Abstract
Objective
For most common infections requiring hospitalization, antibiotic treatment is completed after hospital discharge. Post-discharge therapy is often unnecessarily broad-spectrum and prolonged. We developed an intervention to improve antibiotic selection and shorten treatment durations.
Design
Single center, quasi-experimental retrospective cohort study.
Methods
Patients prescribed oral antibiotics at hospital discharge before (July 2012 – June 2013) and after (October 2014 – February 2015) an intervention consisting of: 1) institutional guidance for oral step-down antibiotic selection and duration of therapy, and 2) pharmacy audit of discharge prescriptions with real-time prescribing recommendations to providers. The primary outcomes were total prescribed duration of therapy and use of antibiotics with broad gram-negative activity (fluoroquinolones or amoxicillin-clavulanate).
Results
300 cases from the pre-intervention period and 200 from the intervention period were included. Compared with the pre-intervention period, use of antibiotics with broad gramnegative activity decreased during the intervention (51% vs 40%, p = 0.02), particularly fluoroquinolones (38% vs 25%, p = 0.002). The difference in total duration of therapy did not reach statistical significance (10 days [interquartile range (IQR) 7–13] vs 9 [IQR 6–13], p = 0.13); however, the duration prescribed at discharge declined from 6 days (IQR 4–10) to 5 (IQR 3–7) (p = 0.003). During the intervention, there was a non-significant increase in the overall appropriateness of discharge prescriptions (52% vs 66%, p = 0.15).
Conclusions
A multifaceted intervention to optimize antibiotic prescribing at hospital discharge was associated with less frequent use of antibiotics with broad gram-negative activity and shorter post-hospital treatment durations.
Keywords: antibiotic stewardship, hospital discharge, transition of care, oral antibiotics
Introduction
Improving antibiotic use to slow the emergence of antibiotic resistance and decrease Clostridium difficile infections is a national priority [1]. The Infectious Diseases Society of America, Society for Hospital Epidemiology of America, and Centers for Disease Control and Prevention (CDC) provide recommendations for implementing antibiotic stewardship interventions in hospitals [2, 3]. Most of these interventions are geared toward improving antibiotic use during the hospitalization itself; however, a number of studies have demonstrated that for common infections, approximately two-thirds of the total treatment course is completed after the patient is discharged [4–8]. In addition, antibiotics dispensed at the time of hospital discharge are frequently suboptimal due to an overly broad spectrum of activity or excessive duration [8]. This suggests that antibiotic stewardship interventions focused solely on inpatient antibiotic use ignore a substantial component of antibiotic misuse. New interventions are necessary to address this important opportunity for antibiotic stewardship. We developed an intervention specifically focused on improving hospital discharge antibiotic prescribing. We hypothesized that such an intervention would decrease unnecessary antibiotic exposure by reducing use of antibiotics with broad gram-negative activity and by shortening treatment durations.
Methods
Study setting and population
Denver Health is an urban, academic, integrated healthcare system with a 477-bed teaching hospital. Most inpatients are admitted to medical or surgical teaching services; a minority are managed by a non-teaching hospitalist service. For patients admitted to teaching services, resident housestaff are primarily responsible for management orders, including antibiotic prescriptions at hospital discharge.
Intervention
Based on data from our institution demonstrating substantial opportunity to improve discharge prescribing [8], we implemented a multifaceted intervention with two main components: 1) the development and dissemination of an institutional guideline for oral-step down antibiotic selection and duration of therapy for common infections, and 2) prospective audit of discharge prescriptions with real-time recommendations to providers to promote adherence to the institutional guideline.
-
Guideline for discharge therapy: We developed a table with institution-specific recommendations for oral step-down therapy and treatment duration for the following conditions: community-acquired pneumonia (CAP); urinary tract infection (UTI) including uncomplicated cystitis, complicated cystitis, uncomplicated pyelonephritis, and catheter-associated infection; skin infections including cellulitis, cutaneous abscess, or wound infection; healthcare-associated and hospital-acquired pneumonia; chronic obstructive pulmonary disease (COPD) exacerbation; Clostridium difficile infection; and Helicobacter pylori infection (eFigure 1). The recommendations were obtained from a combination of pre-existing institutional clinical practice guidelines and national guidelines [9–15]. The final version of the guideline was approved by the Antimicrobial Subcommittee, the Pharmacy and Therapeutics committee, the hospital’s Clinical Guidelines Committee, and the Chief Clinical Officer.
In September 2014, data supporting the rationale for the intervention and the discharge prescribing guidance was presented to housestaff, attending physicians, and pharmacists. Laminated pocket-sized copies of the guideline were provided to all clinicians and pharmacists, and an electronic copy was posted on the hospital intranet. Throughout the intervention period, the chief medical resident and Infectious Diseases (ID) pharmacist distributed the pocket cards to all incoming housestaff and faculty during orientations. An institutional smartphone application containing all of Denver Health’s antibiotic treatment recommendations, including for the target conditions listed above, was made available to all providers in November 2014.
Prospective audit with real-time feedback to prescribes: Staff pharmacists were trained by the ID pharmacist to cross-reference prescriptions for oral antibiotics submitted to the discharge pharmacy with the above institutional guideline. Beginning in September 2014, when the prescription did not appear to be consistent with the guideline, the ID pharmacist was contacted who then performed a review of pertinent clinical and laboratory data. When appropriate, the prescribing physician was contacted to recommend modifications to the discharge prescription to promote adherence to the institutional guidance. The frequency and result of these audit and feedback episodes were recorded using a standardized reporting form. Prior to the start of the intervention, the audit and feedback process was pilot-tested for a 3-week period to determine feasibility and the impact on the discharge process.
Study design
To determine the effects of the intervention, we performed a quasi-experimental, retrospective cohort study including adult inpatients prescribed an oral antibiotic at the time of hospital discharge before and after the intervention. The pre-intervention cohort was a previously published cohort discharged between1 July 2012 and 30 June 2013 [8]. The intervention cohort included patients discharged between 1 October 2014 and 28 February 2015.
Data Collection
Identical case-finding methods and data collection procedures were used for both time periods [8]. Patients 18 years or older who filled a prescription for oral antibiotic(s) at a Denver Health pharmacy within 7 days of hospital discharge were initially identified through the institution’s data warehouse. To derive the study cohorts, electronic health records were manually reviewed by a single investigator (N.Y.) to determine eligibility, and for included cases, to abstract data. Cases were excluded that involved antibiotic prescriptions unrelated to the hospital stay, intravenous antibiotics at discharge, long-term prophylactic or suppressive antibiotics, absence of documentation of the indication for antibiotics, transfer to or from an outside institution, leaving against medical advice, failure to fill prescribed antibiotics within 48 hours of hospital discharge, infection with non-bacterial pathogens, and multiple hospitalizations for the same ongoing infection. For patients with multiple hospitalizations over the course of the study, only the initial hospitalization resulting in an oral antibiotic prescription was included. A standardized data abstraction form was used to record demographic and clinical characteristics, microbiologic data, inpatient antibiotic therapy, discharge antibiotics (indication, agent, dose, and treatment duration), and clinical encounters within the Denver Health system during the 30 days following hospital discharge. The study was approved by the Colorado Multiple Institutional Review Board.
Appropriateness review
To assess the appropriateness of discharge prescriptions, two ID physicians and an ID pharmacist (K.S., H.Y., T.J.) reviewed the data abstraction form and discharge summary for a random sample of 100 cases from the pre-intervention and intervention periods (50 per period). To blind these investigators to the time period of each case, the lead investigator (N.Y.) redacted service dates and other potentially identifying information from the documents reviewed. After discussion of each case among the three investigators, a prescription was classified as appropriate when at least two judged the indication, antibiotic selection, dose, and prescribed duration to be consistent with institutional guidance. With respect to duration of therapy, prescribed durations two or more days longer or shorter than recommended in the institutional guidance were classified as inappropriate. For infections without institutional guidance, the assessment of appropriateness was based on national guideline recommendations, or if none existed, expert opinion.
Outcomes
All endpoints were specified prior to the study. The primary outcomes were changes in the total prescribed duration of therapy, defined as the number of calendar days of inpatient antibiotics plus the duration prescribed at discharge, and the proportion of patients prescribed antibiotics with a broad spectrum of gram-negative activity, defined as fluoroquinolones or amoxicillin/clavulanate. Secondary end-points included change in the appropriateness of discharge prescriptions and rates of dermatologic, gastrointestinal or nephrotoxic adverse drug events, re-hospitalization, C. difficile infection, and treatment failure within 30 days of discharge. Treatment failure was defined as a change in antibiotic regimen or extension of planned treatment duration due to inadequate clinical response. Given their relative frequency, subgroup analyses for cases involving CAP, UTI, and skin infection were performed to evaluate for changes in antibiotic selection and duration of therapy.
Statistical Analysis
Based on pre-intervention period data [8] and the results of prior antibiotic stewardship interventions at our institution [4, 16], we hypothesized the current intervention would reduce prsecription of antibiotics with broad gram-negative activity from 51% of cases to 38% (25% relative reduction) and the median treatment duration from 10 days to 8 (20% reduction). We calculated that by including the 300 existing cases from the pre-intervention period, a minimum of 200 cases in the intervention period would be necessary to provide at least 80% power at an alpha of 0.05 to detect the hypothesized differences. To determine the number of cases that would need to be reviewed to assess for a change in the appropriateness of discharge prescriptions, we hypothesized the proportion of discharge prescriptions classified as appropriate would increase from 47% to 66% (40% relative increase) and determined that 100 cases (50 in each group) were needed to provide 80% power at an alpha of 0.05 to demonstrate the hypothesized difference. The Pearson χ2, Fisher exact, or Wilcoxon rank-sum test were used to perform comparisons between the periods. P values <0.05 were considered statistically significant. All analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC).
Results
For the 12-month pre-intervention period, the initial electronic search yielded 1825 cases with an oral antibiotic prescribed at discharge, of which 376 were manually reviewed and 300 were included for analysis (Figure 1). For the 5-month intervention period, 777 cases were identified by the initial search, of which 288 cases were manually reviewed and 200 were included for analysis. The frequency of reasons for exclusion were similar between the two periods (Figure 1). Nearly all antibiotics were filled within 24 hours after hospital discharge (295 [98%] pre-intervention, 197 [99%] intervention).
Figure 1.
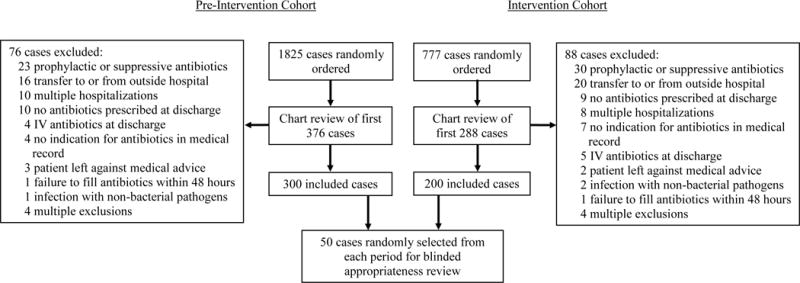
Study diagram. In the pre-intervention study period, 1825 adult cases were identified electronically. A total of 376 charts were manually reviewed until 300 included cases was reached. In the intervention period, 777 adult cases were identified electronically. A total of 288 charts were manually reviewed until 200 included cases was reached. 50 included cases from each period were randomly selected for blinded appropriateness review.
There were no significant differences between the two groups in baseline demographic and clinical characteristics (Table 1). The most common indications for the discharge antibiotic prescriptions were UTI, CAP, and skin infections (Table 1). The frequency of indications was similar among the two groups; however, there were significantly more COPD exacerbations during the intervention period (8% vs 18%, p = 0.001).
Table 1.
Demographic and clinical characteristics of patients discharged on oral antibiotics
| Pre-intervention period n=300 a |
Intervention period n=200 a |
|
|---|---|---|
| Age, mean (standard deviation) | 52.8 (15.8) | 52.6 (17.8) |
| Male | 171 (57) | 110 (55) |
| Comorbidities | ||
| Diabetes mellitus | 87 (29) | 66 (33) |
| Antibiotic use within 6 months | 67 (22) | 57 (29) |
| COPD | 48 (16) | 36 (18) |
| Hospitalization within 90 days | 46 (15) | 33 (17) |
| HIV infection | 17 (6) | 3 (2) |
| Pregnancy | 13 (4) | 6 (3) |
| Cirrhosis | 13 (4) | 11 (6) |
| History of multi-drug resistant organism b | 12 (4) | 5 (3) |
| End-stage renal disease | 9 (3) | 5 (3) |
| Failed outpatient antibiotics c | 17 (6) | 7 (4) |
| Hospital length of stay, median days (IQR) | 4 (3–5) | 4 (3–6) |
| ICU admission | 53 (18) | 35 (18) |
| Infectious Diseases consultation | 42 (14) | 29 (15) |
| Indications for discharge antibiotics | ||
| Urinary tract infection | 72 (24) | 42 (21) |
| Community-acquired pneumonia | 52 (17) | 35 (18) |
| Skin and soft tissue infection | 62 (21) | 34 (17) |
| Gastrointestinal infection | 46 (15) | 22 (11) |
| Osteoarticular infection | 22 (7) | 16 (8) |
| COPD exacerbation d | 23 (8) | 36 (18) |
| Head and neck infection | 15 (5) | 18 (9) |
| Bacteremia | 15 (5) | 9 (5) |
| Other | 40 (13) | 26 (13) |
| ≥2 indications for therapy | 37 (12) | 25 (13) |
No significant differences between pre-intervention and intervention cohort (p>0.05 for all subgroups) with the exception of COPD exacerbation
Defined as prior infection with methicillin-resistant Staphylococcus aureus, vancomycin resistant Enterococci, or extended spectrum beta-lactamase producing Enterobacteriaceae.
Defined as lack of clinical response to outpatient therapy requiring hospital admission.
COPD exacerbations significantly more frequent in intervention cohort (p=0.001) IQR, interquartile range; ICU, intensive care unit; COPD, chronic obstructive pulmonary disease; HIV, human immunodeficiency virus
Microbiology testing and results were similar among the two groups: 74% of patients in the pre-intervention period and 76% in the intervention period had at least one specimen collected for culture, and 31% and 29%, respectively, had a positive culture. The most common organisms identified were Escherichia coli (10% vs 11%), streptococcal species (8% vs 11%), and Staphylococcus aureus (7% vs 4%).
In total, the proportion of patients discharged with antibiotics with broad gram-negative activity significantly declined during the intervention (51% vs 40%, p = 0.02); this was primarily due to a reduction in prescription of fluoroquinolones (38% vs 25%, p = 0.002) (Table 2). Prescription of azithromycin increased during the intervention period (12% vs 20%, p = 0.03). There was a non-significant reduction in the total prescribed duration of therapy (median 10 days [interquartile range (IQR) 7–13] to 9 days [IQR 6–13], p = 0.13); however, the duration prescribed at hospital discharge significantly declined (median 6 days [IQR 4–10] to 5 days [IQR 3–7], p = 0.003).
Table 2.
Antibiotic selection and prescribed duration of therapy
| Pre-intervention period n=300 |
Intervention period n=200 |
p-value | |
|---|---|---|---|
| Broad gram-negative antibiotic | 152 (51) | 80 (40) | 0.02 |
| Fluoroquinolone | 115 (38) | 50 (25) | .002 |
| Amoxicillin/clavulanate | 37 (12) | 30 (15) | 0.39 |
| Azithromycin | 37 (12) | 39 (20) | 0.03 |
| Metronidazole | 29 (10) | 11 (6) | 0.09 |
| Clindamycin | 26 (9) | 23 (12) | 0.30 |
| Trimethoprim/sulfamethoxazole | 20 (7) | 11 (6) | 0.60 |
| Doxycycline | 19 (6) | 16 (8) | 0.47 |
| Penicillin or amoxicillin | 15 (5) | 6 (3) | 0.27 |
| Clarithromycin | 11 (4) | 2 (1) | 0.07 |
| Nitrofurantoin | 8 (3) | 5 (3) | 0.91 |
| 2nd- and 3rd-generation cephalosporins a | 4 (1) | 4 (2) | 0.72 |
| Other | 10 (3) | 2 (1) | 0.14 |
| Received ≥2 antibiotics | 50 (17) | 21 (11) | 0.05 |
| Total prescribed duration of therapy, median (IQR) | 10 (7–13) | 9 (6–13) | 0.13 |
| Duration of inpatient therapy, median (IQR) | 3 (3–5) | 4 (3–5) | 0.01 |
| Duration of prescribed at discharge, median (IQR) | 6 (4–10) | 5 (3–7) | 0.003 |
includes cefdinir, cefixime, cefpodoxime, and cefuroxime
In subgroup analyses of 179 cases of CAP, UTI, or skin infection from the pre-intervention period and 110 cases from the intervention period, the findings were similar but more pronounced (Table 3). Discharge prescriptions for antibiotics with broad gram-negative activity (50% vs 35%, p = 0.01) and for multiple antibiotics (9% vs. 2%, p = 0.02) declined. There was a non-significant reduction in the total prescribed duration of therapy (median 9 days [IQR 7–13] to 8 days [IQR 6–11], p = 0.09), but the duration prescribed at discharged decreased from a median of 6 days (IQR 4–10) to 4 days (IQR 3–6) (p = 0.002). The changes in discharge prescribing were particularly pronounced in cases of CAP and skin infections; in cases of UTI, little change occurred (Table 3).
Table 3.
Subgroup analyses of cases of community-acquired pneumonia, urinary tract infection, or skin infection
| Pre-Intervention period | Intervention period | p-value | |
|---|---|---|---|
| CAP, UTI, or skin infection | n=179 | n=110 | |
| Antibiotic prescribed at discharge | |||
| Broad gram-negative antibiotic | 89 (50) | 38 (35) | 0.01 |
| Fluoroquinolone | 71 (40) | 27 (25) | 0.008 |
| Amoxicillin/clavulanate | 18 (10) | 11 (10) | 0.99 |
| Azithromycin | 16 (9) | 20 (18) | 0.02 |
| Received ≥2 antibiotics | 16 (9) | 2 (2) | 0.02 |
| Total prescribed duration of therapy, median (IQR) | 9 (7–13) | 8 (6–11) | 0.09 |
| Duration of inpatient therapy, median (IQR) | 4 (3–5) | 4 (3–5) | 0.44 |
| Duration prescribed at discharge, median (IQR) | 6 (4–10) | 4 (3–6) | 0.002 |
| Community-acquired pneumonia | n=52 | n=35 | |
| Antibiotic prescribed at discharge | |||
| Broad gram-negative antibiotic | 31 (60) | 11 (31) | 0.01 |
| Fluoroquinolone | 26 (50) | 9 (26) | 0.02 |
| Amoxicillin/clavulanate | 5 (10) | 2 (6) | 0.70 |
| Azithromycin | 16 (31) | 20 (57) | 0.01 |
| Received ≥2 antibiotics | 3 (6) | 0 | 0.27 |
| Total prescribed duration of therapy, median (IQR) | 8 (6–9) | 6 (5–7) | 0.003 |
| Duration of inpatient therapy, median (IQR) | 4 (3–5) | 4 (3–5) | 0.64 |
| Duration prescribed at discharge, median (IQR) | 4 (3–5) | 3 (2–4) | 0.02 |
| Urinary tract infection | n=72 | n=42 | |
| Antibiotic prescribed at discharge | |||
| Broad gram-negative antibiotic | 43 (60) | 23 (55) | 0.60 |
| Fluoroquinolone | 41 (57) | 18 (43) | 0.15 |
| Amoxicillin/clavulanate | 2 (3) | 5 (12) | 0.10 |
| Received ≥2 antibiotics | 6 (8) | 1 (2) | 0.26 |
| Total prescribed duration of therapy, median (IQR) | 10 (8–13) | 9 (7–12) | 0.58 |
| Duration of inpatient therapy, median (IQR) | 4 (3–5) | 4 (3–5) | 0.79 |
| Duration prescribed at discharge, median (IQR) | 6 (4–9) | 5 (3–7) | 0.35 |
| Skin infection | n=62 | n=34 | |
| Antibiotic prescribed at discharge | |||
| Broad gram-negative antibiotic | 21 (34) | 5 (15) | 0.04 |
| Fluoroquinolone | 10 (16) | 1 (3) | 0.09 |
| Amoxicillin/clavulanate | 11 (18) | 4 (12) | 0.44 |
| Received ≥2 antibiotics | 9 (15) | 1 (3) | 0.09 |
| Total prescribed duration of therapy, median (IQR) | 12 (8–15) | 9 (7–12) | 0.02 |
| Duration of inpatient therapy, median (IQR) | 4 (3–5) | 4 (3–5) | 0.96 |
| Duration prescribed at discharge, median (IQR) | 7 (6–12) | 5 (4–7) | <0.001 |
Appropriateness of discharge prescriptions
In the random sample of 100 cases for which the Infectious Diseases specialists performed a blinded assessment of the appropriateness of the discharge prescriptions, there was a nonsignificant increase in the overall appropriateness of the prescriptions during the intervention period (52% vs 66%, p = 0.15) (Table 4). Antibiotic selection was more frequently appropriate during the intervention (72% vs 90%, p = 0.02).
Table 4.
Appropriateness of discharge prescriptions as classified during blinded Infectious Diseases panel review
| Pre-intervention period n=50 |
Intervention period n=50 |
p-value | |
|---|---|---|---|
| Discharge antibiotic appropriate | 26 (52) | 33 (66) | 0.15 |
| Condition warrants antibiotics at discharge | 46 (92) | 48 (96) | 0.68 |
| Antibiotic selection appropriate | 36 (72) | 45 (90) | 0.02 |
| Correct dose of antibiotic(s) | 42 (84) | 46 (92) | 0.22 |
| Duration of therapy appropriate | 30 (65) | 34 (71) | 0.56 |
Pharmacy audit and feedback of discharge prescriptions
During the 5-month intervention period, a total of 918 patients were prescribed oral antibiotics at discharge. Of those, the prescriptions from 363 (40%) cases were reviewed by a pharmacist. In 99 (27%) of the 363 cases reviewed, a provider was contacted by telephone to discuss the prescription. A recommendation to change the discharge prescription was made in 85 cases, of which 66 (67%) were accepted. The most common reasons prompting a recommendation to change the prescription were opportunities to optimize antibiotic choice (n=29, 44%) or dose (n=28, 42%), and to reduce treatment duration (n=20, 30%).
Clinical outcomes
A follow-up encounter within 30 days of hospital discharge was documented in 209 (70%) cases in the pre-intervention period and 144 (72%) in the intervention period. There were no significant differences in the incidence of treatment failure (15% vs 15%), re-admission for the same condition (8% vs 8%), C. difficile infection (2% vs 0%), or adverse drug events (7% vs 3%).
Discussion
To our knowledge, this is the first description of an antibiotic stewardship intervention designed specifically to optimize oral antibiotic prescriptions at the time of hospital discharge. A number of previous studies have demonstrated that for infections commonly managed in the hospital, 60–70% of the total antibiotic course is completed after discharge [4–8]. Thus, ensuring appropriate antibiotic selection at discharge represents an important opportunity to reduce use of antibiotics with overly broad-spectrum activity. Furthermore, since the total duration of therapy is determined by the duration of the discharge prescription, the discharge prescription is the critical point at which to intervene to shorten treatment durations.
Clinical care guidelines often include recommendations for inpatient therapy, the transition to oral therapy, and treatment durations. Our intervention was unique in that it did not attempt to alter inpatient prescribing but rather focused on the discharge prescription. The tools that we developed provided clinicians with point-of-care access to the most appropriate discharge antibiotic and shortest effective duration of therapy for common infections. To our knowledge, this is the first description of a stewardship intervention incorporating prospective audit with provider feedback of discharge prescriptions. The audit and feedback process was intended to correct prescriptions that did not adhere to institutional guidance. Our data show that less than half of all prescriptions were reviewed by pharmacy; this may have been in part due to evening and weekend discharges when prescriptions were not reviewed. It is also noteworthy that of the prescriptions reviewed, a recommendation to change therapy was required in less than a quarter of cases. Ideally, as more providers become familiar with the institutional discharge prescribing guidance, even fewer interventions by pharmacy would be required over time; however, the longer-term sustainability and effectiveness of this intervention is not known and requires additional study.
It is worth noting that the intervention had a more robust impact on prescribing for the subgroup of cases involving CAP, UTI, or skin infections. Interestingly, the greatest changes in prescribing were observed in CAP and skin infections, conditions for which successful syndrome-specific antibiotic stewardship interventions had been implemented in our hospital prior to the pre-intervention period of this study [4, 16]. These prior interventions had led to substantial reductions in use of antibiotics with broad gram-negative activity and shorter treatment durations. It is therefore noteworthy that despite these prior improvements, the current intervention led to additional reductions in broad-spectrum antibiotic use and treatment durations. In contrast, the current intervention was not associated with significant changes in prescribing for UTIs, which had not previously been a focus of intervention in our hospital. Although the reasons for the variable success of this intervention on particular infections cannot be determined from this study, our findings suggest that the intervention may be most effective as a complementary approach to syndrome-specific stewardship interventions. A multilevel intervention that actively targets both inpatient and discharge prescribing may therefore have the greatest impact on antibiotic use.
Our study has several limitations. First, it was performed at a single academic safety-net hospital which limits its generalizability. Second, by including all types of infections in the analysis, the effect of the intervention was likely diluted since the intervention was most likely to affect prescribing for a target group of infections. We designed the study in this manner because it provides the most complete assessment of the overall impact of the intervention on discharge antibiotic use, to avoid the potential selection bias associated with limiting the analysis to certain infections, and because the intervention had the potential to impact prescribing for all types of infections. Third, there was a relatively long gap between the pre-intervention period and the start of the intervention. This is because the intervention itself was designed and implemented after collection of and in response to the pre-intervention period data [8]. We are not aware of any factors that would have impacted hospital discharge prescribing practices during this time. Fourth, although interrupted time series analysis would have been the preferred statistical approach in this setting, this was not felt to be feasible given the lack of sufficient time points over the short intervention period. Fifth, seasonal variation in infections (and therefore prescribing patterns) was a potential confounding factor since the intervention spanned the winter months. This is likely why COPD exacerbations were more common during the intervention period; this may have biased our results toward the null since COPD exacerbations are routinely treated with 5-day courses of azithromycin and were unlikely to have been impacted by the intervention. Sixth, the study was not designed to determine the effects of the individual components of the intervention but rather the effects of the bundled intervention. The impact of audit and feedback of discharge prescriptions, the component of the intervention requiring ongoing personnel time, is therefore not known. Finally, the study may have been underpowered to detect differences in total duration of therapy and appropriateness of discharge prescriptions.
In summary, a novel intervention combining institutional guidance for oral-step down antibiotic selection and duration of therapy with pharmacy-led audit of discharge prescriptions with feedback to providers led to a decrease in use of antibiotics with broad gram-negative activity and shorter post-hospital treatment courses. The intervention was particularly effective in cases of CAP and skin infections. Antibiotic prescribing at the time of hospital discharge may be an underappreciated opportunity to improve overall antibiotic use for common infections, and intervening at this point in time may be an important complement to currently recommended antibiotic stewardship interventions.
Supplementary Material
eFigure 1: Summary of recommendations for initial empiric therapy, oral step-down therapy, and duration of therapy for common infections utilized in the intervention.
Acknowledgments
We are grateful to Allison Nitsch, Elsbeth Jensen-Otsu, and the hospitalist group at Denver Health for their contributions to this project.
Financial support: This work was funded in part by the Department of Patient Safety and Quality, Denver Health. Dr. Jenkins was supported by the National Institute of Allergy and Infectious Diseases, National Institute of Health (TCJ: K23 AI099082).
Footnotes
Conflicts of interest: No relevant conflicts of interest to disclose.
References
- 1.National action plan for combating antibiotic-resistant bacteria. 2015 Mar; Available at: https://www.whitehouse.gov/sites/default/files/docs/national_action_plan_for_combating_antibotic-resistant_bacteria.pdf. Accessed 6 April 2015.
- 2.Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62(10):e51–77. doi: 10.1093/cid/ciw118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.CDC. Core Elements of Hospital Antibiotic Stewardship Programs. Atlanta, GA: US Department of Health and Human Services, CDC; 2014. Available at: http://www.cdc.gov/getsmart/healthcare/implementation/core-elements.html. Accessed 6 April 2015. [Google Scholar]
- 4.Jenkins TC, Knepper BC, Sabel AL, et al. Decreased antibiotic utilization after implementation of a guideline for inpatient cellulitis and cutaneous abscess. Arch Intern Med. 2011;171(12):1072–9. doi: 10.1001/archinternmed.2011.29. [DOI] [PubMed] [Google Scholar]
- 5.Avdic E, Cushinotto LA, Hughes AH, et al. Impact of an antimicrobial stewardship intervention on shortening the duration of therapy for community-acquired pneumonia. Clin Infect Dis. 2012;54(11):1581–7. doi: 10.1093/cid/cis242. [DOI] [PubMed] [Google Scholar]
- 6.Jenkins TC, Stella SA, Cevantes L, et al. Targets for antibiotic and healthcare resource stewardship in inpatient community-acquired pneumonia: a comparison of management practices with National Guideline Recommendations. Infection. 2013;41(1):135–44. doi: 10.1007/s15010-012-0362-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jenkins TC, Knepper BC, Moore SJ, et al. Antibiotic prescribing practices in a multicenter cohort of patients hospitalized for acute bacterial skin and skin structure infection. Infect Control Hosp Epidemiol. 2014;35(10):1241–50. doi: 10.1086/678056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yogo N, Haas MK, Knepper BC, et al. Antibiotic prescribing at the transition from hospitalization to discharge: a target for antibiotic stewardship. Infect Control Hosp Epidemiol. 2015;36(4):474–8. doi: 10.1017/ice.2014.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59(2):147–59. doi: 10.1093/cid/ciu296. [DOI] [PubMed] [Google Scholar]
- 10.Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52(5):e103–120. doi: 10.1093/cid/ciq257. [DOI] [PubMed] [Google Scholar]
- 11.Chey WD, Wong BC, et al. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 2007;102(8):1808–25. doi: 10.1111/j.1572-0241.2007.01393.x. [DOI] [PubMed] [Google Scholar]
- 12.Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA) Infect Control Hosp Epidemiol. 2010;31(5):431–55. doi: 10.1086/651706. [DOI] [PubMed] [Google Scholar]
- 13.Hooton TM, Bradley SF, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 international clinical practice guidelines from the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:625–663. doi: 10.1086/650482. [DOI] [PubMed] [Google Scholar]
- 14.Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44:S27–72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American Thoracic Society and Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 16.Haas MK, Dalton K, Knepper BC, et al. Effects of a syndrome-specific antibiotic stewardship intervention for inpatient community-acquired pneumonia. Open Forum Infect Dis. 2016;3(4):ofw186. doi: 10.1093/ofid/ofw186. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1: Summary of recommendations for initial empiric therapy, oral step-down therapy, and duration of therapy for common infections utilized in the intervention.


