Abstract
Choline is an essential nutrient for humans. Studies in rats and mice have shown that high choline intake during gestation or the perinatal period improves cognitive function in adulthood, prevents memory decline of old age, and protects the brain from damage and cognitive and neurological deterioration associated with epilepsy and hereditary conditions such as Down’s and Rett syndromes. These behavioral changes are accompanied by modified patterns of expression of hundreds of cortical and hippocampal genes including those encoding proteins central for learning and memory processing. The effects of choline correlate with cerebral cortical changes in DNA and histone methylation, thus suggesting an epigenomic mechanism of action of perinatal choline.
Keywords: brain, choline, DNA methylation, memory, nutrition, pregnancy, epilepsy, Down’s syndrome, Rett syndrome
Choline, an essential nutrient for humans
In 1998 the Food and Nutrition Board (FNB) of the Institute of Medicine of the National Academy of Sciences of the United States of America issued a report entitled “Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline” that for the first time included choline as an essential nutrients for humans among other water-soluble vitamins (1). Because there were insufficient data to generate Recommended Daily Allowance values, the FNB issued Adequate Intake (AI) recommendations (Table 1). The AI calls for the average intake of 7.5 mg of choline daily per kg of body weight. Given the high nutritional needs for pregnant and breast feeding women, the AI is increased for them in order to satisfy the requirements of the fetus and baby whose choline is supplied via placenta (2) and milk (3, 4), respectively. The AI values were established primarily to ensure that dietary choline is sufficient to prevent liver dysfunction associated with low choline consumption observed in adult men (5). Subsequent studies have shown that choline deficiency also causes muscle damage (6) and induces apoptotic death of lymphocytes (7). Since the issuance of the FNB report, the establishment of the United States Department of Agriculture (USDA) Database for the Choline Content of Common Foods (8) – has become one of the most valuable resources for epidemiological studies on choline nutrition and has helped to investigate the relationship between choline nutrition and disease. A common finding in such studies is the realization that even in an affluent country like the United States the majority of people consume less choline than the AI value (9–12) (Fig. 1). The use of the USDA database also revealed that women in the highest quintile of choline and its metabolite, betaine, consumption as adults had reduced risk of breast cancer (10) and that high betaine intake lowers the risk of colorectal adenoma in women (13) and of esophageal cancer in both men and women (14). Moreover, high choline consumption during pregnancy reduced the risk of neural tube defects in offspring (15, 16). The latter studies were the first to provide evidence for the significance of choline nutrition during pregnancy for normal development of the human central nervous system. In this minireview we summarize the results of studies in animal models on the significance of choline nutrition in early development on brain function later in life. The overall message from these investigations is that high choline intake during the perinatal period is neuroprotective in a variety of animal models of neuronal dysfunction, including that evoked by aging (17–19), seizures (20–23), alcohol consumption (24–29) and genetic variation (30–36).
Table 1.
Choline Adequate Intake (AI) (mg/day)
| Life stage | Age | Females | Males |
|---|---|---|---|
| Infants | 0–6 months | 125 | 125 |
| Infants | 7–12 months | 150 | 150 |
| Children | 1–3 years | 200 | 200 |
| Children | 4–8 years | 250 | 250 |
| Children | 9–13 years | 375 | 375 |
| Adolescents | 14–18 years | 400 | 550 |
| Adults | 19 years and older | 425 | 550 |
| Pregnancy | All ages | 450 | – |
| Breast-feeding | All ages | 550 | – |
Figure 1. Americans consume less choline than recommended.
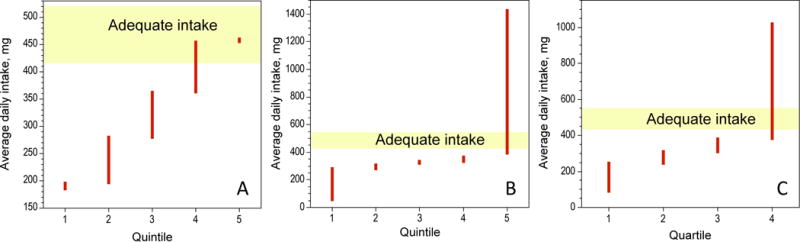
Average daily choline intake reported in three independent studies. The red bars indicate the intake within the bounding values of the quintiles (A, B) or quartiles (C). The yellow strip indicates the Adequate Intake for adults. Data from refs. 10–12 (panels A-C, respectively).
Choline nutrition and cognitive function: protection against age-related memory decline and advancement of hippocampal development
In rats, high maternal choline consumption during pregnancy has profound and long-term cognitive enhancing effects in offspring (19, 37–47). Interestingly, choline is not effective in all periods of pregnancy (pregnancy in rats lasts 20–22 days) but rather exerts its effects during the second half of gestation. This has been established by the studies of Meck and Williams and their colleagues who provided pregnant rats with approximately 4 times more choline than that present in control rodent diets during embryonic days (E) 6–11 and 12–17 and found that the offspring of dams supplemented with choline during the latter (but not the former) period outperformed the control animals in a radial maze spatial memory task (19). This model of choline supplementation or deprivation in pregnant rats during ~E11–17, has become quite common and many investigators adapted it for studies on choline and brain development and cognitive function. The overall observations from these studies is that choline deficiency causes impairments in certain memory tasks (17), whereas choline supplementation improves memory and attention (17, 37, 39–41, 43, 48, 49) and, prevents age-related memory decline (17, 49), i.e. cognitive decline is not an inevitable outcome of old age, but rather can be prevented by increased supply of choline during a critical period of prenatal development. Interestingly, the cognition enhancing effects of high prenatal intake of choline can be seen already at very early age as the animals acquire developmental cognitive milestones. One such milestone is the ability to navigate using relational cues, considered to signal the onset of hippocampal function (50). This ability was assessed by Mellott et al (43), who studied spatial/relational and cued navigational performance of 18–22 day old choline supplemented and control rats using the Morris water maze. At this age, both control and choline-supplemented rats could learn the location of a platform that was directly cued (Fig. 2A) indicating that they had similar visual and swimming abilities. In contrast, at P18–19, only the prenatally choline-supplemented rats were able to use relational cues to remember the hidden platform location during the first 5 trials, while control rats showed no spatial memory ability (Fig. 2B). When these rats were re-tested 3 days later on the same spatial task, both prenatally choline supplemented and control rats shortened their escape latencies over the 5 acquisition trials (43). Thus, prenatal choline-supplementation causes an approximately 3-day advancement in hippocampal development.
Figure 2. Performance of P18–19 prenatally choline supplemented and control rats during cued- and spatial training in the Morris water maze.
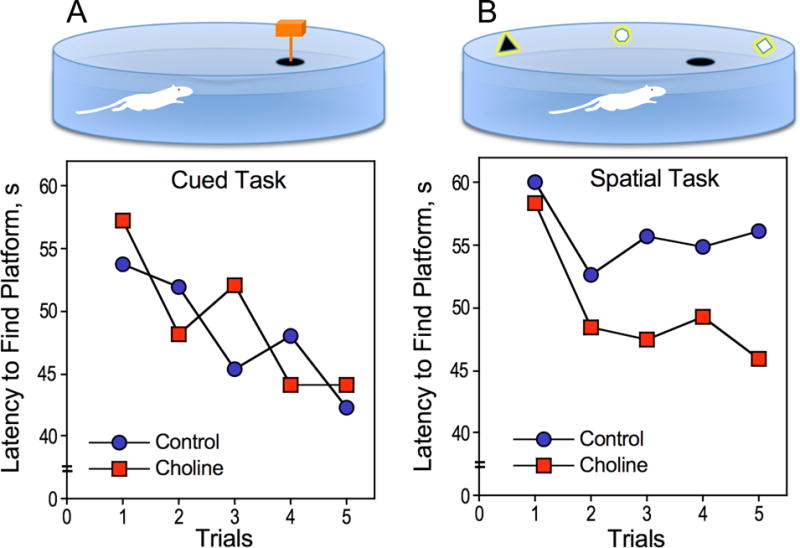
A) Cued training, rats learn the marked platform location (top panel): both choline-supplemented and control rats at P18–19 learned the location of the platform (lower panel). B) Spatial training, rats use relational cues to learn how to navigate to the platform (top panel): only choline supplemented rats showed improved performance across trials (lower panel). Data from ref. 43.
Neuroprotective actions of choline in rat models of epilepsy
Status epilepticus, a period of prolonged seizures, is a neurological condition that produces multiple degenerative and regenerative changes in the hippocampus, that are thought to contribute to the development of temporal lobe epilepsy in humans and in rodent models. Hippocampal pathophysiology following status epilepticus includes neuronal loss, γ-aminobutyric acid (GABA) system alterations, reactive gliosis, altered growth factor levels, and abnormal dentate gyrus cell proliferation and neurogenesis (51). These changes following status epilepticus are accompanied by cognitive deficits in hippocampal-dependent tasks, which are present both before and after the emergence of spontaneous recurrent motor seizures (51). Therapeutic methods that could prevent or reduce this seizure-related brain dysfunction are needed and several studies have addressed this need by testing the effects of high choline intake in rat models of chemically-evoked epilepsy. In pilocarpine- (20) and kainic acid- (21, 23) induced models of status epilepticus, prenatal choline supplementation attenuated the impairments of visual-spatial memory assessed with the Morris water maze test. Moreover, these protective actions of choline were accompanied by markedly attenuated seizure-induced hippocampal neurodegeneration and dentate gyrus cell proliferation (22). Choline supplementation also prevented hippocampal loss of the GAD65 mRNA encoding the GABA-synthesizing enzyme, glutamic acid decarboxylase (22). As in the other rodent models (22, 31, 34, 52–55), choline supplementation also increased the hippocampal levels of nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF) and insulin-like growth factor 1 (IGF1), observed prior to the administration of the seizure-inducing kainic acid (22), indicating that this nutritional treatment may establish a neuroprotective hippocampal microenvironment that dampens the neuropathological response to and/or helps facilitate recovery from status epilepticus to protect cognitive function.
Neuroprotective actions of choline in mouse models of heritable human disease
In addition to studies on the efficacy of dietary choline in models of epilepsy, several investigators tested the hypothesis that high choline intake early in life could be effective in ameliorating the symptoms of genetically-determined neurological disease.
Down’s syndrome is one of the most common forms of mental retardation affecting approximately 1 in 700 births in the United States (56). The disorder is caused by meiotic non-disjunction of chromosome 21 resulting in babies with 3 copies of this chromosome in their cells (trisomy 21). Moon et al (35) used a mouse model of Down’s syndrome (Ts65DN mice) to test the hypothesis that choline supplementation from conception to weaning could prevent some of the neurological and cognitive deficits observed in these mice. The genome of Ts65Dn mice was engineered to carry a third copy of the distal region of mouse chromosome 16 which contains approximately 94 genes orthologous to the Down’s syndrome critical region of the human chromosome 21 (57). The adult offspring of choline-supplemented Ts65Dn dams performed significantly better than control Ts65Dn mice in several visual attention tasks (35). In some of these tasks the choline supplemented Ts65DN mice did not differ from the wild type controls (35). These findings indicate that perinatal choline supplementation significantly ameliorates cognitive dysfunction in Down’s syndrome.
Another series of studies (30–34) tested the above hypothesis in mouse models of Rett syndrome (30–34) – a genetic neurological disorder of childhood that also represents a common (approximately 1 in 10,000 births) forms of mental retardation but affecting almost exclusively girls (58). Rett syndrome is typically caused by a mutation in the X-chromosome linked methyl-CpG-binding protein 2 (MECP2) gene, and several mouse models with inactivating mutations of Mecp2 have been developed. As in human disease the mice are born apparently normal but tend to succumb to severe neurological disease within weeks. Unlike the studies described above, the Rett syndrome model mice were supplemented with choline via mothers’ milk from birth to weaning. In Mecp2 null males, choline supplementation improved motor coordination and locomotor activity and enhanced grip strength in females (30). These changes were accompanied by increase in the total brain volume in females, and cerebellar volume in males (32). As in prenatally choline-supplemented rats (see below), postnatal choline supplementation increased striatal NGF expression in both wild-type and Mecp2 null mice (31), suggesting that neuronal proliferation and survival may contribute to improved motor performance in this model of Rett syndrome. Choline supplementation also increased the brain levels of N-acetyl aspartate, a marker of neuronal integrity, as assessed by nuclear magnetic resonance spectroscopy (33). In mice with a different Mecp2 mutation, early postnatal choline treatment prevented deficits in locomotor activity (34), ameliorated the decline in the activity of the acetylcholine-synthesizing enzyme, choline acetyltransferase in the striatum and increased NGF and BDNF expression in the cerebral cortex and hippocampus (34). Together, these data suggest that postnatal nutritional supplementation with choline may improve neuronal function in Rett syndrome patients and thus should be considered as a potential therapy for this disease.
In order to evaluate the possibility that perinatal choline treatment could be useful as in preventing certain psychiatric disorders, Stevens et al (36) studied the effects of choline supplementation in the DBA/2 mouse strain that is frequently used as a model of schizophrenia (59). The mice were supplemented with choline from conception to weaning by providing high-choline diet to pregnant and lactating dams. DBA/2 mice raised on control diets displayed the characteristic abnormality in sensory processing (that is also present in patients with schizophrenia), whereas prenatally choline-supplemented mice had a normal sensory processing phenotype (36) suggesting that this nutritional treatment may reduce the risk of schizophrenia.
Molecular and cellular correlates and possible mechanisms of the neuroprotective actions of choline
The molecular and cellular mechanisms that govern the neuroprotective actions of perinatal choline nutrition remain to be elucidated. However, a great deal is known about the correlative brain alterations that permit the formulation of plausible hypotheses regarding these mechanisms. Already during brain development, i.e. at the time of altered choline supply, changes in brain structure are observed. Choline deficiency during pregnancy inhibits fetal cell proliferation and stimulates apoptosis in the hippocampus (60, 61), whereas gestational choline supplementation stimulates hippocampal cell division (62). These structural changes in prenatal brain subsequently are followed by neuroanatomical, neurochemical, electrophysiological, and molecular differences in the adult and aged animal. Some aspects of learning and memory require adult neurogenesis that occurs in the dentate gyrus of the hippocampus throughout the lifetime (63, 64). Prenatal choline supplementation enhances this process while prenatal choline deficiency impairs it (52–54). This effect of choline supplementation was also seen in aged rats and correlated with a highly trophic microenvironment within the hippocampus of the prenatally choline supplemented rats that included increased concentrations of NGF, BDNF, IGF1,insulin-like growth factor 2 (IGF2), and vascular endothelial growth factor (VEGF) in these animals as compared to controls (22, 52–55). Prenatal choline supplementation increases the size of the basal forebrain cholinergic neurons (65) that participate in the processes of learning and memory (66, 67) and augments acetylcholine synthesis and release from these neurons (49, 68). Prenatal choline supplementation also increases the activation of key molecular components of memory processing (69), such that phosphorylation of hippocampal mitogen-activated protein kinase (MAPK) and cAMP response element binding protein (CREB) in response to activation of glutamatergic receptors (43). Interestingly hippocampal electrophysiological synaptic plasticity measures termed long-term potentiation (LTP), that are considered as a correlates of certain neuronal aspects of memory, were modulated by prenatal choline in a fashion consistent with these molecular alterations. Prenatal choline supplementation enhanced hippocampal LTP in the CA1 region by decreasing the stimulus intensity required for LTP induction (70, 71), possibly due to an augmented N-methyl-D-aspartate receptor-mediated neurotransmission (72). Mellott et al (73) analyzed gene expression patterns in brains of prenatally choline-deficient, choline-supplemented, and control rats using microarrays and found 530 hippocampal and 815 cerebral cortical mRNA species whose levels were modulated by prenatal choline status. The protein products of several of these genes participate in signaling pathways involved in memory processes (73) and thus may mediate the observed choline-induced changes in LTP and behavior.
In addition, recent advances in the field of epigenetics have provided the conceptual and experimental framework to explain how such changes in gene expression patterns can be transmitted following cell mitosis. The central molecular mechanism that permits this type of long-term modulation of cellular phenotypes is methylation of DNA at the 5-position of cytosine residues within CpG sequences to form 5-methylcytosine (5mC). The transcription of genes whose regulatory elements are methylated tends to be different than when the same regions are not methylated due to a concerted change in the interaction of those elements with a complex network of proteins, including transcription factors (74). This change results in an altered phenotype governed by DNA methylation. The pattern of DNA methylation can be propagated through cell divisions because, after DNA replication, the unmethylated daughter strand in hemimethylated DNA becomes symmetrically methylated by the enzyme DNA methyltransferase 1 (DNMT1) (75). The process of DNA methylation is dynamic (76) and responds to the environment, including the availability of nutrients. In particular, DNA methylation is modulated by the availability of nutrients that serve as methyl group donors and cofactors, such as choline, betaine, methionine, folic acid and vitamin B12 (Fig. 3). This effect is explained by the direct relationship between dietary intake of choline (and/or other methyl groups) and tissue levels of S-adenosylmethionine (the methyl group donor for most enzymatic methylation reactions) that is frequently observed (77). The hypothesis that choline intake by pregnant rats might alter DNA methylation in the fetus was tested in a study by Kovacheva et al (77) who evaluated these parameters in liver and cerebral cortex on E17 in rats following altered dietary supply of choline that had begun on E11. The investigators focused on the differentially methylated region 2 (DMR2) of the Igf2 gene because the DMR2 methylation changes during development (78). Choline-deficient embryos had higher degree of DMR2 methylation as compared to the control and choline-supplemented rats. One possible mechanism that leads to changes in the global, as well as gene-specific, DNA methylation is via alteration in the activity of DNMTs. DNMT1 is important for maintaining the methylation pattern of the Igf2 gene and Dnmt1 knockout mice have abnormal expression of Igf2 (79). In liver of choline-deficient embryos, Dnmt1 mRNA was overexpressed by over 50% as compared to control and choline-supplemented fetuses. The data suggested that maternal choline deficiency causes a compensatory induction of Dnmt1 expression in the fetus thus preventing the loss of DNA methylation when limited amounts of choline are present. As noted above, IGF2 expression in developing and adult brain was governed by prenatal choline intake (55, 73, 77) and recent studies implicate IGF2 as a critical component of memory consolidation mechanisms (80) suggesting that high IGF2 levels observed in brains of prenatally choline-supplemented rats (55, 73) may be part of the mechanism of cognitive enhancement that characterizes these animals. Data showing that maternal choline supply during pregnancy modifies fetal DNA methylation suggest that an epigenomic mechanism contributes to the long-term developmental effects of varied choline intake in utero (77, 81, 82). In addition to the central role of DNA methylation in brain development, these processes are highly dynamic in adult brain and there is considerable evidence that they modulate the expression of key genes of synaptic plasticity (83–87) and are involved in mechanisms of learning and memory (88–92). Thus, it is likely that choline nutrition influences brain development and cognitive function via its effects on DNA methylation.
Figure 3. Choline and methyl group metabolism.
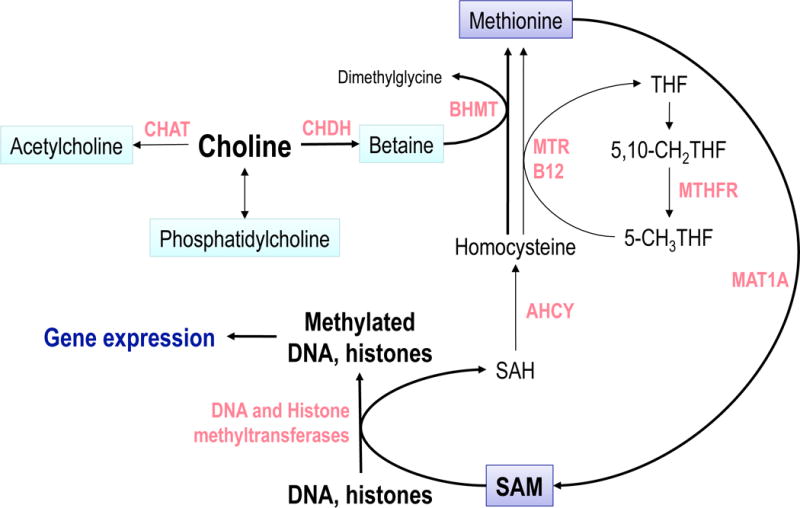
Choline is used as a precursor of phosphatidylcholine, acetylcholine [in a reaction catalyzed by choline acetyltransferase (CHAT)], or betaine [in a reaction catalyzed by choline dehydrogenase (CHDH)]. The methyl groups of betaine are used by betaine:homocysteine S-methyltransferase (BHMT) to regenerate methionine from homocysteine. In an alternative pathway, catalyzed by vit. B12-requiring 5-methyltetrahydrofolate-homocysteine S-methyltransferase (MTR), methyltetrahydrofolate (5-CH3THF) is used as a methyl donor. Methionine is used as a precursor of S-adenosylmethionine (SAM) in a reaction catalyzed by methionine adenosyltransferase(s) (MAT1A). SAM is used by multiple methylating enzymes including DNA and histone methyltransferases that use SAM as a donor of methyl groups to methylate DNA at the 5-position of cytosine residues within the CpG sequences and histones at specific lysine and arginine residues. The DNA methylation state and pattern exerts a modulatory influence on expression of multiple genes (e.g. Igf2). The second product of this, and all other SAM-requiring methylation reactions, S-adenosylhomocysteine (SAH) is hydrolyzed to free homocysteine by SAH hydrolase (AHCY). The metabolic pathway linking choline to DNA and histone methylations is indicated by thick arrows.
Conclusions
High choline intake during gestation and early postnatal period has been repeatedly described as a robust cognitive enhancing regimen and is neuroprotective in a variety of animal models of neuronal damage. Data showing that maternal choline supply during pregnancy modifies fetal DNA and histone methylation suggest that a concerted epigenomic mechanism contributes to these long-term effects of varied choline intake in utero (77, 81, 82, 93, 94). Recent data indicate that choline nutrition in adulthood may also be critical for normal cognitive function in people as suggested by a study performed on 1391 normal adult and elderly people (average age 61 years) that reported that verbal and visual memory function correlated positively with the amount of dietary choline consumption, with poorest performance in individuals with lowest choline intake and best performance in those who were consuming the highest amounts of choline (12).
Acknowledgments
Some of the studies reviewed here were supported by NIH grants AG009525 and CA120488 to JKB
Abbreviations
- AI
adequate intake
- BDNF
brain-derived neurotrophic factor
- CREB
cAMP response element binding protein
- DMR
differentially methylated region
- DNMT
DNA methyltransferases
- FNB
Food and Nutrition Board
- GABA
γ-aminobutyric acid
- IGF
insulin-like growth factor
- LTP
termed long-term potentiation
- MAPK
mitogen-activated protein kinase
- MECP2
methyl-CpG-binding protein 2
- NGF
nerve growth factor
- USDA
United States Department of Agriculture
- VEGF
vascular endothelial growth factor
References
- 1.FNB. Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, panthotenic acid, biotin, and choline. Washington, D.C.: National Academy Press; 1998. [PubMed] [Google Scholar]
- 2.Garner SC, Mar MH, Zeisel SH. Choline distribution and metabolism in pregnant rats and fetuses are influenced by the choline content of the maternal diet. J Nutr. 1995;125:2851–8. doi: 10.1093/jn/125.11.2851. [DOI] [PubMed] [Google Scholar]
- 3.Holmes-McNary MQ, Cheng WL, Mar MH, Fussell S, Zeisel SH. Choline and choline esters in human and rat milk and in infant formulas. Am J Clin Nutr. 1996;64:572–6. doi: 10.1093/ajcn/64.4.572. [DOI] [PubMed] [Google Scholar]
- 4.Zeisel SH, Char D, Sheard NF. Choline, phosphatidylcholine and sphingomyelin in human and bovine milk and infant formulas. J Nutr. 1986;116:50–8. doi: 10.1093/jn/116.1.50. [DOI] [PubMed] [Google Scholar]
- 5.Zeisel SH, Da Costa K-A, Franklin PD, Alexander EA, Lamont JT, Sheard NF, Beiser A. Choline, an essential nutrient for humans. FASEB J. 1991;5:2093–8. [PubMed] [Google Scholar]
- 6.da Costa KA, Badea M, Fischer LM, Zeisel SH. Elevated serum creatine phosphokinase in choline-deficient humans: mechanistic studies in C2C12 mouse myoblasts. Am J Clin Nutr. 2004;80:163–70. doi: 10.1093/ajcn/80.1.163. [DOI] [PubMed] [Google Scholar]
- 7.da Costa KA, Niculescu MD, Craciunescu CN, Fischer LM, Zeisel SH. Choline deficiency increases lymphocyte apoptosis and DNA damage in humans. Am J Clin Nutr. 2006;84:88–94. doi: 10.1093/ajcn/84.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patterson KY, Bhagwat AS, Williams JR, Howe JC, Holden JM, Zeisel SH, et al. USDA database for the choline content of common foods. Release two. http://www.ars.usda.gov/Services/docs.htm?docid=6232 (Accessed August 15 2011)
- 9.Jensen HH, Batres-Marquez SP, Carriquiry A, Schalinske KL. Choline in the diets of the US population: NHANES, 2003–2004. FASEB J. 2007;21:LB219. [Google Scholar]
- 10.Xu X, Gammon MD, Zeisel SH, Lee YL, Wetmur JG, Teitelbaum SL, et al. Choline metabolism and risk of breast cancer in a population-based study. FASEB J. 2008;22:2045–52. doi: 10.1096/fj.07-101279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cho E, Holmes MD, Hankinson SE, Willett WC. Choline and betaine intake and risk of breast cancer among post-menopausal women. Br J Cancer. 2010;102:489–94. doi: 10.1038/sj.bjc.6605510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poly C, Massaro JM, Seshadri S, Wolf PA, Cho E, Krall E, et al. The relation of dietary choline to cognitive performance and white-matter hyperintensity in the Framingham Offspring Cohort. Am J Clin Nutr. 2011;94:1584–91. doi: 10.3945/ajcn.110.008938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho E, Willett WC, Colditz GA, Fuchs CS, Wu K, Chan AT, et al. Dietary choline and betaine and the risk of distal colorectal adenoma in women. J Natl Cancer Inst. 2007;99:1224–31. doi: 10.1093/jnci/djm082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ibiebele TI, Hughes MC, Pandeya N, Zhao Z, Montgomery G, Hayward N, et al. High intake of folate from food sources is associated with reduced risk of esophageal cancer in an Australian population. J Nutr. 2011;141:274–83. doi: 10.3945/jn.110.131235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaw GM, Carmichael SL, Yang W, Selvin S, Schaffer DM. Periconceptional dietary intake of choline and betaine and neural tube defects in offspring. Am J Epidemiol. 2004;160:102–9. doi: 10.1093/aje/kwh187. [DOI] [PubMed] [Google Scholar]
- 16.Shaw GM, Finnell RH, Blom HJ, Carmichael SL, Vollset SE, Yang W, Ueland PM. Choline and Risk of Neural Tube Defects in a Folate-Fortified Population. Epidemiology. 2009;20:714–9. doi: 10.1097/EDE.0b013e3181ac9fe7. [DOI] [PubMed] [Google Scholar]
- 17.Meck WH, Williams CL. Simultaneous temporal processing is sensitive to prenatal choline availability in mature and aged rats. Neuroreport. 1997;8:3045–51. doi: 10.1097/00001756-199709290-00009. [DOI] [PubMed] [Google Scholar]
- 18.Meck WH, Williams CL. Metabolic imprinting of choline by its availability during gestation: implications for memory and attentional processing across the lifespan. Neurosci Biobehav Rev. 2003;27:385–99. doi: 10.1016/s0149-7634(03)00069-1. [DOI] [PubMed] [Google Scholar]
- 19.Meck WH, Williams CL, Cermak JM, Blusztajn JK. Developmental periods of choline sensitivity provide an ontogenetic mechanism for regulating memory capacity and age-related dementia. Front Integr Neurosci. 2007;1:7. doi: 10.3389/neuro.07.007.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang Y, Liu Z, Cermak JM, Tandon P, Sarkisian MR, Stafstrom CF, et al. Protective effects of prenatal choline supplementation on seizure-induced memory impairment. J Neurosci. 2000;20:RC109. doi: 10.1523/JNEUROSCI.20-22-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holmes GL, Yang Y, Liu Z, Cermak JM, Sarkisian MR, Stafstrom CE, et al. Seizure-induced memory impairment is reduced by choline supplementation before or after status epilepticus. Epilepsy Res. 2002;48:3–13. doi: 10.1016/s0920-1211(01)00321-7. [DOI] [PubMed] [Google Scholar]
- 22.Wong-Goodrich SJ, Mellott TJ, Glenn MJ, Blusztajn JK, Williams CL. Prenatal choline supplementation attenuates neuropathological response to status epilepticus in the adult rat hippocampus. Neurobiol Dis. 2008;30:255–69. doi: 10.1016/j.nbd.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong-Goodrich SJ, Mellott TJ, Liu B, Blusztajn JK, Williams CL. Water maze experience and prenatal choline supplementation differentially promote long-term hippocampal recovery from seizures in adulthood. Hippocampus. 2011;21:584–608. doi: 10.1002/hipo.20783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas JD, La Fiette MH, Quinn VR, Riley EP. Neonatal choline supplementation ameliorates the effects of prenatal alcohol exposure on a discrimination learning task in rats. Neurotoxicol Teratol. 2000;22:703–11. doi: 10.1016/s0892-0362(00)00097-0. [DOI] [PubMed] [Google Scholar]
- 25.Thomas JD, Biane JS, O’Bryan KA, O’Neill TM, Dominguez HD. Choline supplementation following third-trimester-equivalent alcohol exposure attenuates behavioral alterations in rats. Behav Neurosci. 2007;121:120–30. doi: 10.1037/0735-7044.121.1.120. [DOI] [PubMed] [Google Scholar]
- 26.Ryan SH, Williams JK, Thomas JD. Choline supplementation attenuates learning deficits associated with neonatal alcohol exposure in the rat: effects of varying the timing of choline administration. Brain Res. 2008;1237:91–100. doi: 10.1016/j.brainres.2008.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomas JD, Abou EJ, Dominguez HD. Prenatal choline supplementation mitigates the adverse effects of prenatal alcohol exposure on development in rats. Neurotoxicol Teratol. 2009;31:303–11. doi: 10.1016/j.ntt.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomas JD, Idrus NM, Monk BR, Dominguez HD. Prenatal choline supplementation mitigates behavioral alterations associated with prenatal alcohol exposure in rats. Birth defects research. 2010;88:827–37. doi: 10.1002/bdra.20713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomas JD, Tran TD. Choline supplementation mitigates trace, but not delay, eyeblink conditioning deficits in rats exposed to alcohol during development. Hippocampus. 2011;22:619–30. doi: 10.1002/hipo.20925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nag N, Berger-Sweeney JE. Postnatal dietary choline supplementation alters behavior in a mouse model of Rett syndrome. Neurobiol Dis. 2007;26:473–80. doi: 10.1016/j.nbd.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 31.Nag N, Mellott TJ, Berger-Sweeney JE. Effects of postnatal dietary choline supplementation on motor regional brain volume and growth factor expression in a mouse model of Rett syndrome. Brain Res. 2008;1237:101–9. doi: 10.1016/j.brainres.2008.08.042. [DOI] [PubMed] [Google Scholar]
- 32.Ward BC, Agarwal S, Wang K, Berger-Sweeney J, Kolodny NH. Longitudinal brain MRI study in a mouse model of Rett Syndrome and the effects of choline. Neurobiol Dis. 2008;31:110–9. doi: 10.1016/j.nbd.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 33.Ward BC, Kolodny NH, Nag N, Berger-Sweeney JE. Neurochemical changes in a mouse model of Rett syndrome: changes over time and in response to perinatal choline nutritional supplementation. J Neurochem. 2009;108:361–71. doi: 10.1111/j.1471-4159.2008.05768.x. [DOI] [PubMed] [Google Scholar]
- 34.Ricceri L, De Filippis B, Fuso A, Laviola G. Cholinergic hypofunction in MeCP2-308 mice: beneficial neurobehavioural effects of neonatal choline supplementation. Behav Brain Res. 2011;221:623–9. doi: 10.1016/j.bbr.2011.03.051. [DOI] [PubMed] [Google Scholar]
- 35.Moon J, Chen M, Gandhy SU, Strawderman M, Levitsky DA, Maclean KN, Strupp BJ. Perinatal choline supplementation improves cognitive functioning and emotion regulation in the Ts65Dn mouse model of Down syndrome. Behav Neurosci. 2010;124:346–61. doi: 10.1037/a0019590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stevens KE, Adams CE, Yonchek J, Hickel C, Danielson J, Kisley MA. Permanent improvement in deficient sensory inhibition in DBA/2 mice with increased perinatal choline. Psychopharmacology (Berl) 2008;198:413–20. doi: 10.1007/s00213-008-1170-3. [DOI] [PubMed] [Google Scholar]
- 37.Meck WH, Smith RA, Williams CL. Pre- and postnatal choline supplementation produces long-term facilitation of spatial memory. Dev Psychobiol. 1988;21:339–53. doi: 10.1002/dev.420210405. [DOI] [PubMed] [Google Scholar]
- 38.Schenk F, Brandner C. Indirect effect of peri- and postnatal choline treatment on place-learning abilities in rat. Psychobiology. 1995;23:302–13. [Google Scholar]
- 39.Meck WH, Williams CL. Characterization of the facilitative effects of perinatal choline supplementation on timing and temporal memory. Neuroreport. 1997;8:2831–5. doi: 10.1097/00001756-199709080-00005. [DOI] [PubMed] [Google Scholar]
- 40.Meck WH, Williams CL. Perinatal choline supplementation increases the threshold for chunking in spatial memory. Neuroreport. 1997;8:3053–9. doi: 10.1097/00001756-199709290-00010. [DOI] [PubMed] [Google Scholar]
- 41.Meck WH, Williams CL. Choline supplementation during prenatal development reduces proactive interference in spatial memory. Dev Brain Res. 1999;118:51–9. doi: 10.1016/s0165-3806(99)00105-4. [DOI] [PubMed] [Google Scholar]
- 42.Tees RC, Mohammadi E. The effects of neonatal choline dietary supplementation on adult spatial and configural learning and memory in rats. Dev Psychobiol. 1999;35:226–40. [PubMed] [Google Scholar]
- 43.Mellott TJ, Williams CL, Meck WH, Blusztajn JK. Prenatal choline supplementation advances hippocampal development and enhances MAPK and CREB activation. FASEB J. 2004;18:NIL412–NIL27. doi: 10.1096/fj.03-0877fje. [DOI] [PubMed] [Google Scholar]
- 44.Buhusi CV, Lamoureux JA, Meck WH. Prenatal choline supplementation increases sensitivity to contextual processing of temporal information. Brain Res. 2008;1237:204–13. doi: 10.1016/j.brainres.2008.08.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheng RK, MacDonald CJ, Williams CL, Meck WH. Prenatal choline supplementation alters the timing, emotion, and memory performance (TEMP) of adult male and female rats as indexed by differential reinforcement of low-rate schedule behavior. Learn Mem. 2008;15:153–62. doi: 10.1101/lm.729408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cheng RK, Scott AC, Penney TB, Williams CL, Meck WH. Prenatal-choline supplementation differentially modulates timing of auditory and visual stimuli in aged rats. Brain Res. 2008;1237:167–75. doi: 10.1016/j.brainres.2008.08.062. [DOI] [PubMed] [Google Scholar]
- 47.Lamoureux JA, Meck WH, Williams CL. Prenatal choline availability alters the context sensitivity of Pavlovian conditioning in adult rats. Learn Mem. 2008;15:866–75. doi: 10.1101/lm.1058708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meck WH, Smith RA, Williams CL. Organizational changes in cholinergic activity and enhanced visuospatial memory as a function of choline administered prenatally or postnatally or both. Behav Neurosci. 1989;103:1234–41. doi: 10.1037//0735-7044.103.6.1234. [DOI] [PubMed] [Google Scholar]
- 49.Meck WH, Williams CL, Cermak JM, Blusztajn JK. Developmental periods of choline sensitivity provide an ontogenetic mechanism for regulating memory capacity and age-related dementia. Front Integr Neurosci. 2008;1:7. doi: 10.3389/neuro.07.007.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Castro CA, Rudy JW. Early-life malnutrition selectively retards the development of distal- but not proximal-cue navigation. DevPsychobiol. 1987;20:521–37. doi: 10.1002/dev.420200506. [DOI] [PubMed] [Google Scholar]
- 51.Acharya MM, Hattiangady B, Shetty AK. Progress in neuroprotective strategies for preventing epilepsy. Prog Neurobiol. 2008;84:363–404. doi: 10.1016/j.pneurobio.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Glenn MJ, Kirby ED, Gibson EM, Wong-Goodrich SJ, Mellott TJ, Blusztajn JK, Williams CL. Age-related declines in exploratory behavior and markers of hippocampal plasticity are attenuated by prenatal choline supplementation in rats. Brain Res. 2008;1237:110–23. doi: 10.1016/j.brainres.2008.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Glenn MJ, Gibson EM, Kirby ED, Mellott TJ, Blusztajn JK, Williams CL. Prenatal choline availability modulates hippocampal neurogenesis and neurogenic responses to enriching experiences in adult female rats. Eur J Neurosci. 2007;25:2473–82. doi: 10.1111/j.1460-9568.2007.05505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wong-Goodrich SJ, Glenn MJ, Mellott TJ, Blusztajn JK, Meck WH, Williams CL. Spatial memory and hippocampal plasticity are differentially sensitive to the availability of choline in adulthood as a function of choline supply in utero. Brain Res. 2008;1237:153–66. doi: 10.1016/j.brainres.2008.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Napoli I, Blusztajn JK, Mellott TJ. Prenatal choline supplementation in rats increases the expression of IGF2 and its receptor IGF2R and enhances IGF2-induced acetylcholine release in hippocampus and frontal cortex. Brain Res. 2008;1237:124–35. doi: 10.1016/j.brainres.2008.08.046. [DOI] [PubMed] [Google Scholar]
- 56.Parker SE, Mai CT, Canfield MA, Rickard R, Wang Y, Meyer RE, et al. Updated National Birth Prevalence estimates for selected birth defects in the United States, 2004–2006. Birth defects research. 2010;88:1008–16. doi: 10.1002/bdra.20735. [DOI] [PubMed] [Google Scholar]
- 57.Reeves RH, Irving NG, Moran TH, Wohn A, Kitt C, Sisodia SS, et al. A mouse model for Down syndrome exhibits learning and behaviour deficits. Nat Genet. 1995;11:177–84. doi: 10.1038/ng1095-177. [DOI] [PubMed] [Google Scholar]
- 58.Ricceri L, De Filippis B, Laviola G. Rett syndrome treatment in mouse models: Searching for effective targets and strategies. Neuropharmacology. 2012 doi: 10.1016/j.neuropharm.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 59.Singer P, Feldon J, Yee BK. Are DBA/2 mice associated with schizophrenia-like endophenotypes? A behavioural contrast with C57BL/6 mice. Psychopharmacology (Berl) 2009;206:677–98. doi: 10.1007/s00213-009-1568-6. [DOI] [PubMed] [Google Scholar]
- 60.Albright CD, Friedrich CB, Brown EC, Mar MH, Zeisel SH. Maternal dietary choline availability alters mitosis, apoptosis and the localization of TOAD-64 protein in the developing fetal rat septum. Dev Brain Res. 1999;115:123–9. doi: 10.1016/s0165-3806(99)00057-7. [DOI] [PubMed] [Google Scholar]
- 61.Albright CD, Tsai AY, Friedrich CB, Mar MH, Zeisel SH. Choline availability alters embryonic development of the hippocampus and septum in the rat. Dev Brain Res. 1999;113:13–20. doi: 10.1016/s0165-3806(98)00183-7. [DOI] [PubMed] [Google Scholar]
- 62.Craciunescu CN, Albright CD, Mar MH, Song J, Zeisel SH. Choline availability during embryonic development alters progenitor cell mitosis in developing mouse hippocampus. J Nutr. 2003;133:3614–8. doi: 10.1093/jn/133.11.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Clelland CD, Choi M, Romberg C, Clemenson GD, Jr, Fragniere A, Tyers P, et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–3. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kitamura T, Saitoh Y, Takashima N, Murayama A, Niibori Y, Ageta H, et al. Adult neurogenesis modulates the hippocampus-dependent period of associative fear memory. Cell. 2009;139:814–27. doi: 10.1016/j.cell.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 65.Williams CL, Meck WH, Heyer D, Loy R. Hypertrophy of basal forebrain neurons and enhanced visuospatial memory in perinatally choline-supplemented rats. Brain Res. 1998;794:225–38. doi: 10.1016/s0006-8993(98)00229-7. [DOI] [PubMed] [Google Scholar]
- 66.Fibiger HC. Cholinergic mechanisms in learning, memory and dementia: A review of recent evidence. TINS. 1991;14:220–3. doi: 10.1016/0166-2236(91)90117-d. [DOI] [PubMed] [Google Scholar]
- 67.Sarter M, Parikh V. Choline transporters, cholinergic transmission and cognition. Nat Rev Neurosci. 2005;6:48–56. doi: 10.1038/nrn1588. [DOI] [PubMed] [Google Scholar]
- 68.Cermak JM, Holler T, Jackson DA, Blusztajn JK. Prenatal availability of choline modifies development of the hippocampal cholinergic system. FASEB J. 1998;12:349–57. doi: 10.1096/fasebj.12.3.349. [DOI] [PubMed] [Google Scholar]
- 69.Sweatt JD. The neuronal MAP kinase cascade: a biochemical signal integration system subserving synaptic plasticity and memory. J Neurochem. 2001;76:1–10. doi: 10.1046/j.1471-4159.2001.00054.x. [DOI] [PubMed] [Google Scholar]
- 70.Jones JP, Meck WH, Williams CL, Wilson WA, Swartzwelder HS. Choline availability to the developing rat fetus alters adult hippocampal long-term potentiation. Dev Brain Res. 1999;118:159–67. doi: 10.1016/s0165-3806(99)00103-0. [DOI] [PubMed] [Google Scholar]
- 71.Pyapali GK, Turner DA, Williams CL, Meck WH, Swartzwelder HS. Prenatal dietary choline supplementation decreases the threshold for induction of long-term potentiation in young adult rats. J Neurophysiol. 1998;79:1790–6. doi: 10.1152/jn.1998.79.4.1790. [DOI] [PubMed] [Google Scholar]
- 72.Montoya D, Swartzwelder HS. Prenatal choline supplementation alters hippocampal N-methyl-D-aspartate receptor-mediated neurotransmission in adult rats. Neurosci Lett. 2000;296:85–8. doi: 10.1016/s0304-3940(00)01660-8. [DOI] [PubMed] [Google Scholar]
- 73.Mellott TJ, Follettie MT, Diesl V, Hill AA, Lopez-Coviella I, Blusztajn JK. Prenatal choline availability modulates hippocampal and cerebral cortical gene expression. FASEB J. 2007;21:1311–23. doi: 10.1096/fj.06-6597com. [DOI] [PubMed] [Google Scholar]
- 74.Jones PA. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat Rev Genet. 2012;13:484–92. doi: 10.1038/nrg3230. [DOI] [PubMed] [Google Scholar]
- 75.Jones PA, Liang G. Rethinking how DNA methylation patterns are maintained. Nat Rev Genet. 2009;10:805–11. doi: 10.1038/nrg2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen ZX, Riggs AD. DNA methylation and demethylation in mammals. J Biol Chem. 2011;286:18347–53. doi: 10.1074/jbc.R110.205286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kovacheva VP, Mellott TJ, Davison JM, Wagner N, Lopez-Coviella I, Schnitzler AC, Blusztajn JK. Gestational choline deficiency causes global and Igf2 gene DNA hypermethylation by up-regulation of Dnmt1 expression. J Biol Chem. 2007;282:31777–88. doi: 10.1074/jbc.M705539200. [DOI] [PubMed] [Google Scholar]
- 78.Lopes S, Lewis A, Hajkova P, Dean W, Oswald J, Forne T, et al. Epigenetic modifications in an imprinting cluster are controlled by a hierarchy of DMRs suggesting long-range chromatin interactions. Hum Mol Genet. 2003;12:295–305. doi: 10.1093/hmg/ddg022. [DOI] [PubMed] [Google Scholar]
- 79.Li E, Beard C, Jaenisch R. Role for DNA methylation in genomic imprinting. Nature. 1993;366:362–5. doi: 10.1038/366362a0. [DOI] [PubMed] [Google Scholar]
- 80.Chen DY, Stern SA, Garcia-Osta A, Saunier-Rebori B, Pollonini G, Bambah-Mukku D, et al. A critical role for IGF-II in memory consolidation and enhancement. Nature. 2011;469:491–7. doi: 10.1038/nature09667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Niculescu MD, Craciunescu CN, Zeisel SH. Dietary choline deficiency alters global and gene-specific DNA methylation in the developing hippocampus of mouse fetal brains. FASEB J. 2006;20:43–9. doi: 10.1096/fj.05-4707com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mehedint MG, Niculescu MD, Craciunescu CN, Zeisel SH. Choline deficiency alters global histone methylation and epigenetic marking at the Re1 site of the calbindin 1 gene. FASEB J. 2010;24:184–95. doi: 10.1096/fj.09-140145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen WG, Chang Q, Lin YX, Meissner A, West AE, Griffith EC, et al. Derepression of BDNF transcription involves calcium-dependent phosphorylation of MeCP2. Science. 2003;302:885–9. doi: 10.1126/science.1086446. [DOI] [PubMed] [Google Scholar]
- 84.Martinowich K, Hattori D, Wu H, Fouse S, He F, Hu Y, et al. DNA methylation-related chromatin remodeling in activity-dependent Bdnf gene regulation. Science. 2003;302:890–3. doi: 10.1126/science.1090842. [DOI] [PubMed] [Google Scholar]
- 85.Levenson JM, Roth TL, Lubin FD, Miller CA, Huang IC, Desai P, et al. Evidence that DNA (cytosine-5) methyltransferase regulates synaptic plasticity in the hippocampus. J Biol Chem. 2006;281:15763–73. doi: 10.1074/jbc.M511767200. [DOI] [PubMed] [Google Scholar]
- 86.Nelson ED, Kavalali ET, Monteggia LM. Activity-dependent suppression of miniature neurotransmission through the regulation of DNA methylation. J Neurosci. 2008;28:395–406. doi: 10.1523/JNEUROSCI.3796-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yossifoff M, Kisliouk T, Meiri N. Dynamic changes in DNA methylation during thermal control establishment affect CREB binding to the brain-derived neurotrophic factor promoter. Eur J Neurosci. 2008;28:2267–77. doi: 10.1111/j.1460-9568.2008.06532.x. [DOI] [PubMed] [Google Scholar]
- 88.Miller CA, Sweatt JD. Covalent modification of DNA regulates memory formation. Neuron. 2007;53:857–69. doi: 10.1016/j.neuron.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 89.Lubin FD, Roth TL, Sweatt JD. Epigenetic regulation of BDNF gene transcription in the consolidation of fear memory. J Neurosci. 2008;28:10576–86. doi: 10.1523/JNEUROSCI.1786-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Miller CA, Gavin CF, White JA, Parrish RR, Honasoge A, Yancey CR, et al. Cortical DNA methylation maintains remote memory. Nat Neurosci. 2010;13:664–6. doi: 10.1038/nn.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gupta S, Kim SY, Artis S, Molfese DL, Schumacher A, Sweatt JD, et al. Histone methylation regulates memory formation. J Neurosci. 2010;30:3589–99. doi: 10.1523/JNEUROSCI.3732-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Feng J, Zhou Y, Campbell SL, Le T, Li E, Sweatt JD, et al. Dnmt1 and Dnmt3a maintain DNA methylation and regulate synaptic function in adult forebrain neurons. Nat Neurosci. 2010;13:423–30. doi: 10.1038/nn.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Davison JM, Mellott TJ, Kovacheva VP, Blusztajn JK. Gestational choline supply regulates methylation of histone H3, expression of histone methyltransferases G9a (Kmt1c) and Suv39h1 (Kmt1a) and DNA methylation of their genes in rat fetal liver and brain. J Biol Chem. 2009;284:1982–9. doi: 10.1074/jbc.M807651200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kovacheva VP, Davison JM, Mellott TJ, Rogers AE, Yang S, O’Brien MJ, Blusztajn JK. Raising gestational choline intake alters gene expression in DMBA-evoked mammary tumors and prolongs survival. FASEB J. 2009;23:1054–63. doi: 10.1096/fj.08-122168. [DOI] [PMC free article] [PubMed] [Google Scholar]


