Abstract
Purpose of review
In this article, we review the indications and latest management of high-risk penetrating keratoplasty.
Recent findings
Despite the immune-privilege status of the cornea, immune-mediated graft rejection still remains the leading cause of corneal graft failure. This is particularly a problem in the high-risk graft recipients, namely patients with previous graft failure due to rejection and those with inflamed and vascularized corneal beds. A number of strategies including both local and systemic immunosuppression are currently used to increase the success rate of high-risk corneal grafts. Moreover, in cases of limbal stem cell deficiency, limbal stem cells transplantation is employed.
Summary
Corticosteroids are still the top medication for prevention and treatment in cases of corneal graft rejection. Single and combined administration of immunosuppressive agents e.g. tacrolimus, cyclosporine and mycophenolate are promising adjunctive therapies for prolonging graft survival. In the future, cellular and molecular therapies should allow us to achieve immunologic tolerance even in high-risk grafts.
Keywords: Ocular surface disorder, Cornea, Penetrating keratoplasty, High risk, Graft rejection, Immunosuppressive drugs
Introduction
Corneal transplantation is the most prevalent type of human organ transplantation. Primary corneal grafts with no complications that are placed in avascular “low risk” beds have extremely high success rates. However, in cases of vascularized corneas and/or history of multiple grafts, as “high risk” beds, there is up to 70% rejection rate despite intense local immunosuppressive therapy [1–3]. Although lamellar keratoplasty has augmented graft survival rate, immunological reasons are still the most common cause of graft failure [4, 5]. A number of factors including inflammation, corneal neovascularization and niche disturbance lead to breakdown of the immune privilege of cornea followed by graft rejection [6••]. Immunosuppressive therapy is currently the main strategy available for preventing graft rejection in immunologically high-risk graft recipients.
Immune status of the cornea
The crucial factor that determines the success and failure of corneal transplantation in different cases is the immune privilege status of the cornea. Several factors are responsible for this immune privilege. Immune cells are restricted in reaching the cornea owing to deficiency of vascular system. Lacking corneal lymphatic system avoids high transfer of antigens and antigen presenting cells (APCs) to T-cell pools like lymph nodes (LNs). Moreover, the cornea expresses low levels of MHC antigens, which results in fewer targets for immune cells. T cell and complement activation is also suppressed by production of a special series of immunomodulatory factors and neuropeptides, e.g. α-melanocyte stimulating hormone (α-MSH) and transforming growth factor (TGF)-β [7], and stimulated FAS+ T cells are induced to undergo apoptosis by CD95 (Fas) ligand. Although, the cornea is endowed with resident dendritic cells (DCs), they are universally MHC class II negative; however, they are capable of expressing class II antigens after inflammation or transplantation [8, 9]. Finally, the anterior chamber contains antigens that are responsible for selective and adoptive transferable inhibition of the systemic immune reaction, called “anterior chamber-associated immune deviation (ACAID)” [7].
High risk cornea
The characteristics of a high-risk cornea includes vascularization of cornea, the creation of lymphatic drainage to cervical LNs, corneal migration of LCs, and maturation of local epithelial LCs and stromal DCs [10], and up-regulation of pro-inflammatory cytokines such as interleukin (IL)-1 and tumor necrosis factor (TNF)-α [8–10]. It has been shown that IL-1 and TNF-α inhibit immunomodulatory pathways such as anterior chamber associated immune deviation (ACAID); increase expression of MHCs and maturation of DCs; and also augment the expression of adhesion and chemotactic factors [11, 12].
Risk Factors
Corneal vascularization
Vascularized corneas have a much higher risk of graft rejection compared to avascular corneas. According to Collaborative Corneal Transplantation Studies (CCTs), corneas with deep stromal vascularization in two or more quadrants are considered ‘high risk’. The onset and severity of rejection is determined by degree and depth of preoperative corneal vascularization. Furthermore, once corneal rejection occurs, the likelihood of reversal also depends on the degree of corneal vascularization [13]. Figure 1 showed complete corneal vascularization and conjunctivalization (limbal stem cell deficiency - LSCD) after a severe alkaline chemical burn.
Figure 1.
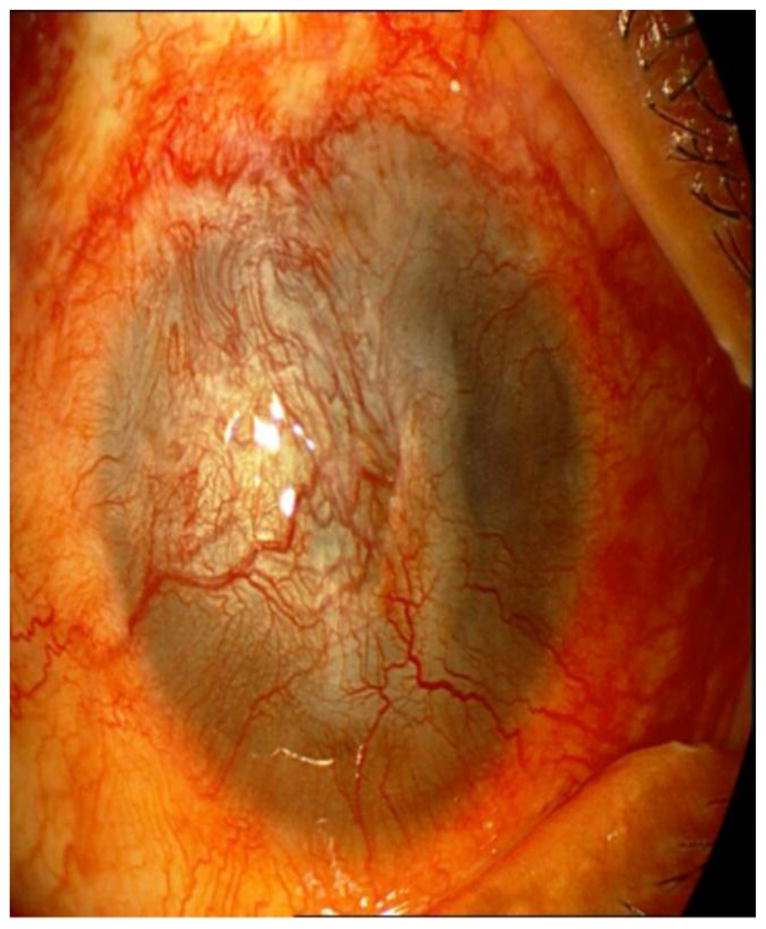
Total corneal conjunctivalization and vascularization after a severe alkaline chemical burn.
Prior graft rejection and failure
Prior corneal graft failure is a significant risk factor for subsequent failure, especially if it is a result of an allograft rejection [14]. Immune mediators released following previous graft rejection, may predispose to more efficient recognition and rejection of corneal graft. Moreover, presensitization may occur when antigens of donor and recipient are shared such that the afferent blockade is circumvented. It is believed that a sympathetic reaction following corneal surgery results in everlasting omission of immune privilege for future corneal allografts, even for transplantations in contralateral eye or using allograft with different expression of foreign histocompatibility antigens from previous graft [15••]. The residua of previous surgery including corneal neovascularization and peripheral anterior synechiae may increase the chance of subsequent graft failure. The Cornea Donor Study (CDS) has shown an increased risk of graft failure in those with a history of a definite graft rejection episode [16]. The results of CCTS indicated that the quantity of prior corneal grafts is a major risk factor for next graft failure, which the probability of rejection increases approximately 1.2 folds with each extra graft [3]. Graft rejection occurs earlier and follows a much more fulminant course in regrafts than in first grafts.
Past intraocular surgery
CCTS results showed association of prior intraocular surgery with graft survival [13]. The recognized risk factors for graft failure include lensectomy, vitrectomy, and any procedure for lowering the intraocular pressure (IOP) [13]. It has been claimed that concurrent vitrectomy with keratoplasty results in rise in risk of immunologic rejection [17••, 18].
Herpes simplex and herpes zoster keratitis
It has been shown that herpetic keratitis predisposes an eye to immunologic graft failure. Graft failure following rejection episodes is significantly more frequent in corneal transplantations due to herpetic keratitis than keratoconus [19]. The use of oral antivirals for prophylaxis has significantly reduced the recurrence rate of herpes simplex; [20] nonetheless, these grafts remain at higher risk of failure from immunologic rejection. Herpes simplex cases treated with systemic acyclovir and immunosuppression with cyclosporin A (CsA) or mycophenolate mofetil showed a rate of graft survival comparable with normal-risk keratoplasty [21]. Favorable outcomes with penetrating keratoplasty (PKP) in patients with herpes zoster ophthalmicus have been reported [22].
Past history of anterior segment inflammatory disorder and/or anterior synechia
A major risk factor of corneal graft failure is the presence of inflammation. Each inflammatory reaction in the anterior segment may accelerate the afferent and efferent arms of allograft response. Auto-immune diseases that have higher risk of rejection include uveitis, ocular mucous membrane pemphigoid (MMP), eye-involved collagen vascular disorders, Steven-Johnson syndrome (SJS), and atopic keratoconjunctivitis.
Anterior synechiae, which can develop following inflammatory events, can likewise impair ocular immune privilege and increase the risk of graft rejection in the presence of three or four quadrants of iris synechiae [13]. In the CCTS, the failure rate from any cause doubled if the eye had three or four quadrants of anterior synechiae [13]. Eyes with anterior synechiae have an increased incidence of glaucoma [14]. Both glaucoma and traction induced by synechiae on the corneal endothelium, may lead to endothelial cell loss and graft failure [14]. Figure 2 shows high risk PKP in a patient with extensive peripheral anterior synechiae formation.
Figure 2.
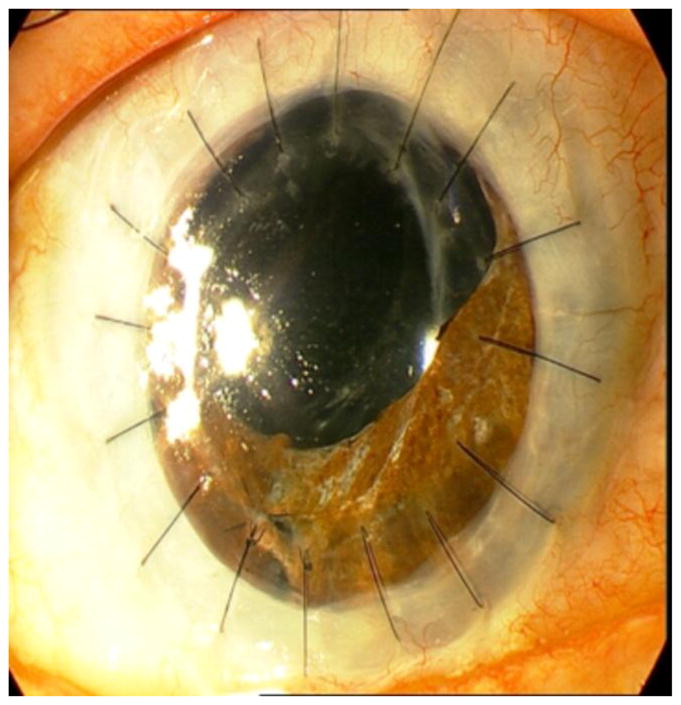
High risk penetrating keratoplasty in a patient with extensive peripheral anterior synechiae formation.
Ocular surface disease
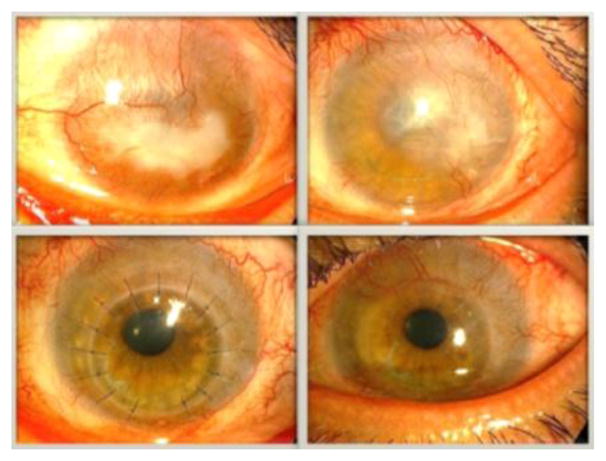
Chemical injuries, SJS, MMP, and congenital aniridia have been considered as risk factors for corneal graft failure, mostly due to surface failure. However, these patients are also at higher risk for immunologic rejection [13]. In general, PKP is not performed as a primary procedure in the presence of LSCD. The appropriate strategy is first to perform a limbal stem cell transplant followed by a later keratoplasty if still necessary. Figure 3 showed PKP in a patient after limbal stem cell transplantation.
Figure 3.
Penetrating keratoplasty in a patient after cultivated limbal epithelial transplantation (CLET). (a) Before CLET. (b) Six months after CLET. (c) Three months after PKP. (d) A year and a half after PKP.
Recipient Age
CCTS results indicate that the age of the host is a major risk factor for transplant rejection. Individuals less than 40 years old had double the risk of graft rejection and failure compared to older patients [13]. Furthermore, a considerable risk of transplant failure has been shown in pediatric population. The graft survival rate in children younger than 15 years was found to be approximately 60–70% at 2 years for first-time grafts [23]. Immunologic and several non-immunologic factors including inadequate follow-up and child’s inability to report symptoms are responsible for graft failure. Interestingly, CDS showed trends toward a higher rate of graft failure in patients 70 years or older versus those younger than 60 years [17].
Glaucoma
Although a higher rate of overall graft failure has been demonstrated in glaucoma patients, there is less evidence for a higher risk of immune rejection; nonetheless, some studies have suggested an association [24]. A report by the CDS found that glaucoma was a risk factor for endothelial rejection in univariate but not in multivariate analysis [25]. CDS group also found that previous glaucoma filtering surgery and glaucoma medication use were risk factors for immune rejection. The highest risk was with both filtering surgery and glaucoma medication use, with a 10-year rejection incidence of 35% versus 14% in those with no glaucoma treatment history [13]. The overall high rate of graft failure in patients with previous glaucoma surgery (e.g. related to endothelial cell loss) may confound determination of the influence of pre-existing glaucoma on rejection.
Strategies to Reduce the Risk of Rejection in High Risk Keratoplasty
Preoperative considerations
Transplantation is delayed until ocular inflammation has been controlled for a minimum of 6 months. In severe cases including SJS, MMP and Mooren’s ulcer, any surgical manipulation may be delayed for at least 1 year (except for emergency tectonic grafts). Patients with atopic or vernal keratoconjunctivitis may benefit from treatment with topical +/− systemic CsA or tacrolimus [26]. Pre-operative (or intraoperative) treatment of vessels with an anti-VEGF agent has also been reported, but remains under study.
A healthy ocular surface is essential for the success of corneal transplantation. Tear film status should be optimized; lubricant gels/ointments and artificial tears may benefit the ocular surface. Blepharitis and meibomian gland dysfunction is treated; adnexal abnormalities including entropion, ectropion, and trichiasis/distichiasis are corrected. Punctal occlusion and tarsorrhaphy are likewise important adjunctive measures to improve the tear film. Partial LSCD need conservative managements. Total LSCD needs surgical intervention. After limbal stem cell transplantation, subsequent PKP should be delayed for at least 3 months.
Tissue matching
The CCTS was unable to detect any beneficial effect of tissue matching on the rate of immune reactions, graft failure or the rate of failure caused by rejection [13]. However, the effect of HLA matching in high-risk cases may have been masked by the using too much steroids, which can suppress HLA expression. On the other hand, the CCTS indicated a measurable increase risk of failure, owing to rejection or other causes, in ABO incompatible cases [27]. The estimated rate of failure was 31% and 41% in ABO compatible and ABO incompatible patients, respectively. These results corroborate other studies showing that non-MHC antigens may be crucial elements in corneal allograft rejection [27, 28].
Other studies, however, have found that matching of donors and recipients for class I histocompatibility antigens may reduce the likelihood of rejection in high-risk PKP [29]. The results of randomized, single-blinded Functional Antigen Matching in Corneal Transplantation (FANCY) trial started in 2009 will provide further information on the role of different class I histocompatibility antigens [30••] . The effect of class II matching remains controversial at the present time [31, 32]. After excluding CCTS data, a meta-analysis suggested a beneficial effect of MHC class II mismatching in high-risk eyes [33]. The Corneal Transplant Follow-up Study II (CTFS II) is evaluating the role of HLA DR-matching in high-risk keratoplasty cases [30••]. Moreover, the effect of gender on the corneal transplant failure or rejection was evaluated in a large sample study. It is indicated that male to female corneal transplants are more prone to graft rejection or failure [34].
Postoperative management
The outcome of immunologically high-risk keratoplasty is largely dependent on the postoperative care. The primary aims of postoperative managements include prevention, early diagnosis and proper treatment of rejection. To establish the early diagnosis, it is useful to educate every patient with rejection symptoms. Frequent follow-up visits allow the identification of early signs of inflammation. Postoperative graft vascularization increases the risk of allograft rejection [35]. In the case of corneal vascularization interrupted sutures can be selectively removed.
Immunosuppressive Therapy
Local and systemic immunosuppressive therapies are often necessary for preventing transplant rejection in immunologically high-risk hosts. While corticosteroids are still the main choice for both prevention and treatment of corneal transplant rejection; there are more targeted therapies that can modulate the immune response in high-risk patients.
Corticosteroids
In high-risk patients, topical steroids are used frequently in the postoperative period followed by long-term indefinite use when no contraindications exist. Typically they are used every 2 to 4 hours for the first few weeks with a gradual decrease over the next several months. Topical difluprednate has been used in the prevention and management of graft rejection in high-risk grafts [36••]. In high-risk cases particularly with concomitant systemic inflammatory diseases, systemic steroids are frequently used peri-operatively. Prednisone (1 mg/kg) is usually started around the time of surgery, which can then be tapered on an individualized schedule within 1–2 months. Because of their adverse effects, every attempt should be made to avoid long-term usage of systemic steroids.
Calcineurin inhibitors
Cyclosporin A
Cyclosporine (CsA) has potent anti-inflammatory and non-myelotoxic immunosuppressive effects that are mediated by binding to two cytoplasmic proteins called cyclophilin A and cyclophilin D [37••], which are critical in T cell activation and proliferation. The beneficial effects of topical CsA in keratoplasty include attenuating the migration of T lymphocytes into the grafts, suppression of corneal neovascularization, optimizing the ocular surface by inhibiting lymphocyte infiltration into lacrimal/accessory glands and conjunctiva, and raising the numbers of goblet cells and also tear production [13].
Topical CsA has been shown in a number of clinical studies to be effective in high-risk keratoplasty [38, 39]. In a study by Inoue et al., cases with high-risk PKP treated with CsA 2% (86 eyes) were retrospectively compared with controls (97 eyes). The rate of rejection-free survival of transplants was 70% and 45% in CsA and control groups, respectively. However, it has been shown that the long-term survival was the same in two groups [40]. The additive effect of topical CsA and topical steroids has also been demonstrated [41].
In very high-risk cases, particularly in monocular patients, systemic CsA is effective in preventing corneal graft rejection [42]. Some have found systemic CsA to have only a moderate and short-term effect on reducing corneal graft rejection [43]. Even, one study found no benefit from systemic CsA [40]. There are a few points to consider when using systemic CsA.
A vital issue is that this strategy must be taken in long-term. It seems that short-term administration of systemic CsA for 6 to 12 months cannot result in long-term prevention of graft rejection. The optimal duration of treatment is best individualized based on the severity and incidence of rejection attacks.
A starting dose of 3–4 mg/kg/day has been recommended [44]. With appropriate monitoring, the risks of therapy can be offset by the potential restoration of vision in patients who would otherwise remain blind. Combination therapy with another immunosuppressive agent such as azathioprine or mycophenolate may be necessary in patients who continue to experience corneal graft rejection on CsA. In addition, the use of a sustained intracameral CsA delivery system has been reported in high-risk keratoplasty patients [45]. The results of this study showed long-term survival of corneal grafts in addition to no significant change in density of corneal endothelial cells after 6 months [45]. Although no complication by implanting the developed CsA drug delivery system was reported, more evaluations are required for replacing such strategies with current therapeutic methods.
The complications of topical CsA are generally related to the discomfort (e.g. burning) associated with its application. For systemic CsA, hypertension, nephrotoxicity, hepatotoxicity, hirsuitism, gingival hyperplasia, neurotoxicity, sensory abnormalities and reactivation of latent tuberculosis have all been reported as a complication in high risk keratoplasty [46, 47]. Many of these complications are uncommon if the patient is appropriately screened before and monitored during therapy [43].
Tacrolimus
Tacrolimus is a macrolide antibiotic with potent immunosuppressive activity. The mechanism of action is very similar to CsA —that is, it binds to calcineurin and inhibits the T cell receptor-mediated signal transduction required for transcription of interleukin-2 and other lymphokines. In recent years, tacrolimus has been used increasingly as an alternative to CsA in liver and renal transplantation [48].
Topical tacrolimus (0.03%, 2– 4 times daily) has been used as a powerful second-line immunosuppressive agent after normal and high-risk PKP [49]. Topical tacrolimus might be more potent than topical CsA for prevention of graft rejection [50]. Likewise, systemic tacrolimus in high-risk cases results in fewer graft rejection episodes and longer graft survival compared with CsA [51•, 52••]. In a study, oral tacrolimus was initiated at a dose of 2 mg/day (1 mg twice daily) on the day of surgery and trough levels are measured 12 h after the last dose of tacrolimus. The mean tacrolimus dosage was 2.5 mg/day per patient (range 2–8 mg/day) [53]. Additionally, in another study the oral tacrolimus was started at 0.03 mg/kg per day in 2 divided doses and then adjusted based on clinical efficacy up to 0.08 mg/kg per day [54]. Like CsA, the duration of therapy should be individualized and it is best combined with other agents. The choice between systemic tacrolimus and CsA remains a matter of experience and personal preference, although there is some recent evidence for superiority of tacrolimus. Its complications are paraesthesia, tremors, headache, fatigue, increased blood pressure, reversible rise in serum creatinine (nephrotoxicity), and diabetes [53].
Agents with Anti-Proliferative Effects
Mycophenolate mofetil
Mycophenolate mofetil blocks the proliferation of T and B cells by inhibiting guanosine synthesis. It has been shown to prolong the survival of high-risk keratoplasty [55]. In one study the effects of mycophenolate (1 g twice daily) was comparable to CsA (adjusted to blood trough level of 120–150 ng/ml) in its efficacy [56]. In a retrospective evaluation by Birnbaum et al, 417 high-risk cases treated with systemic CsA or mycophenolate were enrolled [57]. The rejection-free survival rate was 75% at one year, and 60% at 3 years for CsA compared to the mycophenolate group which had 89% and 72% rejection-free survival rate after 1 and 3 years, respectively. In a different study, mycophenolate was administered at an initial dose of 2×1000 mg and was tapered to 2×500 mg one month after surgery. It was continued another 6 months at 2×250 mg then discontinued a year after surgery [58]. It has been used as a single agent or in combination with CsA, tacrolimus or sirolimus [59]. Reversible adverse effects observed with mycophenolate included gastrointestinal toxicity, bone marrow suppression, arthralgia, and rarely infection [55]. Given its safety profile, it may be considered as first line agent for high-risk keratoplasty.
Rapamycin
Rapamycin is a bacterial macrolide with both immunosuppressive and antifungal effect. It inhibits proliferation and activation of T helper cells and expands T regulatory cells. Rapamycin can effectively prevent neovascular proliferation and immune rejection of organ transplants [60]. Its efficacy has been shown in ameliorating autoimmune uveoretinitis and increasing high-risk corneal graft survival [60, 61]. Rapamycin may be considered as an effective alternative for immunosuppression in high-risk patients, similar to mycophenolate, particularly for long-term maintenance. Patients on rapamycin experience various side effects ranging from hypercholesterolemia to gingivitis; however, most of these are reversible.
Azathioprine
Azathioprine is a purine analog with immunosuppressive activities at the level of DNA. It is no longer considered as a single agent in corneal transplantation. Nowadays, its role in high-risk corneal transplantation is limited to systemic therapy as an adjunct to CsA (or tacrolimus) in resistant cases.
Experimental Approaches
Blocking the activation and action of T cells
Monoclonal antibodies to T-cell antigens
The primary advantage of monoclonal antibodies is their specificity towards the target antigen, and their safety profile. By blocking the effect of IL-2, they inhibit the proliferation of T cells. Monoclonal antibodies against CD3, CD4, CD8, IL-12, and αβ T-cell receptors have beneficial effects after parenteral administration in experimental animal models [62]. Daclizumab (Zenapax) and basiliximab (Simulect) are both directed against the alpha subunit (Tac/CD25) of the IL-2 receptor of activated T cells. When compared to systemic CsA, a lower efficacy with improved side-effect profile was found for basiliximab [63]. As experience accumulates, these biologically active agents are likely to play a more important role in immunosuppressive regimens in high risk corneal transplant.
Blocking of co-stimulatory signals
The activation of T-cells is dependent on co-stimulatory signals. A main co-stimulatory signal is between T-cell CD28 with APCs-B7 molecules. Inhibiting the CD28 and B7 interaction using a B7 high affinity bonded recombinant fusion protein, CTLA4-Ig, has resulted in increasing the allograft survival [64]. Both topical administration of CTLA4-Ig in rats and systemic anti-CD28 monoclonal antibody have graft-surviving effects [65]. In addition, it has been shown that former incubation of rabbit corneal graft with CTLA4-Ig, improves the graft survival in high-risk, but not low-risk, cases [66].
Regulating the immune reaction using cytokines and peptides
Transferring both IL-4 and IL-10 genes into graft epithelial layer led to increased corneal graft survival. These cytokines inhibit the activation of Th1 cells, and turn the T cells toward a Th2 phenotype [67–69]. Hamrah and colleagues reported that regional treatment of host mouse eyes with α-melanocyte stimulating hormone (as an immunomodulatory peptide) resulted in prolonged corneal allograft survival [70].
Inhibition of the antigen-presenting cells
An approach for inhibiting presentation of antigens is to decrease the amount of donor LCs and DCs in corneal grafts. This method, in animal models, has decreased the incidence of graft rejection [71]. The effects of donor graft epithelium removal in low-risk keratoplasty, which leads to eliminating donor LCs in addition to diminishing HLA introduction, were examined in a randomized clinical trial but found to have no effect on the rate of immune rejection [72].
Inhibiting immune access to the graft
Immune cell trafficking
The host effector immune cells invade the transplant via cell adhesion molecules found at corneal inflammation sites [73]. Systemic administration of antibodies against intercellular adhesion molecule (ICAM)-1, leukocyte function antigen (LFA)-1, very late antigen (VLA)-1 and VLA-4 in mice has resulted in inhibition of graft rejection [74–76]. It will be interesting to see if an upcoming new topical therapy for dry eyes, (Lifitegrast), which similarly inhibits adhesion molecules [77], will have any effect on graft rejection.
Chemokines are similarly recognized as important mediators of immune cell trafficking in corneal graft rejection. In a study by Hamrah et al, the survival rates of corneal grafts were measured in various types of chemokine and chemokine receptor knockout mouse models. The results demonstrated that corneal allografts in CCR1 knockout mice survive better than grafts in wild-type recipients [12]. Recently, CCR7 (−/−) grafts led to fewer migrating donor antigen-presenting cells and IFN-gamma producing T-cells in draining lymph nodes when compared to wild-type grafts, demonstrating the importance of CCR7 in host T-cell priming [71].
Attenuating corneal neovascularization
Hemangiogenesis and lymphangiogenesis of the cornea enhance the migration of immune cells and the development of rejection. Attenuating corneal neovascularization by argon laser photoablation, cryotherapy, and fine needle diathermy have resulted in decline in vessel formation, but re-treatment is often necessary owing to the short-term effects. In recent years, with availability of anti-angiogenic agents has led to significant interest in treating corneal neovascularization with anti-VEGF locally (topical, subconjunctival and intrastromal) [6, 78–83•]. In animal models, VEGF neutralization improves the survival of corneal allografts [84–86••]. Clinically, bevacizumab, a monoclonal antibody inhibiting VEGF, has been used most frequently for the treatment of corneal neovascularization in the pre- and postoperative period [13, 78, 81, 83, 87–89••]. It can potentially reduce the inflammatory process and prevent corneal graft rejection [90•]. It has been shown that bevacizumab inhibits not only angiogenesis but also lymphangiogenesis in mice [91]. Some authors have described the use of bevacizumab for interface and/or stromal vessels after deep anterior lamellar keratoplasty (DALK) [92–94]. Anti VEGF therapy can be combined with other anti-angiogenesis modalities including fine-needle diathermy [81].
In one study, the efficacy of topical bevacizumab (5 mg/ml-five times/day on average) was examined in 30 eyes of 27 patients with progressive corneal neovascularization owing to various corneal illnesses. All of these patients were not responding to routine anti-inflammatory treatments. On average, 61% of vascularization area and 24% of vessel diameter were decreased following topical bevacizumab therapy [95]. Furthermore, meta-analysis of human clinical studies revealed that the corneal neovascularization area attenuated by 32% after subconjunctival injection, 48% after topical treatment and 36% overall following anti-VEGF administration [96•]. Thus, simultaneous administration of topical and subconjunctival bevacizumab might result in better corneal graft survival rate in most of high-risk patients [88]. In several animal studies application of topical and subconjunctival ranibizumab caused regression of corneal neovascularization and prevented graft rejection [97–100]. While studies to determine the precise role of anti-VEGF agents in high risk keratoplasty are still ongoing, safety is also concern. There is a report of recipient bed melt and wound dehiscence after penetrating keratoplasty and subconjunctival bevacizumab injection [101].
Induction of the allospecific tolerance
Oral immunization with donor-specific alloantigens
Oral administration of antigen is an efficient way to diminish the immune reaction against several alloantigens. Feeding of animals with cells containing alloantigens led to tolerance induction. The mouse corneal epithelial and endothelial cells derived from donors were cultivated and orally administrated to recipients, which led to a 50% attenuation of the graft rejection rate [102].
Lymphadenectomy
The duration of corneal graft survival after high-risk corneal transplantation was doubled following investigational removal of mouse submandibular lymph nodes [103]. It was attributed to ‘immunologic ignorance,’ as prior sensitization abolished the effect of lymph node resection. Furthermore, migration of APCs to lymph nodes was inhibited by blocking the vascular endothelial growth factor receptor-3 (a procedure known as “molecular nonsurgical lymphadenectomy”), which led to uniform graft survival in all cases [104].
Gene therapy
Genetic modulation of donor grafts using recombinant viral vectors has been shown to improve graft survival rates in animal models [105]. For instance, ex-vivo transfer of programmed cell death ligand 1 (PD-L1) gene using lentivirus into corneas significantly attenuated graft rejection via modulating immune cells that are infiltrating the graft. This outcome was associated with attenuation of cytotoxic CD8+ and natural killer T cells, as well as reduction of pro-inflammatory cytokine expression [106]. Therefore, gene transfer approaches may provide an opportunity to reduce the immunologic response to allograft without significantly compromising endothelial cell viability.
Conclusion
Immune mediated graft rejection is the most frequent cause of corneal transplantation failure. The risk factors for graft failure are corneal vascularization, prior graft rejection and failure, young age, ocular surface disease, previous intraocular surgery, history of anterior segment inflammatory disease and synechia, history of herpes simplex or zoster keratitis and glaucoma. The current recommended approach is summarized in Table 1. Preoperative MHC and non-MHC antigens matching are still controversial. New modalities including anti VEGF therapy, modulating the immune response with biological agents are exciting approaches that remain under investigation.
Table 1.
Stepwise management of High-risk keratoplasty
| 1. Potent and frequent topical steroids -- continued indefinitely on a tapering dose |
| 2. Topical tacrolimus (or CsA) as adjunctive therapy -- continued indefinitely |
| 3. Systemic steroids peri-operatively |
| 4. Systemic mycophenolate (azathioprine or rapamycin alternative) |
| 5. Systemic tacrolimus (or cyclosporine) in addition to mycophenolate |
Acknowledgments
Special thanks to Dr. Victor Perez for helping to review this paper.
Footnotes
Compliance with Ethical Guidelines
Conflict of Interest
Ali Djalilian, Sayena Jabbehdari, Alireza Baradaran Rafii, Ghasem Yazdanpanah, Pedram Hamrah and Edward Holland declare no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Chong EM, Dana MR. Graft failure IV. Immunologic mechanisms of corneal transplant rejection. Int Ophthalmol. 2008;28:209–22. doi: 10.1007/s10792-007-9099-9. [DOI] [PubMed] [Google Scholar]
- 2.Reis A, Reinhard T. Cornea and External Eye Disease. Springer; 2006. Current systemic immunosuppressive strategies in penetrating keratoplasty; pp. 109–22. [Google Scholar]
- 3.The collaborative corneal transplantation studies (CCTS) Effectiveness of histocompatibility matching in high-risk corneal transplantation. The Collaborative Corneal Transplantation Studies Research Group. Arch Ophthalmol. 1992;110:1392–403. [PubMed] [Google Scholar]
- 4.Guilbert E, Bullet J, Sandali O, Basli E, Laroche L, Borderie VM. Long-term rejection incidence and reversibility after penetrating and lamellar keratoplasty. Am J Ophthalmol. 2013;155:560–9. e2. doi: 10.1016/j.ajo.2012.09.027. [DOI] [PubMed] [Google Scholar]
- 5.Borderie VM, Sandali O, Bullet J, Gaujoux T, Touzeau O, Laroche L. Long-term results of deep anterior lamellar versus penetrating keratoplasty. Ophthalmology. 2012;119:249–55. doi: 10.1016/j.ophtha.2011.07.057. [DOI] [PubMed] [Google Scholar]
- 6••.Qazi Y, Hamrah P. Corneal Allograft Rejection: Immunopathogenesis to Therapeutics. J Clin Cell Immunol. 2013;2013 doi: 10.4172/2155-9899.S9-006. A comprehensive review illustrating the immunological principles of graft rejection following allograft PKP. This article also reviewed the management strategies of corneal allograft rejection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Streilein JW. Immunological non-responsiveness and acquisition of tolerance in relation to immune privilege in the eye. Eye (Lond) 1995;9( Pt 2):236–40. doi: 10.1038/eye.1995.46. [DOI] [PubMed] [Google Scholar]
- 8.Hamrah P, Dana MR. Corneal antigen-presenting cells. Chem Immunol Allergy. 2007;92:58–70. doi: 10.1159/000099254. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y, Hamrah P, Zhang Q, Taylor AW, Dana MR. Draining lymph nodes of corneal transplant hosts exhibit evidence for donor major histocompatibility complex (MHC) class II-positive dendritic cells derived from MHC class II-negative grafts. J Exp Med. 2002;195:259–68. doi: 10.1084/jem.20010838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamrah P, Zhang Q, Dana MR. Expression of vascular endothelial growth factor receptor-3 (VEGFR-3) in the conjunctiva--a potential link between lymphangiogenesis and leukocyte trafficking on the ocular surface. Adv Exp Med Biol. 2002;506:851–60. doi: 10.1007/978-1-4615-0717-8_120. [DOI] [PubMed] [Google Scholar]
- 11.Yamagami S, Miyazaki D, Ono SJ, Dana MR. Differential chemokine gene expression in corneal transplant rejection. Invest Ophthalmol Vis Sci. 1999;40:2892–7. [PubMed] [Google Scholar]
- 12.Hamrah P, Yamagami S, Liu Y, et al. Deletion of the chemokine receptor CCR1 prolongs corneal allograft survival. Invest Ophthalmol Vis Sci. 2007;48:1228–36. doi: 10.1167/iovs.05-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maguire MG, Stark WJ, Gottsch JD, et al. Risk factors for corneal graft failure and rejection in the collaborative corneal transplantation studies. Collaborative Corneal Transplantation Studies Research Group. Ophthalmology. 1994;101:1536–47. doi: 10.1016/s0161-6420(94)31138-9. [DOI] [PubMed] [Google Scholar]
- 14.Wilson SE, Kaufman HE. Graft failure after penetrating keratoplasty. Surv Ophthalmol. 1990;34:325–56. doi: 10.1016/0039-6257(90)90110-h. [DOI] [PubMed] [Google Scholar]
- 15••.Paunicka KJ, Mellon J, Robertson D, Petroll M, Brown JR, Niederkorn JY. Severing corneal nerves in one eye induces sympathetic loss of immune privilege and promotes rejection of future corneal allografts placed in either eye. Am J Transplant. 2015;15:1490–501. doi: 10.1111/ajt.13240. An orginal study indicating the role of substance P, secreted following primary corneal trnasplantations owing to severing corneal nerves, which lead to disturabnce of T regulatory cell functions; so, develop graft rejection in second transplantations even in fellow eye. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bersudsky V, Blum-Hareuveni T, Rehany U, Rumelt S. The profile of repeated corneal transplantation. Ophthalmology. 2001;108:461–9. doi: 10.1016/s0161-6420(00)00544-3. [DOI] [PubMed] [Google Scholar]
- 17••.Writing Committee for the Cornea Donor Study Research G. Sugar A, Gal RL, et al. Factors associated with corneal graft survival in the cornea donor study. JAMA Ophthalmol. 2015;133:246–54. doi: 10.1001/jamaophthalmol.2014.3923. A large multi-center double-blind controlled clinical trail assessed the effects of both donor and recipient characteristics on corneal graft survival in Cornea Donor Study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sit M, Weisbrod DJ, Naor J, Slomovic AR. Corneal graft outcome study. Cornea. 2001;20:129–33. doi: 10.1097/00003226-200103000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Epstein RJ, Seedor JA, Dreizen NG, et al. Penetrating keratoplasty for herpes simplex keratitis and keratoconus. Allograft rejection and survival. Ophthalmology. 1987;94:935–44. doi: 10.1016/s0161-6420(87)33356-1. [DOI] [PubMed] [Google Scholar]
- 20.Barney NP, Foster CS. A prospective randomized trial of oral acyclovir after penetrating keratoplasty for herpes simplex keratitis. Cornea. 1994;13:232–6. doi: 10.1097/00003226-199405000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Maier AK, Ozlugedik S, Rottler J, et al. Efficacy of postoperative immunosuppression after keratoplasty in herpetic keratitis. Cornea. 2011;30:1398–405. doi: 10.1097/ICO.0b013e31821e65b3. [DOI] [PubMed] [Google Scholar]
- 22.Kosker M, Duman F, Suri K, Hammersmith KM, Nagra PK, Rapuano CJ. Long-term results of keratoplasty in patients with herpes zoster ophthalmicus. Cornea. 2013;32:982–6. doi: 10.1097/ICO.0b013e318289897e. [DOI] [PubMed] [Google Scholar]
- 23.Stulting RD, Sumers KD, Cavanagh HD, Waring GO, 3rd, Gammon JA. Penetrating keratoplasty in children. Ophthalmology. 1984;91:1222–30. doi: 10.1016/s0161-6420(84)34171-9. [DOI] [PubMed] [Google Scholar]
- 24.Aldave AJ, Chen JL, Zaman AS, Deng SX, Yu F. Outcomes after DSEK in 101 eyes with previous trabeculectomy and tube shunt implantation. Cornea. 2014;33:223–9. doi: 10.1097/ICO.0000000000000028. [DOI] [PubMed] [Google Scholar]
- 25.Stulting RD, Sugar A, Beck R, et al. Effect of donor and recipient factors on corneal graft rejection. Cornea. 2012;31:1141–7. doi: 10.1097/ICO.0b013e31823f77f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leonardi A. Management of vernal keratoconjunctivitis. Ophthalmol Ther. 2013;2:73–88. doi: 10.1007/s40123-013-0019-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sano Y, Ksander BR, Streilein JW. Minor H, rather than MHC, alloantigens offer the greater barrier to successful orthotopic corneal transplantation in mice. Transpl Immunol. 1996;4:53–6. doi: 10.1016/s0966-3274(96)80035-9. [DOI] [PubMed] [Google Scholar]
- 28.Streilein JW, Arancibia-Caracamo C, Osawa H. The role of minor histocompatibility alloantigens in penetrating keratoplasty. Dev Ophthalmol. 2003;36:74–88. doi: 10.1159/000067655. [DOI] [PubMed] [Google Scholar]
- 29.Sanfilippo F, MacQueen JM, Vaughn WK, Foulks GN. Reduced graft rejection with good HLA-A and B matching in high-risk corneal transplantation. N Engl J Med. 1986;315:29–35. doi: 10.1056/NEJM198607033150105. [DOI] [PubMed] [Google Scholar]
- 30••.van Essen TH, Roelen DL, Williams KA, Jager MJ. Matching for Human Leukocyte Antigens (HLA) in corneal transplantation - to do or not to do. Prog Retin Eye Res. 2015;46:84–110. doi: 10.1016/j.preteyeres.2015.01.001. Of importance review elucidating the beneficical consequences of HLA matching to prevent corneal graft rejections. In addition, illustrating new therapeutic strategies for decreasing probability of transplant rejection. [DOI] [PubMed] [Google Scholar]
- 31.Baggesen K, Lamm LU, Ehlers N. Significant effect of high-resolution HLA-DRB1 matching in high-risk corneal transplantation. Transplantation. 1996;62:1273–7. doi: 10.1097/00007890-199611150-00017. [DOI] [PubMed] [Google Scholar]
- 32.Bradley BA, Vail A, Gore SM, et al. Negative effect of HLA-DR matching on corneal transplant rejection. Transplant Proc. 1995;27:1392–4. [PubMed] [Google Scholar]
- 33.Gore SM, Vail A, Bradley BA, Rogers CA, Easty DL, Armitage WJ. HLA-DR matching in corneal transplantation. Systematic review of published evidence. Corneal Transplant Follow-up Study Collaborators. Transplantation. 1995;60:1033–9. [PubMed] [Google Scholar]
- 34.Hopkinson CL, Romano V, Kaye RA, et al. The Influence of Donor and Recipient Gender Incompatibility on Corneal Transplant Rejection and Failure. Am J Transplant. 2017;17:210–7. doi: 10.1111/ajt.13926. [DOI] [PubMed] [Google Scholar]
- 35.Paque J, Poirier RH. Corneal allograft reaction and its relationship to suture site neovascularization. Ophthalmic Surg. 1977;8:71–4. [PubMed] [Google Scholar]
- 36••.Kharod-Dholakia B, Randleman JB, Bromley JG, Stulting RD. Prevention and treatment of corneal graft rejection: current practice patterns of the Cornea Society (2011) Cornea. 2015;34:609–14. doi: 10.1097/ICO.0000000000000403. [DOI] [PubMed] [Google Scholar]
- 37••.Ziaei M, Ziaei F, Manzouri B. Systemic cyclosporine and corneal transplantation. Int Ophthalmol. 2015 doi: 10.1007/s10792-015-0137-8. A comparative study indicating that although topical prednisolone is still the drug of choice after peneterating and ednothelial keratoplasty, there is a growing usage of topical difluprednate for preventing or treatment of graft rejection. [DOI] [PubMed] [Google Scholar]
- 38.Belin MW, Bouchard CS, Frantz S, Chmielinska J. Topical cyclosporine in high-risk corneal transplants. Ophthalmology. 1989;96:1144–50. doi: 10.1016/s0161-6420(89)32756-4. [DOI] [PubMed] [Google Scholar]
- 39.Holland EJ, Olsen TW, Ketcham JM, et al. Topical cyclosporin A in the treatment of anterior segment inflammatory disease. Cornea. 1993;12:413–9. doi: 10.1097/00003226-199309000-00008. [DOI] [PubMed] [Google Scholar]
- 40.Inoue K, Kimura C, Amano S, et al. Long-term outcome of systemic cyclosporine treatment following penetrating keratoplasty. Jpn J Ophthalmol. 2001;45:378–82. doi: 10.1016/s0021-5155(01)00339-2. [DOI] [PubMed] [Google Scholar]
- 41.Goichot-Bonnat L, Chemla P, Pouliquen Y. Cyclosporin A eyedrops in the prevention of high-risk corneal graft rejection. II. Postoperative clinical results. J Fr Ophtalmol. 1987;10:213–7. [PubMed] [Google Scholar]
- 42.Hill JC. Systemic cyclosporine in high-risk keratoplasty. Short- versus long-term therapy. Ophthalmology. 1994;101:128–33. doi: 10.1016/s0161-6420(13)31253-6. [DOI] [PubMed] [Google Scholar]
- 43.Rumelt S, Bersudsky V, Blum-Hareuveni T, Rehany U. Systemic cyclosporin A in high failure risk, repeated corneal transplantation. Br J Ophthalmol. 2002;86:988–92. doi: 10.1136/bjo.86.9.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Panda A, Vanathi M, Kumar A, Dash Y, Priya S. Corneal graft rejection. Surv Ophthalmol. 2007;52:375–96. doi: 10.1016/j.survophthal.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 45.Shi W, Chen M, Xie L, et al. A novel cyclosporine a drug-delivery system for prevention of human corneal rejection after high-risk keratoplasty: a clinical study. Ophthalmology. 2013;120:695–702. doi: 10.1016/j.ophtha.2012.09.035. [DOI] [PubMed] [Google Scholar]
- 46.Ziaei M, Manzouri B. Topical cyclosporine in corneal transplantation. Cornea. 2015;34:110–5. doi: 10.1097/ICO.0000000000000291. [DOI] [PubMed] [Google Scholar]
- 47.Kashani S, Mearza AA. Uses and safety profile of ciclosporin in ophthalmology. Expert Opin Drug Saf. 2008;7:79–89. doi: 10.1517/14740338.7.1.79. [DOI] [PubMed] [Google Scholar]
- 48.Knoll GA, Bell RC. Tacrolimus versus cyclosporin for immunosuppression in renal transplantation: meta-analysis of randomised trials. BMJ. 1999;318:1104–7. doi: 10.1136/bmj.318.7191.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dhaliwal JS, Mason BF, Kaufman SC. Long-term use of topical tacrolimus (FK506) in high-risk penetrating keratoplasty. Cornea. 2008;27:488–93. doi: 10.1097/ICO.0b013e3181606086. [DOI] [PubMed] [Google Scholar]
- 50.Wang M, Lin Y, Chen J, Liu Y, Xie H, Ye C. Studies on the effects of the immunosuppressant FK-506 on the high-risk corneal graft rejection. Yan Ke Xue Bao. 2002;18:160–4. [PubMed] [Google Scholar]
- 51•.Yamazoe K, Yamazoe K, Yamaguchi T, Omoto M, Shimazaki J. Efficacy and safety of systemic tacrolimus in high-risk penetrating keratoplasty after graft failure with systemic cyclosporine. Cornea. 2014;33:1157–63. doi: 10.1097/ICO.0000000000000258. A clinical study indicating the safe and effective administration of systemic tacrolimus after transplant failure with systemic cyclosporine in high-risk peneterating keratoplasties. [DOI] [PubMed] [Google Scholar]
- 52••.Shimmura-Tomita M, Shimmura S, Satake Y, et al. Keratoplasty postoperative treatment update. Cornea. 2013;32(Suppl 1):S60–4. doi: 10.1097/ICO.0b013e3182a2c937. This manuscript elucidates three important clinical trials about post-operative managment of patients undergoing conreal trnasplantation. It has highlighted the role of non-steroid immusuppressive agents such as tacrolimus. [DOI] [PubMed] [Google Scholar]
- 53.Joseph A, Raj D, Shanmuganathan V, Powell RJ, Dua HS. Tacrolimus immunosuppression in high-risk corneal grafts. Br J Ophthalmol. 2007;91:51–5. doi: 10.1136/bjo.2006.097428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chow SP, Cook SD, Tole DM. Long-Term Outcomes of High-Risk Keratoplasty in Patients Receiving Systemic Immunosuppression. Cornea. 2015;34:1395–9. doi: 10.1097/ICO.0000000000000615. [DOI] [PubMed] [Google Scholar]
- 55.Reinhard T, Mayweg S, Sokolovska Y, et al. Systemic mycophenolate mofetil avoids immune reactions in penetrating high-risk keratoplasty: preliminary results of an ongoing prospectively randomized multicentre study. Transpl Int. 2005;18:703–8. doi: 10.1111/j.1432-2277.2005.00126.x. [DOI] [PubMed] [Google Scholar]
- 56.Reinhard T, Reis A, Bohringer D, et al. Systemic mycophenolate mofetil in comparison with systemic cyclosporin A in high-risk keratoplasty patients: 3 years’ results of a randomized prospective clinical trial. Graefes Arch Clin Exp Ophthalmol. 2001;239:367–72. doi: 10.1007/s004170100285. [DOI] [PubMed] [Google Scholar]
- 57.Birnbaum F, Bohringer D, Sokolovska Y, Sundmacher R, Reinhard T. Immunosuppression with cyclosporine A and mycophenolate mofetil after penetrating high-risk keratoplasty: a retrospective study. Transplantation. 2005;79:964–8. doi: 10.1097/01.tp.0000158022.62059.f2. [DOI] [PubMed] [Google Scholar]
- 58.Szaflik JP, Major J, Izdebska J, Lao M, Szaflik J. Systemic immunosuppression with mycophenolate mofetil to prevent corneal graft rejection after high-risk penetrating keratoplasty: a 2-year follow-up study. Graefes Arch Clin Exp Ophthalmol. 2016;254:307–14. doi: 10.1007/s00417-015-3200-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chatel MA, Larkin DF. Sirolimus and mycophenolate as combination prophylaxis in corneal transplant recipients at high rejection risk. Am J Ophthalmol. 2010;150:179–84. doi: 10.1016/j.ajo.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 60.Shi W, Gao H, Xie L, Wang S. Sustained intraocular rapamycin delivery effectively prevents high-risk corneal allograft rejection and neovascularization in rabbits. Invest Ophthalmol Vis Sci. 2006;47:3339–44. doi: 10.1167/iovs.05-1425. [DOI] [PubMed] [Google Scholar]
- 61.Yuan LF, Li GD, Ren XJ, Nian H, Li XR, Zhang XM. Rapamycin ameliorates experimental autoimmune uveoretinitis by inhibiting Th1/Th2/Th17 cells and upregulating CD4+CD25+ Foxp3 regulatory T cells. Int J Ophthalmol. 2015;8:659–64. doi: 10.3980/j.issn.2222-3959.2015.04.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thiel MA, Coster DJ, Williams KA. The potential of antibody-based immunosuppressive agents for corneal transplantation. Immunol Cell Biol. 2003;81:93–105. doi: 10.1046/j.0818-9641.2002.01145.x. [DOI] [PubMed] [Google Scholar]
- 63.Birnbaum F, Jehle T, Schwartzkopff J, et al. Basiliximab following penetrating risk-keratoplasty--a prospective randomized pilot study. Klin Monbl Augenheilkd. 2008;225:62–5. doi: 10.1055/s-2008-1027129. [DOI] [PubMed] [Google Scholar]
- 64.Konig Merediz SA, Zhang EP, Wittig B, Hoffmann F. Ballistic transfer of minimalistic immunologically defined expression constructs for IL4 and CTLA4 into the corneal epithelium in mice after orthotopic corneal allograft transplantation. Graefes Arch Clin Exp Ophthalmol. 2000;238:701–7. doi: 10.1007/s004170000144. [DOI] [PubMed] [Google Scholar]
- 65.Thiel MA, Steiger JU, O’Connell PJ, Lehnert AM, Coster DJ, Williams KA. Local or short-term systemic costimulatory molecule blockade prolongs rat corneal allograft survival. Clin Experiment Ophthalmol. 2005;33:176–80. doi: 10.1111/j.1442-9071.2005.00974.x. [DOI] [PubMed] [Google Scholar]
- 66.Gebhardt BM, Hodkin M, Varnell ED, Kaufman HE. Protection of corneal allografts by CTLA4-Ig. Cornea. 1999;18:314–20. doi: 10.1097/00003226-199905000-00013. [DOI] [PubMed] [Google Scholar]
- 67.Klebe S, Sykes PJ, Coster DJ, Krishnan R, Williams KA. Prolongation of sheep corneal allograft survival by ex vivo transfer of the gene encoding interleukin-10. Transplantation. 2001;71:1214–20. doi: 10.1097/00007890-200105150-00006. [DOI] [PubMed] [Google Scholar]
- 68.Pleyer U, Bertelmann E, Rieck P, Hartmann C, Volk HD, Ritter T. Survival of corneal allografts following adenovirus-mediated gene transfer of interleukin-4. Graefes Arch Clin Exp Ophthalmol. 2000;238:531–6. doi: 10.1007/pl00007896. [DOI] [PubMed] [Google Scholar]
- 69.Gong N, Pleyer U, Volk HD, Ritter T. Effects of local and systemic viral interleukin-10 gene transfer on corneal allograft survival. Gene Ther. 2007;14:484–90. doi: 10.1038/sj.gt.3302884. [DOI] [PubMed] [Google Scholar]
- 70.Hamrah P, Haskova Z, Taylor AW, Zhang Q, Ksander BR, Dana MR. Local treatment with alpha-melanocyte stimulating hormone reduces corneal allorejection. Transplantation. 2009;88:180–7. doi: 10.1097/TP.0b013e3181ac11ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Saban DR, Chauhan SK, Zhang X, El Annan J, Jin Y, Dana R. ‘Chimeric’ grafts assembled from multiple allodisparate donors enjoy enhanced transplant survival. Am J Transplant. 2009;9:473–82. doi: 10.1111/j.1600-6143.2008.02535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stulting RD, Waring GO, 3rd, Bridges WZ, Cavanagh HD. Effect of donor epithelium on corneal transplant survival. Ophthalmology. 1988;95:803–12. doi: 10.1016/s0161-6420(88)33120-9. [DOI] [PubMed] [Google Scholar]
- 73.Whitcup SM, Nussenblatt RB, Price FW, Jr, Chan CC. Expression of cell adhesion molecules in corneal graft failure. Cornea. 1993;12:475–80. doi: 10.1097/00003226-199311000-00003. [DOI] [PubMed] [Google Scholar]
- 74.Hori J, Isobe M, Yamagami S, Mizuochi T, Tsuru T. Specific immunosuppression of corneal allograft rejection by combination of anti-VLA-4 and anti-LFA-1 monoclonal antibodies in mice. Exp Eye Res. 1997;65:89–98. doi: 10.1006/exer.1997.0316. [DOI] [PubMed] [Google Scholar]
- 75.Guymer RH, Mandel TE. Monoclonal antibody to ICAM-1 prolongs murine heterotopic corneal allograft survival. Aust N Z J Ophthalmol. 1991;19:141–4. doi: 10.1111/j.1442-9071.1991.tb00642.x. [DOI] [PubMed] [Google Scholar]
- 76.Yamagami S, Tsuru T, Isobe M, Obata H, Suzuki J. The role of cell adhesion molecules in allograft rejection after penetrating keratoplasty in mice. Clinical and immunohistochemical study. Graefes Arch Clin Exp Ophthalmol. 1996;234:382–7. doi: 10.1007/BF00190715. [DOI] [PubMed] [Google Scholar]
- 77.Donnenfeld ED, Karpecki PM, Majmudar PA, et al. Safety of Lifitegrast Ophthalmic Solution 5.0% in Patients With Dry Eye Disease: A 1-Year, Multicenter, Randomized, Placebo-Controlled Study. Cornea. 2016;35:741–8. doi: 10.1097/ICO.0000000000000803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cho YK, Zhang X, Uehara H, Young JR, Archer B, Ambati B. Vascular Endothelial Growth Factor Receptor 1 morpholino increases graft survival in a murine penetrating keratoplasty model. Invest Ophthalmol Vis Sci. 2012;53:8458–71. doi: 10.1167/iovs.12-10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stevenson W, Cheng SF, Dastjerdi MH, Ferrari G, Dana R. Corneal neovascularization and the utility of topical VEGF inhibition: ranibizumab (Lucentis) vs bevacizumab (Avastin) Ocul Surf. 2012;10:67–83. doi: 10.1016/j.jtos.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cheng SF, Dastjerdi MH, Ferrari G, et al. Short-term topical bevacizumab in the treatment of stable corneal neovascularization. Am J Ophthalmol. 2012;154:940–8. e1. doi: 10.1016/j.ajo.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Koenig Y, Bock F, Kruse FE, Stock K, Cursiefen C. Angioregressive pretreatment of mature corneal blood vessels before keratoplasty: fine-needle vessel coagulation combined with anti-VEGFs. Cornea. 2012;31:887–92. doi: 10.1097/ICO.0b013e31823f8f7a. [DOI] [PubMed] [Google Scholar]
- 82.Dastjerdi MH, Al-Arfaj KM, Nallasamy N, et al. Topical bevacizumab in the treatment of corneal neovascularization: results of a prospective, open-label, noncomparative study. Arch Ophthalmol. 2009;127:381–9. doi: 10.1001/archophthalmol.2009.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83•.Dunn SP, Gal RL, Kollman C, et al. Corneal graft rejection 10 years after penetrating keratoplasty in the cornea donor study. Cornea. 2014;33:1003–9. doi: 10.1097/ICO.0000000000000212. This article has examined the features of donor and recepient important in corneal graft rejection and also the probability of improving graft rejection to graft failure in Cornea Donor Study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yatoh S, Kawakami Y, Imai M, et al. Effect of a topically applied neutralizing antibody against vascular endothelial growth factor on corneal allograft rejection of rat. Transplantation. 1998;66:1519–24. doi: 10.1097/00007890-199812150-00016. [DOI] [PubMed] [Google Scholar]
- 85.Bachmann BO, Bock F, Wiegand SJ, et al. Promotion of graft survival by vascular endothelial growth factor a neutralization after high-risk corneal transplantation. Arch Ophthalmol. 2008;126:71–7. doi: 10.1001/archopht.126.1.71. [DOI] [PubMed] [Google Scholar]
- 86••.Dohlman TH, Omoto M, Hua J, et al. VEGF-trap aflibercept significantly improves long-term graft survival in high-risk corneal transplantation. Transplantation. 2015;99:678–86. doi: 10.1097/TP.0000000000000512. This animal study shows the effectiveness of VEGF neutralization via sub-conjunctival administration of VEGF-trap, anti-VEGF-C, or sVEGFR-3 for reducing neovascularization and improving graft survival in high-risk PKPs. [DOI] [PubMed] [Google Scholar]
- 87.Skeens HM, Holland EJ. Large-diameter penetrating keratoplasty: indications and outcomes. Cornea. 2010;29:296–301. doi: 10.1097/ICO.0b013e3181b6489e. [DOI] [PubMed] [Google Scholar]
- 88.Dekaris I, Gabric N, Draca N, Pauk-Gulic M, Milicic N. Three-year corneal graft survival rate in high-risk cases treated with subconjunctival and topical bevacizumab. Graefes Arch Clin Exp Ophthalmol. 2015;253:287–94. doi: 10.1007/s00417-014-2851-8. [DOI] [PubMed] [Google Scholar]
- 89••.Detry B, Blacher S, Erpicum C, et al. Sunitinib inhibits inflammatory corneal lymphangiogenesis. Invest Ophthalmol Vis Sci. 2013;54:3082–93. doi: 10.1167/iovs.12-10856. This study shows the efficacy of sunitinib, an oral multi-targeted receptor tyrosine kinase inhibitor, on attenuating hemangiogenesis, lymphangiogenesis, and F4/80+ cell infiltration. [DOI] [PubMed] [Google Scholar]
- 90•.Fasciani R, Mosca L, Giannico MI, Ambrogio SA, Balestrazzi E. Subconjunctival and/or intrastromal bevacizumab injections as preconditioning therapy to promote corneal graft survival. Int Ophthalmol. 2015;35:221–7. doi: 10.1007/s10792-014-9938-4. A clinical study shows the safety and efficacy of subconjunctival and/or intrastromal bevacizumab in improving prognosis of high-risk peneterating keratoplasty. [DOI] [PubMed] [Google Scholar]
- 91.Bock F, Onderka J, Dietrich T, et al. Bevacizumab as a potent inhibitor of inflammatory corneal angiogenesis and lymphangiogenesis. Invest Ophthalmol Vis Sci. 2007;48:2545–52. doi: 10.1167/iovs.06-0570. [DOI] [PubMed] [Google Scholar]
- 92.Jarrin E, Ruiz-Casas D, Mendivil A. Efficacy of bevacizumab against interface neovascularization after deep anterior lamellar keratoplasty. Cornea. 2012;31:188–90. doi: 10.1097/ICO.0b013e31820ca19e. [DOI] [PubMed] [Google Scholar]
- 93.Hashemian MN, Zare MA, Rahimi F, Mohammadpour M. Deep intrastromal bevacizumab injection for management of corneal stromal vascularization after deep anterior lamellar keratoplasty, a novel technique. Cornea. 2011;30:215–8. doi: 10.1097/ICO.0b013e3181e291a6. [DOI] [PubMed] [Google Scholar]
- 94.Foroutan A, Fariba B, Pejman B, et al. Perilimbal bevacizumab injection for interface neovascularization after deep anterior lamellar keratoplasty. Cornea. 2010;29:1268–72. doi: 10.1097/ICO.0b013e3181d92834. [DOI] [PubMed] [Google Scholar]
- 95.Koenig Y, Bock F, Horn F, Kruse F, Straub K, Cursiefen C. Short- and long-term safety profile and efficacy of topical bevacizumab (Avastin) eye drops against corneal neovascularization. Graefes Arch Clin Exp Ophthalmol. 2009;247:1375–82. doi: 10.1007/s00417-009-1099-1. [DOI] [PubMed] [Google Scholar]
- 96•.Papathanassiou M, Theodoropoulou S, Analitis A, Tzonou A, Theodossiadis PG. Vascular endothelial growth factor inhibitors for treatment of corneal neovascularization: a meta-analysis. Cornea. 2013;32:435–44. doi: 10.1097/ICO.0b013e3182542613. An important meta-analysis, evaluated clinical and expermintal studies, which indicates that both topical and subconjunctival bevasizumab is reducing corneal neovascularization. [DOI] [PubMed] [Google Scholar]
- 97.Cho KJ, Choi JS, Choi MY, Joo CK. Effects of subconjunctival ranibizumab in a presensitized rat model of corneal graft. Exp Eye Res. 2013;107:74–9. doi: 10.1016/j.exer.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 98.Liarakos VS, Papaconstantinou D, Vergados I, Douvali M, Theodossiadis PG. The effect of subconjunctival ranibizumab on corneal and anterior segment neovascularization: study on an animal model. Eur J Ophthalmol. 2014;24:299–308. doi: 10.5301/ejo.5000391. [DOI] [PubMed] [Google Scholar]
- 99.Bucher F, Parthasarathy A, Bergua A, et al. Topical Ranibizumab inhibits inflammatory corneal hem- and lymphangiogenesis. Acta Ophthalmol. 2014;92:143–8. doi: 10.1111/j.1755-3768.2012.02525.x. [DOI] [PubMed] [Google Scholar]
- 100.Turkcu FM, Cinar Y, Turkcu G, et al. Topical and subconjunctival ranibizumab (lucentis) for corneal neovascularization in experimental rat model. Cutan Ocul Toxicol. 2014;33:138–44. doi: 10.3109/15569527.2013.813030. [DOI] [PubMed] [Google Scholar]
- 101.Bhasin P, Gujar P, Bhasin P. A case of recipient bed melt and wound dehiscence after penetrating keratoplasty and subconjunctival injection of bevacizumab. Cornea. 2012;31:1342–3. doi: 10.1097/ICO.0b013e318245c044. [DOI] [PubMed] [Google Scholar]
- 102.Ma D, Mellon J, Niederkorn JY. Oral immunisation as a strategy for enhancing corneal allograft survival. Br J Ophthalmol. 1997;81:778–84. doi: 10.1136/bjo.81.9.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Plskova J, Holan V, Filipec M, Forrester JV. Lymph node removal enhances corneal graft survival in mice at high risk of rejection. BMC Ophthalmol. 2004;4:3. doi: 10.1186/1471-2415-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen L, Hamrah P, Cursiefen C, et al. Vascular endothelial growth factor receptor-3 mediates induction of corneal alloimmunity. Nat Med. 2004;10:813–5. doi: 10.1038/nm1078. [DOI] [PubMed] [Google Scholar]
- 105.Ritter T, Wilk M, Nosov M. Gene therapy approaches to prevent corneal graft rejection: where do we stand? Ophthalmic Res. 2013;50:135–40. doi: 10.1159/000350547. [DOI] [PubMed] [Google Scholar]
- 106.Nosov M, Wilk M, Morcos M, et al. Role of lentivirus-mediated overexpression of programmed death-ligand 1 on corneal allograft survival. Am J Transplant. 2012;12:1313–22. doi: 10.1111/j.1600-6143.2011.03948.x. [DOI] [PubMed] [Google Scholar]