Abstract
INTRODUCTION
The use of scalp cooling for the prevention of chemotherapy-induced alopecia (CIA) is increasing. Cold caps are placed onto the hair-bearing areas of the scalp for varying time periods before, during, and after cytotoxic chemotherapy cycles. Although not yet reported, improper application procedures could result in undesirable adverse events (AEs). At present, there are no evidence-based scalp cooling protocols, and there is no regulatory oversight of their use.
OBJECTIVE
To report the occurrence of cold thermal injury (frostbite) on the scalp, following the use of cold caps for the prevention of CIA.
MATERIALS AND METHODS
We identified four patients who developed cold thermal injuries on the scalp following the application of cold caps. Medical records were analyzed to retrieve the demographic, clinical, and histologic characteristics.
RESULTS
The cold thermal injuries in our patients were grade 1/2 in severity and improved with topical interventions, although mild persistent alopecia ensued in 3 patients. The true incidence of such injuries in this setting however, remains unknown.
CONCLUSIONS
Cold thermal cold injuries are likely an infrequent and preventable AE that may result from improper device application procedures during scalp cooling. Although these untoward events are usually mild to moderate in severity, the potential occurrence of long-term sequelae (e.g. permanent alopecia, scarring) are not known. Future prospective studies are needed to further elucidate the risk and standardized delivery methods, and patient/clinical education.
Keywords: cold caps, frostbite, thermal injury, chemotherapy-induced alopecia, cryotherapy, scalp hypothermia
INTRODUCTION
Cold thermal injuries (i.e. frostbite, frostnip) from environmental exposure to cold (e.g. winter sports, military activities) are fairly common, with peripheral tissues such as the fingers and toes as well as unprotected ears, nose, and cheeks being the most prone sites. On the other hand, self-use,1–4 iatrogenic,5,6 and industrial exposure-related7 thermal burns due to cold are infrequent, and generally localized to the sites of exposure. Irrespective of the type of cold-exposure, the scalp remains a rarely described site for frostbite injuries, with only one report (environmental exposure-related) of frostbite injury involving the scalp.8
In the 1970s, crushed ice in plastic bags was introduced as a promising modality for the prevention of chemotherapy-induced alopecia (CIA).9,10 The methodology has evolved since, but the underlying principle remains scalp hypothermia, which induces cutaneous vasoconstriction and impedes the delivery of cytotoxic agents to hair follicles,11 and possibly reduces apoptosis in hair follicle stem cells. In addition, the biochemical activity in cells lining the hair follicles is reduced, thus shielding them from cytotoxic drug toxicity. For the procedure, the caps are maintained at very low temperatures (−25 to −30°C) just before application. This is generally achieved either by the use of a gel-filled cap (glycerine-based, ChemoCap™/Elasto-Gel™/Arctic™; or crylon-based, Penguin™12,13) that is pre-cooled in dry ice/freezer, a chilled air-filled cap (SCSII™),14,15 or a refrigerated cooling system (pumps liquid coolant through a neoprene/silicone cap e.g. the FDA-cleared Dignitana DigniCap cooling system,16,17 or the Paxman scalp cooling system), which are applied to the scalp for varying time periods before, during, and after cytotoxic chemotherapy infusions. Since cold caps have shown efficacy without any apparent risk for increased scalp (micro-)metastases,18,19 their use is expanding.
The procedure is generally administered either by self/family member/friend or by hired trained “cappers” (usually not medically trained), and is typically not covered by health insurance carriers. The adverse events (AEs) hitherto reported are mostly mild, and include coldness, headache, dizziness, pruritus, application-site discomfort, local pain, neck pain, sensation of heaviness, and claustrophobia.17,18 Herein, we expand the spectrum of these AEs by describing cold thermal injuries on the scalp due to glycerol-gel based caps.
MATERIALS AND METHODS (CASE SERIES)
Case 1
A 49-year-old female on adjuvant chemotherapy with docetaxel, carboplatin, trastuzumab and pertuzumab (TCHP) for ER+/PR−/Her2+, stage IIA, invasive ductal carcinoma was using Penguin™ cold caps (made with hypoallergenic plastics) for the prevention of CIA. Shortly after her third chemotherapy cycle/cold cap use, she developed erythema and numbness over the upper mid-forehead (near the hairline) and neighboring hair-bearing frontal scalp, with subsequent blistering and eventual crusting. She applied Neosporin® ointment locally to prevent infection, followed by twice daily applications of Bio-oil®. Three weeks later however, partial alopecia ensued at the hairline (Fig 1A), while the scalp developed a much larger patch of alopecia (Fig 1A, inset). Of note, the patient reported that during her third cold cap session, to protect against potential cold contact injury (frostbite) she had used a paper towel instead of the gauze and moleskin padding used previously. At her recent follow-up, around 5.5 months after the inciting event, mild post-inflammatory hyperpigmentation (forehead) and mild hair thinning (scalp) persisted.
Figure 1A.
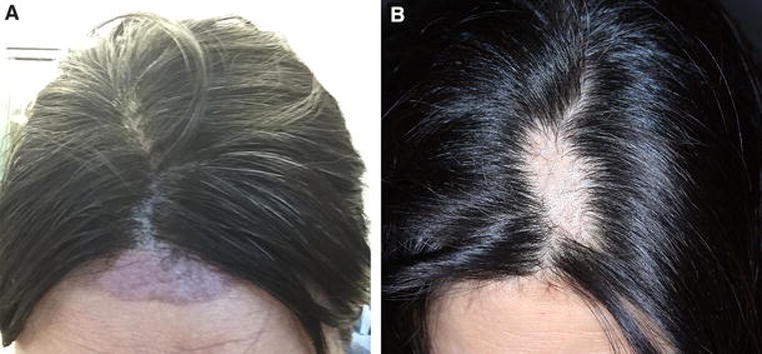
Scalp changes in a 49-year-old woman with stage IIA invasive ductal carcinoma, following improper cold cap application procedures for the prevention of CIA (adjuvant TCHP). Post-inflammatory hyperpigmentation over the upper mid-forehead (distal to the frontal hairline), 1 week after the 3rd chemotherapy cycle/cold cap use. Note the arcuate violaceous patch with sharply demarcated borders and localized alopecia. (Inset) Large patch of alopecia on the frontal scalp, approximately 4 weeks after the 3rd chemotherapy cycle/cold cap use.
Case 2
A 55-year-old female diagnosed with triple-negative, stage IIA, breast cancer, was treated with 4 cycles of adjuvant doxorubicin and cyclophosphamide, which resulted in near-complete alopecia of the scalp. She was subsequently started on paclitaxel and into the 3rd cycle, she resorted to scalp cooling with the Elasto-Gel™ hypothermia cap (made with “rubberized material”) for preventing further alopecia. While the patient felt well, a day later, she noticed a tense blister on the scalp. On exam, a large fluid-filled bulla was appreciated over an erythematous and edematous plaque on the left parietal scalp, with no signs of infection (Fig 1B). The bulla was drained and topical clobetasol and silver sulfadiazine creams were recommended, which led to improvement over the week. At her last follow-up (4 months later), mild hair thinning persisted.
Figure 1B.
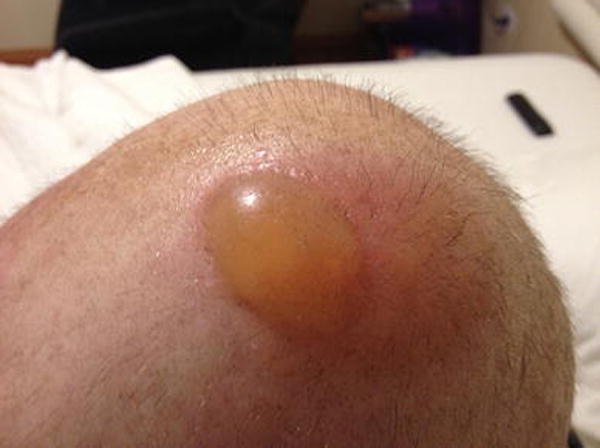
Cold thermal injury in a 55-year-old woman with triple-negative stage IIA invasive ductal carcinoma, following the use of cold caps for the prevention of further alopecia (adjuvant AC-T). Note the fluid-filled bulla (~4 cm) over an erythematous and edematous plaque (10 cm) on the left parietal scalp, appearing after the 3rd chemotherapy cycle (paclitaxel)/cold cap use.
Case 3
A 58-year-old female with ER+/PR+/Her2−, metastatic breast cancer was undergoing taxane-based chemotherapy (PH, paclitaxel and trastuzumab). To prevent CIA, she began using the Penguin cold caps with an intervening layer of moleskin over the entire scalp, starting with the first chemotherapy cycle. During her third cycle however, she experienced a hypersensitivity reaction to paclitaxel, and the infusion was terminated; the cold cap was removed and not used in the post-infusion period. The next day she received albumin-bound paclitaxel (Abraxane®) chemotherapy, and approximately 2.5 weeks later, she developed diffuse alopecia associated with pruritus that progressively worsened over the following 2 weeks.
The patient however, continued cold cap use, and to protect the scalp from cold injury, applied moleskin, a double-layered scarf, and tissue, under the cap. During her 3rd albumin-bound paclitaxel infusion (4 weeks later), she felt extreme cold on her frontal scalp, which was associated with severe nausea. A few days later, there was blistering followed by crusting in the affected area, that eventually resolved with alopecia (Fig 1C). She again developed similar symptoms (with milder nausea) on the frontal scalp during her 6th cycle (10 weeks from initiation), which improved with aloe vera cream. Two months later, at her last follow-up, the hair had regrown to patient’s satisfaction in the affected area.
Figure 1C.
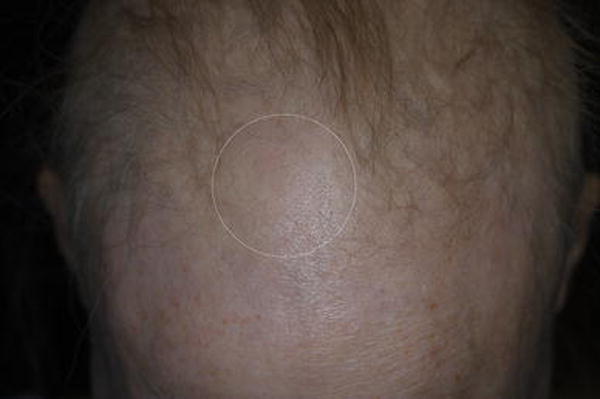
Localized alopecia (white dotted lines) on the background of diffuse hair thinning in a 58-year-old woman with ER+/PR+/Her2−, metastatic breast cancer—approximately 3 weeks after the first cold thermal injury (blistering and crusting). The localized alopecia occurred after one instance of cold cap use on a scalp that had already developed diffuse paclitaxel-induced alopecia.
Case 4
A 50-year-old female with ER+/PR+/Her2−, stage IA, invasive ductal carcinoma of the right breast, was started on chemotherapy (CMF, cyclophosphamide, methotrexate, and fluorouracil). To reduce the chances of developing CIA, she started using the Penguin™ cold caps, but shortly after the first cycle of chemotherapy/cold cap use, she developed tingling and pruritus, and eventually noticed crusting and desquamation on the crown of her scalp. It was described as a “frostnip” by her “capper”, but she consulted an outside dermatologist who diagnosed seborrheic dermatitis (resembling a cradle cap)—no medications were prescribed as the “capper’s instructions” were not to use any topical medications. The symptoms resolved by her 2nd chemotherapy cycle (in 2 weeks), and this time the capper placed a gauze between her scalp and the cap. She however, went on to develop hair thinning and localized alopecia but was unable to recall when it had started. At her most recent follow-up (6 months after the 1st chemotherapy infusion), there was regrowth of hair in the affected area, although less dense and accompanied by sensitivity of the skin (Fig 1D).
Figure 1D.
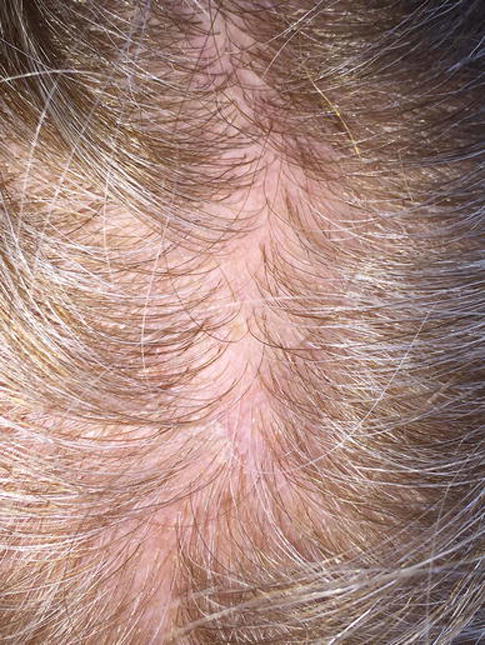
Mild persistent alopecia due to cold thermal injury in a 50-year-old female with stage IA, invasive ductal carcinoma, following the improper application of Penguin cold caps to prevent chemotherapy-induced alopecia. Note the hair thinning in a localized area of the scalp (crown), at 6 months after the 1st chemotherapy infusion/cold cap use.
A summary of the findings related to the use of cold caps in our patients is provided in Table 1.
Table 1.
Characteristics of patients with frostbite on the scalp following the use of cold caps.
| Case No. | Age/Sex | Cancer | Chemo Regimen | Cold Cap Usage Details | Onset | Follow-up†† | Potential contributory factors | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Make | Pre-infusion | During infusion | Post-infusion | |||||||
| 1 | 49/F | Breast | TCHP | Penguin™ | 60′ | 2.5hrs. | 5hrs. | Cycle 3 | Mild persistent alopecia (at 5 months) | Lack of adequate padding between the cold cap and skin; duration of post-infusion cooling |
| 2 | 55/F | Breast | AC➔T | Elasto-Gel™ | 15′ | 60′ | 50mins. | Cycle 3 | Mild persistent alopecia (at 4 months) | Pre-existing alopecia; lack of padding; duration of post-infusion cooling? |
| 3 | 58/F | Breast | PH | Penguin™ | 50′ | 30′ | 2.5hrs. | Cycle 4† | Alopecia resolved (at 2 months) | Pre-existing diffuse alopecia; duration of post-infusion cooling |
| 4 | 50/F | Breast | CMF | Penguin™ | 50′ | 60′ | 4.5hrs. | Cycle 1 | Mild persistent alopecia, skin sensitivity (at 6 months) | Lack of adequate padding; duration of post-infusion cooling |
TCHP – docetaxel, carboplatin, trastuzumab, pertuzumab; AC-T – adriamycin and cyclophosphamide, followed by paclitaxel; PH – paclitaxel, trastuzumab; CMF – cyclophosphamide, methotrexate, fluorouracil
this includes two complete cycles of paclitaxel and two cycles of albumin-bound paclitaxel
it must be noted that the findings occurred in some patients, despite the use of topical minoxidil 5% lotion
DISCUSSION
This is the first report of superficial cold thermal injuries (frostbites) observed during scalp cooling for the prevention of CIA. The scalp receives a rich blood supply from five arteries and numerous anastomoses making it an uncommon site for environmental frostbites, but it is not protected from cold thermal (contact) injury. According to the modern classification by depth,20 freezing cold injuries can be superficial (first- and second-degree; erythema, edema, blisters) or deep (third- and fourth-degree; eschar, ulceration, gangrene). The latter involves tissues beneath the skin (e.g. muscle, tendon, nerve, blood vessel, and bone) and was fortunately not encountered in our patients, but the likelihood seems rare. We are monitoring the long-term outcomes (resolution vs. permanent alopecia and/or scarring) in these patients throughout treatment and long-term follow-up, in our cancer center-based dermatology clinical programs (MSKCC and Brazil).
The injuries in our series were superficial (i.e. limited to the skin), but it is noteworthy that they led to decreased hair regrowth (persistent alopecia) in three patients—a paradoxical outcome for an alopecia prevention strategy, the actual burden of which remains unknown. In addition, there are no evidence-based guidelines for the use of cold caps either, which factor in parameters such as patient characteristics, chemotherapy regimen, scalp protection (e.g. surgical cap/moleskin) duration of use (e.g. post-infusion cooling time), and hair type/amount (e.g. pre-existing alopecia).
Prolonged and direct exposure to sub-zero temperatures causes cutaneous vasoconstriction, anesthesia, endothelial cell injury with platelet aggregation and blood vessel thrombosis, and intracellular ice crystal formation.21,22 These events and their interplay lead to dehydration and protein modification in tissues, worsening tissue damage,23 and eventually resulting in necrotic cell death. Needless to say, rewarming procedures could further aggravate the situation, given the potential for reperfusion injury.24 However, the extent to which these could occur with the (improper) use of cold caps, the approach to effectively managing resultant injury, and whether cold cap use should then continue during subsequent chemotherapy cycles remain unanswered. In addition, the potential for developing permanent alopecia and scarring, and the resultant cosmetic disfigurement can be a huge concern.
A number of animal models have been used to study frostbite injury.21,25–27 Based on findings in a hairless mouse ear model, it appears that the minimum temperature necessary to produce consistent tissue necrosis is −4°C for 3 minutes. Although applied on hair-bearing skin (scalp) in humans, these caps are maintained at much lower temperatures (around −25°C) just before application. The consequences of inadvertent misapplication therefore, could be disastrous. On the other hand, no reports of frostbite have been reported with the refrigerated cooling systems, wherein the temperature is controlled. Thus, more research simulating in-human cold cap use must be undertaken, as the findings may permit streamlining of current usage protocols and the approach in clinic.
Some factors that place patients at risk for the development of frostbites in general, include age, lifestyle (smoking), comorbidities (malnutrition/diabetes/thyroid disease), concomitant medications (beta-blockers), susceptible scalp areas (hair parting/pre-existing alopecia), use of spacer material (moleskin/gauze-piece), the duration of cold cap usage (before/after chemotherapy), capper’s experience, patient education (awareness of prolonged/direct application of ice), and previous frostbites, to name a few. These definitely need to be considered for e.g. only 20 minutes sufficed to offset the development of CIA in patients receiving a docetaxel-based chemotherapy regimen,28 which is contrary to the currently recommended 3–5 hrs. of post-infusion cooling by manufacturers.
In conclusion, there is an urgent need to establish screening programs to exclude at-risk individuals, and develop standardized treatment and education protocols based on the type of cold cap system used. With increasing use of a variety of innovative scalp cooling systems/methodologies, we anticipate that such unusual, yet preventable AEs may be more frequently encountered, especially in patients with high-grade alopecia. Therefore, studies should focus on standardizing the application methodologies, target scalp temperatures to be achieved/maintained, duration of cap usage, delivery mechanisms, continued use post-injury, patient surveys, and better identify the CIA-inducing chemotherapy regimens most amenable to scalp cooling. Proper patient/nurse education, and personnel training and certification procedures in cold cap administration need to be implemented. These are crucial for optimal clinical outcomes and to minimize impairments in health-related quality of life.
Acknowledgments
We thank Ms. Bernadette Murphy for the clinical photography.
M.E.L. and V.R.B are supported by the RJR Oncodermatology Fund at Memorial Sloan Kettering Cancer Center.
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
ABBREVIATIONS
- AC-T
adriamycin + cyclophosphamide + paclitaxel
- AE
adverse event
- CIA
chemotherapy-induced alopecia
- CMF
cyclophosphamide + methotrexate + fluorouracil
- MSKCC
Memorial Sloan Kettering Cancer Center
- PH
pertuzumab + trastuzumab
- TCHP
docetaxel + carboplatin + trastuzumab + pertuzumab
Footnotes
DISCLOSURE STATEMENT
MEL has consulting agreements with Dignitana and Paxman.
KC has consulting agreements with Roche, Amgen, and PER.
The other authors declare that they have no conflicts of interest.
References
- 1.Stevens DM, D’Angelo JV. Frostbite due due to improper use of frozen gel pack. N Engl J Med. 1978;299:1415. doi: 10.1056/nejm197812212992516. [DOI] [PubMed] [Google Scholar]
- 2.Quist LH, Peltier G, Lundquist KJ. Frostbite of the eyelids following inappropriate application of ice compresses. Arch Ophthalmol. 1996;114:226. doi: 10.1001/archopht.1996.01100130220025. [DOI] [PubMed] [Google Scholar]
- 3.Graham CA, Stevenson J. Frozen chips: an unusual cause of severe frostbite injury. Br J Sports Med. 2000;34:382–3. doi: 10.1136/bjsm.34.5.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sohatee MA, Brierley NA, Muir T. “Salt ice dare”: a previously un-described mechanism of rapid frostbite injury. J Plast Reconstr Aesthet Surg. 2014;67:e248–9. doi: 10.1016/j.bjps.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Brown WC, Hahn DB. Frostbite of the feet after cryotherapy: a report of two cases. J Foot Ankle Surg. 2009;48:577–80. doi: 10.1053/j.jfas.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Oksuz S, Eren F, Sever C, et al. Frostbite injury of the breast: a case report. Ann Burns Fire Disasters. 2014;27:105–6. [PMC free article] [PubMed] [Google Scholar]
- 7.Wegener EE, Barraza KR, Das SK. Severe frostbite caused by Freon gas. South Med J. 1991;84:1143–6. doi: 10.1097/00007611-199109000-00022. [DOI] [PubMed] [Google Scholar]
- 8.Orlov AN, Dmitrienko OD, Kozulin DA. Severe frostbite of rare location. Vestn Khir Im I I Grek. 1990;144:76–7. [PubMed] [Google Scholar]
- 9.Luce JK, Raffetto TJ, Crisp IM, et al. Prevention of Alopecia by Scalp Cooling of Patients Receiving Adriamycin. Cancer Chemotherapy Reports Part 1. 1973;57:108–9. [Google Scholar]
- 10.Edelstyn GA, MacDonald M, MacRae KD. Doxorubicin-induced hair loss and possible modification by scalp cooling. Lancet. 1977;2:253–4. doi: 10.1016/s0140-6736(77)92877-x. [DOI] [PubMed] [Google Scholar]
- 11.Bulow J, Friberg L, Gaardsting O, et al. Frontal subcutaneous blood flow, and epi- and subcutaneous temperatures during scalp cooling in normal man. Scand J Clin Lab Invest. 1985;45:505–8. doi: 10.3109/00365518509155250. [DOI] [PubMed] [Google Scholar]
- 12.Peck HJ, Mitchell H, Stewart AL. Evaluating the efficacy of scalp cooling using the Penguin cold cap system to reduce alopecia in patients undergoing chemotherapy for breast cancer. Eur J Oncol Nurs. 2000;4:246–8. doi: 10.1054/ejon.2000.0094. [DOI] [PubMed] [Google Scholar]
- 13.Katsimbri P, Bamias A, Pavlidis N. Prevention of chemotherapy-induced alopecia using an effective scalp cooling system. Eur J Cancer. 2000;36:766–71. doi: 10.1016/s0959-8049(00)00012-5. [DOI] [PubMed] [Google Scholar]
- 14.Ron IG, Kalmus Y, Kalmus Z, et al. Scalp cooling in the prevention of alopecia in patients receiving depilating chemotherapy. Support Care Cancer. 1997;5:136–8. doi: 10.1007/BF01262571. [DOI] [PubMed] [Google Scholar]
- 15.Hillen HF, Breed WP, Botman CJ. Scalp cooling by cold air for the prevention of chemotherapy-induced alopecia. Neth J Med. 1990;37:231–5. [PubMed] [Google Scholar]
- 16.U.S. Food and Drug Administration. FDA Allows Marketing Of Cooling Cap To Reduce Hair Loss During Chemotherapy (12/8/2015) Available at http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm476216.htm. Accessed: 1/18/2016.
- 17.Rugo HS, Klein P, Melin SA, et al. Clinical performance of the DigniCap system, a scalp hypothermia system, in preventing chemotherapy-induced alopecia. ASCO Meeting Abstracts. 2015;33:9518. [Google Scholar]
- 18.Grevelman EG, Breed WP. Prevention of chemotherapy-induced hair loss by scalp cooling. Ann Oncol. 2005;16:352–8. doi: 10.1093/annonc/mdi088. [DOI] [PubMed] [Google Scholar]
- 19.Kadakia KC, Rozell SA, Butala AA, et al. Supportive cryotherapy: a review from head to toe. J Pain Symptom Manage. 2014;47:1100–15. doi: 10.1016/j.jpainsymman.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mills W. Clinical aspects of freezing cold injuries. In: Pandolf K, Burr R, editors. Medical Aspects of Harsh Environments. Office of the Surgeon General, Falls Church; 2001. p. 444. [Google Scholar]
- 21.Bourne MH, Piepkorn MW, Clayton F, et al. Analysis of microvascular changes in frostbite injury. J Surg Res. 1986;40:26–35. doi: 10.1016/0022-4804(86)90141-1. [DOI] [PubMed] [Google Scholar]
- 22.Heggers JP, Robson MC, Manavalen K, et al. Experimental and clinical observations on frostbite. Ann Emerg Med. 1987;16:1056–62. doi: 10.1016/s0196-0644(87)80758-8. [DOI] [PubMed] [Google Scholar]
- 23.Bracker M. Environmental and thermal injury. Clinics in sports medicine. 1992;11:419–36. [PubMed] [Google Scholar]
- 24.Manson PN, Jesudass R, Marzella L, et al. Evidence for an early free radical-mediated reperfusion injury in frostbite. Free Radic Biol Med. 1991;10:7–11. doi: 10.1016/0891-5849(91)90015-u. [DOI] [PubMed] [Google Scholar]
- 25.Goertz O, Baerreiter S, Ring A, et al. Determination of microcirculatory changes and angiogenesis in a model of frostbite injury in vivo. J Surg Res. 2011;168:155–61. doi: 10.1016/j.jss.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 26.Auerbach LJ, Galvez MG, De Clerck BK, et al. A novel mouse model for frostbite injury. Wilderness Environ Med. 2013;24:94–104. doi: 10.1016/j.wem.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 27.Rothenberger J, Held M, Jaminet P, et al. Development of an animal frostbite injury model using the Goettingen-Minipig. Burns. 2014;40:268–73. doi: 10.1016/j.burns.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 28.Komen MM, Breed WP, Smorenburg CH, et al. Results of 20-versus 45-min post-infusion scalp cooling time in the prevention of docetaxel-induced alopecia. Support Care Cancer. 2016:1–7. doi: 10.1007/s00520-016-3084-7. [DOI] [PubMed] [Google Scholar]


