Abstract
Sleep deprivation may lead to increased impulsivity, however, previous literature has focused on examining effects of total sleep deprivation (TSD) rather than the more common condition, partial sleep deprivation (PSD) or ‘short sleep’. Moreover, it has been unclear whether PSD impacts impulse-related cognitive processes, and specifically if it differentially affects impulsive action versus impulsive decision-making. We sought to determine if short compared to long sleep (6 vs. 9 h/night) impacts impulsive action via behavioral inhibition (Go/No-Go), and/or impulsive decision-making processes of risk taking (Balloon Analogue Risk Task [BART]) and preferences for immediate over delayed rewards (Delay Discounting).
In a within-subject design, 34 participants (71% female, mean age = 37.0 years, SD = 10.54) were assigned to four consecutive nights of 6 h/night (short sleep) and 9 h/night (long sleep) in their own home in random counterbalanced order. Sleep was measured via wrist-worn actigraphs to confirm adherence to the sleep schedules (mean short sleep = 5.9 h, SD = 0.3; mean long sleep = 8.6 h, SD = 0.3, p < 0.001). The Go/No-Go, BART, and Delay Discounting tasks were completed following both sleep conditions.
Participants had more inhibition errors on the Go/No-Go task after short (mean false alarms = 19.79%, SD = 14.51) versus long sleep (mean = 15.97%, SD = 9.51, p = 0.039). This effect was strongest in participants reporting longer habitual time in bed (p = 0.04). There were no differences in performance following long- versus short-sleep for either delay discounting or the BART (p’s > 0.4).
Overall, these results indicate that four days of PSD diminishes behavioral inhibition abilities, but may not alter impulsive decision-making. These findings contribute to the emerging understanding of how partial sleep deprivation, currently an epidemic, impacts cognitive ability. Future research should continue to explore the connection between PSD and cognitive functions, and ways to minimize the occurrence and negative consequences of short sleep.
Keywords: Impulsivity, Inhibitory control, Sleep, Partial sleep deprivation, Cognitive function, Actigraphy
1. Introduction
Individuals in the United States are not achieving adequate amounts of sleep [41]. Short sleep duration may negatively impact a wide variety of parameters, including cognitive function and impulsivity [22]. The effect of sleep duration on impulsivity—acting quickly and without thorough consideration of consequences—is of particular interest because impulsivity is associated with many behavioral problems such as attention deficit disorder, oppositional defiant disorder, and conduct disorder [21], addiction [10,17], and overeating [9,23,35,36].
There are a number of cognitive factors contributing to impulse control, many of which are thought to be impacted by sleep deprivation, ranging from vigilance, attention, and perception, to higher order processes such as learning and executive functions [26]. Impulsivity has been further delineated into two separable constructs: impulsive action and impulsive decision-making or choice [38]. Impulsive action is the failure to inhibit inappropriate responses. It is often assessed with the Go/No-Go task, which requires participants to withhold pre-potent responses (i.e., to establish a dominant response to stimuli such as letters and then refrain from responding to only a certain type of those stimuli) [38]. Impulsive decision-making (or impulsive choice) involves decisions based on evaluation of potential outcomes (i.e., risks and rewards) and is associated with the tendency to favor more immediate rather than delayed rewards [11,20,50]. Two different types of tasks are frequently used to assess impulsive decision-making. One is the Delay Discounting Task (DDT), which measures impulsive decision-making as a participant’s tendency to choose smaller immediate rewards over larger delayed rewards. The other is the Balloon Analogue Risk Task (BART), which measures impulsive decision-making as risk-taking, or a choice to risk rewards already gained for the potential of higher rewards. The two tasks have been shown to load on the same factor in principal component analyses; although the constructs of delay discounting and risk-taking are correlated, they are not completely overlapping [38].
Previous research is mixed as to whether sleep deprivation impacts each of these constructs and the extent of sleep loss required to observe changes. Moreover, impulsive action and impulsive decision-making are rarely assessed within the same study under the same deprivation conditions. Most studies to date have examined the effects of total sleep deprivation (TSD; typically 1–2 night of no sleep), on either impulsive action or impulsive decision-making. One night of TSD compared to normal sleep has been consistently shown to worsen performance on the Go/No-Go task [5,15,19]. By contrast, TSD of varying lengths has had inconsistent effects on tasks related to impulsive decision-making. For instance, 49 h of TSD is associated with preference for riskier decisions in the Iowa Gambling Task [27]. However, TSD has been show to both increase [28] and decrease [3,25] risk-taking on the BART task, with the different outcomes potentially due to factors such as the length of sleep deprivation and gender. The extant literature on sleep deprivation and the DDT suggest TSD does not impact preferences for immediate over delayed monetary rewards [3,31]. Taken together, these findings suggest total sleep deprivation may have more consistent effects on impulsive action than impulsive decision-making.
Much less is known about the effects of short sleep, or partial sleep deprivation (PSD), on impulsive action and decision-making despite the substantive prevalence of short sleep duration in the United States [41]. Short sleep is typically defined as restricted sleep of 6 h or less per night [4], and has been linked to deficits in multiple cognitive domains, including sustained attention and working memory [47]. To our knowledge, only one study has investigated the impact of short sleep on both impulsive action and impulsive decision-making per se. This study focused on younger adults (aged 18–24 years) after a single night of restricted sleep in which participants were awakened at 4 am compared to their habitual wake time [39]. Greater risk taking behavior on the BART task was observed following restricted sleep, but no differences in performance accuracy on an emotional version of the Go/No-Go were seen [39]. Since this is the only prior study to assess the effects of PSD on the Go/No-Go task, it remains unclear whether the differences between this PSD study and the TSD studies are due to differences in the extent of sleep deprivation or to the use of the emotional version of the Go/No-Go task and potential differences in how sleep deprivation impacts the cognitive processes supporting behavioral inhibition versus emotional inhibition. No studies have examined whether PSD over several days, which may be typical of what occurs during the common work week, impacts impulse-related cognitive functioning. Given far-reaching implications of impulsive action and decision-making for health, and the marked prevalence of short sleep, additional research is required to delineate the role of PSD in these processes.
We therefore employed a within-subject counterbalanced design to test the effects of short sleep (6 h per night for 4 consecutive nights) versus long sleep (9 h per night for 4 nights) on multiple measures of impulsivity. Importantly, to enhance ecological validity and to build upon previous research, we focused on changes in sleep within participants’ home environments. We used the Go/No-Go task to assess impulsive action and both the BART and the Delayed Discounting task to assess impulsive decision-making. Based on previous TSD literature we expected that, relative to long sleep, short sleep would impact impulsivity, specifically impulsive action via the Go/No-Go, but were unsure about whether PSD would affect Delay Discounting task or the BART.
2. Methods
2.1. Participants
Participants included 37 right-handed healthy adults aged 21–55 years. Individuals were excluded based on the following criteria: self-reported habitual sleep less than 6.5 h or greater than 8.5 h, regular use of tobacco, taking part in a weight loss program, use of prescription or over-the-counter sleep medications or medications affecting sleep such as certain pain medications, antihistamines, and steroids, medications for psychological disorders, self-reported excessive consumption of caffeine or alcohol, no daily access to a computer with internet (needed to upload data from the Sense Wear Armband used for initial validation of adherence to prescribed sleep schedules), commute greater than 30 min with no alternative transportation during short sleep week, a diagnosis or positive assessment of obstructive sleep apnea based on questionnaire, self-reported napping, history of falling asleep while driving, and standard MRI contradictions, as the tasks reported in this manuscript were completed prior to functional neuroimaging which was conducted using a different set of tasks. Study recruitment was conducted from June 2012 to October 2014, through the use of community advertisements. Three subjects were removed from the study after not following the first week of the sleep schedule, which resulted in the final sample of 34 (92%) participants. The protocol was approved by the Miriam Hospital Institutional Review Board. All individuals provided written consent before participating and were compensated $150 for each sleep week (total of $300 per participant) in addition to any money earned during performance of the BART (described below).
2.2. Procedures
Participants attended an initial consenting visit including baseline measures (described below) and scheduling of experimental sleep conditions. Using a randomized, within-subject crossover design, participants completed two experimental conditions: four nights of “long sleep” (9 hour time in bed, TIB) and four nights of “short sleep” (6 hour TIB), in counterbalanced order at home. Definitions for long and short sleep were based, in part, on previous studies (e.g., [32,43, 44] that compared 9 h to 4 h in a laboratory setting. In order to avoid more extreme sleep deprivation within the context of a home-based study a short sleep condition of 6 h/night was utilized. A period of four consecutive nights was selected to be consistent with previous work that aimed to mimic what may occur within a typical work week [32], as well as work by Van Dongen et al. [47]) suggesting that 4–5 nights of short sleep induces perceived sleepiness similar to that of one night of TSD [47]. A washout period of at least 1 week separated experimental conditions. In the late afternoon/evening (mean time across participants of approximately 4:30 pm) following the final consecutive night of the sleep conditions, participants returned to the lab for their assessment.
2.2.1. Baseline visit
Upon consenting, participants completed demographic and baseline questionnaires, including an assessment of self-reported time in bed (TIB) over the past month. They were then randomized to have either their short or long sleep week first and given instructions on the sleep week requirements including prescribed bedtimes and wake times.
2.2.2. Sleep conditions
Each sleep week (short and long) consisted of 4 consecutive nights (Sunday–Wednesday or Monday–Thursday) of either short or long sleep, and concluded with a laboratory session on the fifth day. Participants were instructed to follow the prescribed sleep schedule without napping. They were allowed to consume caffeine according to their typical use throughout the week, with no more than two average sized caffeinated beverages (equivalent to 2 6 oz. cups of coffee) in the 24 h before the laboratory session, and none within 3 h prior. Participants were asked to refrain from consuming alcoholic beverages 24 h prior to laboratory sessions.
To monitor adherence to sleep prescriptions in real time, on each day of prescribed sleep participants wore Sensewear Armbands (BodyMedia, Inc., Pittsburgh, PA, USA) from the hour preceding their bedtime until waking in the morning and used BodyMedia, Inc. software to sync their activity data. This allowed for experimenters to monitor compliance to the sleep schedule each day in real time. If the participant was off by 15 min or more, study staff called to follow-up with the participant on adherence, and, if necessary, to reschedule the laboratory session to ensure 4 consecutive nights of high adherence.
Sleep was formally scored using Mini-Motionlogger actigraphs (AMI, Ardsley, NY, USA), which were worn 24 h a day during each experimental sleep condition on the participant’s non-dominant wrist and only removed in instances where the device would be exposed to water. Using standard procedures for scoring actigraph-estimated sleep [1], participants also completed sleep diaries (including documenting bedtimes and wake times) and called to leave a time-stamped voicemail at the Center twice daily. Discrepancies in reported and objective sleep measures were reviewed with participants during the laboratory sessions to determine accurate sleep and wake times. Activity data were downloaded and scored using the Sadeh algorithm [40] in Action Wversion 2.6.9905 (AMI, Ardsley, NY, USA). The primary sleep variable of interest for confirmation of adherence to the sleep schedule was the actigraph sleep period (time between actigraph-estimated sleep onset and actigraph-estimated wake). Sleep schedule adherence was assessed primarily via actigraphy (N = 27), however, for subjects whose actigraph device malfunctioned on any 1 night out of the 8 total sleep condition nights, adherence was verified with a combination of armband data, sleep diaries, and call-ins (N = 7).
2.2.3. Laboratory assessments
Participants visited the lab for identical assessments following both short and long sleep conditions. These assessments were conducted at approximately the same time of day (late afternoon/evening) for all participants, and when possible, assessments for a given participant were scheduled at the same exact time of day for each sleep week. Twenty-seven participants were able to have both sessions at the exact same time of day for both conditions. For the remaining 7 participants, sessions were within approximately 1 h of each other. During each laboratory session, participants completed the following computer tasks:
2.2.3.1. Go/No-Go
The Go/No-Go task was used to examine impulsive action. The version employed in the present study is a simple Go/No-Go task during which the target No-Go stimulus remained constant and was not dependent upon other stimuli [42]. It is similar to the version used previously by Kiehl et al. [24], except that the Go stimuli were randomly selected letters other than “X” and the inter-stimulus intervals were 900 ms [24]. Specifically, a 1 Hz serial stream of letters were presented for 100 ms each, with an inter-stimulus interval of 900 ms. A total of 512 letters were presented consisting of 80% “Go” stimuli (all letters other than “X”) and 20% “No-Go” stimuli (“X”). Subjects were instructed to press a button for each “Go” stimulus in order to establish a pre-potent response, and inhibit their response when an “x” appeared on the screen. Total percentage correct, percentage of false alarms (impulsive errors: pressing the button during a no-go “X” stimulus), and reaction times for correct “Go” stimuli served as the primary outcomes.
2.2.3.2. Balloon Analogue Risk Task (BART)
The BART Options [30] was used to examine impulsive decision-making. In this computer-administered behavioral assessment of risk-taking, subjects made key presses with their dominant hand to inflate a simulated balloon. In this study, a total of 20 balloon trials were used. On each trial (i.e., each balloon) participants had the option to earn money by inflating the balloon with a key press, but with risk of losing all earnings if the balloon were to pop, or they could refrain from pressing and ensure against loss of money accrued [30]. Each successful pump was worth 2 cents and cumulative money earned across trials was provided to the participant. The primary outcome was the average adjusted number of pumps on un-popped balloons. Additionally, a cost-benefit ratio was computed according to previous studies [25,28]. In this way, the relative proportion of ‘lost opportunity’, i.e. (number of exploded balloons)/(total number of balloons presented), to the relative proportion of ‘profit’ obtained, i.e. (total money cashed in)/(maximum possible money that could be earned) was assessed.
2.2.3.3. Delay discounting
A delay discounting task, originally designed as a questionnaire [29], was adapted to be completed on the computer and was also used to examine impulsive decision-making. Participants made dichotomous choices between smaller immediate rewards and larger, delayed rewards (e.g., “Would you rather have $24 today or $35 in 29 days”) on the same 27 questions employed by Kirby and colleagues and k values were generated according to the methods of this previous work. In short, each item has a predetermined temporal discounting function (k) value and the overall array of responses permits calculation of the individual’s discounting level via identifying indifference points (when the value of the immediate and delayed rewards are equivalent). This was done according to the model and formula described by Mazur [33] in which value of the immediate reward is equal to the amount of the delayed reward divided by the sum of 1 + k* the delay in time [33]. Put simply, using items from Kirby et al. [29], the point at which a participant ‘switches’ and begins selecting immediate over delayed rewards may be identified and assigned the corresponding k value. Again, according to methods of Kirby et al., k values were transformed with a natural log function. Percentage of trials in which delayed rewards were selected over immediate rewards was also calculated.
2.3. Data analytic plan
Baseline participant characteristics were described using means and standard deviations (SD) for continuous variables or percentages for categorical variables using standard methods. Transformations of non-normal variables were applied as necessary. Repeated measures analyses of variance (ANOVAs) were used to assess effects of sleep condition (short vs. long) on the outcomes, with and without covariates (post-hoc analyses were conducted with a covariate for baseline self-reported time in bed (TIB) given variability observed in this measure). All data were analyzed using PASW Statistics software, Version 18.0 (IBM, Armonk, NY).
3. Results
3.1. Sample characteristics
Demographic characteristics and descriptive statistics for the sample (N = 34) are presented in Table 1. Females represented 71% of the sample; 27% of the sample reported minority race or ethnicity. Average self-reported time in bed at baseline was 7.7 h (SD = 0.74).
Table 1.
Demographics and descriptive statistics (N = 34).
| Mean (or %) | SD | |
|---|---|---|
| Age (years) | 37 | 10.54 |
| Sex | ||
| Male | 29% | |
| Female | 71% | |
| Race | ||
| Asian | 9% | |
| African-American | 6% | |
| Caucasian | 79% | |
| Other | 6% | |
| Ethnicity | ||
| Hispanic | 6% | |
| Non-Hispanic | 94% | |
| Time in bed (h) | 7.72 | 0.74 |
3.2. Sleep schedule verification
Consistent with prescriptions for time in bed, the mean actigraph-measured sleep period was 5.91 h (SD = 0.30), and 8.63 h (SD = 0.28) for the short and long-sleep schedules, respectively. As expected, this within-subject difference in sleep time between the two experimental conditions was significant (t = 43.54, p < 0.001).
3.3. Impulsive action: Go/No-Go task
Table 2 shows that participants made more errors on the No-Go trials (when participants were required to refrain from responding; mean false alarms = 19.79, SD = 14.51) during the short sleep week compared to the long week (mean = 15.97, SD = 9.51, p = 0.039), which conversely led to fewer correct rejections. Testing whether this effect of short sleep on performance differed as a function of habitual time in bed (TIB), a significant interaction (sleep condition × habitual TIB) emerged (p = 0.036). As shown in Fig. 1, individuals who reported habitually achieving longer TIB were more negatively affected by the shortened sleep schedule (p = 0.04; Fig. 1) relative to those with shorter habitual TIB.
Table 2.
Impulsivity measures after short and long sleep conditions.
| Short sleep
|
Long sleep
|
p value | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Go-No Go | |||||
| Correct responses (hits) (%) | 99.41 | 1.14 | 99.47 | 0.94 | 0.725 |
| False alarms (%) | 19.79 | 14.51 | 15.97 | 9.51 | 0.039 |
| Reaction time (ms) | |||||
| Hits | 344.61 | 45.50 | 346.43 | 51.02 | 0.753 |
| False alarms | 271.18 | 33.21 | 274.31 | 29.73 | 0.466 |
| Delay Discounting | |||||
| K value (ln) | −4.10 | 0.05 | −4.24 | 1.93 | 0.50 |
| Percent delayed | 46.19 | 20.21 | 46.51 | 22.08 | 0.88 |
| BART | |||||
| Adjusted average pumps | 34.16 | 14.02 | 36.30 | 16.65 | 0.44 |
Fig. 1.
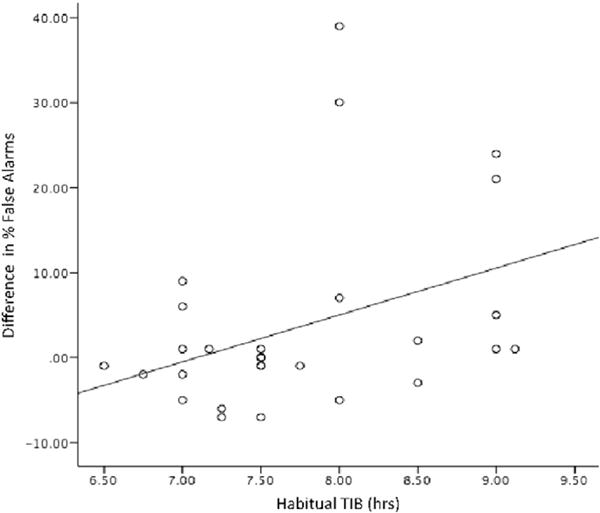
Difference in Go/No-Go performance (percentage false alarms) during short–long sleep as a function of self-reported habitual time in bed (TIB). Longer self-reported habitual TIB was associated with greater inhibition errors in short vs. long sleep (r = 0.38, p = 0.04).
Reaction time on the Go/No Go task did not differ across the two sleep conditions (RT hits p = 0.753, RT false alarms p = 0.466).
3.4. Impulsive decision-making (risk taking): Balloon Analogue Risk Task (BART)
Results are presented in Table 2. No significant difference was observed for the mean adjusted number of pumps between the two experimental conditions (mean short sleep = 34.16, SD = 20.21, mean long sleep = 36.30, SD = 16.65; p = 0.443). Using habitual sleep as a covariate revealed a similar non-significant result (F = 0.009, p = 0.93). Additionally, in analyses of the cost-benefit ratio on the BART [25,28], there were again no significant differences between short and long sleep (p = 0.28; co-varying habitual sleep: F = 0.06, p = 0.81).
3.5. Impulsive decision-making (preference for immediate rewards): Delayed Discounting
For the Delayed Discounting task, k values in the short sleep (mean = −4.10, SD = 1.75) and long sleep conditions (mean=−4.24, SD =1.95) did not differ (p = 0.50), nor did the overall percentage of trials in which participants selected larger delayed rewards over smaller immediate rewards (mean short sleep = 46.2%, SD = 20.2, mean long sleep = 46.5%, SD = 22.1, p = 0.88; Table 2). Again, when habitual TIB was entered into the model as a covariate the results were not significantly different (k value: F = 0.001, p = 0.97; percent delayed: F = 0.25, p = 0.62).
4. Discussion
In this study, we found that short sleep led to increased impulsive action, but not impulsive decision-making. Specifically, short sleep lowered performance on the Go/No-Go task but did not influence performance on measures of impulsive decision-making concerning decisions for immediate versus delayed rewards (DDT) or risk-taking (BART). Thus, our findings add to prior literature suggesting that sleep deprivation, including partial sleep deprivation, has more consistent negative effects on impulsive action than on impulsive decision-making. The second important finding in this study was that the negative impact of short sleep on impulsive action was strongest in those individuals who reported that they typically had longer time in bed at baseline.
Importantly, these results extend prior studies demonstrating lower performance on the Go/No-Go with TSD to PSD. This is particularly compelling given that PSD is on the rise in the US [14,41]. Moreover, findings suggest that the negative effects of short sleep may be most apparent in those who report habitually longer sleep duration. Such a change in sleep duration might occur in situations such as times of increased stress leading up to work/school deadlines or illness of oneself or a family member. This finding is in line with previous research suggesting there are potential individual differences in the degree to which sleep deprivation impairs performance and cognitive function [4,48]. These studies have suggested that differences in tolerance for sleep deprivation or vulnerability to deprivation-induced impairments may go beyond sleep history (i.e., habitual sleep) and be related to other traits, such as self-evaluation of sleepiness, fatigue, mood, cognitive processing capability, and behavioral alertness [46]. It is also possible that those individuals who are most vulnerable to the negative effects of short sleep spend longer time in bed in order to avoid impairments. Further research is necessary to better understand the role of individual differences in the impact of both total and partial sleep deprivation on impulsivity.
In our study we found no effects of PSD on either measure of impulsive decision-making, the BART or the DDT. In prior studies the effects of TSD and PSD on tasks related to impulsive choice have been inconsistent. Studies of TSD have not found effects of sleep on the DDT [3,31] and to our knowledge this is the first study to examine effects of PSD on the DDT. However, as noted, risk-taking per-se, rather than decisions for immediate versus delayed rewards, may be impacted by sleep deprivation, yet it may be specific to longer periods of TSD. Contrary to our findings herein, Rossa and colleagues found that PSD was associated with more impulsive choices on the BART. The difference between our findings and those of Rossa et al. on the BART may reflect differences in methodology in the two studies (e.g. age of participants; methods for restricting sleep, number of trials and payout per pump). Thus the current study found no impact of shortened sleep on either delay discounting or BART, but further research with common BART methods is needed to determine whether the results may differ in younger age groups or with different sleep deprivation regimens.
There are a number of reasons that might explain why sleep deprivation differentially affected impulsive decision-making and impulsive action. Prior literature suggests that dissociable neural systems may support impulsive action and decision-making. Impulsive action tasks have been shown to activate IFG, anterior cingulate cortex, inferior parietal lobule, anterior insula, supplementary motor cortex, and the subthalamic nucleus [7,16]. Recent work has suggested that the IFG in particular may implement the stop or ‘brake’ signal of motor response inhibition via connection with the basal ganglia [6,8,34]. A different network of regions including those involved in value processing such as medial prefrontal cortex, the ventral striatum, orbitofrontal cortex, posterior cingulate cortex, and posterior insula, along with regions involved in autobiographical memory and future planning (e.g., inferior prefrontal cortex, middle temporal gyrus) are thought to be involved in impulsive decision-making tasks[2,12,13,37,49]. Thus it is possible that sleep may affect brain regions required for control of impulsive action more so than those regions involved in impulsive decision-making. Indeed, sleep deprivation has been linked to functional brain alterations specifically in the prefrontal cortex and the inferior frontal gyrus (IFG) [45,51]. That the IFG is thought to be involved in control of impulsive action and is also notably impacted by sleep deprivation suggests a potential functional neuroanatomical explanation for the specific effects of sleep deprivation on impulsive action but not impulsive decision-making observed herein. Functional brain imaging studies are needed to further elucidate this possibility.
An additional pathway through which short sleep may specifically impact impulsive action is via impairments in vigilance and sustained attention. It is well established that these processes are affected by sleep deprivation, while effects on higher order cognitive processes, including decision-making, may be less understood [26]. A great deal of previous research suggests attention suffers with sleep deprivation, as evidenced by lapses in vigilance and decreased reaction time [18]. Performance on the most commonly used task to assess this, the Psychomotor Vigilance Task (PVT), is typically impaired following both TSD and PSD [47]. Relative to the other tasks, the Go/No-Go has more vigilance and attention demands as it is not self-paced, requiring participants to respond quickly. Participants must also attend to each letter stimulus in order to respond accurately. Although impairments in attention may have contributed to the effects we observed, we found no evidence of lapses (failure to respond to a ‘go’ trial), as would be expected with reduced vigilance. Further, reaction times did not differ between short and long sleep on the Go/No-Go task, suggesting something other than vigilance and processing speed may be involved.
Another possible explanation for the observed results may relate to differences in salience or engagement among these tasks that may emerge under conditions of short sleep. For instance, BART and DDT involve actual and hypothetical monetary gains and losses, respectively that may be meaningful to participants while the Go/No-Go does not involve money or rewards for each individual trial. It is possible that motivation, even for hypothetical rewards, may have helped participants compensate for any effects of short sleep on impulsivity.
This study has both strengths and weaknesses. Strengths include the within-subject design, the randomization of order of the short and long sleep prescriptions, the focus on PSD rather than TSD, and excellent adherence to the sleep protocol documented by objective measurement. Weaknesses include a relatively small sample size, which may have potentially precluded detection of smaller effect sizes, and the use of fewer BART trials than some previous studies. Although performance on the BART has been shown to be stable between 11–30 trials [52], to date performance stability has not been assessed specifically under conditions of sleep deprivation. We also assessed habitual sleep only by self-report and compared Short and Long Sleep (defined as 6 hour and 9 hour TIB, respectively). An alternative design would be to compare both of these conditions with a third condition of habitual sleep. Although the use of actigraphy allowed for the objective measurement of sleep within participants home environment, which increases the ecological validity of this study, it is limited in that it is a movement-based assessment of sleep, and does not allow for assessment of effects of specific sleep stages on measured outcomes. However, we employed recommended procedures for querying and scoring of sleep, which improve reliability and validity of actigraphy measurement when compared to polysomnography [1].
Overall, this study contributes to the prior literature by documenting significant increases in impulsive action following consecutive nights of short sleep compared to long sleep. Given that many individuals are required to restrict sleep at times, these findings highlight changes in impulsivity that may have widespread influence over behavior. It is of specific interest that those individuals who report habitual longer time in bed exhibited greater impairments with short sleep. This highlights that there may be important individual differences in tolerance to short sleep, and perhaps those who typically achieve longer time in bed are most at risk for impairment when sleep must be restricted. Alternatively, it is possible that those who are most vulnerable to the negative effects of short sleep typically spend longer time in bed in order to avoid impairments. Future research should continue to explore individual differences in the connection between sleep and cognitive function, the pathways through which short sleep affects impulsive action versus impulsive decision-making, and whether there are ways to diminish the impact of short sleep on impulsivity.
HIGHLIGHTS.
This study extends prior research by focusing on the prevalent problem of short sleep using an ecologically valid paradigm.
A randomized within-subject design compared 4 nights of short (6h/night) vs long (9h/night) sleep in participants’ homes.
Short sleep only negatively impacted impulsive action and did not affect impulsive decision-making tasks.
Negative effects of short sleep on impulsive action were strongest for those reporting longer habitual time in bed.
Acknowledgments
This work was supported by a U01 (5U01CA150387-05) (PI: RRW) from the National Cancer Institute of the National Institute of Health (N1H). Additional funding to support Dr. Demos comes from an N1H National Institute of Diabetes and Digestive and Kidney Diseases KO1 (5K01DK090445-04) awarded to KED.
References
- 1.Acebo C, LeBourgeois MK. Actigraphy, Respir. Care Clin N Am. 2006;12(1):23–30. doi: 10.1016/j.rcc.2005.11.010. http://dx.doi.org/10.1016/j.rcc.2005.11.010 (viii) [DOI] [PubMed] [Google Scholar]
- 2.Acheson A, Farrar AM, Patak M, Hausknecht KA, Kieres AK, Choi S, Richards JB. Nucleus accumbens lesions decrease sensitivity to rapid changes in the delay to reinforcement. Behav Brain Res. 2006;173(2):217–228. doi: 10.1016/j.bbr.2006.06.024. http://dx.doi.org/10.1016/j.bbr.2006.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Acheson A, Richards JB, de Wit H. Effects of sleep deprivation on impulsive behaviors in men and women. Physiol Behav. 2007;91(5):579–587. doi: 10.1016/j.physbeh.2007.03.020. http://dx.doi.org/10.1016/j.physbeh.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 4.Alhola P, Polo-Kantola P. Sleep deprivation: impact on cognitive performance. Neuropsychiatr Dis Treat. 2007;3(5):553–567. [PMC free article] [PubMed] [Google Scholar]
- 5.Almklov EL, Drummond SP, Orff H, Alhassoon OM. The effects of sleep deprivation on brain functioning in older adults. Behav Sleep Med. 2015;13(4):324–345. doi: 10.1080/15402002.2014.905474. http://dx.doi.org/10.1080/15402002.2014.905474. [DOI] [PubMed] [Google Scholar]
- 6.Aron AR. From reactive to proactive and selective control: developing a richer model for stopping inappropriate responses. Biol Psychiatry. 2011;69(12):e55–e68. doi: 10.1016/j.biopsych.2010.07.024. http://dx.doi.org/10.1016/j.biopsych.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aron AR, Durston S, Eagle DM, Logan GD, Stinear CM, Stuphorn V. Converging evidence for a fronto-basal-ganglia network for inhibitory control of action and cognition. J Neurosci. 2007;27(44):11860–11864. doi: 10.1523/JNEUROSCI.3644-07.2007. http://dx.doi.org/10.1523/JNEUROSCI.3644-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex: one decade on. Trends Cogn Sci. 2014;18(4):177–185. doi: 10.1016/j.tics.2013.12.003. http://dx.doi.org/10.1016/j.tics.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Barry D, Clarke M, Petry NM. Obesity and its relationship to addictions: is overeating a form of addictive behavior? Am J Addict. 2009;18(6):439–451. doi: 10.3109/10550490903205579. http://dx.doi.org/10.3109/10550490903205579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320(5881):1352–1355. doi: 10.1126/science.1158136. http://dx.doi.org/10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Broos N, Schmaal L, Wiskerke J, Kostelijk L, Lam T, Stoop N, Goudriaan AE. The relationship between impulsive choice and impulsive action: a cross-species translational study. PLoS One. 2012;7(5):e36781. doi: 10.1371/journal.pone.0036781. http://dx.doi.org/10.1371/journal.pone.0036781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cardinal RN, Pennicott DR, Sugathapala CL, Robbins TW, Everitt BJ. Impulsive choice induced in rats by lesions of the nucleus accumbens core. Science. 2001;292(5526):2499–2501. doi: 10.1126/science.1060818. http://dx.doi.org/10.1126/science.1060818. [DOI] [PubMed] [Google Scholar]
- 13.Carter RM, Meyer JR, Huettel SA. Functional neuroimaging of intertemporal choice models: a review. Journal of Neuroscience, Psychology, and Economics. 2010;3(1):27–45. [Google Scholar]
- 14.CDC, National Health and Nutrition Examination Survey From US Department of Health and Human Sevices. CDC, National Center for Health Statistics, 2007–2010. ( http://www.cdc.gov/nchs/nhanes.htm)
- 15.Chuah YM, Venkatraman V, Dinges DF, Chee MW. The neural basis of interindividual variability in inhibitory efficiency after sleep deprivation. J Neurosci. 2006;26(27):7156–7162. doi: 10.1523/JNEUROSCI.0906-06.2006. http://dx.doi.org/10.1523/JNEUROSCI.0906-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Criaud M, Boulinguez P. Have we been asking the right questions when assessing response inhibition in go/no-go tasks with fMRI? A meta-analysis and critical review. Neurosci Biobehav Rev. 2013;37(1):11–23. doi: 10.1016/j.neubiorev.2012.11.003. http://dx.doi.org/10.1016/j.neubiorev.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Dom G, D’Haene P, Hulstijn W, Sabbe B. Impulsivity in abstinent early- and late-onset alcoholics: differences in self-report measures and a discounting task. Addiction. 2006;101(1):50–59. doi: 10.1111/j.1360-0443.2005.01270.x. http://dx.doi.org/10.1111/j.1360-0443.2005.01270.x. [DOI] [PubMed] [Google Scholar]
- 18.Dorrian J, Rogers NL, Dinges DF. Psychomotor vigilance performance: neurocognitive assay sensitive to sleep loss. In: Kushida CA, editor. Sleep Deprivation: Clinical Issues, Pharmacology and Sleep Loss Effects. Marcel Dekker, Inc.; New York, NY: 2005. pp. 39–70. [Google Scholar]
- 19.Drummond SP, Paulus MP, Tapert SF. Effects of two nights sleep deprivation and two nights recovery sleep on response inhibition. J Sleep Res. 2006;15(3):261–265. doi: 10.1111/j.1365-2869.2006.00535.x. http://dx.doi.org/10.1111/j.1365-2869.2006.00535.x. [DOI] [PubMed] [Google Scholar]
- 20.Evenden JL. Varieties of impulsivity. Psychopharmacology. 1999;146(4):348–361. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- 21.Frick PJ, Nigg JT. Current issues in the diagnosis of attention deficit hyperactivity disorder, oppositional defiant disorder, and conduct disorder. Annu Rev Clin Psychol. 2012;8:77–107. doi: 10.1146/annurev-clinpsy-032511-143150. http://dx.doi.org/10.1146/annurev-clinpsy-032511-143150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harrison Y, Horne JA. The impact of sleep deprivation on decision making: a review. J Exp Psychol Appl. 2000;6(3):236–249. doi: 10.1037//1076-898x.6.3.236. [DOI] [PubMed] [Google Scholar]
- 23.Hou R, Mogg K, Bradley BP, Moss-Morris R, Peveler R, Roefs A. External eating, impulsivity and attentional bias to food cues. Appetite. 2011;56(2):424–427. doi: 10.1016/j.appet.2011.01.019. http://dx.doi.org/10.1016/j.appet.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 24.Kiehl KA, Liddle PF, Hopfinger JB. Error processing and the rostral anterior cingulate: an event-related fMRI study. Psychophysiology. 2000;37(2):216–223. [PubMed] [Google Scholar]
- 25.Killgore WD. Effects of sleep deprivation and morningness-eveningness traits on risk-taking. Psychol Rep. 2007;100(2):613–626. doi: 10.2466/pr0.100.2.613-626. http://dx.doi.org/10.2466/pr0.100.2.613-626. [DOI] [PubMed] [Google Scholar]
- 26.Killgore WD. Effects of sleep deprivation on cognition. Prog Brain Res. 2010;185:105–129. doi: 10.1016/B978-0-444-53702-7.00007-5. http://dx.doi.org/10.1016/B978-0-444-53702-7.00007-5. [DOI] [PubMed] [Google Scholar]
- 27.Killgore WD, Balkin TJ, Wesensten NJ. Impaired decision making following 49 h of sleep deprivation. J Sleep Res. 2006;15(1):7–13. doi: 10.1111/j.1365-2869.2006.00487.x. http://dx.doi.org/10.1111/j.1365-2869.2006.00487.x. [DOI] [PubMed] [Google Scholar]
- 28.Killgore WD, Kamimori GH, Balkin TJ. Caffeine protects against increased risk-taking propensity during severe sleep deprivation. J Sleep Res. 2011;20(3):395–403. doi: 10.1111/j.1365-2869.2010.00893.x. http://dx.doi.org/10.1111/j.1365-2869.2010.00893.x. [DOI] [PubMed] [Google Scholar]
- 29.Kirby KN, Petry NM, Bickel WK. Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. J Exp Psychol Gen. 1999;128(1):78–87. doi: 10.1037//0096-3445.128.1.78. [DOI] [PubMed] [Google Scholar]
- 30.Lejuez CW, Read JP, Kahler CW, Richards JB, Ramsey SE, Stuart GL, Brown RA. Evaluation of a behavioral measure of risk taking: the Balloon Analogue Risk Task (BART) J Exp Psychol Appl. 2002;8(2):75–84. doi: 10.1037//1076-898x.8.2.75. [DOI] [PubMed] [Google Scholar]
- 31.Libedinsky C, Massar SA, Ling A, Chee W, Huettel SA, Chee MW. Sleep deprivation alters effort discounting but not delay discounting of monetary rewards. Sleep. 2013;36(6):899–904. doi: 10.5665/sleep.2720. http://dx.doi.org/10.5665/sleep.2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Markwald RR, Melanson EL, Smith MR, Higgins J, Perreault L, Eckel RH, Wright KP., Jr Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proc Natl Acad Sci U S A. 2013;110(14):5695–5700. doi: 10.1073/pnas.1216951110. http://dx.doi.org/10.1073/pnas.1216951110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mazur JE. An adjusting procedure for studying delayed reinforcement. In: Commons ML, Mazure JE, Nevin A, Rachlin H, editors. Quantitative Analysis of Behavior: Vol 5. The Effect of Delay and Intervening Events on Reinforcement Value. Vol. 5. Erlbaum; Hillsdale, NJ: 1987. pp. 55–73. [Google Scholar]
- 34.Meffert H, Hwang S, Nolan ZT, Chen G, Blair JR. Segregating attention from response control when performing a motor inhibition task: segregating attention from response control. NeuroImage. 2016;126:27–38. doi: 10.1016/j.neuroimage.2015.11.029. http://dx.doi.org/10.1016/j.neuroimage.2015.11.029. [DOI] [PubMed] [Google Scholar]
- 35.Meule A. Impulsivity and overeating: a closer look at the subscales of the Barratt Impulsiveness Scale. Front Psychol. 2013;4:177. doi: 10.3389/fpsyg.2013.00177. http://dx.doi.org/10.3389/fpsyg.2013.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meule A, Lutz AP, Vogele C, Kubler A. Impulsive reactions to food-cues predict subsequent food craving. Eat Behav. 2014;15(1):99–105. doi: 10.1016/j.eatbeh.2013.10.023. http://dx.doi.org/10.1016/j.eatbeh.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 37.Pothuizen HH, Jongen-Relo AL, Feldon J, Yee BK. Double dissociation of the effects of selective nucleus accumbens core and shell lesions on impulsive-choice behaviour and salience learning in rats. Eur J Neurosci. 2005;22(10):2605–2616. doi: 10.1111/j.1460-9568.2005.04388.x. http://dx.doi.org/10.1111/j.1460-9568.2005.04388.x. [DOI] [PubMed] [Google Scholar]
- 38.Reynolds B, Ortengren A, Richards JB, de Wit H. Dimensions of impulsive behavior: personality and behavioral measures. Personal Individ Differ. 2006;40:305–315. [Google Scholar]
- 39.Rossa KR, Smith SS, Allan AC, Sullivan KA. The effects of sleep restriction on executive inhibitory control and affect in young adults. J Adolesc Health. 2014;55(2):287–292. doi: 10.1016/j.jadohealth.2013.12.034. http://dx.doi.org/10.1016/j.jadohealth.2013.12.034. [DOI] [PubMed] [Google Scholar]
- 40.Sadeh A, Sharkey KM, Carskadon MA. Activity-based sleep-wake identification: an empirical test of methodological issues. Sleep. 1994;17(3):201–207. doi: 10.1093/sleep/17.3.201. [DOI] [PubMed] [Google Scholar]
- 41.Schoenborn CA, Adams PE. Health behaviors of adults: United States, 2005–2007. Vital Health Stat. 2010;10(245):1–132. [PubMed] [Google Scholar]
- 42.Simmonds DJ, Pekar JJ, Mostofsky SH. Meta-analysis of Go/No-go tasks demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia. 2008;46(1):224–232. doi: 10.1016/j.neuropsychologia.2007.07.015. http://dx.doi.org/10.1016/j.neuropsychologia.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.St-Onge MP, McReynolds A, Trivedi ZB, Roberts AL, Sy M, Hirsch J. Sleep restriction leads to increased activation of brain regions sensitive to food stimuli. Am J Clin Nutr. 2012;95(4):818–824. doi: 10.3945/ajcn.111.027383. http://dx.doi.org/10.3945/ajcn.111.027383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.St-Onge MP, O’Keeffe M, Roberts AL, RoyChoudhury A, Laferrere B. Short sleep duration, glucose dysregulation and hormonal regulation of appetite in men and women. Sleep. 2012;35(11):1503–1510. doi: 10.5665/sleep.2198. http://dx.doi.org/10.5665/sleep.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thomas M, Sing H, Belenky G, Holcomb H, Mayberg H, Dannals R, Redmond D. Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res. 2000;9(4):335–352. doi: 10.1046/j.1365-2869.2000.00225.x. [DOI] [PubMed] [Google Scholar]
- 46.Van Dongen HP, Baynard MD, Maislin G, Dinges DF. Systematic interindividual differences in neurobehavioral impairment from sleep loss: evidence of trait-like differential vulnerability. Sleep. 2004;27(3):423–433. [PubMed] [Google Scholar]
- 47.Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26(2):117–126. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 48.Van Dongen HP, Vitellaro KM, Dinges DF. Individual differences in adult human sleep and wakefulness: leitmotif for a research agenda. Sleep. 2005;28(4):479–496. doi: 10.1093/sleep/28.4.479. [DOI] [PubMed] [Google Scholar]
- 49.Wesley MJ, Bickel WK. Remember the future II: meta-analyses and functional overlap of working memory and delay discounting. Biol Psychiatry. 2014;75(6):435–448. doi: 10.1016/j.biopsych.2013.08.008. http://dx.doi.org/10.1016/j.biopsych.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Winstanley CA, Eagle DM, Robbins TW. Behavioral models of impulsivity in relation to ADHD: translation between clinical and preclinical studies. Clin Psychol Rev. 2006;26(4):379–395. doi: 10.1016/j.cpr.2006.01.001. http://dx.doi.org/10.1016/j.cpr.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu JC, Gillin JC, Buchsbaum MS, Chen P, Keator DB, Khosla Wu N, Bunney WE. Frontal lobe metabolic decreases with sleep deprivation not totally reversed by recovery sleep. Neuropsychopharmacology. 2006;31(12):2783–2792. doi: 10.1038/sj.npp.1301166. http://dx.doi.org/10.1038/sj.npp.1301166. [DOI] [PubMed] [Google Scholar]
- 52.Wallsten TS, Pleskac TJ, Lejuez CW. Modeling behavior in a clinically diagnostic sequential risk-taking task. Psychological Review. 2005;112(4):862–880. doi: 10.1037/0033-295X.112.4.862. http://dx.doi.org/10.1037/0033-295X.112.4.862. [DOI] [PubMed] [Google Scholar]


