Abstract
Heroin is a highly abused opioid and incurs a significant detriment to society worldwide. In an effort to expand the limited pharmacotherapy options for opioid use disorders, a heroin conjugate vaccine was developed through comprehensive evaluation of hapten structure, carrier protein, adjuvant and dosing. Immunization of mice with an optimized heroin-tetanus toxoid (TT) conjugate formulated with adjuvants alum and CpG oligodeoxynucleotide (ODN) generated heroin ‘immunoantagonism’, reducing heroin potency by >15-fold. Moreover, the vaccine effects proved to be durable, persisting for over eight months. The lead vaccine was effective in rhesus monkeys, generating significant and sustained anti-drug IgG titers in each subject. Characterization of both mouse and monkey anti-heroin antibodies by surface plasmon resonance (SPR) revealed low nanomolar antiserum affinity for the key heroin metabolite, 6-acetylmorphine (6AM), with minimal cross reactivity to clinically-used opioids. Following a series of heroin challenges over six months in vaccinated monkeys, drug-sequestering antibodies caused marked attenuation of heroin potency (>4-fold) in a schedule-controlled responding (SCR) behavioral assay. Overall, these preclinical results provide an empirical foundation supporting the further evaluation and potential clinical utility of an effective heroin vaccine in treating opioid use disorders.
TOC image
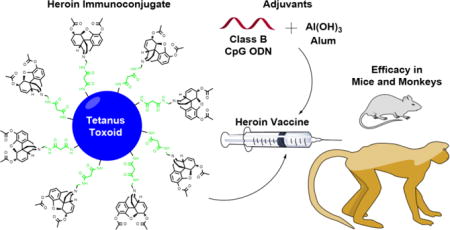
Introduction
Heroin, a semisynthetic opioid, and its parent natural product, morphine, are among the longest known and commonly abused psychoactive drugs. Heroin is a prodrug1 that readily crosses the blood-brain barrier while quickly deacetylating to 6-acetyl morphine (6AM) and then more slowly to morphine (Figure 1).2–3 These two metabolites agonize brain mu-opioid receptors (MORs) to produce heroin’s abuse-related euphoric and reinforcing effects.4–6 Moreover, the robust analgesic effects of opioids have led to their extensive clinical use as prescription painkillers such as OxyContin (oxycodone) and Vicodin (hydrocodone); however, these opioids are also routinely abused and can act as “gateway drugs” to heroin.7–8 Persistent opioid abuse leads to a neuropsychiatric disorder, i.e. opioid use disorder, characterized by compulsive opioid administration despite the negative physical, mental, legal and social consequences of prolonged use.
Figure 1.
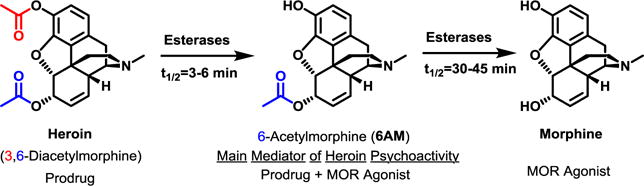
Structures of heroin and its key metabolites which, act as prodrugs and/or MOR agonists.
Currently in the United States, opioid abuse has reached epidemic levels. The number of people who have used heroin in the past 10 years has doubled from 379,000 in 2005 to 828,000 in 2015,9–10 and heroin expenditures have grown steadily to an estimated $27 billion (2010) on drug purchases alone.11 The widespread prevalence of heroin abuse is a significant cost to users and to society as a whole (an estimated total of $22 billion in the US).12–13 Other negative impacts of heroin abuse include HIV or HCV infection, for which injection drug users remain at the highest risk.14 While prescription opioids combined are involved in the most drug-related deaths in the US, compared to any one single drug, heroin is responsible for at least twice as many deaths.15
Abuse of prescription opioids may be mitigated by tightening regulations or by introducing anti-abuse technology during manufacturing. On the other hand, heroin and other synthetic opioids, e.g. acetyl fentanyl, are produced and distributed illegally; therefore, great measures must be taken to curb illicit opioid use. Current treatment options for opioid use disorders include opioid replacement therapy utilizing methadone or buprenorphine as MOR agonists to reduce opioid withdrawal symptoms and maintain heroin abstinence.16–17 Opioid antagonists naloxone and naltrexone (NTX) are other treatment options, FDA-approved for opioid overdose and dependence, respectively.18–19 Pharmacological intervention for heroin abuse has proven to be effective but has a number of drawbacks including high cost of in-patient rehab,12–13 undesirable effects,20–21 and relapse potential following therapy.13, 22–23
Humanity has benefited from vaccines for more than two centuries, and of all the biomedical achievements, immunization for the prevention of infectious diseases ranks highly. The first attempt at translating vaccination to reduce the abuse of psychoactive substances was reported in the early 70s when a conjugate vaccine containing a morphine-like hapten was tested in a single rhesus monkey.24 However, this work was not followed up due to the emergence of pharmacotherapies for opioid use disorders, e.g. methadone, which at the time, appeared more promising.25 Drug conjugate vaccine research re-emerged in the mid-90s, and focused on cocaine26 and nicotine.27 Unfortunately, multiple failures of both cocaine and nicotine vaccines in human trials have called into question the clinical value of vaccination for treating substance use disorders.28–31 Potential problems of these vaccines include poor hapten design32 and adjuvant selection. Moreover, these vaccines lacked rigorous preclinical development, as they have not demonstrated the ability to block a wide range of drug doses in multiple behavioral procedures. Failure to address and ultimately solve these problems has hampered progress in the drug-vaccine field.
The principle design elements behind drug vaccines include a hapten (B-cell epitope), highly congruent in structure to the target drug, and an immunogenic carrier protein (T-cell epitope) such as tetanus toxoid (TT) (Figure 2). Immunization of the hapten-protein conjugate formulated with adjuvants e.g. alum and CpG oligodeoxynucleotide (ODN), triggers an adaptive immune response against the drug-like hapten (Figure 2). Subsequently, when the vaccinated subject receives a drug dose, available polyclonal IgG antibodies in the periphery bind the drug with a high degree of affinity and specificity, precluding drug entry to the brain (Figure 2). Moreover, the vaccine significantly attenuates the pharmacodynamics of the drug dose without modulating receptors in the brain. The unique mechanism of action of drug-conjugate vaccines may offer many advantages for treating substance use disorders such as the potential for reduced side effects,33 convenient and low-cost administration, and long-term efficacy.34
Figure 2.
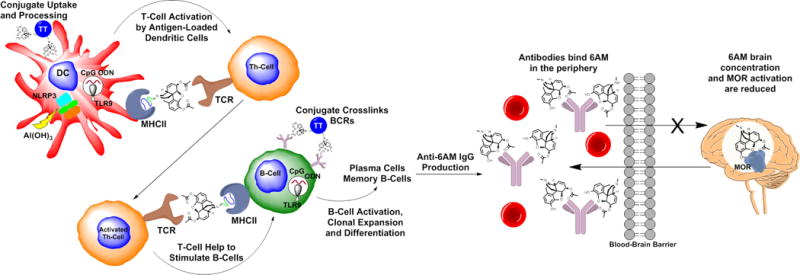
Simplified schematic of heroin conjugate immunological pathway and mechanism of action. Upon vaccination, the conjugate is taken up by dendritic cells (DCs), processed and displayed on the major histocompatibility complex class II (MHCII) as a haptenated peptide for activation of helper T-cells (Th-cells) via the T-cell receptor (TCR). B-cells which have encountered the conjugate via their B-cell receptors (BCR) are stimulated by activated Th-cells. Adjuvants alum and CpG ODN enhance the vaccine response through NLRP3 inflammasome and TLR9 signaling, respectively. Memory B-cells and plasma cells produce high affinity anti-6AM IgG antibodies which bind to an administered heroin dose (metabolized to 6AM) in the periphery, thus mitigating drug effects in the brain.
We envisioned the need for a comprehensive series of preclinical experiments wherein every component of the vaccine would be scrutinized, i.e. hapten, carrier and adjuvant. Moreover, the cornerstone of our development process involved the quantitative evaluation of each vaccine iteration in a rodent antinociception assay. Thus, full heroin dose-response curves were generated to compare ED50 values of vaccinated and non-vaccinated groups of rodents, providing a direct measure of the ‘immunoantagonistic’ capacity of the vaccine.35 A first-generation heroin vaccine produced heroin ED50 ratios of 4–5 in both mice and rats,36 blocking the effects of heroin in a series of behavioral models.37 In this work, a significant leap forward was made in redesigning the vaccine to achieve a greater than 15-fold heroin ED50 shift in rodents, warranting further investigation in a primate behavioral model. The translational potential of the heroin vaccine was confirmed for the first time in rhesus monkeys, supported by statistical analysis in n = 4 subjects.
Results
Vaccine optimization: hapten, carrier and adjuvant
As depicted in Scheme 1, preparation of heroin haptens was accomplished by first demethylating heroin via Olofson’s procedure.38 Reductive amination of Boc-protected 4-aminobutanal followed by TFA deprotection afforded the key intermediate 1 as previously described.36 Amide coupling(s) followed by trityl or t-butyl ester deprotections yielded the first generation thiol hapten (5, HerSH)36 or novel second generation carboxylic acid haptens (2–4). Thiol and carboxylic acid haptens were coupled to surface lysines of carrier proteins via thiol-maleimide or amide couplings, respectively. At a 1:1 w/w ratio of hapten to protein, carboxylate haptens showed higher hapten loading according to MALDI-ToF analysis (Table S1, Figure S1). Additional advantages to carboxylate hapten conjugations included resistance to oxidation and a one-pot coupling procedure. In contrast, thiols can form disulfides and require preparation of maleimide-loaded proteins prior to conjugation, i.e., a two-pot procedure.
Scheme 1.
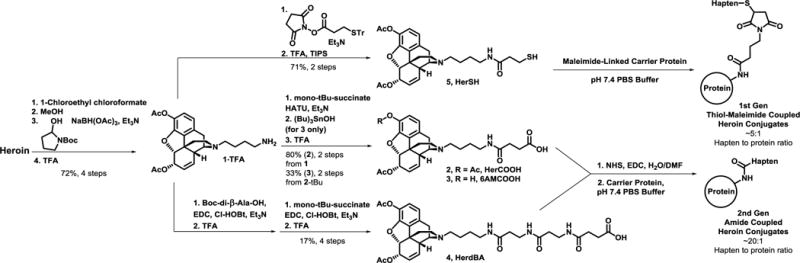
Synthesis and conjugation of heroin haptens.
Three of the most commonly used carrier proteins keyhole limpet hemocyanin (KLH), diphtheria toxoid (DT) and tetanus toxoid (TT) were investigated in the context of the heroin conjugate vaccine. In comparing the proteins as HerSH conjugates, TT, which is routinely used in human-approved tetanus vaccines, showed the best performance in attenuating heroin-induced antinociception in mice (Figure 3A) and produced the greatest anti-hapten titers (Figure S2A). Bioconjugation methods and linker structures were also compared through the testing of TT conjugates of HerSH (5), HerCOOH (2) and HerdBA (4), and the HerCOOH hapten demonstrated the greatest efficacy (Figure 3B, S2B). While the amide coupling method appeared to generate a more efficacious conjugate compared to thiol-maleimide coupling, this benefit appeared to be erased by the presence of the di-beta-alanine (dBA) linker found in the HerdBA hapten. The effect of the 3-acetyl group was probed through a 6AM-like hapten (6AMCOOH, 3), because 6AM is the main mediator of heroin psychoactivity.4–6 Behavioral results indicated that the 6AM and the heroin haptens were comparable in efficacy (Figure 3C). ELISA results corroborated the behavior because sera from both groups bound both 6AM and heroin haptens to a similar degree; however, the 6AM conjugate elicited antibodies with a slightly reduced capacity to bind the heroin hapten (Figure S2C).
Figure 3. Optimization of heroin immunoconjugate and vaccine formulation.
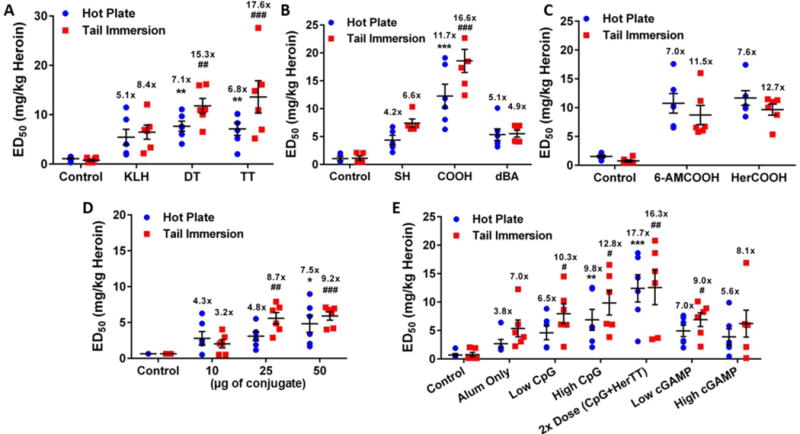
(A) Comparison of carrier proteins with HerSH hapten. ##P < 0.01, ###P < 0.001, **P < 0.01 versus KLH. (B) Evaluation of heroin haptens as TT immunoconjugates. ###P < 0.001, ***P < 0.001 versus HerSH-TT. (C) Comparison study of 6AMCOOH vs. HerCOOH haptens as TT conjugates. (D) Dose- dependency of HerCOOH-TT immunoconjugate on vaccine efficacy. ##P < 0.01, ###P < 0.001, *P < 0.05 versus 10 μg dose. No CpG ODN was used. (E) Adjuvant effects of CpG ODN 1826 and cGaMp on vaccine efficacy. Doses: 4 μg CpG (Low CpG), 30 μg CpG (High CpG), 60 μg CpG + 100 μg HerCOOH-TT (2X dose), 1.3 μg (Low cGAMP), 10 μg (High cGAMP). #P < 0.05, ##P < 0.01, **P < 0.01, ***P < 0.001 versus alum only. Vaccine formulations for all panels contained 0.75 mg alum, 30 μg CpG ODN 1826 and 50 μg HerCOOH-TT unless otherwise noted and were administered to n = 6 mice i.p. at wk 0, 2 and 4. For all panels, the mean heroin ED50 ± SEM (determined via cumulative s.c. dosing) is shown and statistics were evaluated by a one-way ANOVA with Tukey’s post-hoc test. Testing was performed at wk 6 and significance is denoted by (#) for tail immersion and (*) for hot plate. Fold-shifts in ED50 versus n = 6 non-vaccinated control mice are reported above each set of data points. Raw antinociception curves shown in Figures S3A–E.
In continuing studies with the HerCOOH-TT conjugate, the effect of conjugate dosing and adjuvant was explored, using FDA-approved alum adjuvant (Al(OH)3) alone as a benchmark. Conjugate dosing demonstrated a positive effect on titer levels and mitigation of heroin antinociception (Figure S2D, 3D). Addition of CpG ODN 1826, a Toll-like receptor (TLR) 9 agonist, enhanced vaccine potency of HerCOOH-TT (Figure 3E) as previously shown for HerSH-KLH.35 Without alum, CpG ODN was not effective (data not shown). When both conjugate and CpG ODN doses were doubled to 100 μg and 60 μg respectively, heroin ‘immunoantagonistic’ capacity and anti-hapten titers increased dramatically (Figure 3E, S2E). A second DNA-based immunostimulatory adjuvant, 2′3′-cyclic guanosine monophosphate-adenosine monophosphate (cGAMP)39 was also evaluated. Although both cGAMP and CpG ODN target the innate immune system, they activate different receptors and pathways via STING and TLR9, respectively. A mild vaccine improvement was noted with low dose cGAMP, however, this effect was not present at a higher cGAMP dose (Figure 3E). The outcome of these optimization studies was the identification of a lead vaccine formulation containing HerCOOH-TT, CpG ODN and alum.
Long-term duration of lead vaccine efficacy
In order to test the durability of the optimized heroin vaccine, an extended vaccination study was performed over a 37-week period in mice. After an initial immunization, peak anti-heroin titers were observed by ELISA, causing significant potency shifts in heroin ED50 as measured by antinociceptive testing (Figure 4A–C). Although vaccine-mediated shifts in heroin ED50 declined over the next three months, a second and third round of vaccinations at months 5 and 8 maintained vaccine efficacy at approximately 50–70% of the initial level (Figure 4A–C). As a means to corroborate the antinociception results, ELISA titers were evaluated with the caveat that serum binding to an immobilized drug hapten does not necessarily equate to affinity for the actual drug molecule.40–42 To address this potential limitation, individual heroin ED50s were plotted against anti-heroin titers, and a linear relationship was observed (Figure 4D). The correlation implies that the degree of serum antibody binding to the HerCOOH hapten is representative of the degree of binding to the actual opioids (heroin and 6AM) in vivo.
Figure 4. Heroin conjugate vaccine shows robust, long-term efficacy in mice.
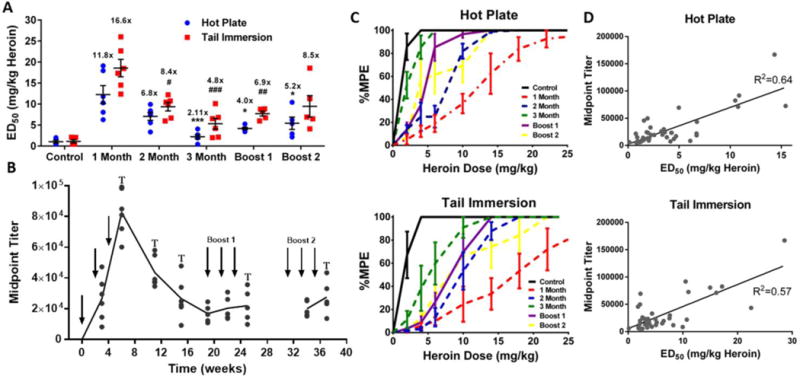
(A) Heroin ED50 in the hot plate and tail immersion tests at various time points following three rounds of vaccination (expressed as mean ± SEM). Mice (n = 6) were vaccinated i.p. with 50 μg HerCOOH-TT + 30 μg CpG 1826 at wk 0, 2 and 4; 25 μg HerCOOH-TT at wk 19, 21 and 23; 25 μg HerCOOH-TT + 15 μg CpG 1826 at wk 31, 33 and 35. All immunizations contained 0.75 mg alum. #P < 0.05, ###P < 0.01, ###P < 0.001 (tail immersion); *P < 0.05, ***P < 0.001(hot plate) versus the 1 month time point. Fold-shifts in ED50 versus non-vaccinated control mice are reported above each set of data points. (B) Timeline of midpoint titers versus HerCOOH-BSA coating antigen over a period of 37 weeks. Arrows indicate times of vaccination, while (T) indicates behavioral tests shown in the above panel. (C) Raw curves corresponding to panel A. Heroin dose-effect curve data expressed as mean ± SEM from six mice were generated via cumulative s.c. heroin dosing (same procedures as Figure 3). (D) Correlation between heroin ED50s and midpoint titers. Pearson’s correlation: P < 0.0001 for both tests.
A possible liability in using TT in the final formulation is that preexisting immunity to TT from the clinically administered DTaP vaccine could reduce the subsequent response to a heroin vaccine, a phenomenon known as carrier-induced epitopic suppression (CIES);43–44 however, no evidence was found for the occurrence of CIES in the context of the current heroin vaccine (Figure S4).
Heroin vaccine-induced antibody response in non-human primates (NHPs)
As an initial assessment of the lead vaccine in NHPs, a pilot vaccine study was performed in which two rhesus monkeys (M1,2) received the heroin conjugate while one monkey (M3) received the unmodified TT carrier protein as a control. Following three injections, a significant and consistent anti-drug IgG antibody response was observed in the conjugate-vaccinated monkeys while anti-heroin antibodies were not observed in the carrier-vaccinated monkey (Figure S5A). Preliminary behavioral assessments suggested that heroin dose-effect curves were right-shifted >3-fold from baseline in both conjugate-vaccinated monkeys (Figure S5B). Surface plasmon resonance (SPR) analysis of both monkey and mouse sera indicated sub-micromolar and low nanomolar competitive IC50s for heroin and 6AM, respectively (Figure 5A), which in contrast to ELISA is representative of actual antibody Kd.45 In comparing binding selectivity for other opioids, antiserum affinity for morphine, oxycodone and methadone was >1000-fold lower (Figure 5B).
Figure 5. Heroin conjugate vaccine elicits a robust anti-drug antibody response in monkeys with high affinity and selectivity for 6AM.
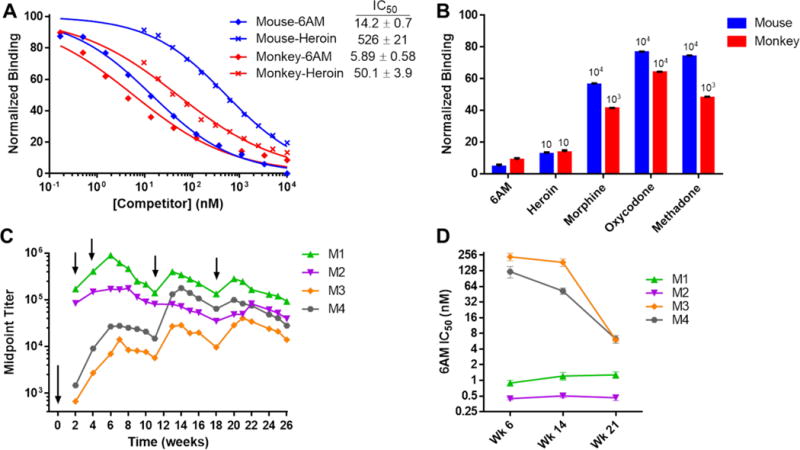
(A) Binding curves of vaccinated monkey and mouse antiserum for heroin and 6AM as determined by SPR in triplicate. Points represents the mean binding to immobilized HerCOOH-BSA following serum incubation with 12 dilutions of heroin or 6AM competitors. Binding values were normalized to serum binding without competitor drug. Listed IC50s ± SEM were derived from a nonlinear fit of the binding curves. Serum was pooled from n = 2 monkeys (M1,2) and n = 6 mice collected after three initial immunizations. (B) Binding selectivity of mouse and monkey antiserum for various opioids at 100 μM ([6AM] = 10 μM) by SPR in triplicate. Minimum selectivity factors relative to 6AM are shown above each bar. (C) Anti-heroin IgG titers of rhesus monkeys (M1–4) vaccinated at the indicated time points (arrows) with 400 μg HerCOOH-TT, 600 μg CpG ODN 2006 and 5 mg alum. Midpoint titers were determined in duplicate by ELISA against a HerCOOH-BSA coating antigen. No anti-heroin IgM titers were detected at any point. Monkeys M1 and M2 were previously vaccinated with the heroin vaccine in a pilot study while M3 received unconjugated TT. M4 was not pretreated. (D) Individual monkey antiserum affinity for 6AM over time by SPR. Points represent mean IC50 ± SEM determined from a 12 point 6AM dilution curve similar to panel A methods. Time points match up with the panel C timeline.
An extended vaccination study involving the same monkeys including one new one (M4) demonstrated that all subjects produced a long-lasting, high IgG titer response to the vaccine (Figure 5C). Interestingly, the two monkeys that received the heroin vaccine in the pilot study (M1,2) showed significantly higher titers than the monkeys that received carrier (M3) or no vaccine (M4). SPR analysis of antiserum from each monkey revealed consistent drug affinity for 6AM of ≤1 nM in monkeys M1,2 while monkeys M3,4 gradually increased 6AM affinity over the course of the study to ~6 nM (Figure 5D). The 6AM affinity of M3,4 matches the 6AM affinity of M1,2 observed after the pilot study (Figure 5A), while 6AM affinity in M1,2 increased further by approximately six-fold following the second immunization study (Figure 5D).
Vaccine-mediated alteration of heroin pharmacology in NHPs
Given the strong presence of 6AM-neutralizing antibodies in vaccinated rhesus monkeys, further experiments were conducted to evaluate the capacity of the antibodies to alter the pharmacodynamics (PD) and pharmacokinetics (PK) of heroin. MOR agonists consistently produce dose-dependent decreases in operant responding that appear to be mediated by pharmacologically similar populations of MORs. These MORs mediate other mu-opioid agonist behavioral effects, such as antinociceptive and discriminative stimulus effects.46–47 Moreover, drug ED50 values can be quantified from operant responding to serve as a potency metric for MOR modulators such as the antagonist NTX;48–50 therefore, an assay of schedule-controlled responding (SCR) was selected as a reliable behavioral measure of opioid pharmacology upon which to examine the ‘immunoantagonist’ vaccine.
Following the double-determination of baseline heroin and oxycodone potencies to decrease rates of responding (represented by ED50 values), monkeys were vaccinated and retested six weeks later, resulting in a clear 3.5-fold heroin ED50 shift (Figure 6A). A greater potency ratio was detected 2–3 weeks following week 11 (4.3-fold) and 18 (4.1-fold) booster injections (Figure 6A, S5C). The heroin ED50 values at these times (weeks 14 and 21) were similar to heroin ED50 values obtained following acute NTX pretreatment (Figure 6B). Not only were heroin potency ratios significantly increased versus baseline levels, they were also selectively elevated compared to the control opioid, oxycodone, throughout the entire study (Figure 6A, S6). A correlation between monkey anti-heroin titers and heroin SCR ED50 values revealed a linear relationship (Figure 6C). Notably, the previously vaccinated monkeys M1 and M2 showed higher titers and ED50s compared to monkeys M3 and M4 in the SCR procedure (Figure S5C); however, normalization of all post-vaccination data to the prevaccine baseline revealed a relatively uniform anti-heroin behavioral effect among all four monkeys (Figure 6A).
Figure 6. Heroin vaccine diminishes heroin potency and alters 6AM pharmacokinetics in rhesus monkeys.
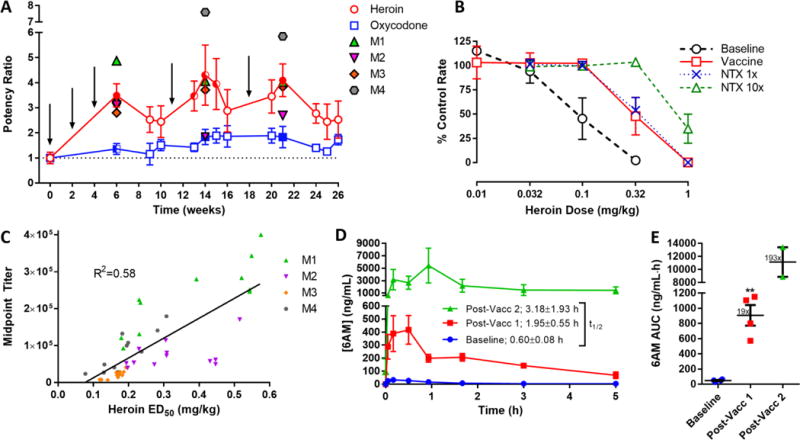
(A) Timeline of changes in either heroin or oxycodone ED50s in monkeys after heroin vaccine administration. Monkeys (M1–4) were vaccinated i.m. at the indicated time points (arrows) with 400 μg HerCOOH-TT, 600 μg CpG ODN 2006 and 5 mg alum. Baseline heroin and oxycodone ED50 values were double-determined in each monkey prior to vaccination in the assay of schedule-controlled responding (SCR). Each point in the figure represents the average ratio of heroin (red) or oxy (blue) ED50 ± SEM relative to the baseline value. Group mean ± SEM baseline ED50 values for heroin and oxycodone were 0.08 ± 0.03 and 0.19 ± 0.06 mg/kg, respectively. RM one-way ANOVA of heroin and oxycodone SCR over time; F12,36 = 2.76 and 1.07, P = 0.0092 and 0.414, respectively; half-filled (P < 0.05) and fully-filled (P < 0.01) circles indicate significance by Dunnett’s post-hoc test vs. heroin baseline. RM two-way ANOVA of heroin versus oxycodone SCR over time; F1,3 = 13.2, P = 0.0358; half-filled (P < 0.05) and fully-filled (P < 0.01) squares indicate significance by Bonferroni’s post-hoc test versus heroin. (B) SCR cumulative heroin dose-effect curves at week 0 (baseline) and 14 (vaccine) in comparison to naltrexone (NTX) treatment at 3.2 μg/kg (1×) and 32 μg/kg (10×). Points represent mean ± SEM for n = 4 monkeys. (C) Correlation between titers against HerCOOH-BSA and SCR heroin ED50 values collected over 26 weeks. P < 0.0001 by Pearson’s correlation. (D) 6AM serum concentrations over time in n = 4 rhesus macaques (M1–4) following 0.32 mg/kg i.m. heroin. The PK study was performed in the same subjects before and after a course of three initial immunizations. F 1,6 = 20.35, P = 0.0041 by a RM two-way ANOVA comparing pre and post vaccination PK (post-vacc 1). After 7 months, monkeys M1 and 2 received three additional immunizations and the PK study was repeated (post-vacc 2). (E) 6AM AUC values corresponding to the PK study. Fold increases versus baseline are reported as mean ± SEM. **P < 0.01 by paired t-test. Cmax values (ng/mL): baseline = 32.5 ± 6.8, post-vacc 1 = 418 ± 110, post-vacc 2 = 5430 ± 2800.
To support the vaccine-mediated effects observed in the SCR assay, a heroin PK study was performed in the four monkeys both before and after vaccination (Figure 6D). Results indicate that anti-drug antibodies bound large amounts of 6AM in the blood causing significant increases in PK metrics such as half-life and especially AUC (19-fold greater) compared to baseline measurements (Figure 6D,E); interestingly, when M1,2 were tested after revaccination, a much larger AUC shift (193-fold) was observed. The Cmax values were similarly shifted by 13- and 167-fold for the first and second vaccination studies, respectively. It should also be noted that no gross undesirable effects to repeated vaccine administration were observed throughout the entire monkey study (Figure S7).
Discussion
Achievement of heroin vaccine effectiveness required mastery of a number of unique challenges. First, the desired immune response from the heroin vaccine is distinct compared to vaccines being developed against pathogens because drug vaccines have a greater requirement for strong humoral immunity; a larger molar quantity of drug relative to pathogens must be neutralized by IgGs for the drug vaccine to achieve efficacy. On the other hand, while cell-mediated immunity is necessary for subduing disease-causing pathogens, it would be counterproductive in the development of immunoantagonists for the treatment of substance use disorders. Second, utilizing TT as a carrier protein at the clinical level could readily be questioned if pre-existing immunity to TT diminished the immune response to the drug-carrier conjugate. Fortunately, pre-existing antibody titers to TT did not suppress heroin vaccine efficacy. Third, as heroin is a prodrug for 6AM/morphine, hapten design was tailored to direct antibody binding toward 6AM followed by heroin but not morphine. Despite being a psychoactive metabolite of heroin, morphine penetrates the blood-brain barrier much less readily, hence, antibody sequestration of heroin/6AM until enzymatic hydrolysis ensues is key to vaccine performance.51 Fourth, while titer is typically the metric used in gauging vaccine success, antibody affinity is also critical. The low nanomolar antibody affinity for 6AM, as observed by SPR, provides a competitive sink with the brain MORs for drug binding based on Le Chatelier’s principle, thus causing heroin potency reduction in behavioral assays.
Heroin hapten design was greatly improved through conversion of the terminal thiol (HerSH) to a carboxylic acid (HerCOOH). This modification enabled more efficient and reliable protein coupling, producing a more efficacious immunoconjugate with higher epitope density. Furthermore, the HerCOOH hapten contains a shorter, more ‘immunologically silent’ linker with less chemical functionalities relative to HerSH and HerdBA. Following antigen processing, the HerCOOH hapten linker likely interferes minimally with immune presentation of a heroin-like epitope. Previous studies have shown that peptidic linkers can increase anti-hapten immune responses,52–54 possibly by enhancing hapten anchoring to the MHCII55 (Figure 2). However, the dBA linker was not effective, which may be explained by the fact that beta-alanine is not naturally found in proteins, thus hampering immune processing and presentation of HerdBA. Because it was not clear whether heroin or 6AM should be emulated for hapten design, as heroin and 6AM are equipotent,4–6 a direct comparison was made between the corresponding haptens (HerCOOH and 6AMCOOH). From an efficacy standpoint, both 6AMCOOH- and HerCOOH-TT conjugates behaved similarly, implying that the heroin hapten likely hydrolyzes in vivo almost completely to the 6AM hapten. This result is not unexpected given the known high lability of the 3-acetyl group and is further supported by the fact that antibodies show drug affinity in the following order: 6AM>heroin>>morphine. Moreover, the probable 3-acetyl hydrolysis of our hapten corroborates the dynamic nature of the vaccine as we had previously posited,36–37 although the 6-acetyl group likely remains mostly intact given the low antiserum affinity for morphine. Finally, while several heroin vaccines containing hydrolytically stable haptens are currently in preclinical development, they have yet to demonstrate blockade of heroin effects beyond a 1 mg/kg dose in rodents and have not been tested in NHPs.51, 56–59
In exploring dosing and adjuvants for the HerCOOH-TT conjugate, a positive correlation was discovered between vaccine efficacy and the amount of immunoconjugate and CpG ODN adjuvant. Although a plateau may exist in the dose-responsive effect of the immunoconjugate and CpG ODN, no such plateau was observed at the doses tested in mice. The implication of this finding is that the vaccine dosage can be increased to achieve a higher degree of efficacy. In fact, conjugate doses as high as 2000 μg were safely used in a clinical cocaine vaccine study.34 By comparison, a relatively low conjugate dose (330–400 μg) was used in our NHP studies. In terms of the primate-compatible TLR9-agonist (CpG ODN 2006), a dose of 400–600 μg was used in our studies similar to a nicotine vaccine study,60 while doses of up to 2 mg have been safely used.61 Promisingly, CpG ODN 2006 has shown success in human vaccine trials (phase I and II) with a wide variety of antigens.62–66 In contrast, we found that cyclic dinucleotide cGAMP only presented mild activity as a vaccine adjuvant at the doses tested, despite sharing structural and mechanistic similarities with CpG ODN – both are DNA-based and act as pathogen-associated molecular patterns (PAMPs) to stimulate the innate immune system. Likely, the strong activation of B-cells via TLR9 to produce an anti-drug humoral response contributes to the success of CpG ODN 1826 and 2006 as adjuvants.67–68 These effects were synergistic with alum-mediated induction of humoral immunity, as shown previously.35, 60, 69–70 As only one dosage of CpG ODN and alum was investigated in the current study, fine tuning of CpG ODN and alum dosing in future monkeys studies would be anticipated to further enhance vaccine efficacy.
Once a heroin vaccine formulation was identified that was efficacious in generating long-term, antibody-mediated protection from heroin PD, studies in rhesus macaques were pursued to evaluate the clinical potential of the vaccine. One major hurdle for drug vaccines in human trials is achieving efficacy in all patients. Although clinical trials for nicotine and cocaine vaccines have failed to demonstrate significant efficacy, they did demonstrate a proof-of-concept that patients who generated a strong anti-drug antibody response displayed protection from the abuse-related drug effects.28–31 In order to improve drug vaccine performance, preclinical testing must employ clinically relevant benchmarks focusing directly on vaccine capacity to selectively alter the behavioral pharmacology of the target drug. Our studies in an outbred strain of mice (Swiss Webster) demonstrated that the vaccine reliably induced anti-drug titers and significantly shifted the heroin dose-effect curves in the antinociception assay. In fact, the only detectable source of immune variability was the degree of hapten conjugation; heroin conjugates with greater haptenation produced greater efficacy upon immunization, which has been observed previously.56, 71
The optimized vaccine formulation identified from the mouse studies translated well to rhesus monkeys, albeit with smaller heroin potency shifts, possibly due to immunological differences between species or vaccine dosages. Moreover, the large initial spike in vaccine response that was only observed in mice can be explained by the differing vaccine injection routes, i.p. in mice vs. i.m. in monkeys, and in the former case, the vaccine can rapidly drain to the spleen to induce a strong, short-lived B-cell response. Regardless, significant anti-heroin IgG titers with low nanomolar 6AM affinity were observed in all four monkeys. In addition, the humoral immune response to the vaccine significantly altered heroin PK, increasing 6AM AUC values by ~19-fold and half-life by ~3-fold, resulting in the observed reduction in heroin PD. The PK results are in agreement with previous studies, which have noted that conjugate-vaccinated animals showed higher drug concentrations in serum due to antibody sequestration leading to lower drug concentrations in the brain relative to controls – a phenomenon observed for opioids45, 52, 72–73 and other drugs such as methamphetamine, cocaine and nicotine.71, 74–77 In a manner that has not been fully studied, the drug-specific antibodies appear to increase drug half-life and diminish drug clearance. This is likely caused by antibody-drug binding, which hampers glomerular filtration of drugs by the kidneys and shields drugs from metabolic destruction. Eventual drug metabolism and clearance does not appear to be mediated by immunological mechanisms but rather by slow dissociation of antibody-drug complexes.72
As a testament to vaccine durability, the two monkeys M1,2 that participated in a second study ~7 months later showed much greater antibody titer, affinity and 6AM AUC, indicating that vaccination in the second study strongly recalled memory immunity initially established during the first study. In considering dosing schedule, the data suggest that at least three vaccinations spaced 6 weeks apart or greater is required for achieving significant immunity against heroin; however, as observed in monkeys M1,2, vaccination on an even longer time scale may be optimal. Importantly, no evidence of immune tolerance to the vaccine was seen at any point despite multiple immunization rounds and frequent administration of heroin as a result of SCR testing. The immunochemical and PK data support the heroin SCR data, the latter of which revealed an approximately 4-fold shift in the heroin dose-effect curve following each immunization. While the decrease in heroin potency was observed via the i.m. route of administration, these results are unlikely to be significantly different than the i.v. route commonly used by humans. The relative ratios of heroin, 6AM, and morphine after i.m. administration were consistent with the PK profile observed following i.v. heroin administration in humans.78 Furthermore, our first generation vaccine was effective in attenuating i.v. heroin self-administration in rats.37 Specifically, the vaccine could block heroin reinstatement following a single bolus of 0.18 mg/kg i.v. heroin, which would translate to a 14.4 mg dose in an 80 kg adult. This dose is within the dose range reported by heroin users (see Erowid.org). As our second generation vaccine has been shown to be at least three-fold better than the first generation vaccine in the mouse antinociception model, we posit the second generation vaccine’s capacity to neutralize i.v. heroin would only be enhanced. Consolidation of our data strongly suggests that the vaccine acts as a heroin immunoantagonist; furthermore, the vaccine effects paralleled the effects of the FDA-approved opioid antagonist NTX. In previous rhesus monkey studies, NTX antagonized the rate suppressant, antinociceptive and discriminative stimulus effects of heroin.46, 79–80 Similarly, reductions in heroin vs. food choice have been demonstrated by administration of another opioid antagonist naloxone in non-opioid dependent monkeys81 and NTX pretreatment in non-opioid dependent rats.82 These results provide empirical evidence that the vaccine-mediated antagonism of heroin in the SCR assay would be predictive of antagonism of heroin effects in other models such as drug discrimination and self-administration. Given the noteworthy vaccine results in the SCR procedure, future studies to determine vaccine effects in more complex NHP behavioral procedures, e.g. i.v. self-administration, are warranted.
Other studies in NHPs have demonstrated the efficacy of optimized nicotine and cocaine vaccines. The second generation nicotine conjugate (NIC7-CRM) was optimized in mice83 and translated well to cynomolgus monkeys in a CpG ODN + alum formulation to elicit sustained (~106) anti-nicotine titers.84 Following a nicotine challenge in vaccinated monkeys, blood nicotine Cmax and AUC increased by 29- and 89-fold, respectively,84 and nicotine brain concentrations were reduced.71 A fully synthetic nicotine vaccine (SEL-068) also translated from mice to monkeys85 and has been shown to attenuate nicotine discrimination in squirrel monkeys while reducing nicotine potency by 3-fold.86 The GNE cocaine hapten conjugated to a disrupted adenovirus (dAd5) and formulated with a proprietary adjuvant (Adjuplex) has consistently generated high anti-cocaine titers (105–106) in a number of rhesus macaque studies.87–90 This vaccine increased cocaine Cmax in serum by ~3.5-fold, mitigated biodistribution of cocaine to the brain and other organs,88–89 and attenuated cocaine self-administration and reacquisition.90 Although these reports provide some context for our NHP heroin vaccine studies, direct comparisons between our studies and others are limited. In all cases, anti-hapten titer levels responded similarly to the vaccines; however, our studies use midpoint titers, which are intrinsically 10–100 fold lower than the endpoint titers used in other studies. Furthermore, hapten-specific titer levels do not necessarily reflect affinity to the actual target drug, and thus are not always indicative of efficacy, although a robust correlation between titer and efficacy was observed for the disclosed heroin vaccine. While previous NHP vaccine studies have not fully investigated drug affinity of monkey antiserum, we have observed 0.5–6 nM affinity to 6AM. Comparatively, dAd5GNE in NHPs was shown to produce 5–120 nM antibody affinity to cocaine.87 In considering behavioral testing, few studies have compared drug ED50s in vaccinated and non-vaccinated animals whereas we have demonstrated heroin dose-effect curve shifting in both mouse and monkey models. For reference, the SEL-068 vaccine reduced nicotine potency by 3-fold in monkeys while we saw a 4-fold reduction in heroin potency. However, results in the more complex self-administration procedures used in a cocaine vaccine study90 and the first heroin vaccine study,24 cannot be related to the ED50 determinations in our study. On the other hand, PK metrics in vaccinated monkeys can be compared with the caveat that drug PK/PD between each of the drugs differ drastically. Vaccine-mediated fold-increases in heroin Cmax for HerCOOH-TT (post-vacc 1) appeared to be half that of the nicotine Cmax for NIC7-CRM84 but four-fold greater than cocaine Cmax for dAd5GNE;89 therefore, our vaccine possesses excellent serum neutralizing capacity for heroin and its psychoactive metabolites relative to other vaccines.
In response to the opioid epidemic that has plagued the United States, the disclosed heroin vaccine could satisfy a dire, unmet need for an opioid use disorder therapeutic. Since the vaccine reduces heroin potency, vaccinated drug users would encounter an increased cost to “getting high”, potentially similar to the effects of clinically available and FDA-approved depot NTX formulations.91–92 Indeed, our previous studies have shown that following a period of drug abstinence, drug-dependent, immunized rats actually extinguish self-administration of heroin,37 and these rats demonstrated 4-fold shifted heroin dose-effect curves in antinociceptive testing. Considering this study and the similar curve shifts observed in monkeys, our vaccine has the potential to be clinically useful; it may serve as a ‘safety catch’ for preventing relapse episodes in former heroin users attempting to maintain drug abstinence, or it may help current heroin abusers to achieve abstinence. This hypothesis is supported by clinical studies showing that long-acting depot NTX mitigated the reinforcing effects of heroin in humans,92–93 and instead of heroin users increasing their drug intake to surmount the antagonist, heroin extinction-like behaviors occurred.91, 94 The obvious major benefits to vaccination over pharmacological antagonists are the potential for increased duration of action and decreased side effects. Co-administration of opioid agonists methadone or buprenorphine as needed along with the vaccine would likely enhance therapeutic efficacy by alleviating opioid cravings. Such combination therapy would be possible due to the vaccine’s selective sequestration of 6AM by >1000-fold over other opioids; moreover, prescription pain medication e.g. oxycodone also would not interact with the heroin vaccine.
In conclusion, an efficacious heroin vaccine has been identified through optimization of the adjuvant (CpG ODN + alum), carrier protein (TT) and hapten (HerCOOH). The vaccine is efficacious in basic pre-clinical mouse and NHP models over a wide range of heroin doses, accomplishing an important milestone in the drug development process to human clinical trials. Forty years after the first report of a heroin/morphine vaccine,24 numerous studies and research groups have advanced the concept of anti-drug vaccines to a functional level, whereby the vaccines act as a long-term ‘immunoantagonist’ to attenuate drug PD. While we have speculated as to how our particular heroin vaccine could be used to treat use disorder, future studies involving more advanced NHP models and clinical trials must be performed to elucidate the true therapeutic utility of this vaccine as well as other opioid vaccines.
Supplementary Material
Acknowledgments
This work was supported by the NIH under grants UH2DA041146, R01DA026625, K99DA037344 and F31DA037709 and the Skaggs Institute for Chemical Biology. This is manuscript # 29467 from The Scripps Research Institute.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/jacs.7b03334.
Materials and methods, conjugate mass data, titer data, additional mouse and monkey vaccine data: Tables S1–2, Figures S1–10.
ORCID
Paul T. Bremer: 0000-0002-8357-1743
Joel E. Schlosburg: 0000-0003-0659-7760
Matthew L. Banks: 0000-0003-4949-5246
Kim D. Janda: 0000-0001-6759-4227
Notes
The authors declare no competing financial interest
References
- 1.Oldendorf WH, Hyman S, Braun L, Oldendorf SZ. Science. 1972;178:984–6. doi: 10.1126/science.178.4064.984. [DOI] [PubMed] [Google Scholar]
- 2.Maurer HH, Sauer C, Theobald DS. Ther Drug Monit. 2006;28:447–53. doi: 10.1097/01.ftd.0000211812.27558.6e. [DOI] [PubMed] [Google Scholar]
- 3.Wright CI. J Pharmacol Exp Ther. 1941;71:164–177. [Google Scholar]
- 4.Andersen JM, Ripel A, Boix F, Normann PT, Morland J. J Pharmacol Exp Ther. 2009;331:153–61. doi: 10.1124/jpet.109.152462. [DOI] [PubMed] [Google Scholar]
- 5.Selley DE, Cao CC, Sexton T, Schwegel JA, Martin TJ, Childers SR. Biochem Pharmacol. 2001;62:447–55. doi: 10.1016/s0006-2952(01)00689-x. [DOI] [PubMed] [Google Scholar]
- 6.Brownstein MJ. Proc Natl Acad Sci U S A. 1993;90:5391–3. doi: 10.1073/pnas.90.12.5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenblum A, Parrino M, Schnoll SH, Fong C, Maxwell C, Cleland CM, Magura S, Haddox JD. Drug Alcohol Depend. 2007;90:64–71. doi: 10.1016/j.drugalcdep.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 8.Jones CM. Drug Alcohol Depend. 2013;132:95–100. doi: 10.1016/j.drugalcdep.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 9.Substance Abuse and Mental Health Services Administration. Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings. Substance Abuse and Mental Health Services Administration; Rockville, MD: Sep, 2013. (NSDUH Series H-46, HHS Publication No (SMA) 13-4795). [Google Scholar]
- 10.Center for Behavioral Health Statistics and Quality. 2015 National Survey on Drug Use and Health: Detailed Tables. Substance Abuse and Mental Health Services Administration; Rockville, MD: Sep, 2016. [Google Scholar]
- 11.Kilmer B, Everingham S, Caulkins J, Midgette G, Pacula R, Reuter P, Burns R, Han B, Lundberg R. What America’s Users Spend on Illegal Drugs: 2000–2010. RAND Corporation; Santa Monica, CA: Feb, 2014. [Google Scholar]
- 12.Mark TL, Woody GE, Juday T, Kleber HD. Drug Alcohol Depend. 2001;61:195–206. doi: 10.1016/s0376-8716(00)00162-9. [DOI] [PubMed] [Google Scholar]
- 13.National Institute on Drug Abuse. Principles of Drug Addiction and Treatment. National Institute on Drug Abuse, National Institutes of Health, U.S. Department of Health and Human Services; Bethesda, MD: Dec, 2012. (NIH Publication No 12-4180). [Google Scholar]
- 14.Di Martino V, Rufat P, Boyer N, Renard P, Degos F, Martinot-Peignoux M, Matheron S, Le Moing V, Vachon F, Degott C, Valla D, Marcellin P. Hepatology. 2001;34:1193–9. doi: 10.1053/jhep.2001.29201. [DOI] [PubMed] [Google Scholar]
- 15.Warner M, Trinidad JP, Bastian BA, Minino AM, Hedegaard H. National Vital Statistics Report. 2016;65:1–15. [PubMed] [Google Scholar]
- 16.Kaur AD, McQueen A, Jan S. J Manag Care Pharm. 2008;14:186–194. doi: 10.18553/jmcp.2008.14.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manchikanti L, Atluri S, Trescot AM, Giordano J. Pain Physician. 2008;11:S155–80. [PubMed] [Google Scholar]
- 18.Maxwell S, Bigg D, Stanczykiewicz K, Carlberg-Racich S. J Addict Dis. 2006;25:89–96. doi: 10.1300/J069v25n03_11. [DOI] [PubMed] [Google Scholar]
- 19.Sporer KA, Kral AH. Ann Emerg Med. 2007;49:172–7. doi: 10.1016/j.annemergmed.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 20.Anton B, Salazar A, Flores A, Matus M. Hum Vaccines. 2009;5:214–29. doi: 10.4161/hv.5.4.7556. [DOI] [PubMed] [Google Scholar]
- 21.Benyamin R, Trescot AM, Datta S, Buenaventura R, Adlaka R, Sehgal N, Glaser SE, Vallejo R. Pain Physician. 2008;11:S105–20. [PubMed] [Google Scholar]
- 22.Gossop M, Stewart D, Browne N, Marsden J. Addiction. 2002;97:1259–67. doi: 10.1046/j.1360-0443.2002.00227.x. [DOI] [PubMed] [Google Scholar]
- 23.Gossop M, Green L, Phillips G, Bradley B. Br J Psychiatry. 1989;154:348–353. doi: 10.1192/bjp.154.3.348. [DOI] [PubMed] [Google Scholar]
- 24.Bonese KF, Wainer BH, Fitch FW, Rothberg RM, Schuster CR. Nature. 1974;252:708–710. doi: 10.1038/252708a0. [DOI] [PubMed] [Google Scholar]
- 25.Martin WR, Jasinski DR, Mansky PA. Arch Gen Psychiatry. 1973;28:784–91. doi: 10.1001/archpsyc.1973.01750360022003. [DOI] [PubMed] [Google Scholar]
- 26.Carrera MR, Ashley JA, Parsons LH, Wirsching P, Koob GF, Janda KD. Nature. 1995;378:727–30. doi: 10.1038/378727a0. [DOI] [PubMed] [Google Scholar]
- 27.Hieda Y, Keyler DE, Vandevoort JT, Kane JK, Ross CA, Raphael DE, Niedbalas RS, Pentel PR. J Pharmacol Exp Ther. 1997;283:1076–81. [PubMed] [Google Scholar]
- 28.Kosten TR, Domingo CB, Shorter D, Orson F, Green C, Somoza E, Sekerka R, Levin FR, Mariani JJ, Stitzer M, Tompkins DA, Rotrosen J, Thakkar V, Smoak B, Kampman K. Drug Alcohol Depend. 2014;140:42–7. doi: 10.1016/j.drugalcdep.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cornuz J, Zwahlen S, Jungi WF, Osterwalder J, Klingler K, van Melle G, Bangala Y, Guessous I, Muller P, Willers J, Maurer P, Bachmann MF, Cerny T. PLoS One. 2008;3:e2547. doi: 10.1371/journal.pone.0002547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martell BA, Orson FM, Poling J, Mitchell E, Rossen RD, Gardner T, Kosten TR. Arch Gen Psychiatry. 2009;66:1116–23. doi: 10.1001/archgenpsychiatry.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoogsteder PHJ, Kotz D, van Spiegel PI, Viechtbauer W, van Schayck OCP. Addiction. 2014;109:1252–1259. doi: 10.1111/add.12573. [DOI] [PubMed] [Google Scholar]
- 32.Lockner JW, Lively JM, Collins KC, Vendruscolo JC, Azar MR, Janda KD. J Med Chem. 2015;58:1005–11. doi: 10.1021/jm501625j. [DOI] [PubMed] [Google Scholar]
- 33.Kosten TR, Rosen M, Bond J, Settles M, Roberts JS, Shields J, Jack L, Fox B. Vaccine. 2002;20:1196–204. doi: 10.1016/s0264-410x(01)00425-x. [DOI] [PubMed] [Google Scholar]
- 34.Martell BA, Mitchell E, Poling J, Gonsai K, Kosten TR. Biol Psychiatry. 2005;58:158–64. doi: 10.1016/j.biopsych.2005.04.032. [DOI] [PubMed] [Google Scholar]
- 35.Bremer PT, Schlosburg JE, Lively JM, Janda KD. Mol Pharm. 2014;11:1075–1080. doi: 10.1021/mp400631w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stowe GN, Vendruscolo LF, Edwards S, Schlosburg JE, Misra KK, Schulteis G, Mayorov AV, Zakhari JS, Koob GF, Janda KD. J Med Chem. 2011;54:5195–204. doi: 10.1021/jm200461m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schlosburg JE, Vendruscolo LF, Bremer PT, Lockner JW, Wade CL, Nunes AA, Stowe GN, Edwards S, Janda KD, Koob GF. Proc Natl Acad Sci U S A. 2013;110:9036–41. doi: 10.1073/pnas.1219159110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olofson RA, Martz JT, Senet JP, Piteau M, Malfroot T. J Org Chem. 1984;49:2081–2082. [Google Scholar]
- 39.Li XD, Wu J, Gao D, Wang H, Sun L, Chen ZJ. Science. 2013;341:1390–4. doi: 10.1126/science.1244040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Collins KC, Janda KD. Bioconjugate Chem. 2014;25:593–600. doi: 10.1021/bc500016k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matyas GR, Rice KC, Cheng K, Li F, Antoline JF, Iyer MR, Jacobson AE, Mayorov AV, Beck Z, Torres OB, Alving CR. Vaccine. 2014;32:1473–9. doi: 10.1016/j.vaccine.2014.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bremer PT, Janda KD. J Med Chem. 2012;55:10776–80. doi: 10.1021/jm301262z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schutze MP, Leclerc C, Jolivet M, Audibert F, Chedid L. J Immunol. 1985;135:2319–22. [PubMed] [Google Scholar]
- 44.Herzenberg LA, Tokuhisa T, Herzenberg LA. Nature. 1980;285:664–667. doi: 10.1038/285664a0. [DOI] [PubMed] [Google Scholar]
- 45.Bremer PT, Kimishima A, Schlosburg JE, Zhou B, Collins KC, Janda KD. Angew Chem Int Edit. 2016;55:3772–3775. doi: 10.1002/anie.201511654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bowen CA, Fischer BD, Mello NK, Negus SS. J Pharmacol Exp Ther. 2002;302:264–273. doi: 10.1124/jpet.302.1.264. [DOI] [PubMed] [Google Scholar]
- 47.Negus SS, Brandt MR, Gatch MB, Mello NK. Drug Alcohol Depend. 2003;70:17–27. doi: 10.1016/s0376-8716(02)00331-9. [DOI] [PubMed] [Google Scholar]
- 48.Negus SS, Burke TF, Medzihradsky F, Woods JH. J Pharmacol Exp Ther. 1993;267:896–903. [PubMed] [Google Scholar]
- 49.Banks ML, Folk JE, Rice KC, Negus SS. Pharmacol Biochem Behav. 2010;97:205–212. doi: 10.1016/j.pbb.2010.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Banks ML, Rice KC, Negus SS. J Pharmacol Exp Ther. 2010;335:497–505. doi: 10.1124/jpet.110.169276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raleigh MD, Pravetoni M, Harris AC, Birnbaum AK, Pentel PR. J Pharmacol Exp Ther. 2013;344:397–406. doi: 10.1124/jpet.112.201194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pravetoni M, Le Naour M, Harmon TM, Tucker AM, Portoghese PS, Pentel PR. J Pharmacol Exp Ther. 2012;341:225–32. doi: 10.1124/jpet.111.189506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gooyit M, Miranda PO, Wenthur CJ, Ducime A, Janda KD. ACS Chem Neurosci. 2017;8:468–472. doi: 10.1021/acschemneuro.6b00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Collins KC, Janda KD. Bioconjugate Chem. 2014;25:593–600. doi: 10.1021/bc500016k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Avci FY, Li XM, Tsuji M, Kasper DL. Nat Med. 2011;17:1602–U115. doi: 10.1038/nm.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jalah R, Torres OB, Mayorov AV, Li FY, Antoline JFG, Jacobson AE, Rice KC, Deschamps JR, Beck Z, Alving CR, Matyas GR. Bioconjugate Chem. 2015;26:1041–1053. doi: 10.1021/acs.bioconjchem.5b00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matyas GR, Rice KC, Cheng K, Li F, Antoline JFG, Iyer MR, Jacobson AE, Mayorov AV, Beck Z, Torres OB, Alving CR. Vaccine. 2014;32:1473–1479. doi: 10.1016/j.vaccine.2014.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bremer PT, Janda KD. J Med Chem. 2012;55:10776–10780. doi: 10.1021/jm301262z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Torres OB, Matyas GR, Rao M, Peachman KK, Jalah R, Beck Z, Michael NL, Rice KC, Jacobson AE, Alving CR. NPJ Vaccines. 2017;2:13. doi: 10.1038/s41541-017-0013-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McCluskie MJ, Pryde DC, Gervais DP, Stead DR, Zhang N, Benoit M, Robertson K, Kim IJ, Tharmanathan T, Merson JR, Davis HL. Int Immunopharmacol. 2013;16:50–6. doi: 10.1016/j.intimp.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 61.Wille-Reece U, Flynn BJ, Lore K, Koup RA, Miles AP, Saul A, Kedl RM, Mattapallil JJ, Weiss WR, Roederer M, Seder RA. J Exp Med. 2006;203:1249–58. doi: 10.1084/jem.20052433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cooper CL, Davis HL, Morris ML, Efler SM, Adhami MA, Krieg AM, Cameron DW, Heathcote J. J Clin Immunol. 2004;24:693–701. doi: 10.1007/s10875-004-6244-3. [DOI] [PubMed] [Google Scholar]
- 63.Mullen GE, Ellis RD, Miura K, Malkin E, Nolan C, Hay M, Fay MP, Saul A, Zhu D, Rausch K, Moretz S, Zhou H, Long CA, Miller LH, Treanor J. PLoS One. 2008;3:e2940. doi: 10.1371/journal.pone.0002940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sogaard OS, Lohse N, Harboe ZB, Offersen R, Bukh AR, Davis HL, Schonheyder HC, Ostergaard L. Clin Infect Dis. 2010;51:42–50. doi: 10.1086/653112. [DOI] [PubMed] [Google Scholar]
- 65.Valmori D, Souleimanian NE, Tosello V, Bhardwaj N, Adams S, O’Neill D, Pavlick A, Escalon JB, Cruz CM, Angiulli A, Angiulli F, Mears G, Vogel SM, Pan L, Jungbluth AA, Hoffmann EW, Venhaus R, Ritter G, Old LJ, Ayyoub M. Proc Natl Acad Sci U S A. 2007;104:8947–52. doi: 10.1073/pnas.0703395104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brody JD, Ai WZ, Czerwinski DK, Torchia JA, Levy M, Advani RH, Kim YH, Hoppe RT, Knox SJ, Shin LK, Wapnir I, Tibshirani RJ, Levy R. J Clin Oncol. 2010;28:4324–32. doi: 10.1200/JCO.2010.28.9793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Verthelyi D, Ishii KJ, Gursel M, Takeshita F, Klinman DM. J Immunol. 2001;166:2372–7. doi: 10.4049/jimmunol.166.4.2372. [DOI] [PubMed] [Google Scholar]
- 68.Hartmann G, Krieg AM. J Immunol. 2000;164:944–53. doi: 10.4049/jimmunol.164.2.944. [DOI] [PubMed] [Google Scholar]
- 69.Davis HL, Weeratna R, Waldschmidt TJ, Tygrett L, Schorr J, Krieg AM. J Immunol. 1998;160:870–6. [PubMed] [Google Scholar]
- 70.Chen X, Pravetoni M, Bhayana B, Pentel PR, Wu MX. Vaccine. 2012;31:159–64. doi: 10.1016/j.vaccine.2012.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McCluskie MJ, Thorn J, Mehelic PR, Kolhe P, Bhattacharya K, Finneman JI, Stead DR, Piatchek MB, Zhang NL, Chikh G, Cartier J, Evans DM, Merson JR, Davis HL. Int Immunopharmacol. 2015;25:518–527. doi: 10.1016/j.intimp.2015.02.030. [DOI] [PubMed] [Google Scholar]
- 72.Hill JH, Wainer BH, Fitch FW, Rothberg RM. J Immunol. 1975;114:1363–8. [PubMed] [Google Scholar]
- 73.Kimishima A, Wenthur CJ, Zhou B, Janda KD. ACS Chem Biol. 2017;12:36–40. doi: 10.1021/acschembio.6b00977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Miller ML, Moreno AY, Aarde SM, Creehan KM, Vandewater SA, Vaillancourt BD, Wright MJ, Jr, Janda KD, Taffe MA. Biol Psychiatry. 2013;73:721–8. doi: 10.1016/j.biopsych.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fox BS, Kantak KM, Edwards MA, Black KM, Bollinger BK, Botka AJ, French TL, Thompson TL, Schad VC, Greenstein JL, Gefter ML, Exley MA, Swain PA, Briner TJ. Nat Med. 1996;2:1129–1132. doi: 10.1038/nm1096-1129. [DOI] [PubMed] [Google Scholar]
- 76.Hieda Y, Keyler DE, VandeVoort JT, Kane JK, Ross CA, Raphael DE, Niedbalas RS, Pentel PR. J Pharmacol Exp Ther. 1997;283:1076–1081. [PubMed] [Google Scholar]
- 77.Byrnes-Blake KA, Laurenzana EM, Carroll FI, Abraham P, Gentry WB, Landes RD, Owens SM. Eur J Pharmacol. 2003;461:119–28. doi: 10.1016/s0014-2999(03)01313-x. [DOI] [PubMed] [Google Scholar]
- 78.Rook EJ, Huitema AD, van den Brink W, van Ree JM, Beijnen JH. Curr Clin Pharmacol. 2006;1:109–18. doi: 10.2174/157488406775268219. [DOI] [PubMed] [Google Scholar]
- 79.Negus SS, Brandt MR, Gatch MB, Mello NK. Drug Alcohol Depend. 2003;70:17–27. doi: 10.1016/s0376-8716(02)00331-9. [DOI] [PubMed] [Google Scholar]
- 80.Rowlett JK, Wilcox KM, Woolverton WL. J Pharmacol Exp Ther. 1998;286:61–9. [PubMed] [Google Scholar]
- 81.Negus SS. J Pharmacol Exp Ther. 2006;317:711–723. doi: 10.1124/jpet.105.095380. [DOI] [PubMed] [Google Scholar]
- 82.Ettenberg A, Pettit HO, Bloom FE, Koob GF. Psychopharmacology. 1982;78:204–9. doi: 10.1007/BF00428151. [DOI] [PubMed] [Google Scholar]
- 83.Pryde DC, Jones LH, Gervais DP, Stead DR, Blakemore DC, Selby MD, Brown AD, Coe JW, Badland M, Beal DM, Glen R, Wharton Y, Miller GJ, White P, Zhang N, Benoit M, Robertson K, Merson JR, Davis HL, McCluskie MJ. PLoS One. 2013;8:e76557. doi: 10.1371/journal.pone.0076557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McCluskie MJ, Thorn J, Gervais DP, Stead DR, Zhang NL, Benoit M, Cartier J, Kim IJ, Bhattacharya K, Finneman JI, Merson JR, Davis HL. Int Immunopharmacol. 2015;29:663–671. doi: 10.1016/j.intimp.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 85.Fraser CC, Altreuter DH, Ilyinskii P, Pittet L, LaMothe RA, Keegan M, Johnston L, Kishimoto TK. Vaccine. 2014;32:2896–903. doi: 10.1016/j.vaccine.2014.02.024. [DOI] [PubMed] [Google Scholar]
- 86.Desai RI, Bergman J. Neuropsychopharmacology. 2015;40:2207–2216. doi: 10.1038/npp.2015.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wee S, Hicks MJ, De BP, Rosenberg JB, Moreno AY, Kaminsky SM, Janda KD, Crystal RG, Koob GF. Neuropsychopharmacology. 2012;37:1083–1091. doi: 10.1038/npp.2011.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Maoz A, Hicks MJ, Vallabhjosula S, Synan M, Kothari PJ, Dyke JP, Ballon DJ, Kaminsky SM, De BP, Rosenberg JB, Martinez D, Koob GF, Janda KD, Crystal RG. Neuropsychopharmacology. 2013;38:2170–8. doi: 10.1038/npp.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hicks MJ, Kaminsky SM, De BP, Rosenberg JB, Evans SM, Foltin RW, Andrenyak DM, Moody DE, Koob GF, Janda KD, Ricart Arbona RJ, Lepherd ML, Crystal RG. Hum Gene Ther Clin Dev. 2014;25:40–9. doi: 10.1089/humc.2013.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Evans SM, Foltin RW, Hicks MJ, Rosenberg JB, De BP, Janda KD, Kaminsky SM, Crystal RG. Pharmacol Biochem Behav. 2016;150:76–86. doi: 10.1016/j.pbb.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Comer SD, Sullivan MA, Yu E, Rothenberg JL, Kleber HD, Kampman K, Dackis C, O’Brien CP. Arch Gen Psychiatry. 2006;63:210–218. doi: 10.1001/archpsyc.63.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sullivan MA, Vosburg SK, Comer SD. Psychopharmacology. 2006;189:37–46. doi: 10.1007/s00213-006-0509-x. [DOI] [PubMed] [Google Scholar]
- 93.Comer SD, Collins ED, Kleber HD, Nuwayser ES, Kerrigan JH, Fischman MW. Psychopharmacology. 2002;159:351–360. doi: 10.1007/s002130100909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jarvis BP, DeFulio A, Long L, Holtyn AF, Umbricht A, Fingerhood M, Bigelow GE, Silverman K. J Subst Abuse Treat. 2017 doi: 10.1016/j.jsat.2017.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


