Abstract
Background
In human heart failure, Ser199 (equivalent to Ser200 in mouse) of cardiac troponin I (cTnI) is significantly hyper-phosphorylated and in vitro studies suggest it enhances myofilament calcium sensitivity and alters calpain-mediated cTnI proteolysis. However, how its hyper-phosphorylation affects cardiac function in vivo remains unknown.
Methods and Results
To address the question, two transgenic mouse models were generated: a phospho-mimetic cTnIS200D and a phospho-silenced cTnIS200A, each driven by the cardiomyocyte-specific α-MHC promoter. Cardiac structure assessed by echocardiography and histology were normal in both transgenic models compared to littermate controls (n=5). Baseline in vivo hemodynamics and isolated muscle studies showed that cTnIS200D significantly prolonged relaxation and lowered left ventricular peak filling rate, whereas ejection fraction and force development were normal (n=5). However, with increased heart rate or beta-adrenergic stimulation, cTnIS200D mice had less enhanced ejection fraction or force development versus controls, whereas relaxation improved similarly to controls (n=5). By contrast, cTnIS200A was functionally normal both at baseline and under the physiological stresses. To test if either mutation impacted cardiac response to ischemic stress, isolated hearts were subjected to ischemia/reperfusion. cTnIS200D were protected, recovering 88±8% of contractile function versus 35±15% in littermate controls and 28±8% in cTnIS200A (n=5). This was associated with less cTnI proteolysis in cTnIS200D hearts.
Conclusions
Hyper-phosphorylation of this Serine in cTnI C-terminus impacts heart function by depressing diastolic function at baseline and limiting systolic reserve under physiological stresses. However, paradoxically it preserves heart function after ischemia/reperfusion injury potentially by decreasing proteolysis of cTnI.
Sarcomeric proteins are the major components of the contractile apparatus of heart muscle. Cardiac muscle contraction is a result of myosin in the thick filament interacting with actin in the thin filament to form a force-generating cross-bridge, which is regulated by cyclic Ca2+ concentration changes in cytosol.1 The molecular regulators of this Ca2+ dependent muscle activation are troponin (Tn) and tropomyosin (Tm), located on thin filament. Tn consists of three subunits; TnI is the inhibitory subunit, which functions via interactions with TnC (the Ca2+- binding subunit), TnT (the Tm-binding subunit) and Tm to inhibit myosin-actin interaction during diastole when [Ca2+] is low and to switch on the contractile machinery during systole when [Ca2+] increases and binds to TnC.2
Cardiac troponin I (cTnI) is the cardiac-specific isoform of TnI and the only TnI isoform expressed in adult hearts. The protein has 210 amino acid residues in human and is highly conserved across species.3, 4 The cTnI knockout is lethal due to severe diastolic dysfunction.5 Post-translational modifications of cTnI regulate cardiac function in response to physiological and pathological stresses.6, 7 For example, phosphorylation of cTnI Ser22/23, primarily mediated by PKA, enhances Ca2+ disassociation from cTnC and increases the amount of Ca2+ required for generating submaximal force (this is referred to as decreasing myofilament Ca2+ sensitivity), and therefore contributes to the enhanced relaxation rate mediated by β-adrenergic stimulation during exercise and stress.6 Additionally, cTnI proteolysis occurs with ischemia/reperfusion (I/R) injury; the truncated cTnI lacking the distal C-terminus fragment is incorporated in the myofilament contributing to I/R related cardiac dysfunction.8
Recent detailed phosphoproteomic studies of human heart failure (HF) myocardium have revealed a novel phosphorylation site on human cTnI at Ser199, which is two-fold hyper-phosphorylated in HF compared to normal donor hearts.9 The phosphorylation level of this site is also significantly increased in a dog model of dyssynchronous HF and restored to normal level after resynchronization therapy.9 The site is also highly conserved across species from frog to human10 suggesting high selection pressure. It is physically located at the cTnI distal C-terminus, a highly mobile region that is crucial for the kinetics of thin filament regulation.11, 12 More than 40% of the known cardiomyopathy-related cTnI mutations are within this region.13 Furthermore, this site is within a peptide cleaved from cTnI via proteolysis following I/R injury.7 Therefore, whether and how human cTnI Ser199 hyper-phosphorylation affects cardiac function in vivo is of great interest. The present study addresses this question by studying two novel transgenic mouse models, a phospho-mimetic model and a phospho-silenced model. We examined cardiac function of both models at rest, under conditions of rate and adrenergic stress, and following I/R.
Methods
Animal models
The transgenic mouse model cTnIS200D overexpresses cTnI Ser200Asp mutation (cTnI Ser200 in mouse sequence is equivalent to cTnI Ser199 in human sequence) to mimic the site-specific hyper-phosphorylation in vivo. The other transgenic model cTnIS200A overexpresses cTnI Ser200Ala mutation to create a non-phosphorylatable site. Both transgenes were cloned into the downstream of a cardiac-specific α-MHC promoter (a kind gift from Dr. Jeffrey Robbins, Children's Hospital Medical Center, Cincinnati, Ohio). After six generations of breeding with wildtype C57BL/6 mice, 5-6 months old male transgenic mice were used and compared with their non-transgenic (NTG) siblings. All protocols concerning animal use were approved by Johns Hopkins University Animal Care and Use Committee.
Echocardiography
Echocardiography studies were performed on conscious non-anesthetized mice by using a Vevo 2100 High-Resolution In Vivo Imaging System with 40MHz transducer (VisualSonics, Toronto, ON, Canada) and analyzed with an Advanced Cardiovascular Package Software (VisualSonics, Toronto, ON, Canada).
In vivo hemodynamics
Mice were ventilated with 6–7 μl/g tidal volume, 130 breaths/min and anesthetized by either isofluorane (1-2%) or intraperitoneal injection of etomidate (250μg/kg), urethane (30mg/kg) and morphine (15ug/kg). Continuous pressure-volume data from left ventricle at baseline and then under varied stresses was recorded by using a 1.4-Fr PV catheter (SPR 839; Millar Instruments Inc.) as previously described.14 An atrial/ventricular pacing wire was placed into the esophagus and connected to a Grass stimulator for heart rate control. Transient inferior vena cava occlusion was used to derive pressure-volume relations for analysis of cardiac function.
Cardiac muscle isometric force measurements
Mice were euthanized with pentobarbital (50 mg/kg) and heparinization (100 U). As previously described,15, 16 right ventricular trabeculae were dissected and mounted between a customized force transducer ((AEM 801, SensoNor) and a motor arm, superfused with modified Krebs-Henseleit solution (in mM: NaCl 120, Na2HCO3 20, KCl 5, MgCl2 1.2, glucose 10, and CaCl2, pH 7.35-7.40) equilibrated with 95% O2 and 5% CO2 at 20-22°C, stimulated at 0.5 Hz. The sarcomere was set and maintained at an optimal length corresponding to 2.2-2.3 μm as determined by laser diffraction.
Myofilament proteins analysis
Myofilament proteins were extracted from freshly dissected ventricles as previously described.17 The expression level of mutant proteins cTnI Ser200Asp and cTnI Ser200Ala in hearts were assessed by Western blotting using two monoclonal antibodies (McAb). McAb 8I-7 (epitope cTnI137-148, International Point of Care Inc, Canada, 1:5000) can detect wildtype cTnI, cTnI Ser200Asp, and cTnI Ser200Ala; while McAb p45-10 (epitope cTnI195-203, HyTest Ltd, 1:5000) cannot recognize cTnI Ser200Asp and has a much lower affinity for cTnI Ser200Ala than wildtype cTnI.
A quantitative mass spectrometry approach called multiple reaction monitoring (MRM) was used to quantify the phosphorylation level of endogenous wildtype cTnI at Ser200 in heart according to the published procedures.9 The standard peptide NITEIADLTQK and its heavy labeled version NITEIADLTQK* were used to quantify total cTnI; the standard peptide NIDAL(p)SGMEGR and its heavy labeled version NIDAL(p)SGMEGR* was used to quantify endogenously phosphorylated cTnI at Ser200. The pan-protein phosphorylation levels of myofilament proteins were determined via Pro-Q Diamond gel staining followed by SYPRO Ruby protein gel stain (Molecular Probes) according to the manufacturer's protocols. To determine the phosphorylation level of cTnI at Ser23 and Ser24, the myofilament proteins were treated with protein kinase A (PKA) catalytic subunit from bovine heart (Sigma-Aldrich, protein: enzyme = 5μg: 1unit) at 30°C for 30 min in a reaction buffer containing (in mM) MOPS 20, KCl 200, MgCl2 10 and DTT 1. The phosphorylation of cTnI Ser23 and Ser24 was detected via Phospho-Troponin I (Cardiac) (Ser23/24) antibody (Cell Signaling, 1:1000).
Global ischemia-reperfusion with Langendorff system and cTnI cleavage analysis
Mice were euthanized with pentobarbital (50 mg/kg) and the hearts were rapidly excised and retrogradely perfused with Krebs-Henseleit buffer (37°C) gassed with 95% O2 and 5% CO2. The function of isolated heart was monitored with a water-filled balloon connected to a pressure transducer according to the published methods.18 After stabilization, the heart was subjected to 30-minute global ischemia followed by 1-hour reperfusion and then quickly removed, frozen with liquid nitrogen, and stored at -80°C for later use. cTnI and its degradation fragments was detected by McAb 8I-7 via western blot in whole heart homogenate.
Statistical analysis
One-way ANOVA followed by Tukey's post hoc test was used to analyze mutant protein expression, phosphorylation of myofilament proteins, cTnI degradation, and basal cardiac/muscle function between the three groups. Analysis of cardiac functional responses to pacing rates or isoproterenol was done with two-way ANOVA mixed design followed by Tukey's post hoc test. Levene's test and Welch's correction was used to check the homogeneity of variance and analyze the data with heterogeneity of means, respectively. Values are presented as mean ± SEM, P<0.05 indicates significance.
Results
cTnI mutants were incorporated into myofilaments of transgenic mice
Transgenic mice reproduced normally and were viable beyond twelve months of age.
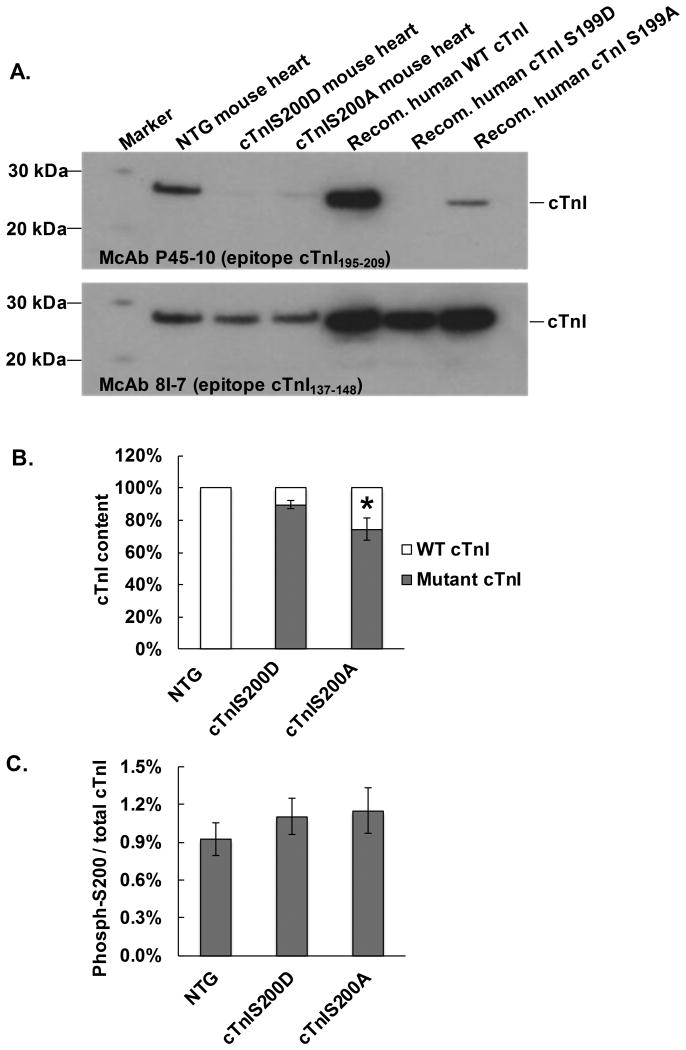
With two cTnI monoclonal antibodies (McAbs), McAb 8I-7 (epitope cTnI137-148) and McAb P45-10 (epitope cTnI195-209), we were able to differentiate the mutant cTnI Ser200Asp and cTnI Ser200Ala from wildtype cTnI via western blot and thus quantify the expression level of mutant cTnI in transgenic mouse hearts. The results showed that ∼88% ± 8% of cTnI in cTnIS200D heart was replaced by mutant protein cTnI Ser200Asp while ∼75 ± 6% of cTnI in cTnIS200A heart was replaced by mutant protein cTnI Ser200Ala (Figure 1A, B). We further quantified the cTnI phosphorylation at Ser200 and total cTnI (including both wildtype and mutant cTnI) via a quantitative mass spectrometry approach called multiple reaction monitoring (MRM) and found that the ratio of phosphorylated cTnI at Ser200 to total cTnI was not altered in either transgenic mice as compared to the non-transgenic (NTG) littermates, indicating that neither pseudo-phosphorylation (cTnI Ser200Asp) nor phosphorylation-silenced (cTnI Ser200Ala) mutation affected the endogenous phosphorylation of cTnI at Ser200 (Figure 1C, Supplemental Figure 1).
Figure 1.
Mutant cTnI expression level and endogenous phosphorylation of cTnI Ser200. A. A representative image of Western blot with two cTnI McAbs, 8I-7 and P45-10 to determine mutant cTnI expression level. McAb 8I-7 recognizes all three cTnI variants, including the wildtype (WT), Ser200Asp (S200D), and Ser200Ala (S200A), and thus demonstrated the total cTnI in myofilament homogenate. McAb P45-10 recognizes WT cTnI but not cTnI S200D and has a very low affinity for cTnI S200A (about 20% of its affinity for WT cTnI). Purified human recombinant cTnI variants were used as positive control. B. The estimated ratio of mutant cTnI to total cTnI, n=5. * P<0.05 compared to cTnIS200D group. C. Quantitative data of cTnI phosphorylation at Ser200 in the hearts of NTG, cTnIS200D, and cTnIS200A mice. n=5.
TnI Ser200 pseudo-phosphorylation mice showed normal histology but impaired relaxation on echocardiography
At age of six months old, both cTnIS200D and cTnIS200A mice had normal heart mass, LV chamber size, and wall thickness as detected by echocardiography (Table 1). Histological examination confirmed normal cardiac structure in both transgenic models (Supplemental Figure 2).
Table 1. Echocardiography data of LV structure and function.
| NTG | cTnIS200D | cTnIS200A | |
|---|---|---|---|
| Age (month) | 6 | 6 | 6 |
| Sex | Male | Male | Male |
| Body Weight (g) | 31.26 ± 1.81 | 28.52 ± 1.40 | 29.84 ± 1.37 |
| Heart Weight/Body Weight (%) | 0.47 ± 0.02 | 0.54 ± 0.05 | 0.50 ± 0.03 |
| Heart Weight/Tibial Length (g/cm) | 0.078 ± 0.005 | 0.077 ± 0.004 | 0.079 ± 0.005 |
| Heart Rate (bpm) | 683 ± 3 | 673 ± 2 | 697 ± 4 |
| Interventricular Septum thickness (mm) | 0.97 ± 0.03 | 0.97 ± 0.01 | 0.98 ± 0.02 |
| LV Posterior Wall Thickness (mm) | 0.95 ± 0.03 | 0.91 ± 0.02 | 0.97 ± 0.01 |
| LV End Diastolic Diameter (mm) | 2.79 ± 0.10 | 2.75 ± 0.06 | 2.86 ± 0.07 |
| LV End Systolic Diameter (mm) | 1.13 ± 0.05 | 1.10 ± 0.04 | 1.21 ± 0.05 |
| FS (%) | 59.53 ± 0.98 | 59.83 ± 1.06 | 57.72 ± 0.78 |
| EF (%) | 83.59 ± 0.79 | 83.82 ± 0.86 | 82.10 ± 0.67 |
| IVCT (m/sec) | 12.88 ± 0.92 | 11.79 ± 0.92 | 10.46 ± 0.84 |
| IVRT (m/sec) | 22.75 ± 0.32 | 25.96 ± 0.84 * | 24.31 ± 1.18 |
| E′(mm/s) | 38.14 ± 3.69 | 22.95 ± 4.40 † | 42.45 ± 3.70 |
| A′(mm/s) | 29.72 ± 3.47 | 23.81 ± 3.05 | 31.92 ± 2.58 |
P<0.05 compared to NTG.
P<0.05 compared to NTG and cTnIS200A. n=5. Abbreviations: FS, fraction shortening; EF, ejection fraction; IVCT, isovolumic contraction time; IVRT, isovolumic relaxation time; E′, peak early diastolic mitral annulus velocity; A′, peak late diastolic (atrial contraction) mitral annulus velocity.
LV functional data obtained by using a parasternal short-axis echocardiographic imaging in conscious mice showed that cTnIS200D mice had normal ejection fraction (EF) but significantly prolonged isovolumic relaxation time (IVRT) as compared to NTG at similar heart rates (Table 1). In addition, Tissue Doppler Imaging demonstrated that early diastolic myocardial relaxation velocity (E′) of cTnIS200D hearts was significantly lower than in NTG (Table 1). The functional data obtained with echocardiography indicate that cTnIS200D hearts, which mimic hyper-phosphorylation, have normal systolic but impaired diastolic function. cTnIS200A mice displayed normal LV function, based on all echocardiographic measurements, indicating that hypo-phosphorylation of cTnI Ser200 does not alter cardiac function at baseline in mice.
TnI Ser200 pseudo-phosphorylation model demonstrated diastolic dysfunction with preserved EF in hemodynamic studies
Pressure-volume analysis is summarized in Table 2. Under basal conditions, cTnIS200D displayed comparable heart rates, preload (end-diastolic volume, EDV), and afterload (arterial elastance) to NTG controls. cTnIS200D hearts had preserved EF and normal dP/dtmax (the maximal rate of pressure rise). To better assess the overall contractility, the slope of the relationship between stroke work and preload, termed preload recruitable stroke work (PRSW), was measured from variably pre-loaded cardiac cycles. PRSW was unchanged between controls and cTnIS200D hearts. However, all relaxation parameters in cTnIS200D were abnormal. cTnIS200D had a less negative peak rate of pressure fall (dP/dtmin), prolonged isovolumic relaxation time (Tau), and slower peak filling rate (PFR/EDV). As in the echocardiographic studies, there were no differences between cTnIS200A and NTG mice in either systolic or diastolic function detected by in vivo pressure-volume analysis. Those data indicate that hyper-phosphorylation rather than hypo-phosphorylation of cTnI Ser200 impairs diastolic function impairs diastolic function but preserves EF and the overall contractility, which agrees with the echocardiography findings. It was notable that, the systolic indexes that were more impacted by contractile kinetics, such as dP/dtmax/iP (maximal rate of pressure rise normalized to instantaneous pressure at the same time during isovolumic contraction) and Powermax/EDV (maximal ventricular power normalized to preload during LV ejection), were altered in the cTnIS200D heart versus controls, although the overall EF was normal in cTnIS200D hearts.
Table 2. In vivo LV hemodynamic measures on mice at baseline.
| NTG | cTnIS200D | cTnIS200A | |
|---|---|---|---|
| Measures | |||
| Heart Rate (beat/min) | 580 ± 12 | 554 ± 14 | 578 ± 16 |
| Main Arterial Pressure (mmHg) | 76.64 ± 4.46 | 67.53 ± 2.75 | 71.54 ± 1.82 |
| End-systolic Pressure (mmHg) | 94.16 ± 5.15 | 82.25 ± 3.54 | 87.40 ± 2.66 |
| End-diastolic Pressure (mmHg) | 4.71 ± 0.39 | 7.34 ± 0.76 * | 4.89 ± 0.51 |
| End-systolic Volume (μl) | 14.69 ± 0.38 | 14.98 ± 0.36 | 14.37 ± 1.18 |
| End-diastolic Volume (μl) | 39.57 ± 0.97 | 40.19 ± 0.54 | 39.38 ± 1.48 |
| Stroke Volume (μl) | 24.88 ± 1.08 | 25.21 ± 0.68 | 25.01 ± 0.61 |
| Cardiac Output (ml/min) | 14.44 ± 0.76 | 13.95 ± 0.43 | 14.44 ± 0.50 |
| Arterial Elastance | 3.82 ± 0.22 | 3.29 ± 0.19 | 3.50 ± 0.10 |
| Systolic Indices | |||
| Ejection Fraction (%) | 62.67 ± 1.34 | 62.66 ± 1.04 | 63.97 ± 1.99 |
| dP/dtmax (mmHg/s) | 11096 ± 1015 | 11628 ± 760 | 10324 ± 374 |
| dP/dtmax/iP (s-1) | 188.61 ± 9.41 | 226.02 ± 7.89 * | 189.57 ± 4.34 |
| Powermax/EDV | 38.27 ± 3.18 | 27.97 ± 2.05 * | 36.38 ± 1.97 |
| SW | 2402 ± 200 | 2114 ± 76 | 2253 ± 99 |
| PRSW | 87.17 ± 6.82 | 93.06 ± 8.36 | 91.79 ± 4.81 |
| Diastolic Indices | |||
| dP/dtmin | 9130 ± 901 | 6255 ± 483 * | 8412 ± 498 |
| Tau (ms) | 5.27 ± 0.36 | 6.90 ± 0.38 * | 5.25 ± 0.21 |
| Peak Filling Rate-EDV | 36.93 ± 2.61 | 28.51 ± 1.79 † | 31.01 ± 1.95 |
P<0.05 compared to NTG and cTnIS200A.
P<0.05 compared to NTG. n=5. End-diastolic volume presents preload. Arterial elastance is a measure of afterload. Abbreviations: EF, ejection fraction; dP/dtmax, peak rate of pressure rise; dP/dtmax/iP, peak rate of pressure normalized to instantaneous pressure, a preload-independent index of contractility during isovolumic contraction; Powermax/EDV, maximal power normalized to EDV reflecting contractility during LV ejection of blood; −dP/dtmin, peak rate of pressure decay during isovolumic relaxation, which is preload dependent; Tau, isovolumic relaxation time constant, a preload-independent index; PFR/EDV, peak ventricular filling rate normalized to EDV.
TnI Ser200 pseudo-phosphorylation model slowed force decay in isolated muscle studies
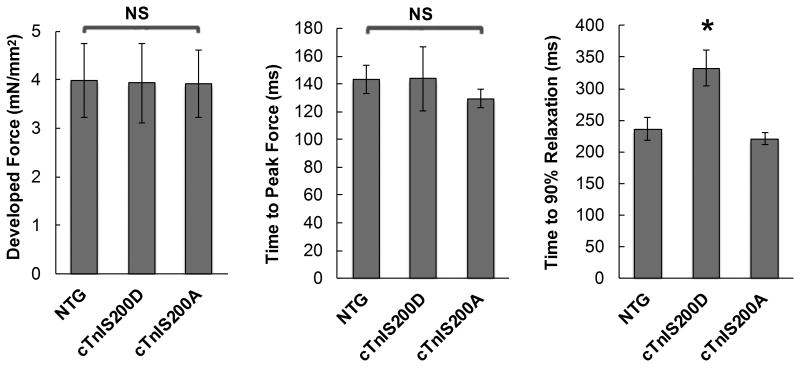
To assess if the chamber level changes represented alterations in the muscle, we tested force development of the trabecular muscle isolated from right ventricles of mice. As shown in Figure 2, muscles from cTnIS200D hearts developed similar peak force and take a similar time to reach the peak force relative to NTG, but relaxation was significantly slower. Consistent with the in vivo findings, muscles from cTnIS200A hearts behaved normally during force development.
Figure 2.
The baseline function of isolated ventricular muscle in vitro. The isometric force development of intact trabeculae muscle isolated from mouse hearts were measured at 1.0 mM external Ca2+, stimulation frequency 0.5 Hz. A. Peak force developed by trabeculae muscle isolated from mouse hearts. B. The time from the start of contraction to force peak. C. The time from force peak to 90% of force decay. *P<0.05 compared to NTG and cTnIS200A. n=5.
Force-frequency relationship was blunted in the TnI Ser200 pseudo-phosphorylation model
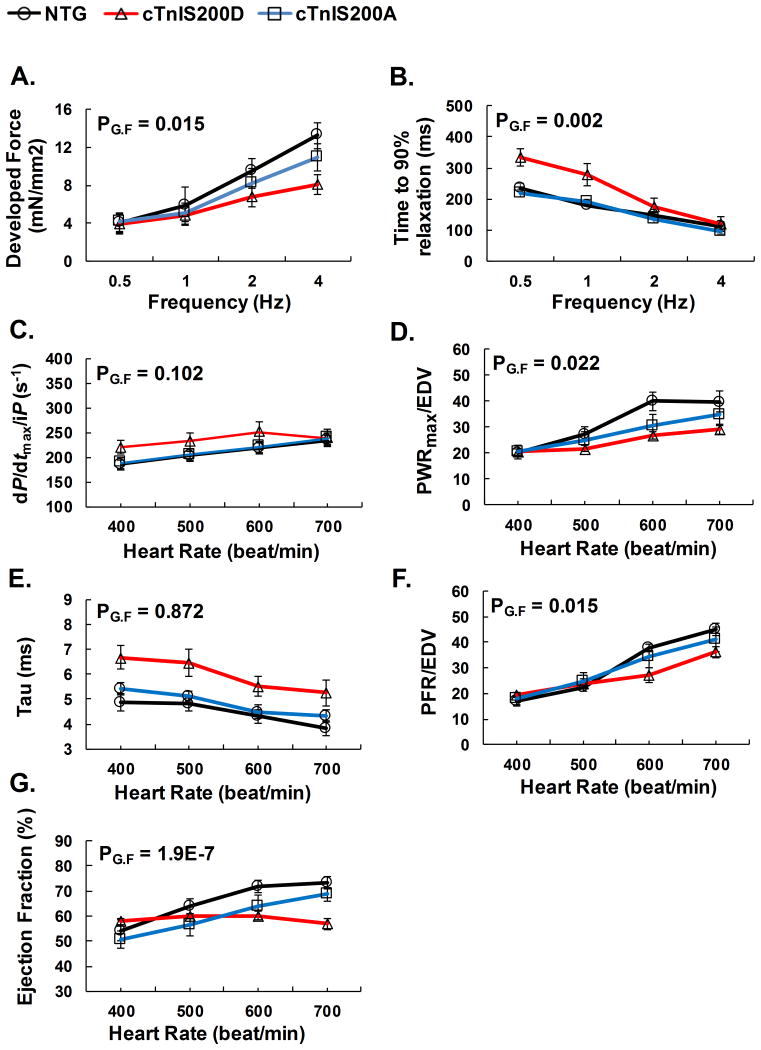
To assess if alterations in TnI Ser200 (pseudo or silenced phosphorylation) impacted cardiac reserve mechanisms, we examined heart function with incremental heart pacing rates. As expected, trabecular muscle from NTG hearts displayed a positive force-frequency relation (Figure 3A). However, the relationship was significantly blunted in cTnIS200D muscle. On the other hand, isometric relaxation of trabecular muscles from NTG and both TG mice was enhanced with increasing pacing rates in the similar fashion (Figure 3B).
Figure 3.
Cardiac responses to increased pacing in vitro and in situ. A-B. In vitro isometric force measurements on isolated intact trabecular muscle over stimulation frequency 0.5-2 Hz, 1mM external Ca2+, n=5. C-G. In situ hemodynamic study of cardiac function over paced heart rates from 400-700 beats/min. n=5. Explanation of abbreviations is the same as Table 2. PG.F<0.05 indicates significance among the interactions between genotype and pacing frequency. cTnIS200D mice with hyper-phosphorylation mutation depressed positive force-frequency relationship both in vitro and in vivo.
Similar analysis was performed in situ with heart rate ranging from 400-700 beat/min. cTnIS200D mice had enhanced isovolumic contraction (reflected by dP/dtmax /iP) and isovolumic relaxation (reflected by dP/dtmin and Tau), in agreement with the in vitro data of isometric measurements of muscle function (Figure 3C, E). While the in vitro muscle measurements were done at fixed length, in vivo muscle length changes during the ejection and ventricular filling phases of the cardiac cycle. During those two phases, cardiac muscle function is reflected by Powermax/EDV and PFR/EDV, respectively. cTnIS200D mice failed to enhance either Powermax/EDV or PFR/EDV to the same extent as NTG (Figure 3D, F). As a result, when heart rate increased from 400 to 700 beats/min, cTnIS200D did not increase EF at all while NTG increased EF from 54.2 ± 4.3% to 73.1 ± 2.5% (Figure 3G), indicating that pseudo-phosphorylation of cTnI Ser200 impairs cardiac reserve in response to incremental heart rate pacing. In contrast to the phospho-mimetic cTnIS200D, the phospho-silenced cTnIS200A does not affect the cardiac reserve.
Inotropic response to β-adrenergic stimulation was depressed in TnI Ser200 pseudo-phosphorylation model
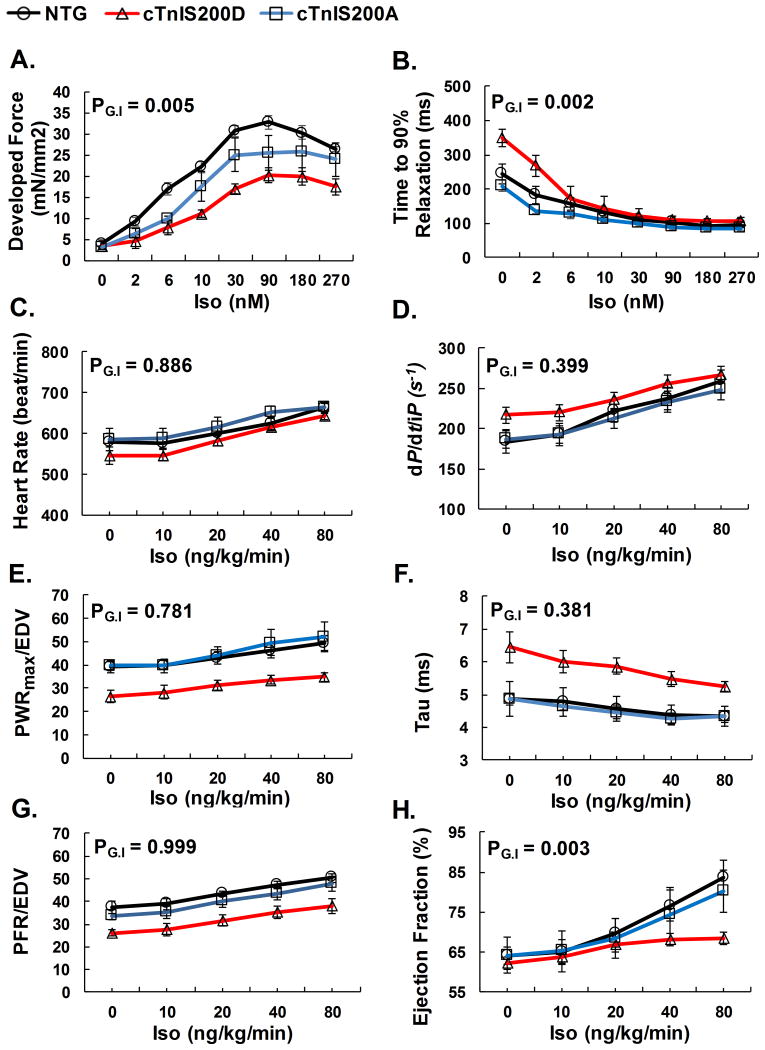
To test the impact of cTnI Ser200 modulation on beta-adrenergic stimulation, we studied the cardiac response to isoproterenol exposure in both isolated muscles in vitro and intact chambers in situ. Isoproterenol increased developed isometric force in trabecular muscles isolated from NTG and cTnIS200A, but had less inotropic effect in cTnIS200D (Figure 4A). Isoproterenol accelerated isometric relaxation similarly in the muscles from all three types of mice, in agreement with the PKA-dependent enhancement of SR calcium cycling and myofilament desensitization via TnI phosphorylation at its N-terminus (Figure 4B). In vivo, cTnIS200D hearts displayed enhanced systolic and diastolic function with isoproterenol, but the net response remained lower than controls (Figure 4 C-H). As a result, β-adrenergic stimulation with isoproterenol augmented EF in NTG, but not in cTnIS200D mice. Since increased heart rate alone is sufficient to alter cardiac function and isoproterenol increases heart rate (chronotropic effect), we further investigated the cardiac responses to isoproterenol at a fixed heart rate of 600 beats/min in order to rule out the interference of frequency-dependent functional changes. The results showed that, with fixed heart rate, NTG mice were still able to enhance systolic and diastolic function. However, such systolic enhancement was completely depressed in cTnIS200D (Supplemental Figure 3). The responses of cTnIS200A mice to isoproterenol were not significantly different from NTG either in vitro or in vivo. Overall, these results indicate that pseudo-phosphorylation of cTnI Ser200 depresses inotropic rather than lusitropic responses to β-adrenergic stimulation.
Figure 4.
Cardiac responses to β-adrenergic stimulation with isoproterenol in vitro and in situ. A-B. In vitro isometric force measurements on isolated intact trabecular muscle perfused with isoproterenol, 1mM Ca2+, stimulation frequency 0.5Hz. n=5. C-H. In situ hemodynamic measures of cardiac responses to isoproterenol (i.v. injection). n=5. Explanation of abbreviations is the same as Table 2. PG.I <0.05 indicates significance among the interactions between genotype and the concentration of isoproterenol. Transgenic mice cTnIS200D demonstrated depressed cardiac inotropic response to isoproterenol both in vitro and in vivo.
The TnI Ser200 pseudo-phosphorylation model was resistant to ischemia-reperfusion injury
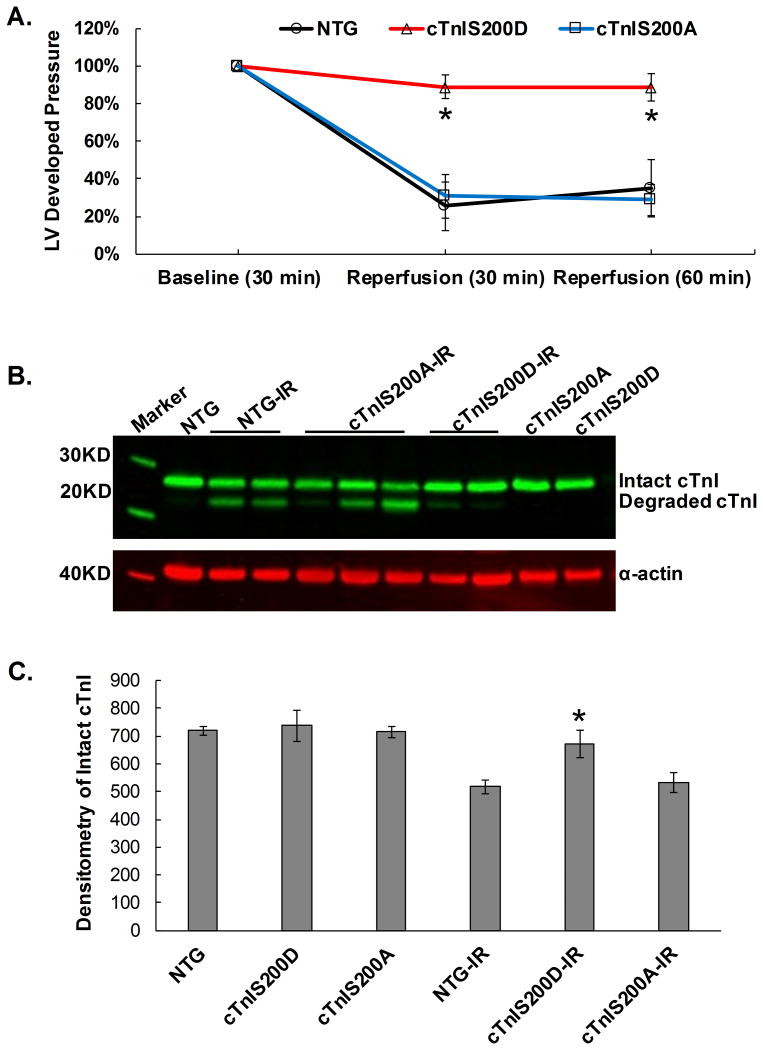
Removal of cTnI (193-210) by proteolysis during I/R contributes to I/R-related myocardium dysfunction. Our prior in vitro data showed that human cTnI Ser199 hyper-phosphorylation appeared to increase cTnI proteolysis mediated by calpain I in vitro,19 suggesting that cTnIS200D hearts might be more vulnerable to I/R injury. To test the impact in the intact heart, we subjected ex-vivo hearts to 30-minute global ischemia followed by 1-hour reperfusion by using a Langendorff perfusion apparatus. After I/R, on average, NTG and cTnIS200A hearts respectively recovered 35 ± 15% and 29 ± 8% of their initial left ventricular developed pressure (LVDP), while surprisingly, cTnIS200D hearts recovered about 88 ± 8% (Figure 5A). Western blot with cTnI McAb 8I-7 (epitope cTnI137-148) showed that after I/R, cTnIS200D retained more intact cTnI than NTG and cTnIS200A hearts (Figure 5B, C), and the cTnI degradation is less likely to be detected in cTnIS200D than NTG and cTnIS200A hearts (Figure 5B). The result indicates that pseudo-phosphorylation of cTnI at Ser200 protects cTnI from proteolysis in vivo during I/R, which is specific for the Ser200Asp mutation because the Ser200Ala mutant hearts have an equivalent degree of TnI proteolysis as the NTG.
Figure 5.
Mouse heart under Langendorff 30min global ischemia followed by 1hr reperfusion. A. Normalized LV developed pressure indicates LV function recovery after ischemia/reperfusion(IR). On average, cTnIS200D hearts recovered better than NTG and cTnIS200A hearts. The baseline time point is 30 min after the isolated heart was cannulated and perfused via Langendorff system when developed pressure was stabilized. B. A representative image of Western blot with anti-cTnI McAb 8I-7 shows that cTnI proteolysis is more likely to be detected in NTG and cTnIS200A than cTnIS200D heats under IR treatment. No cTnI proteolysis was observed in mice without IR as demonstrated in the 2nd, 10th and 11th lane (from left to right). C. Quantitative data of the amount of intact cTnI in the heart tissue without and without I/R. n=5. *P<0.05 indicates significance as compared with NTG and cTnIS200A after IR.
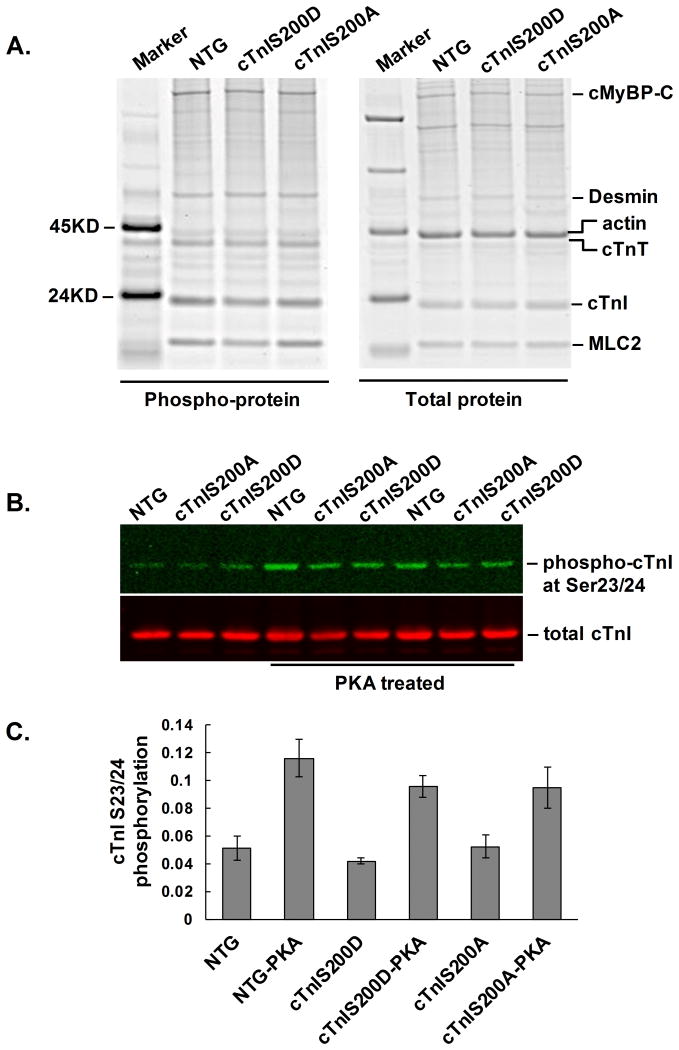
TnI Ser200 mutants did not impact phosphorylation of other sarcomeric proteins
Since phosphorylation of sarcomeric proteins, such as myosin-binding protein C (MyBP-C), desmin, cTnT, and myosin light chain 2 (MLC2), could also impact cardiac function, we assessed if the global phosphorylation of sarcomeric proteins was altered in the two TG mouse models. Pro-Q Diamond staining followed by Sypro-Ruby staining showed the pan-protein phosphorylation level was not significantly changed in any of the sarcomeric proteins listed above (Figure 6A, Supplemental Figure 4) in either TG mouse model. Among all the known phosphorylation sites on cTnI, Ser23 and 24 are the two most abundantly phosphorylated sites, and they can significantly impact cardiac relaxation under adrenergic stimulation. Figures 6B and C show that there is no significant difference in cTnI Ser23/24 phosphorylation level between NTG, cTnIS200D or cTnIS200A group either at baseline or after treated with PKA.
Figure 6.
Phosphorylation of sarcomeric proteins. A. A representative image of protein gel with Pro-Q Diamond staining of pan-protein phosphorylation (left) and with Sypro-Ruby staining of total protein (right). A PeppermintStick Phosphoprotein marker was added on the gel. Abbreviations: cMyBP-C, cardiac myosin-binding protein-C; MLC2, myosin light chain 2. The quantitative data is presented in Supplemental Figure 4. B. A representative western blot image of cTnI Ser23/24 phosphorylation. Myofilament proteins extracted from the mouse hearts were treated with PKA catalytic subunit at 30°C for 30 min. The phosphorylation of cTnI Ser23 and Ser24 was detected via western blot by using Phospho-Troponin I (Cardiac) (Ser23/24) antibody (Cell Signaling, 1:1000). The total cTnI detected by antibody 8I-7 (International Point of Care Inc, Canada, 1:5000) serves as loading control. C. Quantitative data of cTnI Ser23/24 phosphorylation level as detected by Western blot. n=5. At baseline cTnI Ser23/24 phosphorylation did not differ between groups. Phosphorylation increased after PKA treatment, but again did not differ between groups after treatment.
Discussion
The major findings of this work are that cTnI Ser200 pseudo-phosphorylation has divergent effects, impairing basal diastolic function and systolic reserve, but paradoxically protecting against ischemia-reperfusion injury. The study reveals both physiological and pathophysiological relevance to this post-translational modification, as well as its potential relevance in cardiac disease.
Detrimental effect of cTnI Ser200 pseudo-phosphorylation on diastolic function on systolic reserve
The finding that pseudo-phosphorylation at cTnI Ser200 impairs diastolic function in mice in vivo is consistent with our prior finding that human cTnI Ser199 pseudo-phosphorylation lowers cTnI affinity for actin in vitro.19 This site resides in the distal C-terminus of cTnI, a highly mobile region that has been shown to have large Ca2+-induced structural changes during thin filament activation and deactivation.12 According to the fly-casting model of the cTnI C-terminal mobile region, the cTnI distal C-terminus is unstructured in systole when Ca2+ is bound to a regulatory site on TnC; however it senses actin from a large distance and folds upon binding to actin when Ca2+ is absent in order to initiate relaxation.11, 12, 20 The effective interaction of actin and cTnI distal C-terminus including Ser200 is necessary for full inhibition of contraction as well as normal relaxation kinetics.20, 21 Therefore, we speculate that addition of negative charges and/or a phosphate group by phosphorylation to cTnI Ser200 interferes cTnI sensing and binding to actin and thus impairs diastolic function.
Despite the preservation of overall baseline systolic function, pseudo-phosphorylation of cTnI Ser200 significantly and negatively affects cardiac systolic reserve, which is manifested under physiological stresses including rapid heart rate and β-adrenergic stimulation. Frequency-dependent enhancement of cardiac muscle contraction and relaxation is an important intrinsic regulatory mechanism in modulating heart function.22 Although mice usually have less force-frequency reserve than human and larger animals, the positive force-frequency relationship (FFR) is still present in isolated muscle and in vivo hemodynamic studies.23, 24 Our results indicate that pseudo-phosphorylation of cTnI Ser200 impaired FFR with depressed enhancement in systolic function. In addition, cTnI Ser200 pseudo-phosphorylation depresses inotropic response to β-adrenergic signaling, independent of the chronotropic effect of catecholamine. The exact mechanism by which systolic reserve is impaired in this model is not entirely clear. Interestingly, the lusitropic response to β-adrenergic signaling is preserved, which is consistent with the equivalent cTnI Ser23/24 phosphorylation with PKA between cTnIS200D and NTG controls at either baseline or after PKA treatment, given that cTnI Ser23/24 phosphorylation is one of the effectors of lusitropic β-adrenergic signaling.
Unanticipated protective effect of cTnI Ser200 pseudo-phosphorylation in hearts subjected to ischemia/reperfusion injury
The protection of cTnIS200D hearts from I/R injury was striking and was associated with suppression of cTnI proteolysis. These are likely related, given prior data regarding the adverse impact of cTnI cleavage. First, selective clevage of cTnI C-terminus 17 amino acid residues including this serine residue is generated after I/R, and the remaining cTnI1-193 is incorporated into the myofilament leading to depressed myofilament calcium sensitivity and thus cardiac dysfunction.7 Second, crosstalk between protein phosphorylation and its proteolysis has been reported on cTnI,19, 25 although the precise site involved was unknown. Third, protease inhibitor presence in reperfusion solution protects cardiac function after I/R in ex vivo hearts.26 cTnI Ser200 with pseudo-phosphorylation is still susceptible to calpain-mediated proteolysis in vitro in the context of recombinant troponin complex,19 yet it protected the protein from proteolysis ex vivo in the whole heart with I/R. This disparity may imply a role for different accessibility of the protease to cTnI with I/R injury, or a protease other than calpain which cleaves cTnI during I/R, for example, a matrix metalloproteinase-2.27
Implications of cTnI Ser200 pseudo-phosphorylation for human HF
Hyper-phosphorylation of human cTnI Ser199 was originally found in explanted hearts from HF patients.9 cTnIS200D mice, the model of hyper-phosphorylation of this site, share many pathophysiological characteristics with HF patients, in particular the patients with heart failure with preserved EF (HFpEF). HFpEF patients have primarily diastolic dysfunction. Although EF is preserved in HFpEF patients, they have a “dramatic limitation of systolic reserve capacity during stress with blunted increase in ejection fraction.” 28 Prior studies have demonstrated that both FFR and β-adrenergic signaling is depressed and the positive effect of β-adrenergic stimulation on FFR is also diminished or disappeared in HF.29, 30 It is notable that the phosphorylation level of this site is associated with disease status in a canine model of HF; its phosphorylation level increases abnormally during dyssynchronous HF and returns to normal after resynchronization therapy.9
Implications of cTnI Ser200 pseudo-phosphorylation for myofilament regulation
The distal C-terminus of cTnI (cTnI189-210 in human sequence) is suggested to be a highly mobile region with unclear structure and function. Human Ser199 is the only phosphorylatable site found within the region. The present in vivo study together with our previous in vitro study on cTnI Ser199 phosphorylation provide further insight into the function of cTnI C-terminus. In vitro, pseudo-phosphorylation of human cTnI Ser199 decreases cTnI affinity for actin and increases myofilament Ca2+ sensitivity of force-development in skinned human cardiomyocytes in which endogenous cTnI was replaced by recombinant human cTnI Ser199Asp.19 This may explain why we observed the overall unchanged EF and PRSW with increased dP/dtmax/iP during isovolumic contraction and decreased Powermax/EDV during the ejection phase. We speculated that increased myofilament sensitivity would lead to an augmented formation of cross-bridges during isovolumic contraction and less cross-bridges recruitable for the stretch activation during ejection phase. For instance, Davis et al. suggested that the force required to eject blood against aortic pressure is enhanced by stretch activation of endomyocardium.31 There are other reports that stretch activation of the myofilament depends on the number of recruitable cross-bridges and cooperativity of the weakly bound non-force generating cross-bridges transition into strong-binding force-generating states.32, 33 Furthermore, our finding also agrees with the concept that in absence of cTnI193-210, the remaining cTnI1-192 stabilizes Tm equilibrium position toward an “enhanced Ca2+-activated-state,” 34 a state from which myosin heads start to isomerize in order to bind actin strongly and push Tm further to generate force.2, 35
Limitations of the study
Because it is not possible to simply phosphorylate a single site of cTnI in vivo, the overall study is based on the assumption that replacement of serine residue with aspartic acid mimics site-specific phosphorylation in vivo. Although the model may have limitations, aspartic acid has negative charges and a relatively bulky side chain that is analogous to phosphorylation adding a phosphate to serine residue. This strategy has been experimentally validated as a suitable way to study site-specific phosphorylation of cTnI.36-38 Furthermore, the transgenic model cTnIS200A that carries cTnI Ser200Ala is considered as both a de-phosphorylation/non-phosphorylatable model and serves as a control. cTnIS200A mice have morphologically and functionally normal hearts, suggesting that an amino acid substitution without negative charges or bulky side chain, does not impair cardiac function.
Conclusions
This study is the first investigation of the in vivo impact of phosphorylation of cTnI Ser200 on intact cardiac function. The results indicate that HF-related hyper-phosphorylation of cTnI Ser200 depresses diastolic but not systolic function at rest, and also diminishes systolic reserve to both chronotropic and catecholamine stimuli, supporting a role of cTnI Ser200 hyper-phosphorylation in the development of cardiac dysfunction similar to HFpEF. Additionally, in previous work, hyper-phosphorylation of this site was associated with onset of dyssynchronous HF and returned to normal after cardiac resynchronous treatment in a large animal model.9 Therefore, hyper-phosphorylation of human cTnI Ser199 could be seen as a potential biomarker for HF as well as a potential therapeutic target. Paradoxically this phospho site could also mediate some protection from I/R injury. Together, these data provide new functional insights into the post-translational regulation of cTnI and the role of a major modification observed in human HF.
Supplementary Material
What is new.
This study reports the in vivo cardiac impact of the pseudophosphorylation of Ser199 of cardiac troponin I (cTnI).
The study reveals that cardiac-specific expression of cTnI Ser200Asp in mice (equivalent to human Ser199), a phospho-mimic, is sufficient to depress diastolic but not systolic function at baseline with diminished systolic reserve under chronotropic and catecholamine stimulation.
What are the clinical implications.
These findings resemble physiologic changes in human HF, in particular, features of HF with preserved ejection fraction.
The results indicate that excessive phosphorylation on human cTnI Ser199 reduces heart function, likely through increased sensitivity to Ca2+ in the myocardial contractile apparatus and impact on the nuanced interactions between sarcomere proteins on the thin filament.
The study provides new functional insights into the post-translational regulation of cTnI and the role of a post-translational modification observed in human HF might have impact in patients.
Acknowledgments
We sincerely thank John Robinson and Dr. Djahida Bedja for expert technical assistance.
Funding Sources: This study was funded by NIH RO1 HL 63038 (A.M.M) and American Heart Association Fellowship (L.Y.) and by general support to the laboratory from a grant from the Children's Cardiomyopathy Foundation.
Footnotes
Disclosures: Dr. Murphy has a patent application pending on “Novel Phosphorylation of Cardiac Troponin I as a Monitor for Cardiac Injury.” The other authors report no conflicts.
References
- 1.Gordon AM, Homsher E, Regnier M. Regulation of contraction in striated muscle. Physiol Rev. 2000;80:853–924. doi: 10.1152/physrev.2000.80.2.853. [DOI] [PubMed] [Google Scholar]
- 2.Lehrer S. The 3-state model of muscle regulation revisited: Is a fourth state involved? Journal of Muscle Research and Cell Motility. 2011;32:203–208. doi: 10.1007/s10974-011-9263-8. [DOI] [PubMed] [Google Scholar]
- 3.Hunkeler N, Kullman J, Murphy A. Troponin i isoform expression in human heart. Circulation Research. 1991;69:1409–1414. doi: 10.1161/01.res.69.5.1409. [DOI] [PubMed] [Google Scholar]
- 4.Marston SB, Redwood CS. Modulation of thin filament activation by breakdown or isoform switching of thin filament proteins. Circulation Research. 2003;93:1170–1178. doi: 10.1161/01.RES.0000105088.06696.17. [DOI] [PubMed] [Google Scholar]
- 5.Huang X, Pi Y, Lee KJ, Henkel AS, Gregg RG, Powers PA, Walker JW. Cardiac troponin i gene knockout: A mouse model of myocardial troponin i deficiency. Circ Res. 1999;84:1–8. doi: 10.1161/01.res.84.1.1. [DOI] [PubMed] [Google Scholar]
- 6.Solaro RJ, Henze M, Kobayashi T. Integration of troponin i phosphorylation with cardiac regulatory networks. Circ Res. 2013;112:355–366. doi: 10.1161/CIRCRESAHA.112.268672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murphy AM. Heart failure, myocardial stunning, and troponin: A key regulator of the cardiac myofilament. Congest Heart Fail. 2006;12:32–38. doi: 10.1111/j.1527-5299.2006.04320.x. quiz 39-40. [DOI] [PubMed] [Google Scholar]
- 8.Murphy AM, Kogler H, Marban E. A mouse model of myocardial stunning. Mol Med Today. 2000;6:330–331. doi: 10.1016/s1357-4310(00)01732-9. [DOI] [PubMed] [Google Scholar]
- 9.Zhang P, Kirk JA, Ji W, dos Remedios CG, Kass DA, Van Eyk JE, Murphy AM. Multiple reaction monitoring to identify site-specific troponin I phosphorylated residues in the failing human heart. Circulation. 2012;126:1828–1837. doi: 10.1161/CIRCULATIONAHA.112.096388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jin JP, Yang FW, Yu ZB, Ruse CI, Bond M, Chen A. The highly conserved cooh terminus of troponin I forms a Ca2+-modulated allosteric domain in the troponin complex. Biochemistry. 2001;40:2623–2631. doi: 10.1021/bi002423j. [DOI] [PubMed] [Google Scholar]
- 11.Hoffman RMB, Blumenschein TMA, Sykes BD. An interplay between protein disorder and structure confers the Ca2+ regulation of striated muscle. Journal of Molecular Biology. 2006;361:625–633. doi: 10.1016/j.jmb.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 12.Zhou Z, Li KL, Rieck D, Ouyang Y, Chandra M, Dong WJ. Structural dynamics of C-domain of cardiac troponin I protein in reconstituted thin filament. Journal of Biological Chemistry. 2012;287:7661–7674. doi: 10.1074/jbc.M111.281600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Willott RH, Gomes AV, Chang AN, Parvatiyar MS, Pinto JR, Potter JD. Mutations in troponin that cause HCM, DCM and RCM: What can we learn about thin filament function? Journal of Molecular and Cellular Cardiology. 2010;48:882–892. doi: 10.1016/j.yjmcc.2009.10.031. [DOI] [PubMed] [Google Scholar]
- 14.Pacher P, Nagayama T, Mukhopadhyay P, Batkai S, Kass DA. Measurement of cardiac function using pressure-volume conductance catheter technique in mice and rats. Nat Protoc. 2008;3:1422–1434. doi: 10.1038/nprot.2008.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meng T, Bu W, Ren X, Chen X, Yu J, Eckenhoff RG, Gao WD. Molecular mechanism of anesthetic-induced depression of myocardial contraction. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2016;30:2915–2925. doi: 10.1096/fj.201600290RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao WD, Perez NG, Marban E. Calcium cycling and contractile activation in intact mouse cardiac muscle. J Physiol. 1998;507(Pt 1):175–184. doi: 10.1111/j.1469-7793.1998.175bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramirez-Correa GA, Frazier AH, Zhu G, Zhang P, Rappold T, Kooij V, Bedja D, Snyder GA, Lugo-Fagundo NS, Hariharan R, Li Y, Shen X, Gao WD, Cingolani OH, Takimoto E, Foster DB, Murphy AM. Cardiac troponin I Pro82Ser variant induces diastolic dysfunction, blunts beta-adrenergic response, and impairs myofilament cooperativity. Journal of applied physiology. 2015;118:212–223. doi: 10.1152/japplphysiol.00463.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tocchetti CG, Caceres V, Stanley BA, Xie C, Shi S, Watson WH, O'Rourke B, Spadari-Bratfisch RC, Cortassa S, Akar FG, Paolocci N, Aon MA. GSH or palmitate preserves mitochondrial energetic/redox balance, preventing mechanical dysfunction in metabolically challenged myocytes/hearts from type 2 diabetic mice. Diabetes. 2012;61:3094–3105. doi: 10.2337/db12-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wijnker PJ, Li Y, Zhang P, Foster DB, dos Remedios C, Van Eyk JE, Stienen GJ, Murphy AM, van der Velden J. A novel phosphorylation site, serine 199, in the C-terminus of cardiac troponin I regulates calcium sensitivity and susceptibility to calpain-induced proteolysis. J Mol Cell Cardiol. 2015;82:93–103. doi: 10.1016/j.yjmcc.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blumenschein TMA, Stone DB, Fletterick RJ, Mendelson RA, Sykes BD. Dynamics of the C-terminal region of TnI in the troponin complex in solution. Biophysical Journal. 2006;90:2436–2444. doi: 10.1529/biophysj.105.076216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Narolska NA, Piroddi N, Belus A, Boontje NM, Scellini B, Deppermann S, Zaremba R, Musters RJ, dos Remedios C, Jaquet K, Foster DB, Murphy AM, van Eyk JE, Tesi C, Poggesi C, van der Velden J, Stienen GJ. Impaired diastolic function after exchange of endogenous troponin I with C-terminal truncated troponin I in human cardiac muscle. Circ Res. 2006;99:1012–1020. doi: 10.1161/01.RES.0000248753.30340.af. [DOI] [PubMed] [Google Scholar]
- 22.Endoh M. Force-frequency relationship in intact mammalian ventricular myocardium: Physiological and pathophysiological relevance. European journal of pharmacology. 2004;500:73–86. doi: 10.1016/j.ejphar.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 23.Takimoto E, Soergel DG, Janssen PM, Stull LB, Kass DA, Murphy AM. Frequency- and afterload-dependent cardiac modulation in vivo by troponin I with constitutively active protein kinase a phosphorylation sites. Circ Res. 2004;94:496–504. doi: 10.1161/01.RES.0000117307.57798.F5. [DOI] [PubMed] [Google Scholar]
- 24.Georgakopoulos D, Kass DA. Minimal force-frequency modulation of inotropy and relaxation of in situ murine heart. The Journal of Physiology. 2001;534:535–545. doi: 10.1111/j.1469-7793.2001.00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Di Lisa F, De Tullio R, Salamino F, Barbato R, Melloni E, Siliprandi N, Schiaffino S, Pontremoli S. Specific degradation of troponin T and I by mu-calpain and its modulation by substrate phosphorylation. Biochem J. 1995;308(Pt1):57–61. doi: 10.1042/bj3080057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao WD, Atar D, Liu Y, Perez NG, Murphy AM, Marban E. Role of troponin I proteolysis in the pathogenesis of stunned myocardium. Circ Res. 1997;80:393–399. [PubMed] [Google Scholar]
- 27.Ali MA, Fan X, Schulz R. Cardiac sarcomeric proteins: Novel intracellular targets of matrix metalloproteinase-2 in heart disease. Trends in cardiovascular medicine. 2011;21:112–118. doi: 10.1016/j.tcm.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 28.Borlaug BA. The pathophysiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2014;11:507–515. doi: 10.1038/nrcardio.2014.83. [DOI] [PubMed] [Google Scholar]
- 29.Brixius K, Hoischen S, Reuter H, Lasek K, Schwinger RH. Force/shortening-frequency relationship in multicellular muscle strips and single cardiomyocytes of human failing and nonfailing hearts. J Card Fail. 2001;7:335–341. doi: 10.1054/jcaf.2001.29902. [DOI] [PubMed] [Google Scholar]
- 30.Bhargava V, Shabetai R, Mathiasen RA, Dalton N, Hunter JJ, Ross J., Jr Loss of adrenergic control of the force-frequency relation in heart failure secondary to idiopathic or ischemic cardiomyopathy. Am J Cardiol. 1998;81:1130–1137. doi: 10.1016/s0002-9149(98)00133-7. [DOI] [PubMed] [Google Scholar]
- 31.Davis JS, Hassanzadeh S, Winitsky S, Lin H, Satorius C, Vemuri R, Aletras AH, Wen H, Epstein ND. The overall pattern of cardiac contraction depends on a spatial gradient of myosin regulatory light chain phosphorylation. Cell. 2001;107:631–641. doi: 10.1016/s0092-8674(01)00586-4. [DOI] [PubMed] [Google Scholar]
- 32.Stelzer JE, Larsson L, Fitzsimons DP, Moss RL. Activation dependence of stretch activation in mouse skinned myocardium: Implications for ventricular function. J Gen Physiol. 2006;127:95–107. doi: 10.1085/jgp.200509432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Linari M, Reedy MK, Reedy MC, Lombardi V, Piazzesi G. Ca-activation and stretch-activation in insect flight muscle. Biophys J. 2004;87:1101–1111. doi: 10.1529/biophysj.103.037374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galinska A, Hatch V, Craig R, Murphy AM, Van Eyk JE, Wang CL, Lehman W, Foster DB. The C terminus of cardiac troponin I stabilizes the Ca2+-activated state of tropomyosin on actin filaments. Circ Res. 2010;106:705–711. doi: 10.1161/CIRCRESAHA.109.210047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McKillop DF, Geeves MA. Regulation of the interaction between actin and myosin subfragment 1: Evidence for three states of the thin filament. Biophysical Journal. 1993;65:693–701. doi: 10.1016/S0006-3495(93)81110-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wijnker PJ, Foster DB, Tsao AL, Frazier AH, dos Remedios CG, Murphy AM, Stienen GJ, van der Velden J. Impact of site-specific phosphorylation of protein kinase a sites ser23 and ser24 of cardiac troponin i in human cardiomyocytes. Am J Physiol Heart Circ Physiol. 2013;304:H260–268. doi: 10.1152/ajpheart.00498.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang H, Grant JE, Doede CM, Sadayappan S, Robbins J, Walker JW. PKC-beta II sensitizes cardiac myofilaments to Ca2+ by phosphorylating troponin I on threonine-144. J Mol Cell Cardiol. 2006;41:823–833. doi: 10.1016/j.yjmcc.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 38.Wijnker PJ, Sequeira V, Foster DB, Li Y, Dos Remedios CG, Murphy AM, Stienen GJ, van der Velden J. Length-dependent activation is modulated by cardiac troponin I bisphosphorylation at Ser23 and Ser24 but not by Thr143 phosphorylation. Am J Physiol Heart Circ Physiol. 2014;306:H1171–1181. doi: 10.1152/ajpheart.00580.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.