Abstract
Background
Silent myocardial infarction (SMI) accounts for about half of the total number of MIs, and is associated with poor prognosis as is clinically documented MI (CMI). The electrocardiographic (ECG) spatial QRS/T angle has been a strong predictor of cardiovascular outcomes. Whether spatial QRS/T angle also is predictive of SMI, and the easy-to-obtain frontal QRS/T angle will show similar association are currently unknown.
Methods
We examined the association between the spatial and frontal QRS/T angles, separately, with incident SMI among 9498 participants (mean age 54 years, 57% women, and 20% African-American), who were free of cardiovascular disease at baseline (visit 1, 1987–1989) from the Atherosclerosis Risk in Communities (ARIC) study. Incident SMI was defined as MI occurring after the baseline until visit 4 (1996–1998) without CMI. The frontal plane QRS/T angle was defined as the absolute difference between QRS axis and T axis. Values greater than the sex-specific 95th percentiles of the QRS/T angles were considered wide (abnormal).
Results
A total of 317 (3.3%) incident SMIs occurred during a 9-year median follow-up. In a model adjusted for demographics, cardiovascular risk factors and potential confounders, both abnormal frontal (HR 2.28, 95% CI 1.58–3.29) and spatial (HR 2.10, 95% CI 1.44–3.06) QRS/T angles were associated with an over 2-fold increased risk of incident SMI. Similar patterns of associations were observed when the results were stratified by sex.
Conclusions
Both frontal and spatial QRS/T angles are predicative of SMI suggesting a potential use for these markers in identifying individuals at risk.
Keywords: Electrocardiography, QRS/T angle, Silent myocardial infarction
Introduction
Heart disease still is the leading cause of death globally. In 2013, about 14% of total deaths in the United States were due to coronary heart disease (CHD), with over 660,000 new myocardial infarctions (MI) with clinical manifestations (CMI) as well as 160,000 accidentally discovered asymptomatic silent MIs (SMI) [1–3]. Reported SMIs thus accounted for over 20% of all new MIs [3–9]. In a recent analysis from the Atherosclerosis Risk in Communities (ARIC) study [10], we showed that asymptomatic or SMI, accounted for about 45% of the total number of MIs in the study. These SMIs were associated with an increased risk of death comparable to that of CMI.
Abnormally wide spatial QRS/T angle has been repeatedly shown to be among the strongest ECG markers of abnormal repolarization predictive of cardiovascular disease (CVD) events and mortality [11–20]. Nevertheless, computation of the spatial QRS/T angle from the standard 12-lead ECG requires several steps which is a challenge in its wide utilization. On the other hand, the frontal plane QRS/T angle could be easily calculated as the absolute value of the difference between QRS axis and T axis. Whether spatial QRS/T angle is predictive of SMI and whether the easy-to-obtain frontal QRS/T angle will show similar association with SMI are currently unknown. Therefore, the primary objective of the present study was to perform a comparative evaluation of the utility of the frontal plane and spatial QRS/T angle as predictors of incident SMI.
Methods
The Atherosclerosis Risk in Communities (ARIC) study prospectively enrolled 15,792 men and women between 45 and 64 years of age, which is a population-based multicenter study designed to investigate the natural history and cause of atherosclerotic and CVD from 4 US communities: Forsyth County, North Carolina; Jackson, Mississippi; suburbs of Minneapolis, Minnesota; and Washington County, Maryland. Eligible participants were interviewed at home and then invited to a baseline clinical examination between 1987 and 1989. They attended 3 additional clinical examinations at every 3 yearly and a recent 5th examination completed in 2013 for which data are not included here. Participants were interviewed by phone annually. Details of the ARIC Study design, protocol sampling procedures, and selection and exclusion criteria were published previously [21]. The study was approved by each study site’s institutional review board. All participants provided written informed consent.
For the purpose of this analysis, we included all ARIC participants with good quality and complete ECG data at visits 1 to 4 as well as outcome events after visit 4. We excluded the follwing partcipants: 47 with reported race other than African-American or white, 136 with poor quality ECG, 3775 with missing ECG in any of the ARIC first 4 visits including those who died during this period, 429 with ECG diagnosis of bundle branch block, external pacemaker or Wolff–Parkinson–White pattern, and 201 with missing one or more of baseline CVD risk factors. We also excluded 1706 participants with history of CVD at baseline which was defined as the presence of ECG evidence of MI, or a self-reported history of physician-diagnosed MI, coronary artery bypass surgery, coronary angioplasty, heart failure, or stroke. After all exclusions (n = 6294), a total of 9498 remained and were included in the analysis.
Identical electrocardiographs (MAC PC, Marquette Electronics Inc., Milwaukee, WI) were used at all clinic sites, and resting, 10 seconds standard simultaneous 12-lead ECGs were recorded in all participants using strictly standardized procedures. All ECGs were processed in a central ECG laboratory (initially at Dalhousie University, Halifax, Nova Scotia, Canada and later at the EPICARE Center, Wake Forest School of Medicine, Winston-Salem, NC, USA), where all ECGs were visually inspected for technical errors and inadequate quality.
Spatial QRS/T angle was computed from the mean QRS and T vectors of the quasi-orthogonal XYZ leads, and in turn computed using the Kors transform [11]. Frontal QRS/T angle was defined as the absolute value of the difference between the frontal plane QRS axis and T axis adjusted to the minimal angle using (360° – angle) for angles >180° [12] (Supplementary Figures 1 and 2). Values greater than the sex-specific 95th percentile of the frontal and spatial QRS/T angles were considered abnormal while values between 95th and 75th were considered borderline. Left ventricular hypertrophy (LVH) was defined by Cornell voltage criteria (RaVL amplitude + SV3 amplitude) using the following sex-specific cut-off points: ≥2200 microvolt (μV) in women and ≥2800 μV in men.
Incident SMI was defined as ECG evidence of new MI without clinically documented MI (CMI) after the baseline until ARIC visit 4 (1996–1998). ECG evidence of MI was defined by new appearance of a major Q wave or a minor Q wave with major ischemic ST-T changes according to the Minnesota ECG Classification [10,21–23].
Frequency distributions of the variables used in analyses were first inspected to rule out anomalies and outliers. Descriptive statistics were used to determine mean values, standard deviations, and percentile distributions for continuous variables, and frequencies and percentages for categorical variables. Cox’s proportional hazards analysis was used to examine the association between abnormal, borderline and normal (reference) frontal and spatial QRST angle (separately), with SMI and CMI (vs. no MI) occurring from visit 1 to visit 4. Models were adjusted as follows: Model-1 adjusted for demographics (age, sex and race), and Model-2 adjusted for demographics plus study center, body mass index, systolic blood pressure, smoking status, education, hypertension, diabetes mellitus, total ratio of total cholesterol/high-density lipoprotein cholesterol, use of cholesterol lowering medications, use of aspirin, family history of CHD, ECG-LVH by Cornell voltage, and serum creatinine at baseline. Given the known sex difference in QRS/T angle, subgroup analyses by sex using similar models were also conducted. All analyses were performed with SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
A total of 9498 participants (age 54.0 ± 5.7 years, 56.9% women, 20.3% African American) were included in the analysis. Table 1 shows the demographic, clinical, and ECG characteristics of the study participants stratified by spatial QRS/T angles categories at baseline. Compared to those with normal QRS/T angle, participants with wide QRS/T angle were more likely to be older, to be African-American, and to have diabetes and hypertension. They also were more likely to have lower education level, and higher values for body mass index and systolic blood pressure. Table 1 also shows that the participants with wide QRS/T angle had higher rates of incident MI (SMI and CMI).
Table 1.
Baseline (1987–1989) participants characteristic stratified by spatial QRS/T angle groups and outcomes during follow-up (1996–1998).
| N = 9498 | Mean ± SD or n (%),
|
p-Valuec | p-Valued | ||
|---|---|---|---|---|---|
| Normal QRS/T (n = 7167) | Borderline QRS/T (n = 1851) | Wide QRS/T (n = 480) | |||
| Age (years) | 54 ± 5.7 | 54 ± 5.6 | 55 ± 5.7 | <.0001 | <.0001 |
| Women | 4085 (57) | 1038 (56) | 277 (58) | .3789 | .4835 |
| African-American | 1424 (19.9) | 356 (19.2) | 150 (31.3) | <.0001 | <.0001 |
| Education ≤ high school | 3644 (50.8) | 961 (51.9) | 266 (55.4) | .0180 | .2526 |
| Current smoker | 1407 (19.7) | 490 (26.5) | 117 (24.4) | <.0001 | .9251 |
| Body mass index (kg/m2) | 27 ± 4.9 | 27 ± 5.2 | 28 ± 5.8 | <.0001 | <.0001 |
| Systolic blood pressure (mm Hg) | 118 ± 16 | 120 ± 18 | 128 ± 23 | <.0001 | <.0001 |
| Hypertension | 1875 (26.3) | 518 (28.1) | 234 (49.0) | <.0001 | <.0001 |
| Antihypertensive medication | 1607 (22.4) | 408 (22.1) | 176 (36.7) | <.0001 | <.0001 |
| Diabetes | 528 (7.4) | 160 (8.7) | 73 (15.3) | <.0001 | <.0001 |
| Ratio of total/HDL cholesterol | 4.5 ± 1.6 | 4.5 ± 1.9 | 4.7 ± 1.7 | .0020 | <.0001 |
| Cholesterol lowering medication | 168 (2.4) | 43 (2.3) | 10 (2.1) | .5370 | .4862 |
| Aspirin use | 3218 (45.3) | 886 (48.3) | 222 (46.7) | .0100 | .5270 |
| Family history of coronary heart disease | 2861 (39.9) | 751 (40.6) | 187 (39.0) | .4380 | . 2177 |
| Serum creatinine (mg/dL) | 1.1 ± 0.3 | 1.1 ± 0.2 | 1.1 ± 0.2 | <.0001 | .0002 |
| Left ventricular hypertrophya | 90 (1.0) | 9 (2.8) | 7 (1.8) | <.0001 | <.0001 |
| QRS/T angle—spatial (°) | 55 ± 18 | 94 ± 11 | 127 ± 15 | <.0001 | <.0001 |
| QRS/T angle—frontal (°) | 19 ± 16 | 29 ± 24 | 56 ± 42 | <.0001 | <.0001 |
| Heart rate (beats/min) | 65 ± 9.5 | 67 ± 10 | 68 ± 11 | <.0001 | .0046 |
| QRS duration (ms) | 90 ± 9.1 | 93 ± 9.6 | 94 ± 10 | <.0001 | .0026 |
| QTrr interval (ms)b | 414 ± 14 | 416 ± 15 | 418 ± 16 | <.0001 | .0137 |
| Outcomes | |||||
| Silent myocardial infarction | 198 (2.8) | 85 (4.6) | 34 (7.1) | <.0001 | <.0001 |
| Clinical myocardial infarction | 274 (3.8) | 73 (3.9) | 39 (8.1) | <.0001 | <.0001 |
Left ventricular hypertrophy was defined by Cornell voltage criteria.
QTrr interval is the rate-adjusted QT interval as a linear function of the RR interval.
p-Value comparing three groups (Wide QRS/T angle for >95th percentile, Borderline QRS/T angle for >75th and ≤ 95th percentile, and Normal QRS/T angle for ≤75th percentile) with ternary level of QRS/T angle including ≤75th percentile using analysis of variance and Chi-squared for continuous and categorical variables, respectively.
p-Value comparing groups with wide QRS/T angle and borderline QRS/T angle using unpaired Student T test and chi-squared for continuous and categorical variables, respectively.
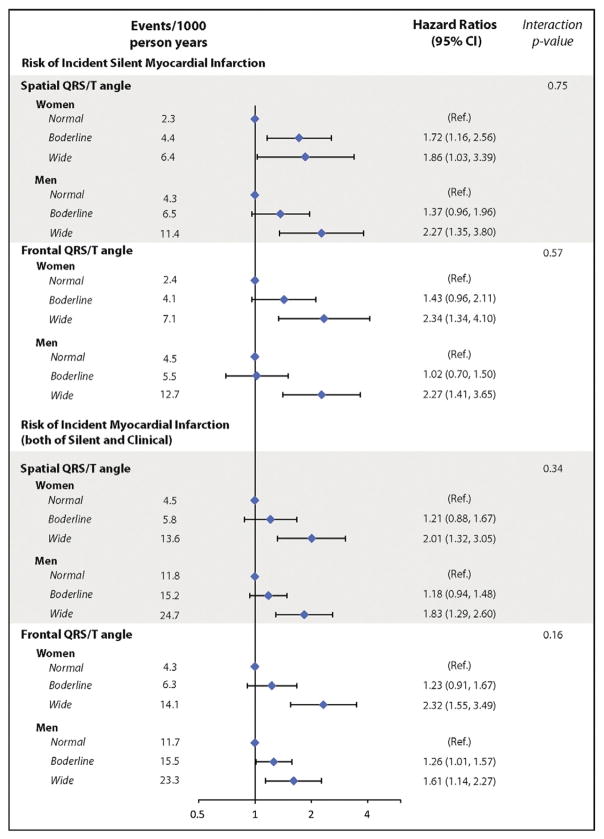
During a median of 8.9 years of follow-up from baseline through the 4th ARIC visit, 317 participants developed SMI while 386 developed CMI. Using a sex-specific cut point at 95th percentile for abnormal QRS/T angle and at 75th percentile cutpoint for borderline angle at baseline (Table 2), participants with wide spatial QRS/T angle as well as frontal plane QRS/T angle had significantly higher rates of incident SMI than participants with normal QRS/T angles (8.5 vs. 3.2, and 9.4 vs. 3.3 per 1000 person-years, respectively). In multivariable adjusted models, wide spatial and frontal QRS/T angles exceeding 95th percentiles were both associated with an over 2-fold increased risk of incident SMI, and near 2-fold increase risk for all incident all MIs (including both of SMI and CMI) (Table 3 and Fig. 1).
Table 2.
The cutpoints for borderline and wide QRS/T angles by gender and race.
| N | Spatial QRS/T angle
|
Frontal QRS/Ta angle
|
|||||
|---|---|---|---|---|---|---|---|
| Mean | BLb | Widec | Mean | BLb | Widec | ||
| All participants | 9498 | 65.9 | 83 | 114 | 22.7 | 31 | 63 |
| Men | 4098 | 73.6 | 91 | 120 | 23.4 | 31 | 66 |
| Women | 5400 | 60.2 | 76 | 104 | 22.1 | 30 | 61 |
Frontal plane QRS/T angle calculated as the absolute value of (QRS axis – T axis). If absolute value >180°, then subtract it from 360°.
BL (borderline) is for QRS/T angles >75th percentile and ≤ 95th percentile.
Wide for QRS/T angles >95th percentile.
Table 3.
Hazard ratios with 95% confidence intervals for risk of incident myocardial infarction by QRS/T angle category.
| Events/1000 person years | Model-1a, HR (95% CI) | p Value | Model-2b, HR (95% CI) | p Value | |
|---|---|---|---|---|---|
| Risk of incident silent myocardial infarction (N = 9112) | |||||
| Spatial QRS/T angle | |||||
| Normal | 3.2 | 1 (ref.) | 1 (ref.) | ||
| Borderline | 5.3 | 1.66 (1.29–2.15) | <.0001 | 1.51 (1.16–1.96) | .0023 |
| Wide | 8.5 | 2.59 (1.79–3.73) | <.0001 | 2.00 (1.35–2.96) | .0005 |
| Frontal QRS/T angle | |||||
| Normal | 3.3 | 1 (ref.) | 1 (ref.) | ||
| Borderline | 4.7 | 1.31 (1.01–1.71) | .0437 | 1.23 (0.94–1.61) | .1372 |
| Wide | 9.4 | 2.72 (1.92–3.84) | <.0001 | 2.28 (1.59–3.27) | <.0001 |
| Risk of incident myocardial infarction (both of silent and clinical, N = 9498) | |||||
| Spatial QRS/T angle | |||||
| Normal | 7.6 | 1 (ref.) | 1 (ref.) | ||
| Borderline | 9.8 | 1.30 (1.09–1.56) | .0039 | 1.17 (0.97–1.41) | .1018 |
| Wide | 18.2 | 2.39 (1.87–3.07) | <.0001 | 1.87 (1.43–2.44) | <.0001 |
| Frontal QRS/T angle | |||||
| Normal | 7.4 | 1 (ref.) | 1 (ref.) | ||
| Borderline | 10.4 | 1.35 (1.13–1.60) | .0009 | 1.25 (1.05–1.49) | .0147 |
| Wide | 17.9 | 2.25 (1.75–2.89) | <.0001 | 1.86 (1.43–2.41) | <.0001 |
Cutpoints listed in Table 2: normal, QRS/T angle ≤ 75th percentile; borderline, >75th percentile and ≤ 95th percentile; wide, >95th percentile.
Model 1: adjusted for age, sex, and race.
Model 2: adjusted for the variables in Model-1 plus study site, body mass index, education, smoking status, systolic blood pressure, blood pressure-lowering medications, diabetes mellitus, ratio of total cholesterol to high-density lipoprotein, use of cholesterol-lowering medications, use aspirin, family history of coronary heart disease, ECG-LVH by Cornell voltage, and serum creatinine at baseline.
Fig. 1.
Risk of incident myocardial infarction by QRS/T angle category and gender. CI = confidence interval. Cutpoints listed in Table 2: normal, QRS/T angle ≤ 75th percentile; borderline, >75th percentile and ≤95th percentile; wide, >95th percentile. Model adjusted for age, sex, race, study site, body mass index, education, smoking status, systolic blood pressure, blood pressure lowering medications, diabetes mellitus, ratio of total cholesterol to high density lipoprotein, use of cholesterol-lowering medications, use of aspirin, family history of coronary heart disease, ECG-LVH by Cornell voltage, and serum creatinine at baseline.
In addition to the new Q wave development, about 18.3% of the participants in the SMI group had other major ECG abnormality during follow up (28 new complete bundle branch blocks, 28 ECG-LVH by Cornell voltage criteria, and 6 new atrial fibrillations). Among those, 29% were found on the ECG after SMI event, and 52% were shown on the same ECG with Q wave SMI event.
In multivariable adjusted models, common ECG markers of ventricular depolarization or repolarization were not associated with increased risk of SMI in our study. The association of SMI with prolonged QRS duration and prolonged QT interval had hazard ratios of 1.20 (95% confidence Intervals 0.79–1.83) and 1.35 (0.89–2.03), respectively.
Discussion
The purpose of this investigation was to examine and compare the frontal plane and spatial QRS/T angle as predictors of incident SMI. The key finding from our study is that both wide spatial and frontal QRS/T angles are equally predictive of incident SMI.
The QRS/T angle is a sensitive and reproducible marker of abnormal ventricular depolarization and repolarization which is able to detect depolarization and repolarization abnormalities before other common markers are apparent as QRS duration or QT interval [13–16]. Abnormal ventricular repolarization is an established mechanism for arrhythmogenesis. Wide QRS/T angles might be associated with myocardial structure and functional change. Over the years, a wide spatial QRS/T angle has been repeatedly shown to be associated with increased risk of all-cause mortality, cardiac mortality, and sudden cardiac death in several cohort studies [11–20]. To our knowledge this is the first report documenting that spatial QRS/T angles also is associated with risk of incident SMI.
Despite the interest in spatial QRS/T angle and the repeated evidence of its usefulness as a predictor of CVD outcomes, its wide application has been challenged by being not readily available as an output from ECG machine currently in use, and being not familiar to most physicians. Conversely, the frontal QRS/T angle could be readily available from routine 12-lead ECGs, and simpler to calculate. Similar to spatial QRS/T angle, frontal QRS/T angle also reflects both ventricular depolarization and repolarization. A decade ago, we first reported that the frontal plane QRS/T angle was a ‘suitable clinical substitute’ for spatial QRS/T angle with respect to CHD risk prediction in the ARIC study [12]. Since then, the frontal QRS/T angle has been intensively studied in various populations, as a predictor of different outcomes including heart failure, atrial fibrillation, sudden cardiac death, and all-cause mortality [16–18,24–26]. Our study adds SMI to this list of outcomes, for the first time.
Our results should be read in the context of certain limitations. The population studied was restricted to those with white and African-American ethnicity, which impact the generalizability if the results to other races/ethnicities. Also, analyses of subgroups by sex might have been limited by the small numbers in the subgroups, and hence the interaction results should read with caution. Finally and similar to other studies with similar design, residual confounding is always a possibility. SMI in the present study is MI with ECG evidence of a major Q wave or a minor Q wave with major ischemic ST-T changes, and CMI is clinically documented MI with or without Q wave. It is not possible to detect NQWMIs with the ECGs alone. Our study has several strengths. The ARIC study is a large, well-designed prospective cohort study with long term follow-up. The study included highly standardized ECG procedures and carefully documented events ascertained by an independent adjudication committee.
Conclusions
Wide spatial and frontal plane QRS/T angles are both significant predictors of incident SMI suggesting a potential use for these markers in identifying individuals at risk. Hence, the frontal plane QRS/T angle could be used as a practical substitute for the more complex spatial QRS/T angle for early identification of individuals at risk for silent MI.
Supplementary Material
Acknowledgments
Funding sources
The ARIC Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C).
The authors thank the staff and participants of the ARIC study for their important contributions.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.jelectrocard.2017.05.001.
Disclosures
The authors have no conflicts of interests to disclose.
References
- 1.Thygesen K, Alpert JS, White HD on behalf of the Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction. Circulation. 2007;116:2634–53. doi: 10.1161/CIRCULATIONAHA.107.187397. [DOI] [PubMed] [Google Scholar]
- 2.Thygesen K, Alpert JS, Jaffe AS, et al. The writing group on behalf of the Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction. Third universal definition of myocardial infarction. Circulation. 2012;126:2020–35. doi: 10.1161/CIR.0b013e31826e1058. [DOI] [PubMed] [Google Scholar]
- 3.Mozaffarian D, Benjamin EJ, Go AS, et al. American Heart Association Statistics Committee; Stroke Statistics Subcommittee. Heart disease and stroke statistics—2016 update. A report from the American Heart Association. Circulation. 2016;133:e255–69. [Google Scholar]
- 4.Sheifer SE, Gersh BJ, Yanez ND, III, et al. Prevalence, predisposing factors, and prognosis of clinically unrecognized myocardial infarction in the elderly. J Am Coll Cardiol. 2000;35:119–26. doi: 10.1016/s0735-1097(99)00524-0. [DOI] [PubMed] [Google Scholar]
- 5.Kannel WB, Abbott RD. Incidence and prognosis of unrecognized myocardial infarction. An update on the Framingham study. Med. 1984;311:1144–7. doi: 10.1056/NEJM198411013111802. [DOI] [PubMed] [Google Scholar]
- 6.Sigurdsson E, Thorgeirsson G, Sigvaldason H, et al. Unrecognized myocardial infarction: epidemiology, clinical characteristics, and the prognostic role of angina pectoris: the Reykjavik Study. Ann Intern Med. 1995;122:96–102. doi: 10.7326/0003-4819-122-2-199501150-00003. [DOI] [PubMed] [Google Scholar]
- 7.Jonsdottir LS, Sigfusson N, Sigvaldason H, et al. Incidence and prevalence of unrecognized and recognized myocardial infarction in women: the Reykjavik Study. Eur Heart J. 1998;19:1011–8. doi: 10.1053/euhj.1998.0980. [DOI] [PubMed] [Google Scholar]
- 8.Schelbert EB, Cao JJ, Sigurdsson S, et al. Prevalence and prognosis of unrecognized myocardial infarction determined by cardiac magnetic resonance in older adults. JAMA. 2012;308(9):890–6. doi: 10.1001/2012.jama.11089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Torbal A, Boersma E, Kors JA, et al. Incidence of recognized and unrecognized myocardial infarction in men and women aged 55 and older: the Rotterdam Study. Eur Heart J. 2006;27(6):729–36. doi: 10.1093/eurheartj/ehi707. [DOI] [PubMed] [Google Scholar]
- 10.Zhang ZM, Rautaharju PM, Prineas RJ, et al. Race and sex differences in the incidence and prognostic significance of silent myocardial infarction in the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2016;133:2141–8. doi: 10.1161/CIRCULATIONAHA.115.021177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kors JA, van Herpen G, Sittig AC, et al. Reconstruction of the frank vectorcardiogram from standard electrocardiographic leads: diagnostic comparison of different methods. Eur Heart J. 1990;11:1083–92. doi: 10.1093/oxfordjournals.eurheartj.a059647. [DOI] [PubMed] [Google Scholar]
- 12.Zhang ZM, Prineas RJ, Case D, et al. For the ARIC Research Group: comparison of the prognostic significance of the electrocardiographic QRS/tangles in predicting incident coronary heart disease and total mortality (from the Atherosclerosis Risk in Communities study) Am J Cardiol. 2007;100:844–9. doi: 10.1016/j.amjcard.2007.03.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kardys I, Kors JA, van der Meer IM, et al. Spatial QRS-T angle predicts cardiac death in a general population. Eur Heart J. 2003;24:1357–64. doi: 10.1016/s0195-668x(03)00203-3. [DOI] [PubMed] [Google Scholar]
- 14.Yamazaki T, Froelicher VF, Myers J, et al. Spatial QRS-T angle predicts cardiac death in a clinical population. Heart Rhythm. 2005;2:73–8. doi: 10.1016/j.hrthm.2004.10.040. [DOI] [PubMed] [Google Scholar]
- 15.Rautaharju PM, Kooperberg C, Larson JC, et al. Electrocardiographic abnormalities that predict coronary heart disease events and mortality in postmenopausal women. The Women’s Health Initiative. Circulation. 2006;113:473–80. doi: 10.1161/CIRCULATIONAHA.104.496091. [DOI] [PubMed] [Google Scholar]
- 16.Jogu HR, O’Neal WT, Broughton ST, et al. Frontal QRS-T angle and the risk of atrial fibrillation in the elderly. Ann Noninvasive Electrocardiol. 2017;22(2):e12388. doi: 10.1111/anec.12388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whang W, Shimbo D, Levitan EB, et al. Relations between QRS|T angle, cardiac risk factors, and mortality in the third National Health and Nutrition Examination Survey (NHANES III) Am J Cardiol. 2012;109:981–7. doi: 10.1016/j.amjcard.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang ZM, Rautaharju PM, Prineas RJ, et al. Usefulness of electrocardiographic QRS/T angles with versus without bundle branch blocks to predict heart failure (from the Atherosclerosis Risk in Communities study) Am J Cardiol. 2014;114:412–8. doi: 10.1016/j.amjcard.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strauss DG, Mewton N, Verrier RL, et al. Screening entire health system ECG databases to identify patients at increased risk of death. Circ Arrhythm Electrophysiol. 2013;6:1156–62. doi: 10.1161/CIRCEP.113.000411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang X, Zhu Q, Zhu L, et al. Spatial/frontal QRS/T angle predicts all-cause mortality and cardiac mortality: a meta-analysis. PLoS One. 2015;10(8):e0136174. doi: 10.1371/journal.pone.0136174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Investigators ARIC. The Atherosclerosis Risk in Communities (ARIC) study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 22.Prineas RJ, Crow RS, Zhang ZM. The Minnesota code manual of electrocardiographic findings. 2. Springer-London; 2009. Published by. [Google Scholar]
- 23.ARIC Investigators. Manual 3: surveillance component procedures manual of operations. Version 6.4. 2015 http://www2.cscc.unc.edu/aric/surveillance-manuals.
- 24.Pavri BB, Hillis MB, Subacius H, et al. Defibrillators in Nonischemic Cardiomyopathy Treatment Evaluation (DEFINITE) Investigators. Prognostic value and temporal behavior of the planar QRS-T angle in patients with nonischemic cardiomyopathy. Circulation. 2008;117(25):3181–6. doi: 10.1161/CIRCULATIONAHA.107.733451. [DOI] [PubMed] [Google Scholar]
- 25.Aro AL, Huikuri HV, Tikkanen JT, et al. QRS-T angle as a predictor of sudden cardiac death in a middle-aged general population. Europace. 2012;14:872–6. doi: 10.1093/europace/eur393. [DOI] [PubMed] [Google Scholar]
- 26.Gotsman I, Keren A, Hellman Y, et al. Usefulness of electrocardiographic frontal QRS-T angle to predict increased morbidity and mortality in patients with chronic heart failure. Am J Cardiol. 2013;111(10):1452–9. doi: 10.1016/j.amjcard.2013.01.294. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.