCs, CD141+ mDCs, CD14+ DC and pDCs in steady state. The relative contributions of these subclasses, and the overall number, of DCs in lymphoid tissue can vary widely during inflammatory conditions [13].
In vitro/Ex vivo-cultured DCs For their relevance to future discussion in this review, we also want to highlight in vitro-expanded DCs. Similar to their in vivo counterparts, these DCs are separated based on lineage with the two major sub-classes termed myeloid-derived and plasmacyotoid-derived DCs. Myeloid-derived DCs can be obtained by culturing either hematopoetic stems from bone marrow or blood monocytes in the presence of granulocyte macrophage colony-stimulating factor (GM-CSF) and IL-4 [14, 15]. On the other hand, lymphoid progenitors, including T-cell precursors from the thymus [16] and CD19+ committed B-cell precursors [17], can be used to generate DCs that are phenotypically similar to plasmacytoid DCs. The heterogeneity of lymphoid-derived DC is normally broad and dependent on the in vitro cocktail of factors used to induce DC development as well as the progenitor cell type. Overall, DCs are a heterogeneous cell population, and this diversity may be beneficial for the collective functionality of DCs to initiate adaptive immunity against plethora of invaders, whilst regulating self-immunity.
Cytokine supplementation has been employed to generate a large number of DCs in vitro. These methods have been tremendously helpful in understanding the immunobiology of dendritic cells, as well as in therapeutic applications of DCs [18]. Despite these positive outcomes from in vitro DC culture, significant differences in phenotype from in vivo DCs have been highlighted and raise caution on the interpretation of results garnered using in vitro-derived DCs [19]. For example, in vitro-generated lymphoid-lineage, thymic DC lack CD8α marker expression, that is characteristic of their ex vivo counterparts. Further concerns on in vitro-derived DCs include the purity of DC cultures, varied stages of development within cultures and DC plasticity, if a specific maturation state is desired. Another critical factor is the tissue source (e.g., blood, bone marrow, etc.) of the progenitors which could result in variable functionality depending on the cytokine cocktail [20–22]. Ostensibly, it is imperative that the protocol, starting cell and phenotype of the final cultures are well documented wherever in vitro DCs are applied.
Dendritic Cell Receptors and Antigen Capture
Dendritic cells continuously patrol peripheral tissues, sampling the microenvironment for foreign and pathogenic materials. Immature DCs entrap potential threats by one of three size-dependent, mechanisms: phagocytosis, receptor-mediated endocytosis and macropinocytosis. Moreover, DCs are equipped with a plethora of receptors to identify, seize and engulf a broad spectrum of self and non-self entities. Notably, DC endocytic and phagocytic receptors for antigen uptake include C-type lectins (e.g., DEC205, CD206), toll-like receptors (e.g., TLR4), Fcγ receptors (e.g., CD32, CD64) and integrins (e.g. αVβ5, CD11c) [23–26]. Many of these receptors also direct intracellular signaling, adhesion, motility and maturation of dendritic cells. Additionally, receptors may be differentially expressed on different DC subsets and be intricately tied to their function and anatomical location. For instance, human interstitial DCs can rapidly internalize mannosylated antigens and direct these antigens for processing and presentation via major histocompatibility complex (MHC)-II pathways. On the other hand, Langerhans cells have poor expression of mannose receptors such as DC-SIGN (CD209), and therefore have difficulty internalizing mannosylated entities [27, 28].
Dendritic Cell Mobility, Recruitment and Migration
Prior to antigen capture, DCs along with other phagocytic cells accumulate at the site of inflammation, in response to the release of chemokines in this area. Inflammatory chemokines, such as MIP-1α, MIP-3α, MIP-1β, MCP-2, MCP-4 and stromal cell-derived factor-1 (SDF-1), recruit DCs, which express cognate receptors for these potent chemokines. As such, different DC subsets have varied motile reactions to different chemokines [29, 30].
Chemokine gradients are also critical for the homing of DCs to tissue-draining lymph nodes following antigen uptake. Critical to this process is the chemokine receptor CCR7 which is upregulated after antigen uptake and activation. The primary CCR7 ligands are MIP-3β and CCL21 [31, 32]. Additionally, DCs in lymphoid tissue secrete the naïve and memory T-cell chemoattractants, CCL18 and CCL22, to help promote DC-T-cell interactions. It should be again noted that migration capacity, pattern and chemokine production vary between DC subtypes, indicative of the diverse functional roles of this cell type in generating immunity.
Chemokine binding to the cognate receptor initiates signaling within the DC that results in reorganization of the actin cytoskeleton and locomotion of the entire cell. Also integral to this motility are adhesion events, controlled by integrins, which allow DCs to transit between different tissue types. Integrin binding to extracellular matrix (ECM) proteins results in the formation of adhesion complexes (often called focal adhesions) containing both structural (e.g., vinculin, talin) and signaling proteins (e.g., focal adhesion kinase [FAK], paxillin) which are sites for mechanical signal transduction [33, 34]. For instance, Kohl et al. demonstrated that monocyte-derived DCs and CD34+ stem cell-derived DCs employ α5β1 and α6β1 integrins, for initial adhesion to fibronectin and laminin adhesive substrates, respectively [35]. Interestingly, other reports have indicated that integrins may also play a role in activation of DCs. Acharya et al., demonstrated that DC adhesion to various material surface-adsorbed ECM proteins – collagen, fibrinogen, fibronectin, laminin, and vitronectin [36]. In particular, DCs cultured on collagen and vitronectin produce more IL-12. In comparison, DCs cultured on albumin and serum-coated tissue culture-treated polystyrene produce more IL-10. Additionally, DCs cultured on different substrates differentially mediated allogeneic CD4+ T-cell proliferation and T-helper cell type responses. Furthermore, in a complementary study, DCs derived from non-obese diabetic mice, which are predisposed to type 1 autoimmune diabetes, demonstrate altered responses to these same surface-adsorbed ECM proteins [37]. Lastly, Acharya et al., also investigated C57BL/6 mouse bone marrow-derived DCs adherent to click chemistry functionalized surface-density gradients of the cell adhesive peptide, RGD. Dendritic cell αV integrin binding levels were quantified by a cross-linking/extraction method, and tightly followed RGD density, above a minimum threshold value. Activation markers, MHC-II and CD86, moderately correlated to αV integrin binding, while cytokine production of IL-10 and IL-12 were highly correlated to αV integrin binding [38].
Dendritic Cell Antigen Presentation
As DCs traverse toward lymphoid tissue, captured antigen is degraded, processed and presented on the cell surface via receptors referred to as the major histocompatibility complex (MHC). DCs primarily load captured antigen onto two unique MHC molecules which results in the interaction of DCs with different subsets of T cells. For MHC-I loading of antigenic peptide, endogenous and self-peptide is processed via the classical endogenous pathway and presented. This cytosolic pathway involve antigen ubiquitination, proteasome degradation, transport via TAPs (transporters for antigen) and finally, insertion into MHC class I molecules for presentation [39, 40]. Peptide-MHC-I complexes interact with CD8+ T cells. On the other hand, CD4+ T cells form immunological synapses via interaction with peptide-loaded MHC-II molecules, which display exogenously-acquired antigen following endosomal degradation and cathepsin S-assisted loading [41]. An alternative pathway of antigen presentation exists, where subsets of DCs efficiently present exogenously-derived antigen fragments onto MHC-I molecules. This phenomenon, known as cross-presentation, may be important for enlisting all the facets of the adaptive immune system and, mounting effective tolerogenicity [42, 43]. Another non-classical antigen display pathway involves the family of CD1 molecules. Interestingly, both endogenous and exogenous antigen can be presented on CD1 molecules. Moreover, antigen presentation on these molecules is thought to be important for microbial immunity, as well as, autoimmunity [44, 45].
Dendritic Cell Maturation States and Interactions with Adaptive Immune Cells
Antigen process and presentation can be accompanied by DC maturation, where DCs differentiate into a functional phenotype capable of activating resting naïve adaptive immune cells. This morphogenesis is normally marked by a number of coordinated events including, (a) upregulation of peptide-MHC-I and -II complexes, (b) increased expression of co-stimulatory molecules (e.g., CD40, CD80, and CD86), adhesion molecules (CD54, CD58), and chemokine receptors (CCR1, CCR7), (c) secretion of inflammatory cytokines (e.g. IL-12, IFN-γ), (d) shift in lysosomal compartment type with increased expression of DC-lysosome-associated membrane protein (DC-LAMP) and (e) significant decrease in antigen uptake capacity. Morphologically, DCs lose their adhesive structures, undergo cytoskeleton reorganization noted by an increase in filamentous actin and the appearance of veils [6]. In addition to inflammatory cytokines secreted by T cells (e.g., IFN-γ), DC receptor ligation to a range of molecules can induce progression of DCs from an immature to mature state. Pathogen-derived ‘danger signal’ molecules include lipopolysaccharide [LPS] and bacterial DNA (CpG), and endogenous damage-associated molecules include heat-shock proteins, uric acid and ATP [25].
The phenotypical changes observed during DC maturation are important for T- and B-cell priming in the lymphoid organs including the spleen, lymph nodes and gut-associated lymphoid tissue (GALT). DC–T-cell interactions are typically marked by the formation of an immunological synapse. The molecules involved in this bridge between the two cell types include, i) adhesive mediators (e.g., β1 and β2 integrins), ii) peptide-MHC complexes and the T-cell receptor (TCR), which together are termed ‘signal one’, iii) the ‘second signal’ which constitutes co-stimulatory molecules on the DC (e.g., CD80, CD86, CD40) ligated their respective receptors on the surfaces of T-cells (e.g. CD28, CD40L), and iv) ‘signal 3’ or the ‘polarizing’ signal which can be either soluble or membrane-bound cytokines (e.g., IL-12) [5]. Polarization refers to the differentiation of naïve T-cells to a distinct T-cell type (e.g., Th1, Th2, regulatory T cell [TReg]). Dendritic cells not only control the magnitude, but also the direction of T-cell responses based on the following factors: a) the surface density of peptide-MHC complexes, b) the affinity of the TCR for the corresponding peptide-MHC complex, c) DC maturation state and, d) the type of maturation stimulus [46, 47].
Dendritic Cell Responses to Implanted Materials
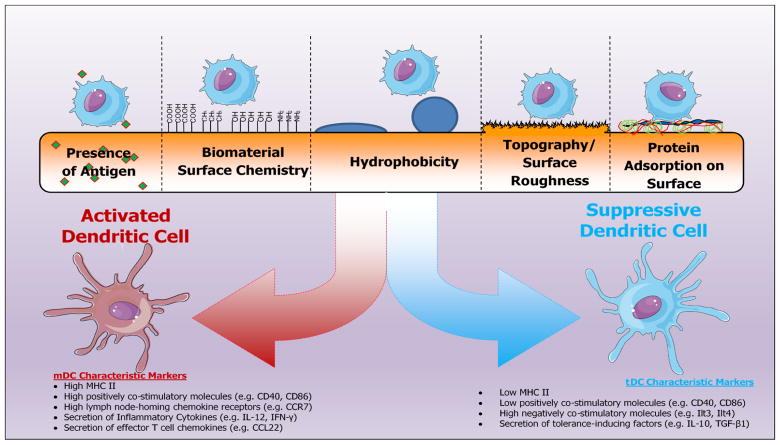
Biomaterials in implanted devices or tissue engineering scaffolds serve a number of purposes, including i) architectural and mechanical support, ii) cellular transport and inhabitation, and iii) localized delivery of instructive and inductive molecules [48]. The range of materials investigated as tissue engineering scaffolds is quite extensive and has been excellently reviewed [49, 50]. Briefly, these include natural materials (e.g., fibrin, dextran, decellularized matrix) which can be formed into hydrogels with similar mechanical properties as native tissues, have intrinsic biological properties that help to support cellular recruitment, differentiation and integration, and are biodegradable allowing for cellular penetration and scaffold remodeling. On the other hand, biomaterials in scaffolds can often be synthetic (e.g., poly[lactide-co-glycolide] [PLGA], polypropylene, polyethylene terephthalate). Synthetic materials typically offer greater mechanical strength than naturally-derived scaffolds, as well as, precise control over the material tunability [49].
For synthetic biomaterials, irrespective of the type, bulk material implantation into soft tissue characteristically induces the foreign body response, denoted by the chronic persistence of macrophages around the implant site. It is now clear that macrophages are the central mediators of this immunological response. However, as noted by Vasilijic et al., DCs also infiltrate the implant area and potentially play role in the development and resolution of the foreign body response. Dendritic cell potency to stimulate adaptive immune responses has been well characterized, but an understanding of their role in the foreign body reaction to biomaterials is incomplete. To the best of our knowledge, Vasilijic et al., is perhaps the only investigation of the role of the DC in acute and chronic inflammation, and wound healing around an implanted biomaterial. It is estimated that as much as 25% of the monocytes recruited to the site of tissue damage differentiate into DCs [51]. Additionally, it is believed that local interstitial DCs and Langerhans cells also infiltrate the implanted material. Taking a closer look at accumulation of DCs in implanted polyvinyl sponges, Vasilijic and coworkers found that DC number gradually increased and peaked at day 10 after implantation. Phenotypically, these DCs expressed normal markers including MHC II, CD11c, CD11b and CD68. One particular observation from this study was that the number of DCs staining for His 24 or His 48 (putative pDC markers) was higher at the day 14 time point than at day 6. Another finding was that DCs isolated at a later stage of inflammation (day 14) were more hyporesponsive to allogeneic T cells than DCs isolated from sponges at 6 d after implantation. This lower allostimulatory capacity of late stage DCs correlated with downregulation of stimulatory molecules (CD80 and CD86), a change in the material-associated DC type from mDC to pDC and increased level of anti-inflammatory cytokines (IL-10 and TGF-β1) in culture supernatants and sponge exudate. The dynamics demonstrated in this study suggest DCs could be involved in suppressing chronic inflammation and/or autoimmune reactions to damaged tissue and may prove to be important to the resolution of this process [51]. It should be noted that this study, as with many of the studies that are described in this review, was performed in a rodent model. As such there are limitations in predicting the DCs response to biomaterials in humans. On the other hand, a study by Takao et al. demonstrated that, at the gene expression level, mouse models of inflammation closely mimicked the same conditions in humans and provided strong evidence for conducting immune-related simulations in rodents [52].
The study outlined above was a rare foray into elucidating the role, if any, that DCs play in the FBR. Instead, most studies focus on dissecting the adjuvant effects of biomaterials in promoting immune responses to biomaterial-biological combination products used in tissue engineering. An ‘adjuvant’ is defined as a substance that can amplify immune responses to an accompanying antigen but alone, does not elicit adaptive immune responses [53, 54]. The adjuvant effect may exacerbate inflammatory responses toward combination products with tissue engineering scaffolds and promote the degradation of the biomaterial. The primary mechanism through which biomaterial adjuvants exert this effect is thought to be the maturation of APCs, particularly DCs [55]. Ostensibly, biomaterials that are non-adjuvanting and obstructive to DC maturation could be beneficial to scaffold integrity, and might be explored for advantageous tissue regeneration. As such, researchers have begun to investigate the effects of materials purposed for tissue engineering on DC phenotype and humoral responses, in the presence or absence of antigen. For instance, Matzelle et al., demonstrated that the presence of a biomaterial resulted in an enhancement of the humoral immune response to co-delivered antigen using a simplified model system [56]. In this study, a model antigen, ovalbumin (OVA) was co-localized with different polymeric biomaterial carriers and administered to C57BL/6 mice to assess whether the presence of the biomaterial enhanced immune responses to the antigen. Specifically, total anti-OVA IgG serum levels were used as the measure to compare the biomaterial (PLGA, polystyrene [PS]) vehicles (microparticles and scaffolds) against the well-known standard, complete Freund’s adjuvant. The primary outcome of this study was that that OVA-adsorbed or co-delivered with carrier biomaterials including non-biodegradable, polystyrene microparticles (MPs) and 75:25 PLGA MPs, supported moderate humoral immune responses for an 18 week period. Further, the presence of IgG1 titers indicate the humoral response is TH2-dependent [56]. A follow-up study further corroborated these initial conclusions by assessing the in vivo proliferation levels of fluorescently-labeled, OVA-specific CD4+ T-cells from transgenic OT-II mice in the presence or OVA delivered by biomaterial carriers, which were comparable to that of OVA combined with complete Freund’s adjuvant. The common link between the biomaterial carriers, co-delivered antigen and the observed adaptive immunity is the maturation of APCs, particularly DC maturation [56].
One implication from the Matzelle studies was that 75:25 PLGA (copolymer whose composition is 75% lactic acid and 25% glycolic acid) may be immunogenic and present danger signals to provoke DC maturation. Yoshida et al., further examined this hypothesis and looked at the phenotypic responses of human monocyte-derived DCs to treatment with 75:25 PLGA MPs or films in comparison to lipopolysaccharide (LPS)-treated DC (for a positive control of matured DCs) and untreated or immature DCs (iDCs; negative control) [57]. Summarily, at early time points (6 h, 24 h), stimulatory molecules (CD40, CD80, CD83, CD86 and MHC-II) were markedly upregulated on DCs, in comparison to the iDC negative control, but lower than that of LPS-matured DCs. Moreover, 75:25 PLGA MP-treated DCs display a ‘stellate’ morphology with extended cellular processes, similar to that of mature DCs, and MP-treated DCs enhanced proliferation of allogeneic T-cells in a mixed lymphocyte reaction (MLR). This report advocates that 75:25 PLGA can stimulate maturation of human monocyte-derived DCs, and maturation is dependent on the form of the biomaterial [57]. Yoshida et al., also reported that DC responses to 75:25 PLGA films and MPs were independent of species and DC subtype, as comparable reactions to those described above with human monocyte-derived DCS were also observed using bone marrow-derived DCs from C57BL/6 mice. Further analysis of DC cytokine secretion and transmigration capacity revealed that the observed biomaterial effect may be contact-mediated [57, 58]. Contact-mediated maturation of DCs was also alluded to in a manuscript by Sharp et al., on the activation of the NALP3 inflammasome in DCs due to particulate engulfment. They reported that, again at early time points (30 min to 24 h) both PLGA and PS particulates significantly enhanced the secretion of IL-1β. The secretion of IL-1β requires the assembly and activation of the inflammasome, which is a cytoplasmic complex composed of NOD-like receptors (NLRs) and caspase 1 (protease). They also noted that either TLR agonists or endogenous tissue factors are needed in conjunction with the particulate adjuvant to promote IL-1β release [59]. Building on these reports, Park et al., comprehensively investigated the different phenotypic changes in human monocyte-derived DCs following exposure to a library of commonly employed biomaterials in tissue engineering including films of alginate, agarose, chitosan, hyaluronic acid, and 75:25 PLGA. Immuno-phenotyping was performed on biomaterial-treated DCs by comparing morphology, stimulatory molecule expression, mixed lymphocyte reaction, NF-κB activity and pro-inflammatory cytokine secretion. Overall, differential effects of DC maturation were induced by different biomaterial films after 24 h exposure time period. More specifically, PLGA or chitosan films induced DC maturation, with higher levels of DC allo-stimulatory capacity, pro-inflammatory cytokine release, and expression of CD80, CD86, CD83, HLA-DQ and CD44 compared to iDC levels. Alginate films evoked an increase in pro-inflammatory cytokine release as well as a decrease in CD44 expression. Whereas, hyaluronic acid films elicited suppressive effects on DC phenotype, with reduced expression of CD40, CD80, CD86 and HLA-DR observed, compared to iDCs [60].
Although the reports described above strongly suggested that PLGA, particularly the 75:25 composition, has intrinsic adjuvant activity, others have reported DCs as being unresponsive or even suppressive responses to PLGA treatment [61, 62]. These tangible differences may be due to the formulations tested (75:25 vs. 50:50), as well as, the time of the immune-phenotyping analysis. Notably, reports that supported the adjuvant activity of PLGA did not assess DC responses beyond 24 h, whereas others tested at later time points and found that DC maturation markers were either similar or decreased levels in comparison to iDCs [61, 62]. Furthermore, there are reports that the degradation products of PLGA into it constituent monomers, particularly lactic acid, can down regulate stimulatory molecules on DCs following exposure, which suggests that the time of DC exposure to this polymer as it degrades over time may also be a factor [63, 64].
Effect of Biomaterial Chemical Properties – Surface Chemistry/Hydrophobicity
Blood contact with a material after implantation results in protein deposition and formation of a provisional matrix, which can influence subsequent leukocyte adhesion and monocyte recruitment [4]. Evidently, the chemical and physical properties of the surface of the biomaterial influence the foreign body reaction. Along this line, studies by Park et al., suggest that different surface chemistries could evoke differential functional responses in DCs [60]. To this extent, Shankar et al., investigated how well-defined surface chemistries modulated DC phenotype. DCs were cultured on self-assembled monolayers (SAM) surfaces of alkanethiols terminated with defined chemical groups, of either -CH3, -OH, -COOH, and -NH2. Response output for this study included DC morphology, expression of positively stimulatory molecules and allo-stimulatory capacity. By measure of expression of stimulatory markers, treatment with -OH, -COOH, or -NH2 terminated SAMs showed moderate maturation, while DCs treated with -CH3 SAMs were least activated. Somewhat contradictorily, -CH3 SAMS elicited the highest levels of inflammatory cytokines (IL-6 and tumor necrosis factor-alpha; TNF-α). Additionally, increased levels of apoptotic markers were observed for DCs and T cells in contact with -CH3 SAMs. Various reports have shown that phagocytosis of apoptotic DCs has strong immunosuppressive effects on DCs, therefore the increased number of apoptotic DCs on -CH3 SAMs may account for lower DC maturation [65]. Finally, higher expression of cytotoxic T lymphocyte associated antigen receptor-4 (CTLA-4) on T cells was shown, suggesting a mechanism of T cell inhibition on -CH3 SAMs [66, 67].
Similarly, the adjuvant activity of standard biomaterials as a factor of surface hydrophobicity, linked to the surface chemistry, was investigated the Ma group. They examined the effects of poly(D,L-lactic acid) (PLA)-, PLGA-, and poly(monomethoxypolyethylene glycol-co-D,L-lactide) (mPEG-PLA, PELA)-based microparticles, which were similar in morphology and size but differed in surface hydrophobicity, on the maturation status of DCs in vitro and in vivo immune responses. Based on contact angle measurement, the surface hydrophobicity of the studies materials increased as follows: PELA < PLGA < PLA. In vitro studies using bone marrow-derived DCs from BALB/c mice showed that increased surface hydrophobicity supports microparticle engulfment and antigen internalization, and boosts expression of stimulatory molecules (CD86, MHC-II). Using atomic force microscopy, they determined that the interaction forces between the cell and adjacent microparticle were strongest for the PLA microparticles, which were the most hydrophobic. Based on these results they argued that MP surface hydrophobicity affects the physical interaction between APCs and MPs, which further influences MP uptake, DC maturation status and antigen display, and downstream immune responses [68]. However, it is important to distinguish that this study involved particulate materials, which may engage different mechanism of response, i.e., cell attachment to a bulk material versus particulate phagocytosis. Along this line, a study by the Babensee group considered the effects of hydrophilicity on DC responses using unphagocytosable material constructs [69]. This work compared the differential biomaterial effects, including hydrophilicity, of PLGA and agarose on the maturation state of human monocyte-derived DCs. Summarily, they found that DCs seeded on PLGA films expressed higher levels of costimulatory and MHC molecules than those exposed to the more hydrophilic, agarose substrates.
Dendritic Cell Responses to Biomaterial Physical properties
Biomaterial surface microarchitecture also influences the orientation of proteins on the surface of biomaterials, and therefore leukocyte adhesion. Acharya et al., demonstrated that DC adhesive cues influence the activation state of DCs [37, 38]. Moreover, the Babensee group elucidated the role of integrins in the recognition and response of DCs to biomaterials. Succinctly, antibody-blocking techniques were used to demonstrate β2 integrin signaling in DC-mediated CD86 upregulation [74]. Another report indicates that DCs cultured on adhesive protein substrates and simultaneously exposed to cyclic mechanical strain develop a semi-mature immuno-phenotype [75]. These studies begin to illustrate that the biophysical and biomechanical microenvironment is influential on DC responses to implanted scaffolds. A more conclusive study that elucidates the effects of material dimensionality on DC immunophenotype was performed by van de Vries et al. The aim of this study was primarily to examine how biomaterial architecture governs DC podosome formation. But, included in this study was an investigation on the influence of dimensionality on the maturation status of DCs. With regards to the latter studies, 3-D micropatterns with widths of 2, 5, 10 and 20 μm were created in the well-studied, biomaterials - polystyrene, teflon, poly(methy methacylate) and polyethylene naphtalate. The differentiation status of iDCs seeded on these 3-D micropatterns was compared to that of DCs cultured on 2-D substrates of the same chemistries. This group found that surface expression of MHC-II molecules in DCs on 3-D micropatterns were significantly higher compared to flat substrates, indicating that substrate dimensionality may play a role in regulating the activation of DCs [73].
Surface roughness may also be an influential factor in DC responses to implanted materials. Kou and coworkers examined this relationship using clinical dental titanium (Ti) surfaces with defined chemistries and surface roughness [72]. The surface roughness (Ra) of tested substrates were (in the increasing order): tissue culture polystyrene (control; TCPS) < pre-treated titanium (PT; smooth finish) (0.6 μm) < grit-blasted and acid-etched titanium (SLA) = (hydrophilic SLA; modSLA) (3.97 μm). Comparison of the surface energies of these various surfaces was also performed, via water-air contact angle measurement, and were ~ 96°, 138°, and 0° for PT, SLA and modSLA respectively. Differences in expression of stimulatory surfaces molecules on DCs were detected following incubation on these experimental surfaces. More specifically, DCs cultured on PT and SLA Ti surfaces showed increased expression levels of CD86 in comparison to TCPS-seeded iDCs. The morphology of DCs cultured on PT and SLA Ti surfaces closely reflected that of LPS-matured DCs, suggesting that these surfaces may promote DC activation. Interestingly, PT surfaces stimulated increased secretion of the anti-inflammatory IL-1ra from DCs in comparison to all other investigated substrates (SLA, modSLA). Taken altogether, these results suggest that surface energy (related to surface chemistry) is a more definitive factor in the polarization of DC maturation by biomaterial surfaces. Moreover, principal component analysis (PCA) analysis based on surface chemical composition indicated that surface chemistry maybe a stronger factor and more predictive of DC activation state. Summarily, PCA analysis showed that increasing surface carbon or nitrogen induces mature characteristics, whereas increasing surface oxygen or titanium promotes an immature DC phenotype [72, 76].
Implant-based modulation of DC activity
As inflammatory activity around an implanted material may be detrimental to the half-life of the devices, scientists are now looking into ways to design implants that modulate DC responses. For instance, Hume at al., functionalized poly(ethylene glycol) (PEG) hydrogels with immobilized anti-inflammatory cytokines – transforming growth factor beta 1 (TGF-β1) and IL-10, to control the maturation status of interacting DCs [77]. Briefly, inflammatory cytokine secretion, as well as, stimulatory marker expression (MHC-II, CD80, CD86) were significantly decreased in primary DCs seeded on ECM-coated, functionalized hydrogels, in comparison to the non-functionalized control substrate. Moreover, DCs cultured on this immunomodulatory PEG hydrogel resisted maturation upon LPS challenge [77]. This strategy represents a newer trajectory in biomaterial design, where immunomodulatory factors are incorporated into the biomaterial to limit maturation of implant-localized DCs and thereby dampen adaptive immune responses.
An emerging, biomaterial-based strategy for manipulation of the immune system is controlled release of immunomodulatory agents from particulates [78]. Along this line, Lewis and coworkers have developed biomaterial-based systems for targeting and tolerogenic conditioning of DCs [70, 79, 80]. One approach developed by this scientific team, consists of two types of PLGA MPs which differ in size. One type is large, unphagocytosable MPs and purposed for the extracellular delivery of DC recruitment and immuno-suppressive biological factors (GM-CSF and TGF-β1). The other MP type is small, phagocytosable MPs and encapsulates either encapsulated antigen or vitamin D3 (tolerogenic activity) for intracellular delivery to DCs. Moreover, this combinatorial system induced a robust, immunosuppressive phenotype in bone marrow-derived DCs and prevented the development of type 1 diabetes in NOD mice [70]. Mechanistically, results indicated that protection was conferred by an increase in the number of FoxP3+ regulatory T cells, which can be induced by tolerogenic DCs [81]. Evidently, infusion of biomaterial implants with controlled-release, DC-instructive factors could be another novel method to mitigate inflammatory responses around the implant, particularly if antigen is present.
Immunomodulatory strategies should be guided by studies on how implanted materials shape the local immune microenvironment. In this regard, recent work by Sadtler et al., indicating that developing a pro-regenerative biomaterial scaffold microenvironment requires TH2 cells may be instructive. Specifically, the Elisseeff group revealed that ECM-derived scaffolds drive the recruitment and generation of DCs and macrophages with immunoregulatory profiles (decreased stimulatory marker expression [CD86]) during tissue remodeling. Moreover, the immunoregulatory profile is induced by IL-4 derived from mTORC2-dependent CD4+ TH2 T cells in the vicinity of the implant [82]. This regulatory immune microenvironment ultimately promotes the faster healing of muscle around an initial injury. These basic biology studies may be crucial to future biomaterials design.
Conclusion
In tissue engineering, biomaterials are exploited to create a local environment that potentiates tissue growth; however, the injury incurred at the site of implantation and accompanying host inflammatory response to the implanted material can negatively impact the success of this therapeutic aim. Dendritic cells have recently been implicated in influencing many of aspects of these responses, and can be guided by the choice of material. This is an active and fruitful area of investigation. Some research goals in this area include gaining a fuller understanding of fundamental DC-biomaterial interactions, as well as, the development of strategies to actively engage either inflammatory or suppressive mechanisms, as required for a specific application.
Figure 1.
Implant materials factors that influence the fate of local dendritic cell responses and shape the implant immune microenvironment
Table 1.
Impact of material properties on dendritic cell phenotype.
| Biomaterial Property | Resulting DC Phenotype | DC Responses from published studies | Reference |
|---|---|---|---|
|
| |||
| Presence of Antigen | Variable; dependent on local immunomodulatory microenvironment at the time of antigen interception | (1) A novel PLGA-based, microparticle system providing concurrent delivery of multiple encapsulated immuno- suppressive factors and antigen drove tolerance- promoting DCs to protect from the onset of insulitis in NOD mice. | [70] |
| (2) Model antigen (OVA) delivered in either polymeric scaffolds or microparticles resulted in time-dependent generation of OVA-specific IgG, suggesting activation of DCs and downstream TH2 engagement. | [56] | ||
|
| |||
| Surface Chemistry | Variable/Inconclusive | (1) Murine BMDCs cultured with OVA antigen coated multi-walled carbon nanotubes of varying surface charges (zeta potentials ranging from −39 mV to +5. 8 mV) and length showed an activation state similar to that of iDCs. However, DCs incubated with more negatively charged MWNTs stimulated greater proliferation of OVA- specific T cells | [71] |
| (2) Human monocyte- derived DCs were cultured on self-assembled monolayers (SAM) surfaces of alkanethiols terminated with defined chemical groups, of either -CH3, -OH, -COOH, and - NH2. By measure of expression of stimulatory markers, treatment with - OH, -COOH, or -NH2 terminated SAMs showed moderate maturation, while DCs treated with - CH3 SAMs were least activated. | [55] | ||
|
| |||
| Hydrophobicity | Dendritic Cell Maturation | In vitro studies using murine bone marrow- derived DCs showed that increased surface hydrophobicity supports microparticle engulfment and antigen internalization, and boosts expression of stimulatory molecules (CD86, MHC-II) | [68] |
| Topography/Surface Roughness | Dendritic Cell Maturation | (1) Human peripheral blood-derived DCs cultured on relatively high roughness resemble LPS- activated DCs in morphology, and high expression of CD86; | [72] |
| (2) Mature murine BMDCs resulted following culture on 3-D micropatterns with widths of 2, 5, 10 and 20 μm on well-studied, biomaterials. Surface expression of MHC-II molecules in DCs on 3-D micropatterns were significantly higher compared to flat substrates. | [73] | ||
|
| |||
| Protein Adsorption | Variable – dependent on type and configuration of protein deposited | (1) This study demonstrated that murine BMDC DC maturation status (based on morphology and differential production of pro- and anti- inflammatory cytokines [IL-12p40 and IL-10, respectively]) is adhesive substrate-dependent. For instance, DCs grown on collagen and vitronectin substrates generate higher levels of IL-12p40. Conversely, DCs cultured on albumin surfaces produce the higher levels of IL-10 indicating a tolerogenic phenotype. | [36] |
| (2) This study revealed the role of integrins in the recognition and response of DCs to biomaterials. Succinctly, antibody- blocking techniques were used to demonstrate that β2 integrin signaling mediated increased expression of CD86 on human peripheral blood- derived DCs, when cultured on PLGA films. | [74] | ||
Acknowledgments
Support is gratefully acknowledged by grants from the National Institute of Diabetes and Digestive and Kidney Diseases, R01 DK091658, and R01 DK098589 (to B.G.K.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Khademhosseini A, Langer R. A decade of progress in tissue engineering. Nature Protocols. 2016;11(10):1775–1781. doi: 10.1038/nprot.2016.123. [DOI] [PubMed] [Google Scholar]
- 2.Hotaling NA, Tang L, Irvine DJ, Babensee JE. Biomaterial Strategies for Immunomodulation. In: Yarmush ML, editor. Annual Review of Biomedical Engineering. Vol. 172015. pp. 317–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Babensee JE, Anderson JM, McIntire LV, Mikos AG. Host response to tissue engineered devices. Advanced Drug Delivery Reviews. 1998;33(1–2):111–139. doi: 10.1016/s0169-409x(98)00023-4. [DOI] [PubMed] [Google Scholar]
- 4.Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Seminars in Immunology. 2008;20(2):86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 6.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YT, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annual Review of Immunology. 2000;18:767. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 7.Steinman RM, Witmer MD, Nussenzweig MC, Chen LL, Schlesinger S, Cohn ZA. DENDRITIC CELLS OF THE MOUSE - IDENTIFICATION AND CHARACTERIZATION. Journal of Investigative Dermatology. 1980;75(1):14–16. doi: 10.1111/1523-1747.ep12521052. [DOI] [PubMed] [Google Scholar]
- 8.Dopheide JF, Obst V, Doppler C, Radmacher M-C, Scheer M, Radsak MP, Gori T, Warnholtz A, Fottner C, Daiber A, Muenzel T, Espinola-Klein C. Phenotypic characterisation of pro-inflammatory monocytes and dendritic cells in peripheral arterial disease. Thrombosis and Haemostasis. 2012;108(6):1198–1207. doi: 10.1160/TH12-05-0327. [DOI] [PubMed] [Google Scholar]
- 9.Summers KL, Hock BD, McKenzie JL, Hart DNJ. Phenotypic characterization of five dendritic cell subsets in human tonsils. American Journal of Pathology. 2001;159(1):285–295. doi: 10.1016/S0002-9440(10)61694-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kassianos AJ, Jongbloed SL, Hart DNJ, Radford KJ. Isolation of Human Blood DC Subtypes. In: Naik SH, editor. Dendritic Cell Protocols. 2. 2010. pp. 45–54. [DOI] [PubMed] [Google Scholar]
- 11.Cao W. Molecular Characterization of Human Plasmacytoid Dendritic Cells. Journal of Clinical Immunology. 2009;29(3):257–264. doi: 10.1007/s10875-009-9284-x. [DOI] [PubMed] [Google Scholar]
- 12.Schmitt N, Cumont M-C, Nugeyre M-T, Hurtrel B, Barre-Sinoussi F, Scott-Algara D, Israel N. Ex vivo characterization of human thymic dendritic cell subsets. Immunobiology. 2007;212(3):167–177. doi: 10.1016/j.imbio.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 13.Mrsic G, Godic V, Janjatovic AK, Spiranec K, Spoljaric D, Grskovic B, Crnjac J, Mihelic D, Popovic M. Immunophenotyping of dendritic cells of the residing porcine gut-associated lymphoid tissues. Veterinarski Arhiv. 2014;84(6):637–648. [Google Scholar]
- 14.Jacobs B, Wuttke M, Papewalis C, Selssler J, Schott M. Dendritic cell subtypes and in vitro generation of dendritic cells. Hormone and Metabolic Research. 2008;40(2):99–107. doi: 10.1055/s-2007-1022561. [DOI] [PubMed] [Google Scholar]
- 15.Naik SH. Generation of Large Numbers of Pro-DCs and Pre-DCs In Vitro. In: Naik SH, editor. Dendritic Cell Protocols. 2. 2010. pp. 177–186. [DOI] [PubMed] [Google Scholar]
- 16.Ardavin C, Wu L, Li CL, Shortman K. THYMIC DENDRITIC CELLS AND T-CELLS DEVELOP SIMULTANEOUSLY IN THE THYMUS FROM A COMMON PRECURSOR POPULATION. Nature. 1993;362(6422):761–763. doi: 10.1038/362761a0. [DOI] [PubMed] [Google Scholar]
- 17.Bjorck P, Kincade PW. Cutting edge: CD19(+) pro-B cells can give rise to dendritic cells in vitro. Journal of Immunology. 1998;161(11):5795–5799. [PubMed] [Google Scholar]
- 18.O’Neill HC, Wilson HL. Limitations with in vitro production of dendritic cells using cytokines. Journal of Leukocyte Biology. 2004;75(4):600–603. doi: 10.1189/jlb.0903446. [DOI] [PubMed] [Google Scholar]
- 19.Lutz MB. IL-3 in dendritic cell development and function: a comparison with GM-CSF and IL-4. Immunobiology. 2004;209(1–2):79–87. doi: 10.1016/j.imbio.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 20.Triozzi PL, Aldrich W. Phenotypic and functional differences between human dendritic cells derived in vitro from hematopoietic progenitors and from monocytes macrophages. Journal of Leukocyte Biology. 1997;61(5):600–608. doi: 10.1002/jlb.61.5.600. [DOI] [PubMed] [Google Scholar]
- 21.Herbst B, Kohler G, Mackensen A, Veelken H, Mertelsmann R, Lindemann A. CD34(+) peripheral blood progenitor cell and monocyte derived dendritic cells: a comparative analysis. British Journal of Haematology. 1997;99(3):490–499. doi: 10.1046/j.1365-2141.1997.4283238.x. [DOI] [PubMed] [Google Scholar]
- 22.Meierhoff G, Krause SW, Andreesen R. Comparative analysis of dendritic cells derived from blood monocytes or CD34(+) hematopoietic progenitor cells. Immunobiology. 1998;198(5):501–513. doi: 10.1016/S0171-2985(98)80074-0. [DOI] [PubMed] [Google Scholar]
- 23.Bardoel BW, van Strijp JAG. Molecular battle between host and bacterium: recognition in innate immunity. Journal of Molecular Recognition. 2011;24(6):1077–1086. doi: 10.1002/jmr.1156. [DOI] [PubMed] [Google Scholar]
- 24.Zhu Y, Ng PML, Wang L, Ho B, Ding JL. Diversity in lectins enables immune recognition and differentiation of wide spectrum of pathogens. International Immunology. 2006;18(12):1671–1680. doi: 10.1093/intimm/dxl101. [DOI] [PubMed] [Google Scholar]
- 25.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124(4):783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 26.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annual Review of Immunology. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 27.Mommaas AM, Mulder AA, Jordens R, Out C, Tan M, Cresswell P, Kluin PM, Koning F. Human epidermal Langerhans cells lack functional mannose receptors and a fully developed endosomal/lysosomal compartment for loading of HLA class II molecules. European Journal of Immunology. 1999;29(2):571–580. doi: 10.1002/(SICI)1521-4141(199902)29:02<571::AID-IMMU571>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 28.Sousa CRE, Stahl PD, Austyn JM. PHAGOCYTOSIS OF ANTIGENS BY LANGERHANS CELLS IN-VITRO. Journal of Experimental Medicine. 1993;178(2):509–519. doi: 10.1084/jem.178.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dieu MC, Vanbervliet B, Vicari A, Bridon JM, Oldham E, Ait-Yahia S, Briere F, Zlotnik A, Lebecque S, Caux C. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. Journal of Experimental Medicine. 1998;188(2):373–386. doi: 10.1084/jem.188.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annual Review of Immunology. 2000;18:217–243. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- 31.Saeki H, Moore AM, Brown MJ, Hwang ST. Cutting edge: Secondary lymphoid-tissue chemokine (SLC) and CC chemokine receptor 7 (CCR7) participate in the emigration pathway of mature dendritic cells from the skin to regional lymph nodes. Journal of Immunology. 1999;162(5):2472–2475. [PubMed] [Google Scholar]
- 32.Vulcano M, Albanesi C, Stoppacciaro A, Bagnati R, D’Amico G, Struyf S, Transidico P, Bonecchi R, Del Prete A, Allavena P, Ruco LP, Chiabrando C, Girolomoni G, Mantovani A, Sozzani S. Dendritic cells as a major source of macrophage-derived chemokine/CCL22 in vitro and in vivo. European Journal of Immunology. 2001;31(3):812–822. doi: 10.1002/1521-4141(200103)31:3<812::aid-immu812>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 33.Romer LH, Birukov KG, Garcia JGN. Focal adhesions - Paradigm for a signaling nexus. Circulation Research. 2006;98(5):606–616. doi: 10.1161/01.RES.0000207408.31270.db. [DOI] [PubMed] [Google Scholar]
- 34.Keselowsky BG, Garcia AJ. Quantitative methods for analysis of integrin binding and focal adhesion formation on biomaterial surfaces. Biomaterials. 2005;26(4):413–418. doi: 10.1016/j.biomaterials.2004.02.050. [DOI] [PubMed] [Google Scholar]
- 35.Kohl K, Schnautz S, Pesch M, Klein E, Aumailley M, Bieber T, Koch S. Subpopulations of human dendritic cells display a distinct phenotype and bind differentially to proteins of the extracellular matrix. European Journal of Cell Biology. 2007;86(11–12):719–730. doi: 10.1016/j.ejcb.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 36.Acharya AP, Dolgova NV, Clare-Salzler MJ, Keselowsky BG. Adhesive substrate-modulation of adaptive immune responses. Biomaterials. 2008;29(36):4736–4750. doi: 10.1016/j.biomaterials.2008.08.040. [DOI] [PubMed] [Google Scholar]
- 37.Acharya AP, Dolgova NV, Xia CQ, Clare-Salzler MJ, Keselowsky BG. Adhesive substrates modulate the activation and stimulatory capacity of non-obese diabetic mouse-derived dendritic cells. Acta Biomaterialia. 2011;7(1):180–192. doi: 10.1016/j.actbio.2010.08.026. [DOI] [PubMed] [Google Scholar]
- 38.Acharya AP, Dolgova NV, Moore NM, Xia C-Q, Clare-Salzler MJ, Becker ML, Gallant ND, Keselowsky BG. The modulation of dendritic cell integrin binding and activation by RGD-peptide density gradient substrates. Biomaterials. 2010;31(29):7444–7454. doi: 10.1016/j.biomaterials.2010.06.025. [DOI] [PubMed] [Google Scholar]
- 39.Inaba K, Inaba M. Antigen recognition and presentation by dendritic cells. International Journal of Hematology. 2005;81(3):181–187. doi: 10.1532/IJH97.04200. [DOI] [PubMed] [Google Scholar]
- 40.Steinman RM, Inaba K, Turley S, Pierre P, Mellman I. Antigen capture, processing and presentation by dendritic cells: Recent cell biological studies. Human Immunology. 1999;60(7):562–567. doi: 10.1016/s0198-8859(99)00030-0. [DOI] [PubMed] [Google Scholar]
- 41.Lewis JS, Roy K, Keselowsky BG. Materials that harness and modulate the immune system. Mrs Bulletin. 2014;39(1):25–34. doi: 10.1557/mrs.2013.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shen H, Ackerman AL, Cody V, Giodini A, Hinson ER, Cresswell P, Edelson RL, Saltzman WM, Hanlon DJ. Enhanced and prolonged cross-presentation following endosomal escape of exogenous antigens encapsulated in biodegradable nanoparticles. Immunology. 2006;117(1):78–88. doi: 10.1111/j.1365-2567.2005.02268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guermonprez P, Amigorena S. Pathways for antigen cross presentation. Springer Seminars in Immunopathology. 2005;26(3):257–271. doi: 10.1007/s00281-004-0176-0. [DOI] [PubMed] [Google Scholar]
- 44.Gumperz JE. The ins and outs of CD1 molecules: Bringing lipids under immunological surveillance. Traffic. 2006;7(1):2–13. doi: 10.1111/j.1600-0854.2005.00364.x. [DOI] [PubMed] [Google Scholar]
- 45.Mori L, De Libero G. Presentation of lipid antigens to T cells. Immunology Letters. 2008;117(1):1–8. doi: 10.1016/j.imlet.2007.11.027. [DOI] [PubMed] [Google Scholar]
- 46.de Jong EC, Smits HH, Kapsenberg ML. Dendritic cell-mediated T cell polarization. Springer Seminars in Immunopathology. 2005;26(3):289–307. doi: 10.1007/s00281-004-0167-1. [DOI] [PubMed] [Google Scholar]
- 47.Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nature Reviews Immunology. 2003;3(12):984–993. doi: 10.1038/nri1246. [DOI] [PubMed] [Google Scholar]
- 48.Boehler RM, Graham JG, Shea LD. Tissue engineering tools for modulation of the immune response. Biotechniques. 2011;51(4):239. doi: 10.2144/000113754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ozdil D, Aydin HM. Polymers for medical and tissue engineering applications. Journal of Chemical Technology and Biotechnology. 2014;89(12):1793–1810. [Google Scholar]
- 50.Tu M. Bioactive materials and tissue engineering. Bioactive Materials in Medicine: Design and Applications. 2011:70–93. [Google Scholar]
- 51.Vasilijic S, Savic D, Vasilev S, Vucevic D, Gasic S, Majstorovic I, Jankovic S, Colic M. Dendritic cells acquire tolerogenic properties at the site of sterile granulomatous inflammation. Cellular Immunology. 2005;233(2):148–157. doi: 10.1016/j.cellimm.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 52.Takao K, Miyakawa T. Genomic responses in mouse models greatly mimic human inflammatory diseases. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(4):1167–1172. doi: 10.1073/pnas.1401965111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Edelman R. VACCINE ADJUVANTS. Reviews of Infectious Diseases. 1980;2(3):370–383. doi: 10.1093/clinids/2.3.370. [DOI] [PubMed] [Google Scholar]
- 54.Edelman R. The development and use of vaccine Adjuvants. Molecular Biotechnology. 2002;21(2):129–148. doi: 10.1385/MB:21:2:129. [DOI] [PubMed] [Google Scholar]
- 55.Babensee JE, Paranjpe A. Differential levels of dendritic cell maturation on different biomaterials used in combination products. Journal of Biomedical Materials Research Part A. 2005;74A(4):503–510. doi: 10.1002/jbm.a.30429. [DOI] [PubMed] [Google Scholar]
- 56.Matzelle MM, Babensee JE. Humoral immune responses to model antigen co-delivered with biomaterials used in tissue engineering. Biomaterials. 2004;25(2):295–304. doi: 10.1016/s0142-9612(03)00531-3. [DOI] [PubMed] [Google Scholar]
- 57.Yoshida M, Mata J, Babensee JE. Effect of poly(lactic-co-glycolic acid) contact on maturation of murine bone marrow-derived dendritic cells. Journal of Biomedical Materials Research Part A. 2007;80A(1):7–12. doi: 10.1002/jbm.a.30832. [DOI] [PubMed] [Google Scholar]
- 58.Bennewitz NL, Babensee JE. The effect of the physical form of poly(lactic-co-glycolic acid) carriers on the humoral immune response to co-delivered antigen. Biomaterials. 2005;26(16):2991–2999. doi: 10.1016/j.biomaterials.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 59.Sharp FA, Ruane D, Claass B, Creagh E, Harris J, Malyala P, Singh M, O’Hagan DT, Petrilli V, Tschopp J, O’Neill LAJ, Lavelle EC. Uptake of particulate vaccine adjuvants by dendritic cells activates the NALP3 inflammasome. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(3):870–875. doi: 10.1073/pnas.0804897106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Park J, Babensee JE. Differential functional effects of biomaterials on dendritic cell maturation. Acta Biomaterialia. 2012;8(10):3606–3617. doi: 10.1016/j.actbio.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lewis JS, Roche C, Zhang Y, Brusko TM, Wasserfall CH, Atkinson M, Clare-Salzler MJ, Keselowsky BG. Combinatorial delivery of immunosuppressive factors to dendritic cells using dual-sized microspheres. Journal of Materials Chemistry B. 2014;2(17):2562–2574. doi: 10.1039/C3TB21460E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Waeckerle-Men Y, Scandella E, Allmen EU, Ludewig B, Gillessen S, Merkle HP, Gander B, Groettrup M. Phenotype and functional analysis of human monocyte-derived dendritic cells loaded with biodegradable poly(lactide-co-glycolide) microspheres for immunotherapy. Journal of Immunological Methods. 2004;287(1–2):109–124. doi: 10.1016/j.jim.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 63.Fischer K, Hoffmann P, Voelkl S, Meidenbauer N, Ammer J, Edinger M, Gottfried E, Schwarz S, Rothe G, Hoves S, Renner K, Timischl B, Mackensen A, Kunz-Schughart L, Andreesen R, Krause SW, Kreutz M. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood. 2007;109(9):3812–3819. doi: 10.1182/blood-2006-07-035972. [DOI] [PubMed] [Google Scholar]
- 64.Peter K, Rehli M, Singer K, Renner-Sattler K, Kreutz M. Lactic acid delays the inflammatory response of human monocytes. Biochemical and Biophysical Research Communications. 2015;457(3):412–418. doi: 10.1016/j.bbrc.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 65.Hart SP, Dransfield I, Rossi AG. Phagocytosis of apoptotic cells. Methods. 2008;44(3):280–285. doi: 10.1016/j.ymeth.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 66.Shankar SP, Chen, Keselowsky BG, Garcia AJ, Babensee JE. Profiles of carbohydrate ligands associated with adsorbed proteins on self-assembled monolayers of defined chemistries. Journal of Biomedical Materials Research Part A. 2010;92A(4):1329–1342. doi: 10.1002/jbm.a.32457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shankar SP, Petrie TA, Garcia AJ, Babensee JE. Dendritic cell responses to self-assembled monolayers of defined chemistries. Journal of Biomedical Materials Research Part A. 2010;92A(4):1487–1499. doi: 10.1002/jbm.a.32487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu Y, Yin Y, Wang L, Zhang W, Chen X, Yang X, Xu J, Ma G. Surface hydrophobicity of microparticles modulates adjuvanticity. Journal of Materials Chemistry B. 2013;1(32):3888–3896. doi: 10.1039/c3tb20383b. [DOI] [PubMed] [Google Scholar]
- 69.Yoshida M, Babensee JE. Differential effects of agarose and poly(lactic-co-glycolic acid) on dendritic cell maturation. Journal of Biomedical Materials Research Part A. 2006;79A(2):393–408. doi: 10.1002/jbm.a.30798. [DOI] [PubMed] [Google Scholar]
- 70.Lewis JS, Dolgova NV, Zhang Y, Xia CQ, Wasserfall CH, Atkinson MA, Clare-Salzler MJ, Keselowsky BG. A combination dual-sized microparticle system modulates dendritic cells and prevents type 1 diabetes in prediabetic NOD mice. Clinical Immunology. 2015;160(1):90–102. doi: 10.1016/j.clim.2015.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hassan H, Smyth L, Rubio N, Ratnasothy K, Wang JTW, Bansal SS, Summers HD, Diebold SS, Lombardi G, Al-Jamal KT. Carbon nanotubes’ surface chemistry determines their potency as vaccine nanocarriers in vitro and in vivo. Journal of Controlled Release. 2016;225:205–216. doi: 10.1016/j.jconrel.2016.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kou PM, Schwartz Z, Boyan BD, Babensee JE. Dendritic cell responses to surface properties of clinical titanium surfaces. Acta Biomaterialia. 2011;7(3):1354–1363. doi: 10.1016/j.actbio.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.van den Dries K, van Helden SFG, te Riet J, Diez-Ahedo R, Manzo C, Oud MM, van Leeuwen FN, Brock R, Garcia-Parajo MF, Cambi A, Figdor CG. Geometry sensing by dendritic cells dictates spatial organization and PGE(2)-induced dissolution of podosomes. Cellular and Molecular Life Sciences. 2012;69(11):1889–1901. doi: 10.1007/s00018-011-0908-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rogers TH, Babensee JE. The role of integrins in the recognition and response of dendritic cells to biomaterials. Biomaterials. 2011;32(5):1270–1279. doi: 10.1016/j.biomaterials.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lewis JS, Dolgova NV, Chancellor TJ, Acharya AP, Karpiak JV, Lele TP, Keselowsky BG. The effect of cyclic mechanical strain on activation of dendritic cells cultured on adhesive substrates. Biomaterials. 2013;34(36):9063–9070. doi: 10.1016/j.biomaterials.2013.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kou PM, Pallassana N, Bowden R, Cunningham B, Joy A, Kohn J, Babensee JE. Predicting biomaterial property-dendritic cell phenotype relationships from the multivariate analysis of responses to polymethacrylates. Biomaterials. 2012;33(6):1699–1713. doi: 10.1016/j.biomaterials.2011.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hume PS, He J, Haskins K, Anseth KS. Strategies to reduce dendritic cell activation through functional biomaterial design. Biomaterials. 2012;33(14):3615–3625. doi: 10.1016/j.biomaterials.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lewis JS, Allen RP. An introduction to biomaterial-based strategies for curbing autoimmunity. Experimental Biology and Medicine. 2016;241(10):1107–1115. doi: 10.1177/1535370216650294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yoon YM, Lewis JS, Carstens MR, Campbell-Thompson M, Wasserfall CH, Atkinson MA, Keselowsky BG. A combination hydrogel microparticle-based vaccine prevents type 1 diabetes in non-obese diabetic mice. Scientific Reports. 2015;5 doi: 10.1038/srep13155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lewis JS, Zaveri TD, Crooks CP, II, Keselowsky BG. Microparticle surface modifications targeting dendritic cells for non-activating applications. Biomaterials. 2012;33(29):7221–7232. doi: 10.1016/j.biomaterials.2012.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Luo XR, Tarbell KV, Yang H, Pothoven K, Bailey SL, Ding RC, Steinman RM, Suthanthiran M. Dendritic cells with TGF-beta 1 differentiate naive CD4+CD25(-) T cells into islet-protective Foxp3(+) regulatory T cells. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(8):2821–2826. doi: 10.1073/pnas.0611646104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sadtler K, Estrellas K, Allen BW, Wolf MT, Fan H, Tam AJ, Patel CH, Luber BS, Wang H, Wagner KR, Powell JD, Housseau F, Pardoll DM, Elisseeff JH. Developing a pro-regenerative biomaterial scaffold microenvironment requires T helper 2 cells. Science. 2016;352(6283):366–370. doi: 10.1126/science.aad9272. [DOI] [PMC free article] [PubMed] [Google Scholar]