Abstract
A 26-year-old woman presented with a 5-day history of fever and 3-day history of left upper quadrant abdominal pain and cough associated with left shoulder tip pain. Initial blood cultures did not display growth. On CT imaging, there was a cyst measuring 7.2×8 cm originally interpreted to be haemorrhagic in nature. Repeat cultures during admission revealed Salmonella Thompson. Percutaneous drainage and antibiotic treatment, rather than splenectomy, was successfully pursued with the patient afebrile and in no pain at 6 weeks follow-up.
Keywords: infectious diseases, medical management
Background
This case report seeks to bring a splenic abscess to the forefront of a clinician’s thoughts when faced with a patient with fever and left shoulder tip pain and to have them first consider a spleen-sparing approach as a means of source control of the splenic abscess.
Case presentation
Our patient is a 26-year-old white woman who presented to the emergency department of a tertiary care centre with a 5-day history of fever and a 3-day history of left upper quadrant abdominal pain and cough associated with left shoulder tip pain. She was a healthy patient with no history of immunodeficiency or recent travel. She had a motor vehicle accident 2 years prior to presentation. This was complicated by self-limiting abdominal pain, for which she never sought medical help. Two weeks prior to presentation, she and a friend ate at a restaurant, soon after which her friend developed a self-limited diarrhoea. On examination, she was febrile and tachycardic. Her abdomen was diffusely tender without signs of peritonitis. No masses could be palpated. The remainder of her physical examination was otherwise unremarkable.
Investigations
Investigations demonstrated a leucocytosis (12.3×109/L). β-human chorionic gonadotropin was negative. Initial blood cultures did not show growth. Urine culture demonstrated no growth and stool cultures were not collected since she had normal bowel movements. A chest X-ray was normal. However, a CT scan of her abdomen demonstrated a 7.2×8 cm cyst in the upper pole of the spleen (figure 1), radiologically interpreted as a haemorrhagic cyst.
Figure 1.
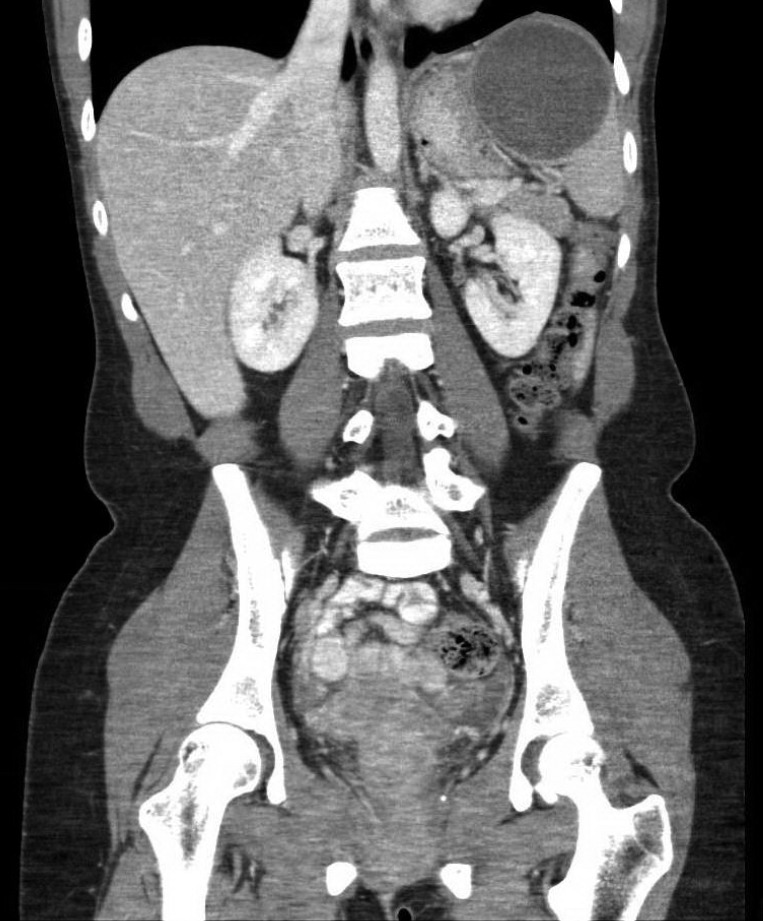
Coronal slice of a CT scan (venous phase) of the abdomen demonstrating a cyst (7.2×8 cm) in the upper pole of the spleen.
Differential diagnosis
The diagnosis of a haemorrhagic cyst, perhaps a distant complication of her motor vehicle accident, was considered the most probable cause of her symptoms. Supportive care, primarily with intravenous fluids and opioid analgesia, was pursued. On the second day of her admission, her abdominal pain became more unbearable and her fevers and rigours would not relent. She developed a salmon-coloured, macular rash, which first appeared on her left upper extremity in close proximity to the peripheral intravenous line and ultimately appeared on her trunk. This was thought to be a minor hypersensitivity reaction to the contrast that she required for the aforementioned CT scan. In light of her clinical decline, another set of blood cultures were drawn. Though the first set grew nothing, the second set grew Gram-negative bacilli, which were ultimately identified as Salmonella Thompson. Her haemorrhagic cyst, though certainly underlying her abdominal pain, diverted attention away from an invasive salmonellosis. Bearing in mind the microbiological diagnosis, her rash was retrospectively diagnosed as ‘rose spots.’ She was started on ceftriaxone. Despite 5 days of antibacterial therapy, her fever and abdominal pain persisted.
Treatment
It was ultimately decided that source control was not achieved. Guided by the hypothesis that her haemorrhagic cyst was rather an abscess, she underwent an ultrasound-guided, percutaneous drainage by interventional radiology. The drained fluid was frank pus. It ultimately grew Salmonella Thompson. Within 24 hours of drainage, defervescence was achieved.
Outcome and follow-up
Her admission was complicated by dyspnoea secondary to a large left pleural effusion confirmed by a repeat chest X-ray on day 10 of admission. It required thoracentesis, which drained more than 1 L of fluid. Pleural fluid analysis demonstrated exudate without bacteria observed on Gram stain or grown on cultures. It was thought to be reactive in aetiology; that is, the abscess resulted in diaphragmatic irritation, which itself produced a sympathetic pleural effusion. Of the 12 documented cases of non-typhoid Salmonella splenic abscesses in adults, five had associated pleural pathology, one of which was an effusion with no bacterial growth,1 one of which was an empyema with growth of the same Salmonella species as was grown in the splenic abscess2 and three of which were called an effusion but were not sampled.3–5 Her admission was also complicated by abdominal pain in her lower quadrants secondary to an 8.3×2.0×3.2 cm pelvic abscess diagnosed by a repeat CT scan of her abdomen on day 16 of admission. It was thought to be a consequence of leakage of the splenic abscess during percutaneous drainage. Because of the difficulty with which this abscess would be percutaneously drained, conservative management was pursued. Our patient was discharged on day 21 of admission, transitioned to ciprofloxacin to achieve a total of 6 weeks of antimicrobial therapy and continued on opioid analgesia for a short-term course. At a follow-up appointment 2 weeks after the completion of her course of antibiotic therapy, she was afebrile and in no pain. She remained asymptomatic both 6 months and 1 year after discharge. As a consequence of her lack of symptoms, no repeat imaging was performed.
Discussion
The incidence of splenic abscesses has been estimated to be between 0.07% and 0.2% in the adult population.6 Splenic abscesses have a mortality rate as high as 15%7 and in a study of 56 such cases, 96% were a consequence of bacteria, of which 1.9% were a consequence of Salmonella species.8 Most patients who develop a splenic abscess have a predisposition to it, including immunodeficiency, diabetes and sickle cell disease.9
This is the first documented case of a splenic abscess secondary to Salmonella Thompson.
This case is peculiar in that the patient was immunocompetent. Of the 12 documented cases of non-typhoid Salmonella splenic abscesses in adults (see online supplementary table 11–5 10–15), 7 did not have a medical history,1–3 10 13–16 2 had a medical history of type 2 diabetes mellitus, to which the extraintestinal Salmonella infection was attributed,5 141 had a medical history of achlorhydria, to which the infection was attributed11 and 2 did not have a medical history reported.12 Our case, in the context of these cases, suggests that an immunocompromised state is not a prerequisite to a disseminated non-typhoid Salmonella infection. Rather, it is hypothesised that her motor vehicle accident resulted in trauma to the spleen, which resulted in the development of her cyst. It is known that up to 56% of non-parasitic splenic cysts are of traumatic origin.17 This cyst evaded detection because she never sought medical attention. Ultimately, she developed a bacteraemia with Salmonella Thompson, substantiated by the presence of ‘rose spots’, acquired from contaminated food consumed at the restaurant. The splenic cyst served as a point of seeding for Salmonella Thompson, culminating to a splenic abscess.
The most common symptoms with which a patient with a splenic abscess may present are left upper quadrant pain and fever and the most common signs are splenomegaly and pleural effusion.18 19 Clinical suspicion of a splenic abscess should therefore drive the collection of blood cultures and radiological confirmation. CT with contrast, rather than ultrasound, should be performed in all patients in whom the suspicion of splenic abscess is raised since it has a very high sensitivity (96%) and specificity (90% to 95%).20–22
The diagnosis of a splenic abscess was delayed in the present case because of an initially negative blood culture and radiological findings that were not entirely consistent with an abscess. Therefore, if a splenic abscess is on the differential diagnosis, we recommend repeat blood cultures and a discussion with the radiologist about its possibility. It has been shown that up to 13% of splenic abscesses are blood culture negative.23 In the nine documented outbreaks of Salmonella Thompson (see online Supplementary file 1),24–31 eight reported information about the clinical status of the patients that constituted the outbreaks. Though mortality was uncommon (4 out of 112 patients in a smoked-salmon outbreak24), hospitalisation was common, ranging from 9% to 35%.24 26–30 Clinical presentation was variable: fever was present in 55% to 89% of the patients, abdominal pain in 53% to 79% of patients and diarrhoea in 71% to 100% of patients. None of the articles reported a splenic abscess or extraintestinal disease. Therefore, though Salmonella Thompson is unlikely to result in mortality, it is capable of causing an infection sufficiently severe to warrant hospitalisation.
bcr-2016-218595supp001.docx (91.1KB, docx)
Early supportive care, including intravenous fluid resuscitation, and empiric antibiotics, generally third or fourth generation cephalosporins, are essential for positive outcomes.32 The spectrum of activity should be narrowed according to culture and sensitivity results. The optimal duration of antibiotics is not well established but can range from 10 to 21 days.33 34 However, antibiotic treatment alone is not sufficient to resolve a splenic abscess and source control must be achieved by means of splenectomy or percutaneous drainage. Splenectomy is considered the gold standard of treatment and is indicated in the following clinical contexts: multiple abscesses, multiloculated abscesses, debris-contained abscess, poorly defined abscess on CT or poor route of percutaneous drainage. Recently, percutaneous drainage and antibiotic therapy, without splenectomy, have been shown to be an effective treatment modality.35 Percutaneous drainage is appropriate in the following clinical contexts: small abscesses (<4 cm), solitary abscesses and patients who are too haemodynamically unstable for surgery or are otherwise not surgical candidates.36
Our patient was successfully treated with appropriate intravenous antibiotics and percutaneous splenic abscess drainage. Of the 12 documented cases of non-typhoid Salmonella splenic abscesses in adults, four were treated without a splenectomy.1 12 16 In the other eight cases, splenectomy was performed after a judged failure of splenic abscess drainage in two cases10 15 and splenectomy was the primary intervention in the remaining six cases.2 3 5 11 13 14 That this is the fifth documented case of successful splenic abscess drainage suggests that a spleen-sparing approach should be primarily attempted to preclude the significant risks of surgery and to circumvent the immunodeficient state that asplenia confers.
Learning points.
Salmonella Thompson can cause splenic abscesses.
Predisposition to a splenic abscess may not only be conferred by an immunological deficiency but also by an anatomical abnormality.
Splenic abscess should be strongly considered in the differential diagnosis with the presentation of fever and left upper quadrant pain. We suggest serial blood cultures and discussion with radiology to confirm diagnoses.
Though splenectomy has been considered the gold standard treatment of a splenic abscess, the present case was effectively treated by percutaneous drainage and antibiotics, the choice of which was driven by culture and sensitivity.
bcr-2016-218595supp002.docx (90KB, docx)
Footnotes
Contributors: MJB and TY are co-first authors. MJB contributed to study design, data collection, data analysis and writing. TY contributed to study design, data collections, data analysis, writing. YP contributed to study design and writing.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Wilmshurst P, Sutcliffe H. Splenic abscess due to Salmonella Heidelberg. Clin Infect Dis 1995;21:1065–6. 10.1093/clinids/21.4.1065 [DOI] [PubMed] [Google Scholar]
- 2.Buscaglia AJ. Empyema due to splenic abscess with Salmonella newport. JAMA 1978;240:1990 10.1001/jama.1978.03290180064031 [DOI] [PubMed] [Google Scholar]
- 3.Sillar JR, Bannan AM, Dahlenburg L. Splenic abscess complicating gastroenteritis due to Salmonella virchow in an immunocompetent host. Med J Aust 2015;203:148–9. 10.5694/mja15.00068 [DOI] [PubMed] [Google Scholar]
- 4.Tappe D, Müller A, Langen HJ, et al. Isolation of Salmonella enterica serotype newport from a partly ruptured splenic abscess in a traveler returning from Zanzibar. J Clin Microbiol 2007;45:3115–7. 10.1128/JCM.00844-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodan BA, Max RJ, Breiman RS, et al. Splenic abscess due to Salmonella typhimurium bacteremia. South Med J 1981;74:382 10.1097/00007611-198103000-00043 [DOI] [PubMed] [Google Scholar]
- 6.Freund R, Pichl J, Heyder N, et al. Splenic abscess-clinical symptoms and diagnostic possibilities. Am J Gastroenterol 1982;77:35–8. [PubMed] [Google Scholar]
- 7.Alvi AR, Kulsoom S, Shamsi G. Splenic abscess: outcome and prognostic factors. J Coll Physicians Surg Pak 2008;18:740–3. doi:12.2008/JCPSP.740743 [PubMed] [Google Scholar]
- 8.Brook I, Frazier EH. Microbiology of liver and spleen abscesses. J Med Microbiol 1998;47:1075–80. 10.1099/00222615-47-12-1075 [DOI] [PubMed] [Google Scholar]
- 9.Fotiadis C, Lavranos G, Patapis P, et al. Abscesses of the spleen: report of three cases. World J Gastroenterol 2008;14:3088 10.3748/wjg.14.3088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharr MM. Splenic abscess due to Salmonella agona. Br Med J 1972;1:546 10.1136/bmj.1.5799.546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scott IH, Thomas HW, Walters RO. Acute splenic abscess due to Salmonella chester. Br Med J 1977;1:688 10.1136/bmj.1.6062.688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crosse BA. Splenic abscess associated with Salmonella infection: successful medical treatment. J Infect 1989;19:80–2. 10.1016/S0163-4453(89)95058-5 [DOI] [PubMed] [Google Scholar]
- 13.Girardin F, Mezger N, Hächler H, et al. Salmonella serovar give: an unusual pathogen causing splenic abscess. Eur J Clin Microbiol Infect Dis 2006;25:272–4. 10.1007/s10096-006-0122-2 [DOI] [PubMed] [Google Scholar]
- 14.Cabadak H, Erbay A, Karaman K, et al. Splenic abscess due to Salmonella enteritidis. Infect Dis Rep 2012;4:4 10.4081/idr.2012.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoff E, Nayeri F. Splenic abscess due to Salmonella schwarzengrund in a previously healthy individual returning from Bali. BMJ Case Rep 2015;2015:bcr2015212969 10.1136/bcr-2015-212969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh AK, Shankar S, Gervais DA, et al. Image-guided percutaneous splenic interventions. Radiographics 2012;32:523–34. 10.1148/rg.322115135 [DOI] [PubMed] [Google Scholar]
- 17.Kojodjojo P, Chew S, Wakeham K, et al. Anaemia and pleural effusion. J R Soc Med 2005;98:23–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Changchien CS, Tsai TL, Hu TH, et al. Sonographic patterns of splenic abscess: an analysis of 34 proven cases. Abdom Imaging 2002;27:739–45. 10.1007/s00261-002-0013-7 [DOI] [PubMed] [Google Scholar]
- 19.Green BT. Splenic abscess: report of six cases and review of the literature. Am Surg 2001;67:80–5. [PubMed] [Google Scholar]
- 20.Ferrucci JT, vanSonnenberg E. Intra-abdominal abscess. radiological diagnosis and treatment. JAMA 1981;246:2728–33. [PubMed] [Google Scholar]
- 21.Nelken N, Ignatius J, Skinner M, et al. Changing clinical spectrum of splenic abscess. Am J Surg 1987;154:27–34. 10.1016/0002-9610(87)90285-6 [DOI] [PubMed] [Google Scholar]
- 22.Ting W, Silverman NA, Arzouman DA, et al. Splenic septic emboli in endocarditis. Circulation 1990;82:IV105–9. [PubMed] [Google Scholar]
- 23.Chang KC, Chuah SK, Changchien CS, et al. Clinical characteristics and prognostic factors of splenic abscess: a review of 67 cases in a single medical center of Taiwan. World J Gastroenterol 2006;12:460 10.3748/wjg.v12.i3.460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friesema I, de Jong A, Hofhuis A, et al. Large outbreak of Salmonella thompson related to smoked salmon in the Netherlands, August to December 2012. Euro Surveill 2014;19:20918 10.2807/1560-7917.ES2014.19.39.20918 [DOI] [PubMed] [Google Scholar]
- 25.Nygård K, Lassen J, Vold L, et al. Outbreak of Salmonella thompson infections linked to imported rucola lettuce. Foodborne Pathog Dis 2008;5:165–73. 10.1089/fpd.2007.0053 [DOI] [PubMed] [Google Scholar]
- 26.Kimura AC, Palumbo MS, Meyers H, et al. A multi-state outbreak of Salmonella serotype thompson infection from commercially distributed bread contaminated by an ill food handler. Epidemiol Infect 2005;133:823 10.1017/S0950268805004127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campbell JV, Mohle-Boetani J, Reporter R, et al. An outbreak of Salmonella serotype thompson associated with fresh cilantro. J Infect Dis 2001;183:984–7. 10.1086/319254 [DOI] [PubMed] [Google Scholar]
- 28.Linares AP, Cohen SH, Goldstein E, et al. Febrile gastroenteritis due to Salmonella thompson. report of an outbreak. West J Med 1984;141:203–5. [PMC free article] [PubMed] [Google Scholar]
- 29.Wright HA, Norval J, Orr A. Salmonella thompson gastro-enteritis; report of two outbreaks. Br Med J 1957;2:69–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williston JB. Salmonella thompson infection in a food handler. N S Med Bull 1966;6:151–2. [PubMed] [Google Scholar]
- 31.Shapiro R, Ackers ML, Lance S, et al. Salmonella thompson associated with improper handling of roast beef at a restaurant in Sioux Falls, South Dakota. J Food Prot 1999;62:118–22. 10.4315/0362-028X-62.2.118 [DOI] [PubMed] [Google Scholar]
- 32.Mustafa M, Jayaram M, Muniandy RK, et al. Clinical spectrum and therapy. IOSR Journal Of Pharmacy 2015;5:37–41. [Google Scholar]
- 33.Caslowitz PL, Labs JD, Fishman EK, et al. The changing spectrum of splenic abscess. Clin Imaging 1989;13:201–7. 10.1016/0899-7071(89)90146-0 [DOI] [PubMed] [Google Scholar]
- 34.Toevs CC, Beilman GJ. Splenic abscess 10 years after splenic trauma: a case report. Am Surg 2000;66:204–5. [PubMed] [Google Scholar]
- 35.Ferraioli G, Brunetti E, Gulizia R, et al. Management of splenic abscess: report on 16 cases from a single center. Int J Infect Dis 2009;13:524–30. 10.1016/j.ijid.2008.08.024 [DOI] [PubMed] [Google Scholar]
- 36.Sammon J, Twomey M, Crush L, et al. Image-guided percutaneous splenic biopsy and drainage. Semin Intervent Radiol 2012;29:301–10. 10.1055/s-0032-1330064 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bcr-2016-218595supp001.docx (91.1KB, docx)
bcr-2016-218595supp002.docx (90KB, docx)


