Abstract
Objective
The aim of this study was to evaluate the effect of polymer carrier, hot melt extrusion (HME) and downstream processing parameters on the water uptake properties of amorphous solid dispersions.
Methods
Three polymers and a model drug were used to prepare amorphous solid dispersions utilizing HME technology. The sorption-desorption isotherms of solid dispersions and their physical mixtures were measured by the Dynamic Vapor Sorption system, and the effect of polymer hydrophobicity, hygroscopicity, molecular weight and the HME process were investigated. FTIR imaging was performed to understand the phase separation driven by the moisture.
Key findings
Solid dispersions with polymeric carriers with lower hydrophilicity, hygroscopicity, and higher molecular weight could sorb less moisture under the high RH conditions. The water uptake ability of polymer-drug solid dispersion systems were decreased compared to the physical mixture after HME, which might be due to the decreased surface area and porosity. The FTIR imaging indicated the homogeneity of the drug molecularly dispersed within the polymer matrix was changed after exposure to high RH.
Conclusion
Understanding the effect of formulation and processing on the moisture sorption properties of solid dispersions is essential for the development of drug products with desired physical and chemical stability.
Keywords: amorphous, polymer carrier, solid dispersion, hot melt extrusion, moisture, downstream processing
Introduction
More than 40% of newly discovered active pharmaceutical ingredients (API) [1] possess unfavorable physical properties, [2] and most of them belong to biopharmaceutics classification system (BCS) class II or IV, characterized by poor aqueous solubility and oral bioavailability. These properties are the biggest challenge for pharmaceutical research and development scientists. In last few decades, amorphous solid dispersions (ASD) have been a very attractive method to deliver the API with potential improvement in the dissolution rate, aqueous solubility and bioavailability [3–5]. The amorphous form has a greater free volume, molecular mobility, and enthalpy compared to the crystalline form resulting in greater chemical reactivity and moisture absorption. Despite numerous publications about solid dispersions, few products have become available in the marketplace [6]. Undesirable stability performance could be a major key factor limiting the development of amorphous solid dispersions. Increasing the stability of solid dispersion formulations has been studied recently with different approaches [5, 7–10].
There are many technologies currently used to prepare solid dispersion systems, which include solvent casting, spray drying, and hot melt extrusion [11–13]. It is argued by many, among all of these techniques that hot melt extrusion (HME) [14], or melt extrusion, is the most promising techniques, which include, advantages such as continuous processing, easy to scale up, solvent free fabrication and lower production costs [2, 8, 15–17].
For most of the HME formulations, polymer carriers entail a large proportion of all of the components, which performs the role of recrystallization inhibitor, as well as that of the dissolution controller [18–20]. However, at the same time, most commonly used polymers in HME processing are hydrophilic polymers or hygroscopic materials. Most amorphous solid dispersion systems containing only the API and polymer carrier, are difficult to be stabilized under the environmental conditions with moisture and temperature near the glass transition temperature (Tg). Moreover, the polymer carriers are hygroscopic in nature and this could aggravate the influence of moisture by lowering the systems Tg, which consequently increases molecular mobility and facilitates the recrystallization of the API.
Evaluation of drug-polymer solubility and miscibility, and the effect of polymer type on the stabilization of amorphous APIs have been reported [19, 21, 22]. However, once exposed to moisture, the drug-polymer binary system will alter to a drug-polymer-water ternary system.
In order to investigate the moisture induced changes in the solid dispersion, utilizing FTIR spectroscopy in the analysis provides several advantages [23]. Sensitive and non-invasive characterization of drug-polymer interactions can be performed quickly, even with the controlled sample temperature and atmosphere. Moreover, FTIR chemical imaging provides straightforward information of miscibility or mixing of the drug and polymer [24, 25]. However, the effect of moisture on the drug dispersion homogeneity is still limited by using this visualized technology.
Previous research has been conducted to investigate the influence of moisture on the formulations containing hygroscopic polymers [26–28]. However, evidence is still deficient as to how polymer properties, HME processing and downstream processing, affects moisture uptake within the systems. In order to achieve a good commercial solid dispersion product, thoroughly understanding the effect of formulation as well as the preparation technology is paramount. It is well recognized that different processing technologies could impart huge differences on the physical properties of solid dispersions [29–32]. However, research on how preparation techniques and downstream processing affects the solid dispersion is still very limited.
Therefore, the main objective of the present study was to investigate the effects of polymeric carrier, HME processing and other downstream processing parameters on the moisture sorption properties of amorphous solid dispersions. Polyethylene glycol (PEG), Hydroxypropyl cellulose (HPC) and Ethyl cellulose (EC) were chosen as polymer carriers and fenofibrate (FF) was used as a model drug. The moisture sorption/desorption isotherms were measured for various molecular weight (MW) grades of the polymer carrier, physical mixture (PM) of polymer and drug, and amorphous solid dispersions prepared by HME technology. To the best of the authors’ knowledge, this is the first study, which compares the moisture uptake ability of melt extruded amorphous solid dispersion with different hygroscopic natured polymer carriers and different molecular weights. Also, the effects of hot melt extrusion processing and downstream processing on the moisture absorption ability of solid dispersions were investigated for the first time.
Materials and methods
Materials
Hydroxypropylcellulose (Klucel™ HPC; grades LF/EF/ELF) and Ethylcellulose (Aqualon® EC; grades N7/N14/N22) were kindly donated by ASHLAND Specialty Products (Wayne, NJ, USA). Polyethylene glycol (3350, 4000, and 6000) were purchased from Sigma–Aldrich (St. Louis, MO, USA). And fenofibrate was purchased from Aurobindo Pharma Ltd. (Hyderabad, India). Reagent grade methanol was purchased from Sigma–Aldrich (St. Louis, MO, USA). All the other reagents used in this study were of the analytical grade.
Methods
Preparation of amorphous solid dispersions using hot-melt extrusion
Amorphous solid dispersions of fenofibrate and model polymer with various MWs were prepared using HME technology. Thermal gravimetric analysis (TGA) and differential scanning calorimetry (DSC) were utilized to determine the extrusion processing temperature range. The API and polymer were mixed in a V-cone blender (MaxiBlend™, GlobePharma) at 30 rpm for 10 min and then extruded with a co-rotating twin-screw extruder (Process 11, ThermoFisher Scientific) into uniform rod extrudates, at an extrusion processing temperature range based on the formulation composition and a screw speed of 100 rpm. The maximum feed rate utilized was 10 g/min, in order to maintain the torque (%) indicator of the extruder within a safe mode range. The extrudates were milled using a comminuting Fitz Mill (Model#L1A, Fitzpatrick Company, IL) at a rotor speed of 3600 rpm.
Dynamic vapor sorption (DVS)
The water sorption behavior of API, physical mixture, milled extrudates, and compressed milled extrudates were determined by Intrinsic DVS (Surface measurement systems, London, UK). 20±0.5 mg samples were exposed to the controlled relative humidity profile (0-90-0% in 10% steps) at a constant temperature (25°C), and the weight changes were measured by a CahnD200 ultra-microbalance (±0.01mg mass resolution). The dm/dt mode was used in all the steps, and the limitation was set at 0.001%/min to detect the equilibrium (the instrument would start next step when the samples dm/dt value equal or less than 0.001%/min). At first step, sample was dried at 0%RH, and the equilibrated mass at 0%RH was used as reference mass. The water sorption isotherms were calculated using the equilibrated sample mass from each step [33].
Thermal gravimetric analysis (TGA)
TGA studies were performed on Perkin Elmer Pyris 1 TGA with the Pyris™ software (PerkinElmer Life and analytical sciences, CT, USA). 3–5 mg of the sample was weighed and heated from 20 °C to 300 °C under an inert nitrogen atmosphere at a flow rate of 20 °C/min. Percent weight loss was plotted against temperature to determine the weight loss. The TGA sample of fenofibrate was held at the highest extrusion temperature (145 °C) for 15 min to test the thermal stability [34].
Differential scanning calorimetry (DSC)
DSC (Perkin Elmer, Diamond DSC) was utilized to measure the melting enthalpy of the solid dispersions. Samples were weighed (3–5 mg) in an aluminum sample pan and hermetically sealed at each time point using a heating rate of 20°C/min from 30°C to 200°C under an inert nitrogen atmosphere at a flow rate was 20 mL/min. An empty pan was used as reference. Measurements were repeated three times. An Indium standard was used for calibration.
HPLC-UV Analysis
A Waters HPLC-UV system (Waters Corp, Milford, MA), equipped with a Luna 5um C18 100Å column (Phenomenex, US), was used to detect fenofibrate at a wavelength of 286 nm. The mobile phase consisted of acetonitrile and phosphoric acid in water (pH=2.5) at a ratio of 85:15 (v/v). The flow rate was maintained at 1.0 mL/min. The injection volume was 20 μL. The observed retention time of fenofibrate was 6 min. The data was acquired and processed using Waters Empower 3 software suite.
Scanning electron microscopy (SEM)
SEM was used to study surface morphology of the solid dispersions. Samples were mounted on aluminum stubs held with a carbon adhesive film. Gold was used to coat the Samples by a Hummer® 6.2 sputtering system (Anatech LTD, VA, USA) in a high vacuum evaporator. The surface topography of the sample was analyzed by a scanning electron microscope operating at an accelerating voltage from 1.0 kV to 5.0 kV (JEOL JSM-5600).
FTIR and Chemical Imaging
Infrared spectra were collected on an FTIR bench (Agilent Technologies Cary 660) fitted with a MIRacle ATR (Pike Technologies) sampling accessory in the spectral range of 4000–650 cm−1. The bench ATR was equipped with a single bounce diamond coated ZnSe internal reflection element. Chemical images were collected using an infrared microscope (Agilent Technologies Cary 620 IR), which was equipped with a 64 x 64 focal plane array (FPA) detector. The images were collected with a germanium micro ATR sampling accessory giving a field of view (FOV) of approximately 70 x 70 microns with 1.1μm spatial resolution.
Statistical Analysis
All statistical analysis was calculated using SPSS v.18.0 (IBM Corp., Armonk, NY, USA). To compare the differences of moisture sorption between solid dispersions with different polymers, one-way ANOVA followed by t-test was used for continuous variables. All significant tests were two-tailed and p<0.05 was considered significant.
Results and discussion
Preparation of amorphous solid dispersions utilizing hot-melt extrusion
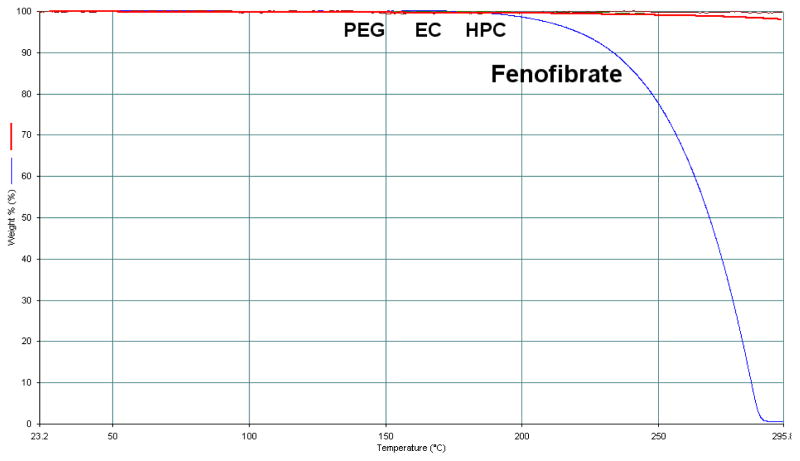
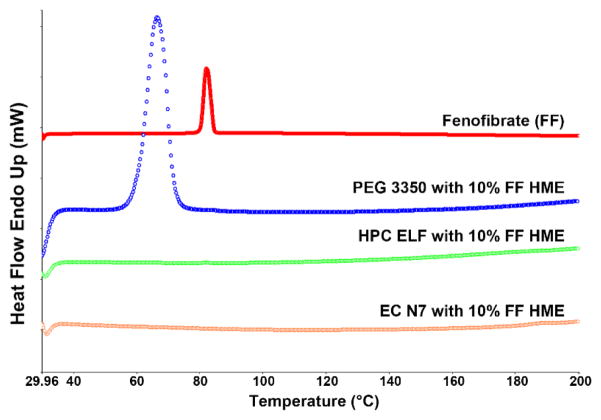
The TGA study results confirmed that all of the polymers and the model drug were stable at the temperature range of 30–200°C, as no degradation peaks were observed (Figure 1). The TGA sample of model drug was held at 145 °C for 15 min, and the weight change was less than 0.5%, which indicated the thermal stability of fenofibrate since the holding time was much longer than retention time in the extruder (less than 5 min). All grades of model polymer with the model drug fenofibrate showed excellent extrudability under the utilized processing parameters (Table 1). Formulations containing higher MW grades of model polymers required higher extrusion temperatures to decrease the torque on the extruder, and pure polymers without fenofibrate needed even higher temperatures for processing. Polymers with higher MW usually have a higher Tg, which requires higher energy input to soften the polymer [35]. However, fenofibrate with a Tg of −20°C could lower the systems Tg by acting as a plasticizer [36]. The extrudates of HPC were difficult to mill due to the polymers high degree of thermoplasticity. Cryomilling, keeping the extrudate in −80°C for several hours before milling, was utilized to resolve this issue. All physical mixture and milled extrudates were passed through the same number sieve to ensure the same particle size. From Figure 2, DSC data confirmed that all of the extruded formulations with the drug were amorphous solid dispersions (PXRD also corroborated the DSC findings, data not shown). HPLC analysis of the freshly extruded solid dispersions showed no reduction in the drug content nor demonstrated a degradation peak from fenofibrate, which indicated that the extrudates were very stable during the processing conditions. All of the extrudates’ content uniformities were well within the range of 85–115%.
Figure 1.
TGA results for all polymeric carriers (PEG, EC and HPC) and the model drug did not show degradation, so the three lines overlapped.
Table 1.
Composition of solid dispersions and hot melt extrusion parameters for each formulation. (For all formulations containing PEG, the last 3 zones temperature were set at 30 °C to avoid liquid extrudate.)
| Polymer Carrier | Drug Content (%) | Temp.(°C) | Screw Speed (rpm) | Torque (%) | ||
|---|---|---|---|---|---|---|
| Type | Content (%) | Molecular weight | ||||
| HPC | LF | 95000 | 10 | 130 | 100 | 35–50 |
| EF | 80000 | 10 | 125 | 100 | 35–45 | |
| ELF | 40000 | 10 | 125 | 100 | 35–40 | |
| EC | N7 | 65000 | 10 | 140 | 100 | 35–45 |
| N14 | 120000 | 10 | 145 | 100 | 35–50 | |
| N22 | 140000 | 10 | 145 | 100 | 40–50 | |
| PEG | 3350 | 3350 | 10 | 75 | 100 | 10–20 |
| 4000 | 4000 | 10 | 80 | 100 | 10–20 | |
| 6000 | 6000 | 10 | 80 | 100 | 10–20 | |
Figure 2.
DSC results for pure model drug and hot melt extruded amorphous solid dispersions. Red line: fenofibrate (FF); blue line: PEG 3350 with 10% FF; green line: HPC ELF with 10% FF; orange line: EC N7 with 10% FF.
Effect of Polymer carrier on the moisture sorption of solid dispersions
For the same model drug, fenofibrate, three different polymers HPC, EC and PEG corresponding to hygroscopic amorphous, hydrophobic amorphous, and hygroscopic semi-crystalline polymer, respectively, were selected to prepare the solid dispersions for testing (Figure 3, 4 and 5). To clarify the comparisons, all of the important parameters are summarized in Table 2.
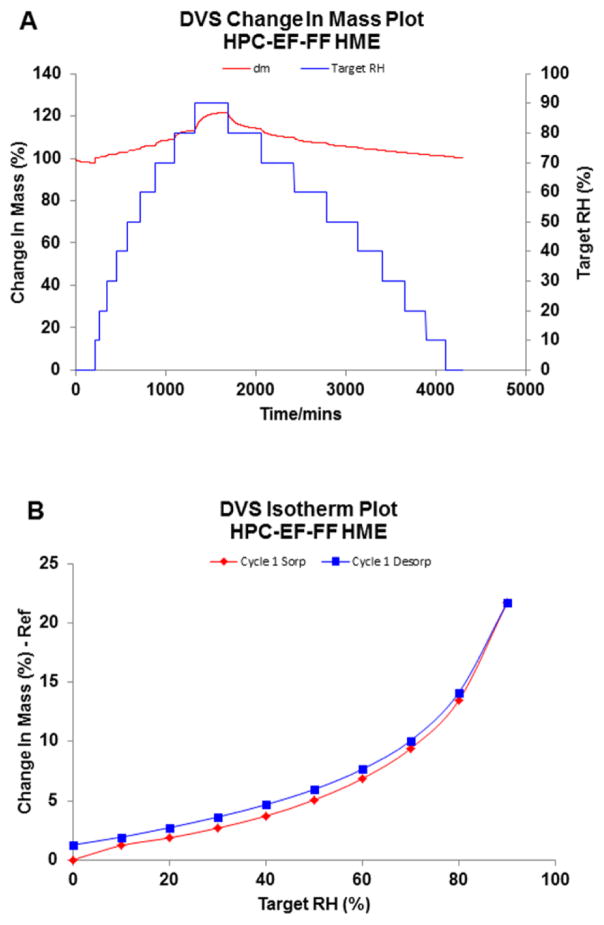
Figure 3.
Dynamic Vapor Sorption measurements of HPC EF-FF amorphous solid dispersions prepared by HME (n=1): (A) change in mass plot and (B) isotherm plot.
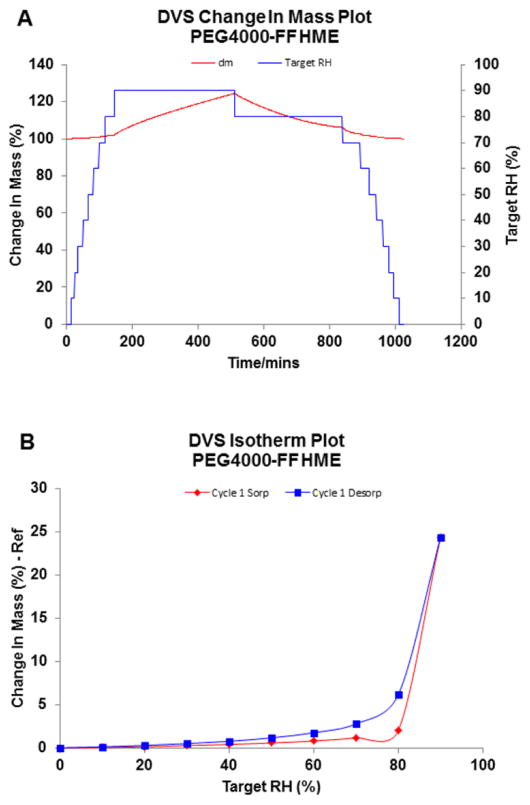
Figure 4.
Dynamic Vapor Sorption measurements of PEG 4000-FF amorphous solid dispersions prepared by HME (n=1): (A) change in mass plot and (B) isotherm plot.
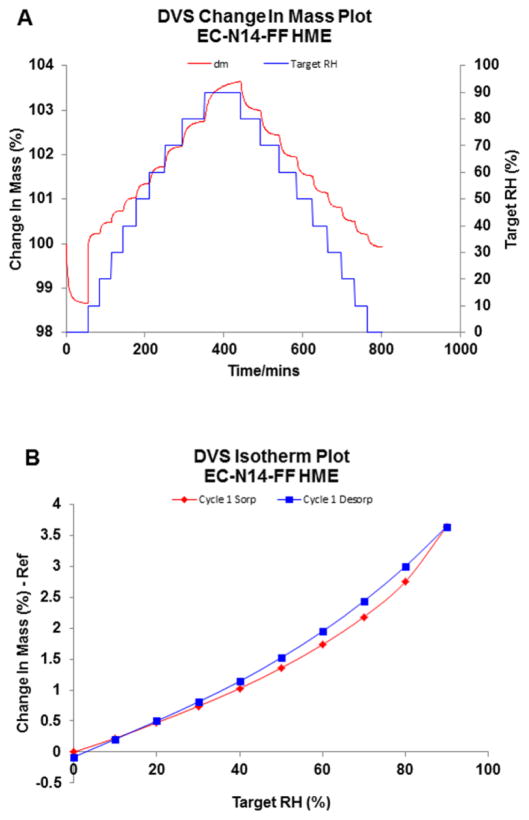
Figure 5.
Dynamic Vapor Sorption measurements of EC N14-FF amorphous solid dispersions prepared by HME (n=1): (A) change in mass plot and (B) isotherm plot.
Table 2.
Important parameters summarized from vapor sorption isotherms of amorphous solid dispersion samples. (Meant ± SD, n=3)
| Polymer Carrier | Maximum Weight Changing (%) | Weight Changing at 60%RH (%) | Hysteresis at 0% RH (%) | ||
|---|---|---|---|---|---|
| Grade | Molecular Weight | ||||
| HPC | ELF | 40000 | 22.81±0.19 | 8.21±0.23 | 1.83±0.16 |
| EF | 80000 | 20.81±0.17 | 8.96±0.21 | 1.93±0.23 | |
| LF | 95000 | 19.23±0.17 | 6.29±0.17 | 0.99±0.05 | |
| EC | N7 | 65000 | 3.42±0.04 | 1.70±0.07 | 0.00 |
| N14 | 120000 | 3.15±0.08 | 1.49±0.05 | 0.00 | |
| N22 | 140000 | 3.10±0.04 | 1.45±0.03 | 0.00 | |
| PEG | 3350 | 3350 | 29.30±0.14 | 0.49±0.10 | 3.69±0.19 |
| 4000 | 4000 | 25.20±0.44 | 0.44±0.03 | 2.53±0.18 | |
| 6000 | 6000 | 23.26±0.27 | 0.39±0.03 | 2.30±0.15 | |
At the 90% RH condition, the pure drug fenofibrate only had a weight change of 0.125%. This would indicate that even at high RH conditions the API absorbs very little water from the moist air. According to the isotherm hysteresis, after desorption processing, the weight change approached 0%, which would suggest that the API had very low ability to hold the moisture.
In the case of the hydrophobic polymer carrier, the EC-FF solid dispersion system showed similar water uptake properties as the pure drug. The low water solubility nature of EC makes it a perfect polymer candidate for sustained and controlled release formulations[26]. Although, EC is considered as water insoluble, the polymer still can sorb some moisture by the mechanism of hydrogen bonding, which was led due to the polarity difference between the oxygen atom and the ethyl group in the ethoxy group [37].
However, the controlled release formulation is only a small part of all drug products. To achieve other dissolution profiles or release mechanisms, water soluble polymer carriers are applicable to be incorporated into a formulation. Indeed, even for the same dissolution type, different dosage forms might require other polymer with special physicochemical properties, such as HPC and PEG for HME extruded films [8, 38].
The hygroscopic characteristic of polymers depend on its structure, hence the water uptake ability for both PEG and HPC polymers depends on either oxygen atoms or hydroxyl –OH groups, which may contribute greatly to the high water solubility by forming hydrogen bonding with water [39].
Both PEG and HPC exhibited high moisture sorption properties, however due to their hygroscopic nature, these two polymers had significantly different sorption isotherm curves. HPC begins water uptake from the environment at low RH conditions, and with the increase in the RH there was an increase in the % weight change. Conversely, PEG did not take up much moisture until the RH exceeded a specific point. This phenomenon was caused by the hydrophilic nature of the polymer and due to the commencement of deliquescence [40]. For both amorphous and semi-crystalline polymers with a hygroscopic nature, after the exposure to high moisture environment, the physical properties are altered by the sorbed moisture, coupled with the visible change such as caking and finally transforming into a semi-liquid state. Previous studies have shown that for semi-crystalline polymers like PEG, the phenomenon of deliquescence would happen once the atmospheric RH exceeds a critical relative humidity RH0 [41, 42].
Effect of Polymer Molecular Weight on the moisture sorption of solid dispersions
For all of the polymers (EC, PEG and HPC) used in this study, three different grades of various MW were used in the ASD formulations to investigate how MW affects the moisture sorption properties of the solid dispersion systems. Statistical analysis showed that maximum weight changing % for the HPC group and PEG group demonstrated significant differences (p<0.05), whereas no significant difference was observed for EC group (p>0.05). ASD containing different grades of EC revealed very low maximum weight changes % and no significant difference in between N7/N14/N22 was observed, which is most likely due to the hydrophobic nature of the polymer (Table 2).
HPC LF exhibited a lesser amount of water sorption as compared to the other grades of HPC with lower MW (LF<EF<ELF). PEG also demonstrated similar MW related sorption properties. However besides these findings, key changes were observed. Each MW of PEG displayed an abrupt increase in weight change when the step RH arrived at one certain point. As stated earlier, this should be the critical RH0 for the semi-crystalline polymer. When the vapor pressure was lower than the critical vapor pressure of the saturated aqueous solution of the PEG, a very small amount of water could be captured by the polymer via surface hydrogen bonding; when it surpassed the RH0, the phase transformation would commence, directing the sample into a semi-solid state. Since at this stage, the aqueous solution is thermodynamically more stable than the semi-crystalline phase, the dissolution of the solid dispersion and water sorption continued until this ternary system arrived at a thermodynamic equilibrium state. As the critical RH was concentrated in the range of 70–90%, the DVS method for PEG was changed by 5% in each step in this range and the critical RH increased as the MW of PEG in the solid dispersion increased. Since the deliquescence process was temperature and RH dependent, the weight change may increase significantly if the formulation is stored at high RH for a longer period of time.
Although this deliquescence and critical RH had been investigated for several small molecules, there are very limited studies for crystalline polymers reported in the literature. Generally speaking, a higher MW polymer always possesses longer polymer chains, which may decrease the mixing entropy of the polymer and water, hence lowering the aqueous solubility of the polymer. At the same time, lower MW polymers tend to be more hydrophilic due to the higher relative fraction of hydroxyl groups. The effect of MW could be limited if increasing MW could not change the fraction of hydrophilic groups.
At the same time, among the polymers utilized in this study, the polymers with higher MW exhibited higher viscosity. Once the moisture saturated the surface of the solid dispersion sample, a polymer-water mixture would form with different viscosities. The polymer with higher MW and viscosity would slow the late stage of moisture absorption as compared with the same kind of polymer with lower MW. With the higher viscosity, longer equilibrium time might be needed since the molecular mobility and phase transaction would be slower. This higher viscosity caused by higher MW might be another factor that results in less hygroscopic solid dispersion samples with higher MW polymer carriers.
FTIR Analysis and Chemical Imaging
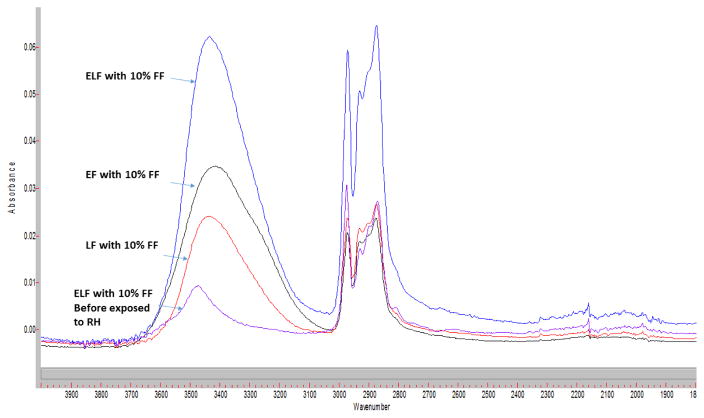
Compared with DVS data, FTIR spectrums provided more detailed information of the solid dispersion systems at the molecular level. Figure 6 illustrates a detailed perspective of how the peak positions and intensity vary with HPC molecular weight after exposure to 90% RH for 24 hours. FTIR imaging (Figure 7) indicated that the API was homogeneously dispersed within the freshly prepared amorphous solid dispersion, however the homogeneity was changed after storage at the high RH condition (25 °C/90% RH). With regard to API homogeneity as illustrated in the chemical images, considering the relatively small field of view (70 x 70 microns) and the marked intensity of absorbance resulting from fenofibrate’s characteristic and spectrally resolved carbonyl centered at 1723cm−1, the API is homogeneously dispersed in the fresh extrudates (Figure 7A; time point 0). The additional accumulation of adsorbed water, resulting from continued exposure to the relative humidity, produced areas of migrated fenorfibrate. In the chemical images (Figures 7A–D), this is graphically illustrated by the growing intensities of both fenofibrate pockets, represented by the color orange and red, and the voids where it is increasingly missing, represented by the color blue.
Figure 6.
FTIR spectrum of HPC-FF solid dispersion after storing at 25 °C/90%RH for 12 hours. Blue line: HPC ELF with 10% FF; black line: HPC EF with 10% FF; red line: HPC LF with 10% FF; and purple line: HPC ELF with 10% FF before exposed to RH.
Figure 7.
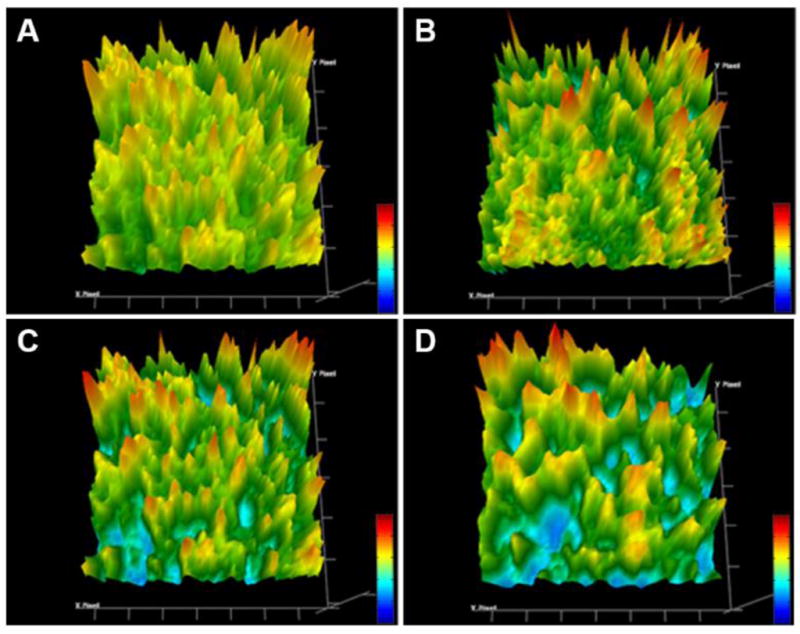
FTIR chemical imaging of HPC LF amorphous solid dispersions after storing at 25 °C/60% RH for (A) 0, (B) 2, (C) 3 and (D) 4 weeks.
As stated previously, the polymers’ hygroscopic nature attributed to sorption of moisture. This may result in many implications such as accelerating degradation by providing a reactant or reaction medium, and also negatively affecting the physical stability of the ASD by increasing molecular mobility and promoting recrystallization [43–45]. As the molecular mobility increases and the miscibility decreases driven by moisture, the drug-rich and polymer-rich amorphous domains could be potentially formed, prior to complete phase separation and drug recrystallization [46]. The uneven distribution of yellow color peaks, as shown in Figure 7, could be evidence of amorphous-amorphous phase separation.
Influence of Hot Melt Extrusion processing
Polymer carriers, APIs, and other additives normally comprise solid dispersion systems. All of these components and their respective interactions determine the characteristics of the system. However even for the same formulation, if prepared by different technologies, the physicochemical properties could be changed significantly. The moisture sorption of polymer and polymeric solid dispersions have been documented in the literature; Konno (2008) found that the polymer in solid dispersion systems could increase the moisture content of the system compared to that of the pure amorphous drug [47]. Rumondor (2009), stated that solid dispersions with different polymer carriers could undergo phase separation at different RH conditions mainly affected by the molecular interaction between API and polymer carrier [48]. To date, no study has reported how processing such as HME affects the water sorption/desorption of amorphous polymeric solid dispersion systems.
The kinetics of water uptake for each physical mixture (P.M.) and corresponding milled HME extrudates were measured at 25 °C by DVS and all of the important parameters are summarized in Table 3.
Table 3.
Important parameters summarized from vapor sorption isotherms and physical properties of amorphous solid dispersions (HME) and physical mixture (P.M.). (Mean ± SD, n=3)
| Drug Content 10% | Maximum Weight Changing (%) | Density (g/cm3) | Surface area (m2/g) | |
|---|---|---|---|---|
| HPC ELF | HME | 22.81±0.19 | 0.43±0.03 | 0.358±0.02 |
| P.M. | 24.2±0.17 | 0.38±0.01 | 0.441±0.02 | |
| EC N7 | HME | 3.42±0.04 | 0.33±0.01 | 0.298±0.03 |
| P.M. | 3.53±0.06 | 0.31±0.03 | 0.301±0.04 | |
| PEG 3350 | HME | 29.30±0.14 | 1.17±0.04 | 1.162±0.13 |
| P.M. | 29.98±0.07 | 1.06±0.03 | 1.217±0.08 | |
The comparison of the sorption isotherms of the physical mixture and milled HME extrudates demonstrated that HME processing could significantly change the maximum moisture sorption of the samples with HPC-FF, EC-FF and PEG-FF (p<0.05). The maximum weight changing % difference was not that much between EC-FF HME and PM. The moisture sorption ability of the EC-FF PM system was already very low (approximately 3%) that the effect of HME processing was limited.
The maximum weight change was always attained at the highest RH, or so called water activity, which was the most important indicator for the potential sorption of moisture by the formulation. The entire sorption isotherm was a process in which the moisture mixed with the solid dispersion by physical adsorption, chemisorption and multilayer condensation was attained. When comparing the isotherms curves of all of the HPC-FF containing samples, an interesting phenomenon was observed. The physical mixture and HME formulations exhibited identical sorption behavior at an early stage, and the differences in weight change for all formulations were observed in the later stages (RH>60%). The properties of water absorbed in the later stages should be similar to those of the free water that was held in the large capillaries or voids. According to the relatively short desorption equilibrium time, the later stage water was loosely bonded to the solid dispersion surface, which indicates that the vaporization enthalpy should be almost the same as the pure water. From this point of view, the authors hypothesized that HME processing may change the volume of the crevices and the space of the large capillaries. To confirm this hypothesis, both physical mixture and HME formulations were characterized by SEM, density meter and surface area measurement (Figure 8). The physical mixture sample had rough surface and lots crevices were observed. And the HME sample exhibited smooth surface and dense structure.
Figure 8.
SEM images for the physical mixture and milled hot melt extrudates. A and B: physical mixture of HPC ELF with 10% FF; C and D: milled solid dispersion of the same formulation.
During the HME processing, the physical mixture would soften and melt under high temperatures and high shear rates. All materials were reshaped by two key steps: first, forcing extrudates through a small round shape die and second milling into small sized particles. By pushing the formulations through the die, the density of the material could be increased, resulting in fewer small cavities in the solid dispersion particles. Also the high condensation polymer could have chains with less flexibility and less space to rotate, which could lead to lower moisture uptake. The milling processing could only affect the surface of the solid dispersed particles, not internally. Compared with the raw material, the milled extrudates tend to have a smoother surface. The physical absorption of moisture could be lowered by the decreased surface area. At the same time, the PM samples with higher surface area would provide more hydrogen bonding sites, such that more water could be taken up by chemisorption. As in the melt extruded solid dispersion, fenofibrate was dispersed in the polymer matrix at the molecular level. The molecular interaction such as hydrogen bonding between the API and polymer could reduce the available hydrogen bonding sites for water, which would lead to slower moisture uptake, less weight change and less hygroscopicity of the system [49].
Effect of different Compression Force
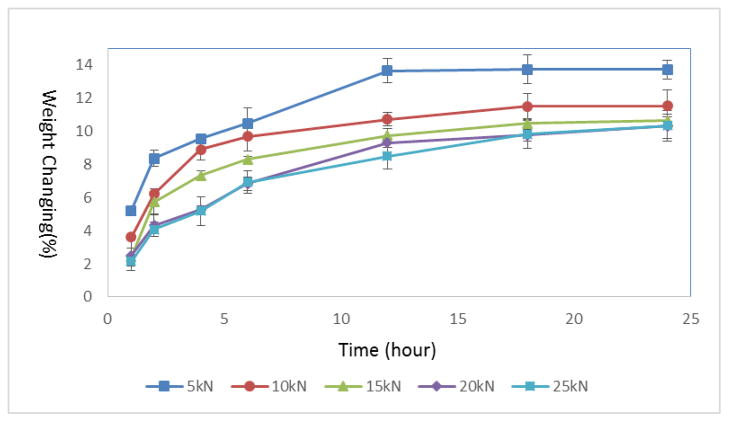
The hot melt extruded solid dispersion can be shaped into many different final dosage forms, such as pellets, films, and suppositories. However, the tablet is still the most popular dosage forms on the market, which means that a few additional downstream processing steps might be needed for the extrudates. In the downstream processing, compression force utilized in the tableting step might have a significant effect on the moisture sorption ability of the tablet. In order to eliminate the effects from other excipients, only milled extrudates were used to compact tablets(200mg) by different compression force (5, 10, 15, 20, 25 kN) using 8mm round flat tooling set with manual compaction machine. The HPC-LF-FF ASD system was chosen as a model system and the water content of the samples were measured under 25°C/90% RH for 48 hours (n=3) (Figure 9).
Figure 9.
Water content (weight changing) plot of tablets containing HPC LF-FF amorphous solid dispersions with different compression force under 25°C/90% RH for 48 hours (Mean ± SD, n=3).
The statistical analysis showed the significant differences between 5, 10, 15 and 20kN force groups (p<0.05). However, the statistical analysis between the 20 and 25kN groups did not indicate a significant difference (p=0.43). Higher compression force can decrease the moisture sorption of the ASD tablet, but this effect arrived at a plateau after 15 kN (samples with 20 and 25 kN compression force did not show any significant difference). Also, high compression force (20 and 25 kN) exhibited a sticking issue during the compression process and previous studies have shown that higher compression force might induce the immiscibility of drug and polymer by weakening and/or disruption of intermolecular hydrogen bonding [50].
The porosity of the compacts was measured to explain the effect of compression force. From low to high compression force groups, the compacts porosities were: 13.6±3.7%, 9.2±2.1%, 7.2±1.3%, 6.5±0.9% and 6.3±1.7% (n=3), respectively. The porosity was decreased as the compression force increased. As the porosity decreased, the surface to absorb moisture would also decrease, which could slow the water uptake process. At the same time, less surface area could lead to less hydrogen bonding sites, which could lower the hygroscopicity of the samples with higher compression force.
Conclusion
In this study, HPC-FF, EC-FF and PEG-FF amorphous solid dispersion systems were successfully prepared by HME technology. This is the first time that the moisture sorption abilities of amorphous solid dispersions prepared by hot melt extrusion technology with different polymeric carriers has been reported. Also, this study investigated the moisture sorption from both the formulation as well as processing approaches, which are very limited as reported in previous literature. The nature of the polymer, molecular weight of the polymer, HME process and downstream processing parameters were found to have a significant effect on the moisture sorption ability of the amorphous solid dispersion systems. As moisture plays an important role in the physical and chemical stability of the solid dispersion, it becomes imperative to understand the effect of both formulation and processing parameters that influence the moisture sorption properties of the solid dispersions. The data, and hence knowledge, attained within this study would be extremely valuable for future commercialization of drug products containing solid dispersions.
Acknowledgments
This project was partially supported by Grant Number P20GM104932 from the National Institute of General Medical Sciences (NIGMS), a component of NIH. The authors also thank Dr Vijayasankar Raman, National Center for Natural Products Research, School of Pharmacy, The University of Mississippi, for his valuable assistance with the SEM imaging studies.
References
- 1.Rojanasthien N, et al. Pharmacokinetics and bioavailability studies of generic ondansetron, and the innovator preparation, in healthy Thai male volunteers. J Med Assoc Thai. 1999;82(7):713–20. [PubMed] [Google Scholar]
- 2.Crowley MM, et al. Pharmaceutical applications of hot-melt extrusion: part I. Drug Dev Ind Pharm. 2007;33(9):909–26. doi: 10.1080/03639040701498759. [DOI] [PubMed] [Google Scholar]
- 3.Repka MA, et al. Pharmaceutical applications of hot-melt extrusion: Part II. Drug Dev Ind Pharm. 2007;33(10):1043–57. doi: 10.1080/03639040701525627. [DOI] [PubMed] [Google Scholar]
- 4.Hancock BC, Zografi G. Characteristics and significance of the amorphous state in pharmaceutical systems. Journal of pharmaceutical sciences. 1997;86(1):1–12. doi: 10.1021/js9601896. [DOI] [PubMed] [Google Scholar]
- 5.Liu X, et al. Improving the chemical stability of amorphous solid dispersion with cocrystal technique by hot melt extrusion. Pharmaceutical research. 2012;29(3):806–817. doi: 10.1007/s11095-011-0605-4. [DOI] [PubMed] [Google Scholar]
- 6.Li Y, et al. Interactions between drugs and polymers influencing hot melt extrusion. J Pharm Pharmacol. 2014;66(2):148–66. doi: 10.1111/jphp.12183. [DOI] [PubMed] [Google Scholar]
- 7.Patwardhan K, et al. A quality by design approach to understand formulation and process variability in pharmaceutical melt extrusion processes. J Pharm Pharmacol. 2015 doi: 10.1111/jphp.12370. [DOI] [PubMed] [Google Scholar]
- 8.Prodduturi S, et al. Solid-state stability and characterization of hot-melt extruded poly(ethylene oxide) films. J Pharm Sci. 2005;94(10):2232–45. doi: 10.1002/jps.20437. [DOI] [PubMed] [Google Scholar]
- 9.Vynckier AK, et al. Hot-melt co-extrusion: requirements, challenges and opportunities for pharmaceutical applications. J Pharm Pharmacol. 2014;66(2):167–79. doi: 10.1111/jphp.12091. [DOI] [PubMed] [Google Scholar]
- 10.Feng X, et al. Evaluation of the recrystallization kinetics of hot-melt extruded polymeric solid dispersions using an improved Avrami equation. Drug development and industrial pharmacy. 2014;(0):1–9. doi: 10.3109/03639045.2014.958755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kapoor B, et al. Solid Dispersion: An evolutionary approach for solubility enhancement of poorly water soluble drugs. Int J Recent Adv Pharm Res. 2012;2:1–16. [Google Scholar]
- 12.Vo CLN, Park C, Lee BJ. Current trends and future perspectives of solid dispersions containing poorly water-soluble drugs. European Journal of Pharmaceutics and Biopharmaceutics. 2013;85(3):799–813. doi: 10.1016/j.ejpb.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 13.Das SK, et al. Solid dispersions: an approach to enhance the bioavailability of poorly water-soluble drugs. International journal of pharmacology and pharmaceutical technology. 2012;1(1):37–46. [Google Scholar]
- 14.Yang D, et al. Effect of the melt granulation technique on the dissolution characteristics of griseofulvin. Int J Pharm. 2007;329(1–2):72–80. doi: 10.1016/j.ijpharm.2006.08.029. [DOI] [PubMed] [Google Scholar]
- 15.Sarode AL, et al. Stability assessment of hypromellose acetate succinate (HPMCAS) NF for application in hot melt extrusion (HME) Carbohydr Polym. 2014;101:146–53. doi: 10.1016/j.carbpol.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 16.Muehlenfeld C, Thommes M. Small-scale twin-screw extrusion - evaluation of continuous split feeding. J Pharm Pharmacol. 2014;66(12):1667–76. doi: 10.1111/jphp.12301. [DOI] [PubMed] [Google Scholar]
- 17.Guo Y, Shalaev E, Smith S. Physical stability of pharmaceutical formulations: solid-state characterization of amorphous dispersions. TrAC Trends in Analytical Chemistry. 2013;49:137–144. [Google Scholar]
- 18.Marsac PJ, et al. Recrystallization of nifedipine and felodipine from amorphous molecular level solid dispersions containing poly(vinylpyrrolidone) and sorbed water. Pharm Res. 2008;25(3):647–56. doi: 10.1007/s11095-007-9420-3. [DOI] [PubMed] [Google Scholar]
- 19.Konno H, et al. Effect of polymer type on the dissolution profile of amorphous solid dispersions containing felodipine. Eur J Pharm Biopharm. 2008;70(2):493–9. doi: 10.1016/j.ejpb.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 20.Patil H, Tiwari RV, Repka MA. Hot-Melt Extrusion: from Theory to Application in Pharmaceutical Formulation. AAPS PharmSciTech. 2015:1–23. doi: 10.1208/s12249-015-0360-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Konno H, Taylor LS. Influence of different polymers on the crystallization tendency of molecularly dispersed amorphous felodipine. J Pharm Sci. 2006;95(12):2692–705. [Google Scholar]
- 22.Marsac PJ, Shamblin SL, Taylor LS. Theoretical and practical approaches for prediction of drug-polymer miscibility and solubility. Pharm Res. 2006;23(10):2417–26. doi: 10.1007/s11095-006-9063-9. [DOI] [PubMed] [Google Scholar]
- 23.Wegiel LA, et al. Mid-infrared spectroscopy as a polymer selection tool for formulating amorphous solid dispersions. The Journal Of Pharmacy And Pharmacology. 2014;66(2):244–255. doi: 10.1111/jphp.12079. [DOI] [PubMed] [Google Scholar]
- 24.Kazarian SG, Chan KA. ATR-FTIR spectroscopic imaging: recent advances and applications to biological systems. Analyst. 2013;138(7):1940–1951. doi: 10.1039/c3an36865c. [DOI] [PubMed] [Google Scholar]
- 25.Saerens L, et al. Process monitoring and visualization solutions for hot-melt extrusion: a review. J Pharm Pharmacol. 2014;66(2):180–203. doi: 10.1111/jphp.12123. [DOI] [PubMed] [Google Scholar]
- 26.Agrawal A, et al. Studies on the interaction of water with ethylcellulose: Effect of polymer particle size. AAPS PharmSciTech. 2003;4(4):469–479. doi: 10.1208/pt040460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mikhailov E, et al. Mass-based hygroscopicity parameter interaction model and measurement of atmospheric aerosol water uptake. Atmospheric Chemistry and Physics. 2013;13(2):717–740. [Google Scholar]
- 28.Saripella KK, Mallipeddi R, Neau SH. Crospovidone interactions with water. I. Calorimetric study of the effect of Polyplasdone particle size on its uptake and distribution of water. J Pharm Sci. 2014;103(2):669–75. doi: 10.1002/jps.23846. [DOI] [PubMed] [Google Scholar]
- 29.Weuts I, et al. Physicochemical properties of the amorphous drug, cast films, and spray dried powders to predict formulation probability of success for solid dispersions: etravirine. Journal of pharmaceutical sciences. 2011;100(1):260–274. doi: 10.1002/jps.22242. [DOI] [PubMed] [Google Scholar]
- 30.Guns S, et al. Comparison between hot-melt extrusion and spray-drying for manufacturing solid dispersions of the graft copolymer of ethylene glycol and vinylalcohol. Pharmaceutical research. 2011;28(3):673–682. doi: 10.1007/s11095-010-0324-2. [DOI] [PubMed] [Google Scholar]
- 31.Patterson JE, et al. Melt extrusion and spray drying of carbamazepine and dipyridamole with polyvinylpyrrolidone/vinyl acetate copolymers. Drug development and industrial pharmacy. 2008;34(1):95–106. doi: 10.1080/03639040701484627. [DOI] [PubMed] [Google Scholar]
- 32.Dong Z, et al. Evaluation of solid state properties of solid dispersions prepared by hot-melt extrusion and solvent co-precipitation. International journal of pharmaceutics. 2008;355(1):141–149. doi: 10.1016/j.ijpharm.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 33.Bley O, Siepmann J, Bodmeier R. Characterization of moisture-protective polymer coatings using differential scanning calorimetry and dynamic vapor sorption. Journal of pharmaceutical sciences. 2009;98(2):651–664. doi: 10.1002/jps.21429. [DOI] [PubMed] [Google Scholar]
- 34.Deng W, et al. Stabilization of fenofibrate in low molecular weight hydroxypropylcellulose matrices produced by hot-melt extrusion. Drug development and industrial pharmacy. 2013;39(2):290–298. doi: 10.3109/03639045.2012.679280. [DOI] [PubMed] [Google Scholar]
- 35.Sathigari SK, et al. Amorphous-state characterization of efavirenz--polymer hot-melt extrusion systems for dissolution enhancement. J Pharm Sci. 2012;101(9):3456–64. doi: 10.1002/jps.23125. [DOI] [PubMed] [Google Scholar]
- 36.Sanganwar GP, Gupta RB. Dissolution-rate enhancement of fenofibrate by adsorption onto silica using supercritical carbon dioxide. International journal of pharmaceutics. 2008;360(1):213–218. doi: 10.1016/j.ijpharm.2008.04.041. [DOI] [PubMed] [Google Scholar]
- 37.Joshi H, Wilson T. Calorimetric studies of dissolution of hydroxypropyl methylcellulose E5 (HPMC E5) in water. Journal of pharmaceutical sciences. 1993;82(10):1033–1038. [PubMed] [Google Scholar]
- 38.Repka MA, et al. Influence of plasticizers and drugs on the physical-mechanical properties of hydroxypropylcellulose films prepared by hot melt extrusion. Drug development and industrial pharmacy. 1999;25(5):625–633. doi: 10.1081/ddc-100102218. [DOI] [PubMed] [Google Scholar]
- 39.Kitano H, et al. Fourier transform infrared study on the state of water sorbed to poly (ethylene glycol) films. Langmuir. 2001;17(6):1889–1895. [Google Scholar]
- 40.Salameh AK, Taylor LS. Deliquescence in Binary Mixtures. Pharmaceutical Research. 2005;22(2):318–324. doi: 10.1007/s11095-005-1563-5. [DOI] [PubMed] [Google Scholar]
- 41.Baird JA, et al. Effect of molecular weight, temperature, and additives on the moisture sorption properties of polyethylene glycol. Journal of pharmaceutical sciences. 2010;99(1):154–168. doi: 10.1002/jps.21808. [DOI] [PubMed] [Google Scholar]
- 42.Marsac PJ, et al. Spontaneous crystallinity loss of drugs in the disordered regions of poly (ethylene oxide) in the presence of water. Journal of pharmaceutical sciences. 2008;97(8):3182–3194. doi: 10.1002/jps.21237. [DOI] [PubMed] [Google Scholar]
- 43.Shamblin SL, Zografi G. The effects of absorbed water on the properties of amorphous mixtures containing sucrose. Pharmaceutical research. 1999;16(7):1119–1124. doi: 10.1023/a:1018960405504. [DOI] [PubMed] [Google Scholar]
- 44.Shibata Y, et al. Effect of storage conditions on the recrystallization of drugs in solid dispersions with crospovidone. Pharmaceutical development and technology. 2013;19(4):468–474. doi: 10.3109/10837450.2013.795168. [DOI] [PubMed] [Google Scholar]
- 45.Shete G, et al. Effect of different “states” of sorbed water on amorphous celecoxib. Journal of pharmaceutical sciences. 2014;103(7):2033–2041. doi: 10.1002/jps.23999. [DOI] [PubMed] [Google Scholar]
- 46.Marsac PJ, et al. Effect of temperature and moisture on the miscibility of amorphous dispersions of felodipine and poly(vinyl pyrrolidone) J Pharm Sci. 2010;99(1):169–85. doi: 10.1002/jps.21809. [DOI] [PubMed] [Google Scholar]
- 47.Konno H, Taylor LS. Ability of different polymers to inhibit the crystallization of amorphous felodipine in the presence of moisture. Pharmaceutical research. 2008;25(4):969–978. doi: 10.1007/s11095-007-9331-3. [DOI] [PubMed] [Google Scholar]
- 48.Rumondor AC, et al. Phase behavior of poly (vinylpyrrolidone) containing amorphous solid dispersions in the presence of moisture. Molecular pharmaceutics. 2009;6(5):1492–1505. doi: 10.1021/mp900050c. [DOI] [PubMed] [Google Scholar]
- 49.Crowley KJ, Zografi G. Water vapor absorption into amorphous hydrophobic drug/poly (vinylpyrrolidone) dispersions. Journal of pharmaceutical sciences. 2002;91(10):2150–2165. doi: 10.1002/jps.10205. [DOI] [PubMed] [Google Scholar]
- 50.Singh A, Van Humbeeck J, Van den Mooter G. A New Twist in the Old Story-can Compression Induce Mixing of Phase Separated Solid Dispersions? A Case Study of Spray-Dried Miconazole-PVP VA64 Solid Dispersions. Pharmaceutical research. 2014;31(11):3191–3200. doi: 10.1007/s11095-014-1411-6. [DOI] [PubMed] [Google Scholar]