Abstract
General theory from aquatic ecology predicts that smaller aquatic habitats have shorter hydroperiods favouring species that are better resource competitors and complete development quickly. Larger habitats are predicted to have longer hydroperiods enabling longer-lived predators to persist. Habitats with long hydroperiods and predators are predicted to favour slower-developing, predator resistant species, rather than competitive species.
In a field experiment, we manipulated independently habitat size and hydroperiod in water-filled containers, to test these hypotheses about processes structuring aquatic communities. We used human-made containers that are dominated by mosquitoes that vary in desiccation resistance, competitive ability, and predation resistance.
Habitat size and drying had significant effects on abundances of larvae of the common species in these communities. There was sorting of species by habitat size and by drying, with species that are better competitors relatively more abundant in smaller, more ephemeral habitats, and predator resistant, slower-developing species relatively more abundant in larger or permanently flooded habitats. There were no detectable effects of habitat size or drying on the dominant predator.
Habitat size and its interaction with drying affected inputs of eggs to containers. Habitat size also affected relative abundances of the two dominant species in the egg population.
Although habitat size and hydroperiod significantly affected composition of these communities, these impacts did not appear to be mediated through effects on predator abundance. Species specific differences in habitat size and drying regime preferences, and habitat-dependent larval performance appear to be the main forces shaping these communities.
Keywords: Aedes, Culex, Toxorhynchites, oviposition choice, hydroperiod
Introduction
Size of aquatic habitats is postulated to be positively related to hydroperiod (=duration of water-filled state; Wellborn et al. 1996). Hydroperiod is also postulated to determine predator abundance, with larger, slower developing, predatory species frequently absent from smaller habitats with shorter hydroperiods (Chase & Knight 2003, Wellborn et al., 1996). General aquatic ecology theory thus predicts that in smaller, more ephemeral habitats, resource competition and the fitness gained from rapid growth and development should be strong determinants of community composition, whereas in larger, more permanent habitats, predation and evasion of, or resistance to, predation should be the primary determinants of community composition (Wellborn et al., 1996; Juliano 2009).
Water-filled containers are discrete and often ephemeral habitats that can either be natural (e.g., tree-holes and bromeliads) or artificial (e.g., discarded tyres and cemetery vases). The associated communities are dominated by detritivorous larval Diptera, mainly mosquitoes (Culicidae), that browse, scrape, and filter microorganisms from the water column, the sides of the containers, and leaf and animal detritus (Merritt et al., 1992). These larvae are thought to experience frequent density-dependent competition for resources, which likely impacts population and community dynamics (Juliano, 2009). Often, these communities include obligate predators such as odonates (Yanoviak, 1999) and mosquitoes in the genus Toxorhynchites (Lounibos, 1985). Predation can alter larval densities and pupal production of prey species and community composition in these habitats (Lounibos, 1985; Bradshaw and Holzapfel, 1988; Copeland and Craig, 1992; Munga et al., 2013; Petermann et al., 2015). Effects of predators can be direct, via consumption altering prey abundances, or indirect, via changes in prey oviposition behavior (Blaustein et al., 2004), or shifts in prey behaviour from risky forging behaviour to less risky resting behaviour (e.g., Kesavaraju et al., 2007, 2011; Wormington and Juliano, 2014). Because of the trade-off of feeding vs. safety, these indirect effects may result in longer development time and smaller size at eclosion for prey exposed to predators, and ultimately, reduced successful completion of larval development (Bolnick and Preisser, 2005).
Container habitats are at the small end of the size spectrum of freshwater habitats, and thus are expected to experience frequent disturbance due to habitat drying (Lounibos, 1985; Bradshaw and Holzapfel, 1988). Drying can have direct effects on larvae through desiccation and death but can also have indirect effects by altering life histories (e.g., Juliano and Stoffregen, 1994), quality of resources in the habitat (Aspbury and Juliano, 1998; Smith et al., 2015), or by altering biotic interactions such as resource competition or predation (Wellborn et al., 1996; Laurila and Kujasalo, 1999; Bridges, 2002; Turner and Montgomery, 2009; Murrell and Juliano, 2013). The species that occupy container habitats vary greatly in their abilities to withstand habitat drying. Container mosquitoes in the genus Aedes lay drought resistant eggs on the sides of containers that hatch when flooded and exhibit plasticity in development rate under conditions of declining water volumes (Juliano and Stoffregen, 1994). Mosquitoes in the genera Culex, Anopheles, Orthopodomyia, and predatory Toxorhynchites have no desiccation resistant stages and lay eggs that hatch immediately and thus are likely to be more negatively impacted by habitat drying (Bradshaw and Holzapfel, 1988). These species also vary in competitive ability, with Aedes species typically superior in resource competition to Culex (Murrell and Juliano 2012, Costanzo et al. 2005, Carrieri et al. 2003), and also to Orthopodomyia (Livdahl 1984, Chambers 1985). Although all these species are vulnerable to predation by Toxorhynchites, Orthopodomyia appears to be least vulnerable (Bradshaw and Holzapfel, 1988, Chambers, 1985), Culex most vulnerable, and Aedes intermediate (Murrell and Juliano 2013).
Observational studies of natural tree holes support these predictions (e.g., Bradshaw and Holzapfel, 1988) but these ideas have not been tested in field experiments. We tested the hypothesis that container habitat size, hydroperiod, and predation impact container communities via oviposition choices and larval success, and thus determine larval densities and pupation. We manipulated independently habitat size and hydroperiod for morphologically similar human-made containers in a factorial design. We predicted that container size and hydroperiod would have interactive effects on community composition and species abundances, with large size and long hydroperiod increasing Toxorhynchites abundance and favouring predator resistant community members.
Materials and Methods
Container habitats were established at Tyson Research Center, near Eureka, MO in April of 2013. Containers were set in six transects along a service road, twenty meters apart and 1 to 5 meters from the road under the canopy of second growth, mixed Oak-Hickory forest. Four sizes of similarly shaped, black plastic containers spanning >2 orders of magnitude in volume were filled 60–75% full with collected rain water to initial water volumes of: 0.35 L (cups; n=48); 3.5 L (buckets; n=24); 35 L (small barrels; n=12); or 140 L (large barrels; n=6) and affixed to trees (cups and barrels) or stakes (buckets). Mosquitoes are known to be vertically distributed (e.g. host seek and oviposit at different heights), but due to the height differences of the containers and declining or stable water volumes, it was not possible to standardise the height of the water volume throughout the course of the experiment. Height differences in this experiment were minimal, from 244mm (buckets staked in the ground) to 921 mm (large barrels attached to trees) and should not have significantly affected the outcome. Observed differences in height preferences are generally at the scale of multiple meters (Novak et al. 1981). Containers on each transect were interspersed systematically so that adjacent containers were of different sizes (Fig. S1A). Each container received 2 g/L of senescent white oak leaves (Quercus alba) collected from the ground (as opposed to aquatic habitats) as initial detritus input. The containers were allowed to be colonised naturally and no attempt was made to exclude additional detritus inputs. To manipulate hydroperiod, rain guards (hardware cloth covered with thick white plastic sheeting) were placed above all containers. Half of the containers were assigned to have stable water volumes and half were assigned dry out steadily over 5 weeks. Containers assigned to the drying treatment had their volumes reduced by 20% of initial volume each week, without removing larvae and macroscopic detritus, until they were dry. They then remained dry for two weeks before being refilled to their initial volume (Fig. S1B). Containers assigned to the stable volume treatment had their volumes raised or lowered to their initial levels weekly as needed. The drying-refilling cycle was completed twice (Fig. S1B).
The sampling and drying schedule resulted in drying containers being sampled for larvae and pupae two weeks after the first refilling and one week after the second refilling. Samples were taken every two weeks. A weighted 153 μ mesh plankton net with a 113 cm2 opening (LaMotte Co. MD, USA) was placed in each barrel and allowed to settle on the bottom. The organisms were given two minutes to resume activity after this disturbance, then the plankton net was hauled vertically and contents were washed into a pan and macro invertebrates collected for identification. Two samples were taken from the small barrels and four samples were taken from the large barrels and the mean densities (individuals/liter) of species in the samples were used in the analysis. The mean densities of larvae and pupae in these samples were determined by dividing the number of individuals in the volume of the water sampled (net opening area × water depth). Buckets were sampled nondestructively every two weeks using a small rectangular aquarium net (42 cm2 opening area) placed and pulled vertically as described for the barrels. The volume of the sample was again estimated as the opening area of the net × water depth. For buckets, small barrels, and large barrels we estimated that the samples represented means (SDs) of: 4.2% (1.9%), 12.0% (0.1%), and 5.1% (0.7%), respectively, of the total container volumes. A different sampling approach was taken with the cups because of their small volume. Half of the 48 total cups were destructively sampled every two weeks, taking all larvae and pupae, and returning water and detritus to the cup. The other half of the 48 cups were sampled two weeks later, so that each cup was sampled destructively every four weeks. The longer sampling interval for each cup was implemented to allow ample time for recolonization and larval development after complete removal of the assemblage.
Pupae in the samples, which we considered to be an estimate of adult production, were allowed to eclose and were identified to genus for Culex or to species for other mosquitoes. Adult Culex were only identified to genus due to the difficulty of distinguishing morphologically female C. restuans Theobald and C. pipiens L. (Harrington and Poulson, 2008), which are the dominant Culex species at Tyson Research Center (Murell and Juliano, 2013).
On the weeks when larval/pupal densities were not quantified, Aedes oviposition was quantified by taping a piece of seed germination paper cut to a standard size (11 × 10 cm) to the container wall, with the bottom 7.5 cm submerged and a standard sample area (2.5 × 10 cm) above the water level and not covered by the tape. This allowed a standardised measure of oviposition intensity (eggs/25 cm2) in the containers of different sizes. One paper was placed in cups and buckets, two papers in small barrels, and three papers in large barrels, and mean eggs per standard sample area was used in analyses. After 7 days in the containers, the papers were returned to lab and eggs within the standard area were counted. Eggs were allowed to embryonate at 25°C and a 14:10 L:D photoperiod for 4 days, then hatched in nutrient broth solution (0.4 g/L). The resulting larvae were raised in the laboratory to the 3rd or 4th instar. We identified to species a sub sample of up to 100 larvae per paper. Because some eggs were laid below the standard sample area (as water levels fell over the week), numbers of hatched larvae sometimes exceeded numbers of eggs in the standard area. We examined the containers every two weeks for Toxorhynchites eggs and Culex egg rafts but did not observe enough of these floating eggs to include in our analysis.
Absolute abundances of larvae and pupae
Larvae per liter was analysed for common species (Aedes triseriatus (Say), Aedes japonicus (Theobald), Orthopodomyia signifera (Coquillett)) or by genus (Culex) using a generalised linear mixed model, repeated measures ANOVA, with the individual containers as subjects. Container size, drying treatment, and their interaction were between-subjects fixed effects. Sample date was a within-subjects fixed effect. Because we used regularly spaced sample dates, we considered sample date a fixed effect, postulating that sample date reflects seasonal time, rather than simply representing randomly chosen dates. We included all possible interactions as fixed effects. Toxorhynchites rutilus (Coquillett) (dominant predator) density was tested as a covariate. Transect and transect-drying treatment interaction were included as random effects, representing spatial variation in conditions We used an autoregressive moving average covariance structure (PROC GLIMMIX, SAS Institute Inc. 2011) for the repeated measures. Sampling weeks where the drying treatment containers were completely dry (and thus yielded no larvae or pupae) were excluded from the analysis for all containers. The data were best fit using a zero-inflated Poisson error distribution which accounts for the large number of zeros (PROC GLIMMIX, SAS Institute Inc. 2011; Leisnham et al., 2014). For weeks early or late in the year in which no larvae were present for a species or genus we considered these absences to be due to phenological effects, and those weeks were excluded from the analysis for the taxon in question. Thus, reported means of larval density reflect means for wet containers when that taxon was active.
We analyzed abundances of A. triseriatus, A. japonicus, Culex spp., and O. signifera pupae as cumulative mean pupae per liter over the sampling period, again excluding early and late weeks when that species was absent. Mean pupae/liter was analysed using the same mixed model and zero-inflated Poisson distribution of error as used in analysis of larvae per liter except that the effect of sample date and its interactions were removed (i.e., there were no repeated measures). We analysed cumulative mean T. rutilus in the same manner as pupae.
Relative abundances of larvae
For each sample, we expressed abundances of larval mosquitoes/liter as proportion of total larval abundance/liter (excluding the predator T. rutilus). These relative abundances of larvae indicate community composition. We subjected all relative abundance data to principal components (PC) analysis (O’Rourke et al. 2005) using varimax rotated loadings to interpret which species’ relative abundance contributed most heavily to each PC. For analysis of treatment effects on relative abundances we focused on PC1 which was unique among the PCs in having strong loadings for >1 species (see Results). We analyzed PC1 scores using mixed model repeated measures ANOVA assuming normally distributed data (PROC MIXED, SAS Institute Inc. 2011) As with analysis of larvae, container size, drying treatment, and their interaction were between-subjects fixed effects, and sample date and its interactions were within-subjects fixed effects. Transect and transect-drying treatment interaction were again random effects, individual container was the subject, and abundance of T. rutilus was tested as a covariate. Analyses of other PCs proved to be largely redundant with analyses of absolute abundances of single species and are not reported.
Total Aedes eggs and egg relative abundances
Number of Aedes eggs laid and relative abundances of A. triseriatus, Aedes albopictus (Skuse), Aedes hendersoni Cockerell and A. japonicus larvae resulting from the eggs were analyzed by repeated measures mixed model ANOVA similar to that used for larvae (PROC GLIMMIX, SAS Institute Inc. 2011), again using a zero-inflated Poisson distribution of error. We did not encounter enough eggs of Culex spp., T. rutilus, or O. signifera to analyse oviposition by those taxa.
For each sample, we expressed counts of larval mosquitoes hatched from eggs as proportion of larvae counted and identified. These relative abundances of hatched larvae indicate composition of the Aedes egg assemblage deposited in each container. As with relative abundances of larvae, we subjected all relative abundances of eggs to principal components (PC) analysis (O’Rourke et al. 2005) and varimax rotated loadings were used to interpret which species’ relative abundances contributed most heavily to each egg PC. We analysed egg PC1 scores (the only PC that loaded strongly on >1 species) using mixed model repeated measures ANOVA as was done with PC scores for larvae (PROC MIXED, SAS Institute Inc. 2011) with container size, drying treatment, sample date, all two way interactions, and transect and as a random effect and individual container as the subject.
Results
Absolute abundances of larvae and pupae
Nine species of mosquitoes, plus the predator T. rutilus occurred in our samples (Table S1). Aedes triseriatus and O. signifera were the two with greatest absolute and relative abundances. Culex restuans had high absolute abundance and moderate relative abundance. Aedes albopictus, A. japonicus and Anopheles barberi Coquillet had moderate absolute and relative abundances. Culex territans Walker, C. pipiens, and A. hendersoni, were rare by both measures.
Sampling date and its interactions with container size (F12,217=2.58, P=0.0032) and drying treatment (F4,217=4.30, P=0.0023) significantly affected A. triseriatus density as did the main effect of sampling date (F4,217=3.97, P=0.004), but all other main effects and interactions were not significant (P>0.05). After adjusting for multiple comparisons there were few significant differences among sizes or treatments within dates (Fig. 1A) and the differences that were significant were mainly between cups and buckets, with small and large barrels differing little (Fig. 1A). The interaction between drying treatment and sample date was driven by greater density in the drying containers than in stable containers after the second refilling, but not after the first (Fig. 1B). Density of T. rutilus had no significant effect on density of A. triseriatus.
Fig. 1.
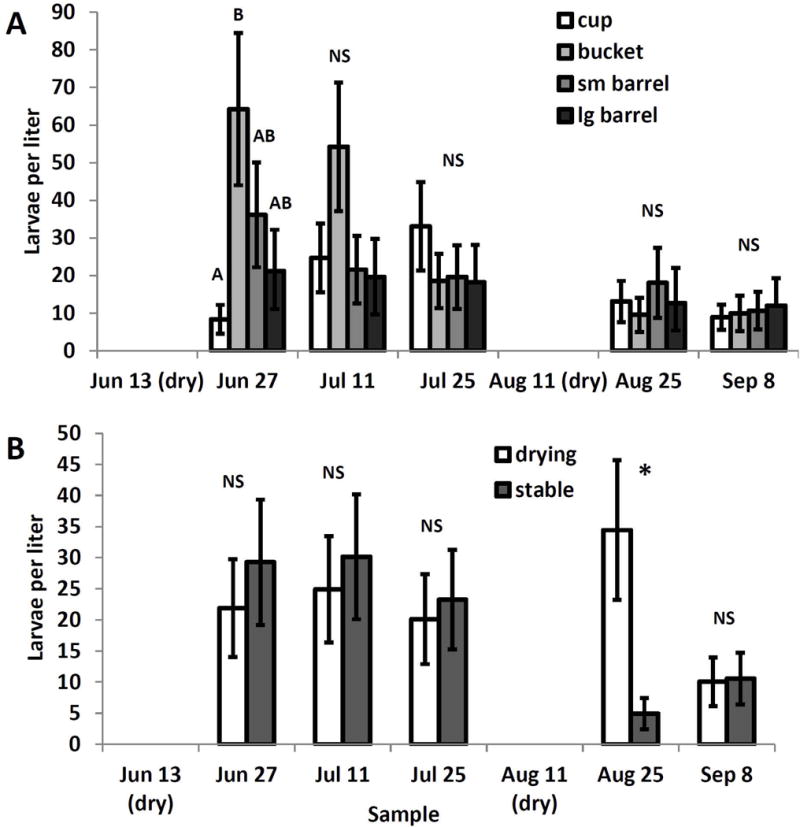
Least squares means (±SE) for significant experimental effects on Aedes triseriatus larval density per sampling unit. A. Significant interaction of container size and sampling date; means for container sizes within a sampling date associated with the same letter are not significantly different at experimentwise α=0.05. B. Significant interaction of drying treatment with sample date. * indicates significant pairwise difference between drying and stable treatments for a sample date.
For A. japonicus, interactions of sampling date with container size (F15, 267=3.25, P<0.0001) and with drying treatment (F5,267=90.84, P<0.0001) were significant. Aedes japonicus were largely absent from the cups, but due to high variability among samples, their densities in cups never differed significantly from those in the larger containers within weeks (Fig 2A). The largest effect was a spike in A. japonicus densities in the buckets after the first refilling but not after the second (Fig. 2A). As with A. triseriatus, densities in the large and small barrels were similar and remained steady over time (Fig. 2A). Although adjustment for multiple comparisons resulted in no detectable pairwise differences between drying treatments within sample dates (Fig. 2B) the source of the significant drying treatment-sample interaction was evident: mean densities across all container sizes for A. japonicus in the drying containers were low prior to drying, and remained low after the first refilling, but then reached a strong peak after the second drying (Fig. 2B). In marked contrast, in the stable containers, A. japonicus densities were greatest after the first drying, and fell to virtually zero after the second drying (Fig. 2B). Density of T. rutilus had no significant effect on density of A. japonicus.
Fig. 2.
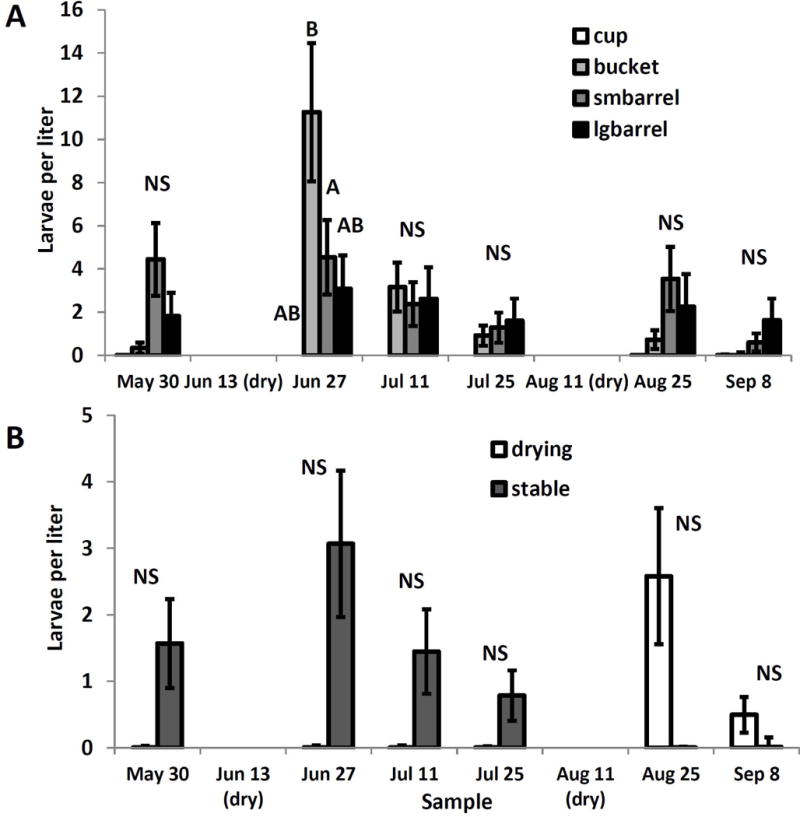
Least squares means (±SE) for significant experimental effects on Aedes japonicus larval density per sampling unit. A. Significant interaction of container size and sampling date; means for container sizes within a sampling date associated with the same letter are not significantly different at experimentwise α=0.05. B. Significant interaction of drying treatment with sample date. * indicates significant pairwise difference between drying and stable treatments for a sample date. After adjusting for multiple comparisons, there were no significant differences within weeks.
The interaction between container size and sample date was the only significant effect for Culex spp. larvae (F11,206=2×1026, P<0.0001). This taxon was absent or nearly absent from cups and very rare in buckets, and was very abundant in barrels in the sampling week before the first drying period (Fig. 3). Densities remained low in the cups and buckets throughout the sampling period (Fig. 3). After drying, Culex abundances were very low in all container sizes (Fig. 3). There were significant pairwise differences between the small barrels and cups or buckets before first drying (May 16) (Fig. 3). Here too, density of T. rutilus had no significant effect.
Fig. 3.
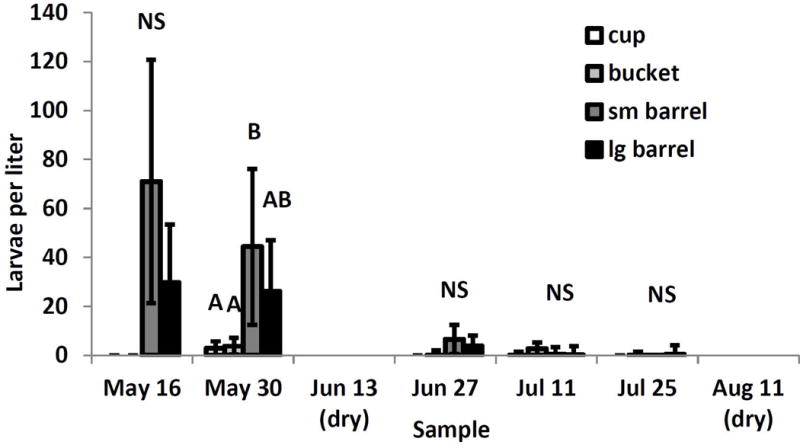
Least squares means (±SE) for significant experimental effects on Culex spp. larval density per sampling unit. Significant interaction of container size and sampling date; means for container sizes within a sampling unit associated with the same letter are not significantly different at experimentwise α=0.05.
The interaction between container size and sample date was the only significant effect on abundance of O. signifera larvae (F12,218=5.99, P<0.0001) though there were no significant pairwise differences within a date after adjusting for multiple comparisons (Fig. 4). Generally, O. signifera abundances appeared to increase with container size (Fig. 4). Density of T. rutilus again had no significant effect.
Fig. 4.
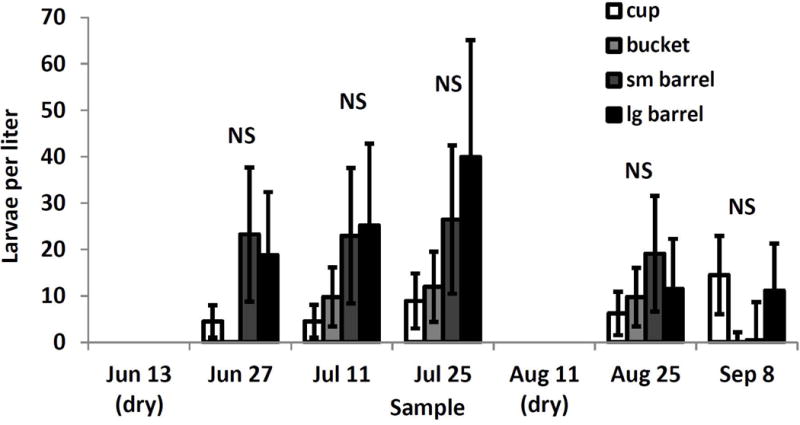
Least squares means (±SE) for significant interaction between sample date and container size on Orthopodomyia signifera larval density. After adjusting for multiple comparisons (experimentwise α=0.05) there were no significant pairwise differences within weeks or among container sizes across weeks.
There was a significant effect of container size on the mean cumulative density of A. triseriatus pupae (F3,78=6.22, P=0.0008), but no significant effect of drying treatment (F1,78=1.43,P=0.2348) or the container size-drying treatment interaction (F3,78=0.44,P=0.9432). Cups produced significantly more pupae/liter than did buckets and small barrels; large barrels were indistinguishable from all other groups (Fig. 5A). For A. japonicus mean cumulative pupal density, the trend was for fewer A. japonicus pupae/liter in the cups but the effects of container size (F3,78=1.32,P=0.2725), drying (F1,78=0.11, P=0.741), and the interaction (F3,78=0.143,P=0.9334) were all nonsignificant (Fig. 5B). There was a significant effect of container size (F3,77=13.15, P<0.0001) but not of drying or drying-container size interaction for mean cumulative density Culex pupae, with significantly fewer pupae per liter in the smaller containers (Fig. 5C). There were no significant effects of container size (F3,78=0.15, P=0.9302), drying treatments (F1,78=3.33, P=0.0717), or the interaction (F2,78=0.07, P=0.9295) on O. signifera pupae (Fig. 5D). There were also no significant effects of container size (F3,78 = 0.25; P = 0.8631), drying treatment (F1, 78 = 0.77; P = 0.385), and interaction (F3, 78 = 0.98; P = 0.4074) on cumulative mean T. rutilus density (Fig. 6).
Fig. 5.
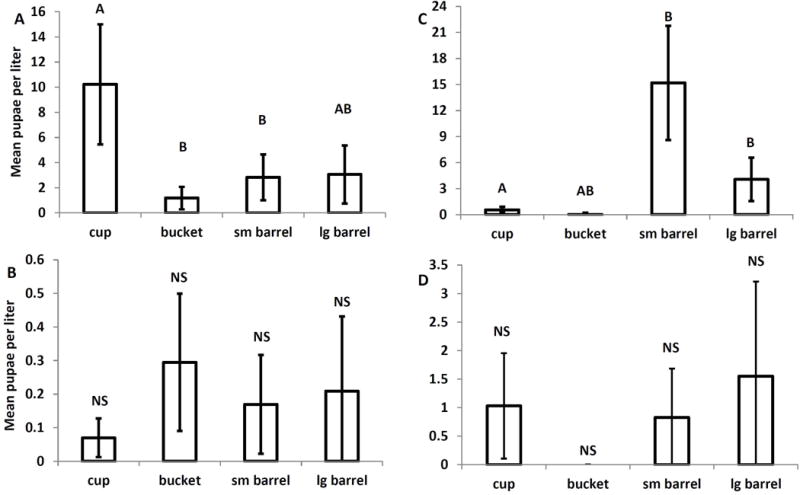
Least squares means and standard errors of the cumulative mean pupae per liter for A. A. triseriatus, B. A. japonicus, C. Culex spp., and O. signifera. Different letters denote container sizes that are significantly different. Adjusted p values were obtained using Tukey’s correction for multiple comparisons.
Fig. 6.
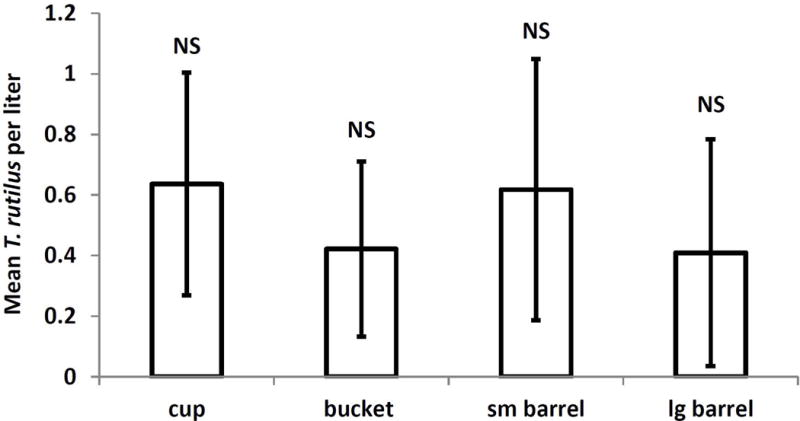
Cumulative mean Toxorhynchites rutilus per liter.
Relative abundances of larvae
Six PCs summarised 79% of the variance in relative abundance and had Eigen values>1. The first three PCs summarised 43% of the variation among samples and the dominant species (A. triseriatus, O. signifera, C. restuans, and A. japonicus) loaded strongly on these PCs (Table 1A). PC1 had a strong positive loading of O. signifera and a strong negative loading of A. triseriatus (Table 1A), meaning that large positive scores for PC1 indicate high relative abundance of O. signifera and large negative scores indicate high relative abundance of A. triseriatus. Analysis of variance on PC1 scores yielded significant effects of size*treatment (F3,80=5.87, P=0.0011), treatment*sample (F5,160=5.33, P=0.0001), sample (F5,160=2.97, P=0.0137), container size (F3,80=4.83, P=0.0038), and treatment (F1,80=24.99, P=0.0001). Other effects, including abundance of T. rutilus, were not significant (P>0.05 in all cases). Pairwise comparisons of mean PC1scores for size-treatment combinations yielded significantly greater values (i.e., greater O. signifera relative abundance) in small and large barrels that had stable water levels, but lower PC1 scores (i.e., greater A. triseriatus relative abundance) in barrels that dried (Fig. 7A). Treatments did not differ in mean PC1 for cups and buckets (Fig. 7A). For the drying treatment mean PC1 score was unaffected by container size, but in stable treatments, barrels had greater PC1 scores (i.e., greater O. signifera relative abundance) than did cups and buckets (Fig. 7A). Pairwise comparisons of treatment mean PC1 scores within sample dates showed significant differences only in the last two sample periods (Fig. 7B), when greater mean scores in stable containers, (i.e., relatively more O. signifera) in contrast to lower PC1 scores in drying containers (i.e., relatively more A. triseriatus; Fig 7B).
Table 1.
Principal component analysis of: A. relative abundances of mosquito larvae in samples (PCs 4–6 omitted); B. Relative abundances of Aedes larvae hatched from egg samples. PC loadings are varimax rotated, multiplied by 100 and rounded to units. Bold face = loadings >60. Resulting PC scores were used in analyses of community compostion of larvae and eggs.
| Relative abundance | A. Larvae/liter in samples | B. Larvae hatching from eggs | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| PC1 | PC2 | PC3 | PC1 | PC2 | PC3 | |
|
|
||||||
| Eigen value | 1.53 | 1.27 | 1.130 | 1.82 | 1.12 | 1.05 |
| Proportion variance | 0.170 | 0.141 | 0.126 | 0.456 | 0.279 | 0.265 |
| Cumulative | 0.170 | 0.311 | 0.437 | 0.456 | 0.735 | 1.000 |
| PC Loadings | ||||||
| Aedes triseriatus | −77 | −36 | −34 | −88 | −36 | −31 |
| Aedes albopictus | 0 | −1 | −2 | 3 | 100 | −3 |
| Aedes japonicus | −6 | 96 | −6 | 98 | −17 | −14 |
| Aedes hendersoni | −7 | −23 | 52 | 3 | −3 | 100 |
| Culex restuans | 4 | 10 | 87 | |||
| Culex pipiens | −3 | 5 | 1 | |||
| Culex territans | 4 | −8 | −3 | |||
| Anopheles barberi | −1 | −10 | −7 | |||
| Orthopodomyia signifera | 92 | −25 | −19 | |||
|
| ||||||
| PC Interpretation | O. signifera versus A. triseriatus | A. japonicus | C. restuans | A. japonicus versus A. triseriatus | A. albopictus | A. hendersoni |
Fig. 7.
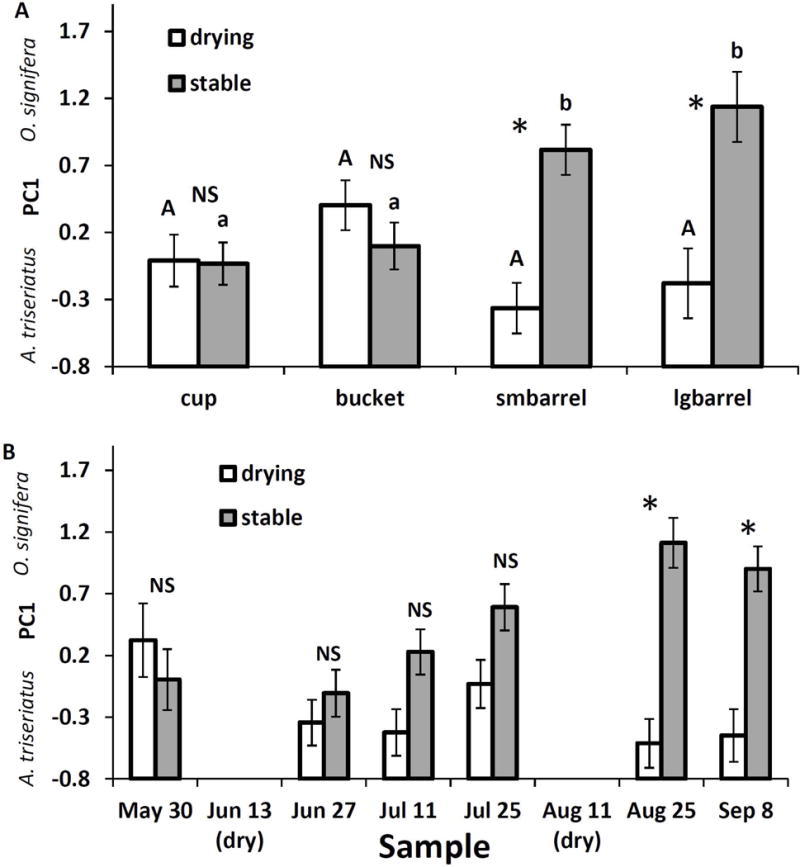
Principal component 1 for species relative abundance of larvae across the experiment. A. Significant interaction of drying treatment with container size. * indicates significant pairwise difference between drying and stable treatments for a container size; means within a drying treatment associated with the same letter are not significantly different at experimentwise α=0.05. B. Significant interaction of drying treatment with sample date. * indicates significant pairwise difference between drying and stable treatments for a date.
Total Aedes eggs and egg relative abundances
Total Aedes eggs/standard sample was significantly affected by a container size by drying treatment interaction (F3,82=4.47, P=0.0059), container size by sample date interaction (F15,410=3.338, P=0.0001) and the main effects of sample date (F5,410=49.10, P=0.0001), and container size (F3,82=29.05, P=0.0001). Other effects, were not significant (P>0.05 in all cases). In the drying treatment number of eggs increased as the size of the container increased, but this trend was not evident in the stable treatment (Fig. 8A). Within a single container size, there were no significant differences between drying and stable treatments (Fig. 8A). The size*sample interaction resulted from significant differences among container sizes for sample dates prior to the second drying (Fig. 8B), with mean Aedes eggs increasing with container size. After the second drying, there were no differences among sizes, and egg numbers were very low (Fig. 8B).
Fig. 8.
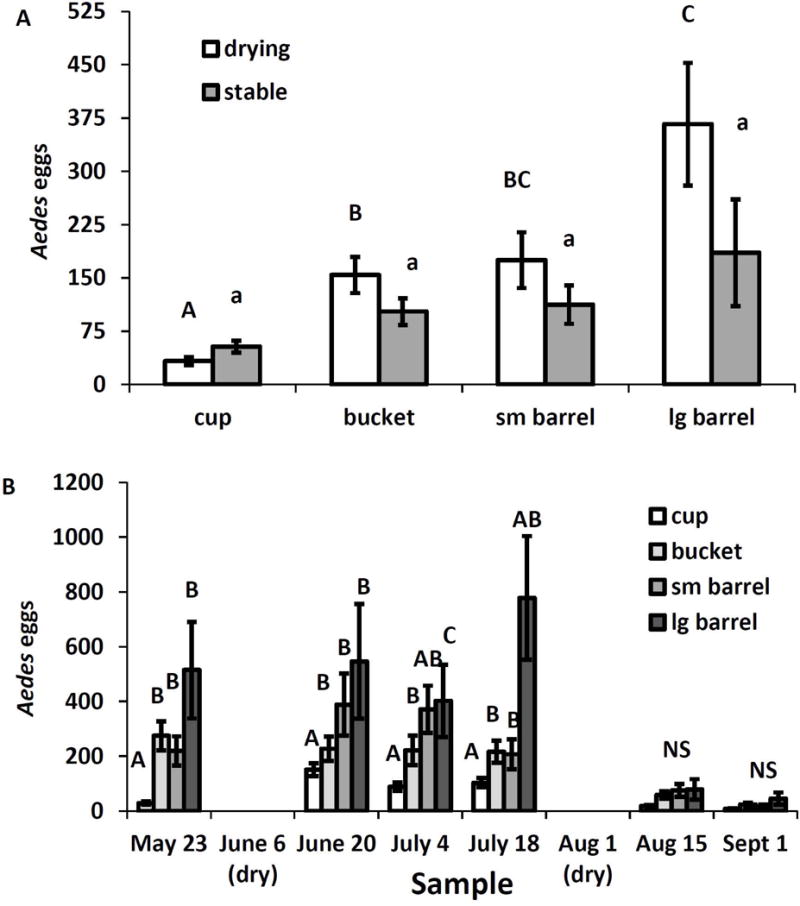
Least squares means (±SE) for significant experimental effects on numbers Aedes eggs per sampling unit. A. Significant interaction of drying treatment with container size; means for container sizes within a treatment associated with the same letter are not significantly different at experimentwise α=0.05. There were no significant differences between drying and stable for any container size. B. Significant interaction of container size with sample date. Within a date, means associated with the same letter are not significantly different at experimentwise α=0.05.
The first three PCs summarised 100% of the variance in egg relative abundance, and had Eigen values>1 (Table 1B). PC1 was the only PC with strong loadings on >1 species abundance (Table 1B) with a strong positive loading of A. japonicus and a strong negative loading of A. triseriatus (Table 1B). Thus, large positive PC1 scores indicated high relative abundance of A. japonicus eggs and large negative PC1 scores indicated a high relative abundance of A. triseriatus eggs. Analysis of variance on egg PC1 scores yielded significant effects of size by sample interaction (F15,324=2.15, P=0.0077), drying treatment (F1,82=5.06, P=0.0271), sample (F5,324=11.71, P=0.0001), and container size (F3,82=37.67, P=0.0001). Other two way interactions were not significant (P>0.05 in all cases), and the three way interaction was omitted from the model to facilitate convergence of the iterative maximum likelihood algorithm. Mean egg PC1 scores indicated significantly greater relative abundance of A. japonicus eggs in stable, and significantly greater relative abundance of A. triseriatus eggs in drying containers (Fig. 9A). Prior to the second drying, egg PC1 mean scores indicated greater relative abundance of A. japonicus for one or both sizes of barrel than for cups, in which A. triseriatus attained greater relative abundance (Fig 9B). After the second drying, this trend was evident but the differences were not significant (Fig. 9B).
Fig. 9.
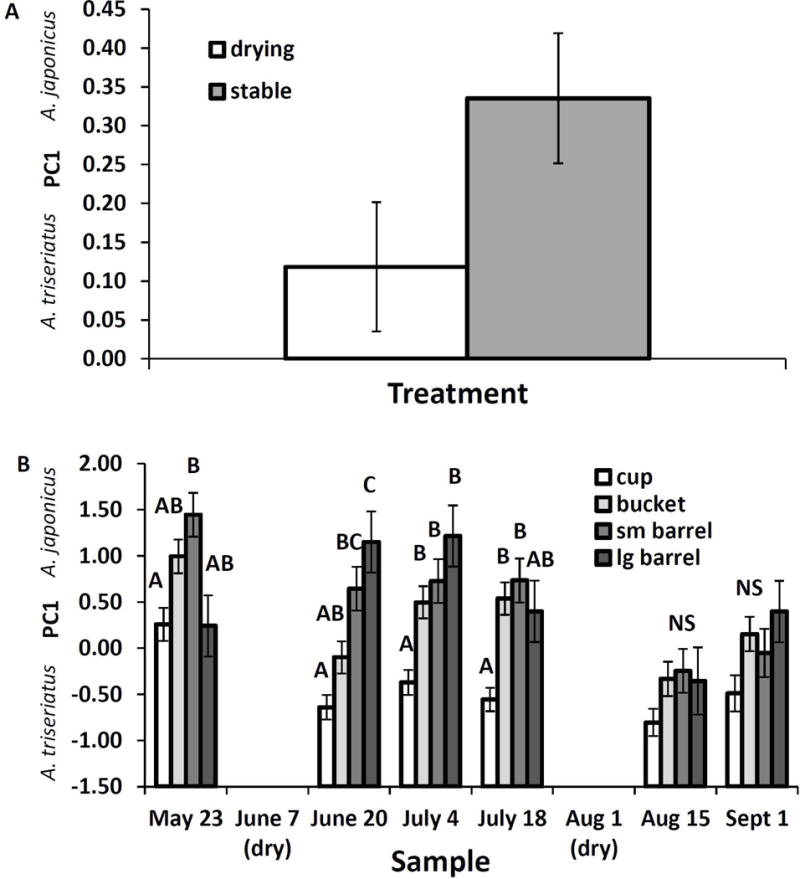
Least squares means (±) for principal component 1 for species relative abundance of larvae hatched from eggs across the experiment. A. Significant main effect of drying treatment B. Significant interaction of container size with sample date. Container size least squares means within a sample date associated with the same letter are not significantly different at experimentwise α=0.05.
Discussion
We find that habitat size and drying have multiple effects on this community of mosquitoes, affecting abundances of the dominant species, and oviposition choices by Aedes. Some of the observed effects are consistent with the prediction that drying and small container size increase relative abundances of less-predator-resistant species, whereas stable water levels and large container size increase the relative abundances of more-predator-resistant species, as predicted by Wellborn et al. (1996). Despite this, we find no evidence that these effects result from impacts of drying and container size on abundances of Toxorhynchites rutilus, the dominant predator in these container habitats. The implication of these results is that container size and hydroperiod – two major components of the abiotic environment – exert substantial effects on absolute and relative mosquito abundances independent of predation.
The significant interactions between sample date and drying treatment for A. triseriatus and A. japonicus result in part from a spike in larval density after refilling the containers after the second drying but not after the first. This difference is likely related to our sampling schedule, in which larval samples were taken two weeks after refilling following the first drying and one week after refilling following the second drying. Refilling likely induced hatching of eggs on the side of the containers, but two weeks after refilling, the densities of these species had returned to levels comparable to those in containers that remained filled to a constant depth. Drying containers maintained steady larval densities even though the volume of water was reduced by 40% over the two weeks between sampling periods. This suggests that egg hatching, larval death, and adult eclosion were roughly in balance, keeping densities stable despite declining volume.
Aedes triseriatus, the numerically dominant species in containers at this site, peaks in abundance in June and July in buckets (Fig. 1). Its abundance per liter is lower in both smaller containers and larger containers. Of all the species involved A. triseriatus is the most uniformly common across sizes, drying regime, and dates. In contrast, for A. japonicus, abundance per liter (Fig. 2) is quite low in the smallest container and only showed one peak of density in buckets in June, immediately following flooding. These patterns contrast with abundances of Aedes eggs (Fig. 8) which increased in abundance as container size increased. Eggs in larger and stable containers yielded a greater relative abundance of A. japonicus than A. triseriatus (or any other species; Fig. 9) suggesting that the assemblage of Aedes species is shaped in part by choice of oviposition sites based on size and permanence.
There were differences in pupal production among container sizes with more A. triseriatus pupae produced per liter in the smaller containers and more A. japonicus and Culex pupae produced per liter in the larger containers. These findings are consistent with observational data about habitat size preferences in these and related species with varying degrees of habitat size association (Bradshaw and Holzapfel, 1988; Sunahara et al., 2002; Bevins, 2007; Gilbert et al., 2008, Laporta et al., 2014) and suggest some species sorting along a gradient of habitat size. As adults of these species are important vectors of human diseases, these data suggest that containers of different sizes may contribute differentially to risk of La Crosse (transmitted by A. triseriatus) and West Nile encephalitis viruses (transmitted primarily by Culex species).
In addition to effects of habitat size and drying on abundances per liter of some dominant species, we find more dramatic effects of these physical factors on relative abundances of larvae of A. triseriatus and O. signifera, the two most abundant species over our sample period (Table S1). In containers with stable water levels, relative abundance of O. signifera increases steadily over the season (Fig. 7B) so that by the end of the season, stable and drying containers differ greatly in relative abundances of these species. Overall, large size and stable water levels enhance relative abundances of O. signifera relative to A. triseriatus (Fig. 7A), and this pattern has been observed in natural tree holes and attributed in part to the impact of predation by T. rutilus being greater in container with stable water levels (Bradshaw and Holzapfel, 1988). This pattern is consistent with the hypothesis that size and drying influence the community via predation because O. signifera has been shown to have low vulnerability to, and is commonly associated with, T. rutuilus (Bradshaw and Holzapfel, 1988, Chambers, 1985, Livdahl, 1984). Our data, yielding no evidence for size or drying effects on T. rutilus abundance, suggest, however, that at least some of this pattern is driven by species specific oviposition preferences and performance differences (e.g, better larval survival, growth, and development) in habitats of different sizes and drying regimes, independent of predation.
We had predicted that drying would be particularly detrimental to T. rutilus as this predator lacks desiccation resistant stages, and requires a longer larval period than its prey. We found that drying and habitat size had no effect on cumulative mean T. rutilus density and we found no evidence that T. rutilus densities impacted prey density or relative abundances. While drying of the habitat surely killed eggs and larvae, Toxorhynchites rapidly recolonised containers via oviposition after refilling and predator densities during wet periods did not differ from those in stable containers. Our data are not consistent with observational data that T. rutilus is more abundant in larger containers with longer hydroperiods (Focks et al., 1983; Bradshaw and Holzapfel, 1988). Thus, the effects of drying and container size we observed on community composition and larval abundances appear to result from active habitat choice by the members of this community and direct effects of these physical variables on community members, rather than indirect effects mediated through predation. Rapid recolonization and similar predator abundances across treatments means that we cannot rule out the possibility that impacts of predation would be evident, perhaps even strong, with greater differences in predator densities. Detecting such an effect in this system is likely to require direct manipulation of Toxorhynchites abundances.
Effects of habitat drying on species abundances in similar communities have been observed before, with drying most likely to have negative impacts on species with no desiccation resistant life stage (e.g., Orthopodomyia, Culex, Toxorhynchites; Lounibos, 1985; Bradshaw and Holzapfel, 1988). The results from our experiment demonstrate that when drying takes place gradually and habitats rapidly refill, these communities are recolonised within a few weeks and species, including predators, attain densities similar to those of stable habitats irrespective of habitat size. Our results suggest that at the scale of a forest, a diversity of container sizes and hydroperiods can support populations and communities of container insects, even with local extinctions due to complete drying of some containers. Only large scale drought that causes drying of most containers in an area seems likely to produce a long lasting shift in the composition of container communities. Our results suggest that container communities, in which the dominant predators have aerial stages adapted to seek out and to oviposit in flooded containers, are organised rather differently than ground water communities that are often dominated by fish predators. Recolonization by predatory fish after drying of ground water habitats likely takes much longer, resulting in longer periods of predator-free community development, and a more pronounced predator-mediated effect of drying on community composition. The effects of drying, and associated predator elimination, on mosquito outbreaks postulated by Chase and Knight (2003) thus seem to be more a property of ponds and wetlands, rather than of natural or human made containers.
Supplementary Material
Fig. S1. A. Schematic representation of container arrangement along transects. B. Schematic representation of drying regime. Volumes shown in the figure are for the small barrels though all container sizes were dried on the same schedule.
Table S1. Summary of abundances of nine larval mosquito species (excluding Toxorhynchites rutilus) across samples from late May through September. Absolute abundances are expressed as larvae/liter. Relative abundances express larvae/liter for a species as a proportion of total mosquito larvae/liter per sample. Sample sizes (N) differ because relative abundances cannot be calculated in samples with 0 total mosquito larvae.
Acknowledgments
We thank P.J. Brabant, L. Garcia, P. McCormick, K.M. McIntire, G.D. Ower, M.K. Schumacher, A. Wiggins, and J.D. Williams for field and laboratory assistance, and R.D. Cassal for assistance with rain guard construction. We also thank K.A. Medley, K. Smith, E. Biro, T. Derton, P. Jamerson, and T. Mohrman at Tyson Research Center for allowing us access to the property and for generous assistance with field set up and equipment. This work was funded by National Institute of Allergy and Infectious Disease grant 1R15AI094322-01A1 to SAJ.
Footnotes
No detectable role for predators mediating effects of aquatic habitat size and permanence on populations and communities of container-dwelling mosquitoes
Contribution of Authors
KMW and SAJ both contributed to the design, implementation, data collection, and analysis of the experiment as well as manuscript preparation.
Conflict of Interest
The authors declare to conflict of interest.
References
- Aspbury AS, Juliano SA. Negative effects of habitat drying and prior exploitation on the detritus resource in an ephemeral aquatic habitat. Oecologia. 1998;115:137–148. doi: 10.1007/s004420050500. [DOI] [PubMed] [Google Scholar]
- Bevins SN. Establishment and abundance of a recently introduced mosquito species Ochleroatus japonicus (Diptera: Culicidae) in the Southern Appalachians, USA. Journal of Medical Entomology. 2007;44:945–952. doi: 10.1603/0022-2585(2007)44[945:eaaoar]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Blaustein L, Kiflawi M, Eitam A, Mangel M, Cohen JE. Oviposition habitat selection in response to risk of predation in temporary pools: Mode of detection and consistency across experimental venue. Oecologia. 2004;138:300–305. doi: 10.1007/s00442-003-1398-x. [DOI] [PubMed] [Google Scholar]
- Bolnick DI, Preisser EL. Resource competition modifies the strength of trait-mediated predator-prey interactions: a meta-analysis. Ecology. 2005;86:2771–2779. [Google Scholar]
- Bradshaw WE, Holzapfel CM. Drought and the organization of tree-hole communities. Oecologia. 1988;74:507–514. doi: 10.1007/BF00380047. [DOI] [PubMed] [Google Scholar]
- Bridges CM. Tadpoles balance foraging and predator avoidance : Effects of predation, pond drying, and hunger. Journal of Herpetology. 2002;36:627–634. [Google Scholar]
- Carrieri M, Bacchi M, Bellini R, Maini S. On the competition occurring between Aedes albopictus and Culex pipiens (Diptera: Culicidae) in Italy. Environmental Entomology. 2003;32:1313–21. [Google Scholar]
- Chambers RC. Competition and predation among larvae of three species of treehole breeding mosquitoes. In: Lounibos LP, Rey JR, Frank JH, editors. Ecology of Mosquitoes: Proceedings of a Workshop. Vero Beach: Florida Medical Entomology Laboratory; 1985. pp. 25–54. [Google Scholar]
- Chase JM, Knight TM. Drought-induced mosquito outbreaks in wetlands. Ecology Letters. 2003;6:1017–1024. [Google Scholar]
- Copeland RS, Craig GB. Interspecific competition, parasitism, and predation affect development of Aedes hendersoni and A. triseriatus (Diptera: Culicidae) in artificial treeholes. Annals of the Entomological Society of America. 1992;85:154–163. [Google Scholar]
- Costanzo KS, Mormann K, Juliano SA. Asymmetrical competition and patterns of abundance of Aedes albopictus and Culex pipiens (Diptera: Culicidae) Journal of Medical Entomology. 2005;42:559–70. doi: 10.1093/jmedent/42.4.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari MCO, Messier F, Chivers DP. Threat-sensitive learning of predators by larval mosquitoes Culex restuans. Behavioral Ecology and Sociobiology. 2008;62:1079–1083. [Google Scholar]
- Focks DA, Sackett SR, Dame DA, Bailey DL. Ability of Toxorhynchites amboinensis (Doleschall) (Diptera: Culicidae) to locate and oviposit in artificial containers in an urban environment. Environmental Entomology. 1983;12:1073–1077. [Google Scholar]
- Gilbert B, Srivastava DS, Kirby KR. Niche partitioning at multiple scales facilitates coexistence among mosquito larvae. Oikos. 2008;117:944–950. [Google Scholar]
- Harrington LC, Poulson RL. Considerations for accurate identification of adult Culex restuans (Diptera: Culicidae) in field studies. Journal of Medical Entomology. 2008;45:1–8. doi: 10.1603/0022-2585(2008)45[1:cfaioa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Juliano SA. Species interactions among larval mosquitoes: Context dependence across habitat gradients. Annual Review of Entomology. 2009;54:37–56. doi: 10.1146/annurev.ento.54.110807.090611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano SA, Stoffregen TL. Effects of habitat drying on size at and time to metamorphosis in the tree hole mosquito Aedes triseriatus. Oecologia. 1994;97:369–376. doi: 10.1007/BF00317327. [DOI] [PubMed] [Google Scholar]
- Kesavaraju B, Damal K, Juliano SA. Threat-sensitive behavioral responses to concentrations of water-borne cues from predation. Ethology. 2007;113:199–206. doi: 10.1111/j.1439-0310.2006.01317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesavaraju B, Khan DF, Gaugler R. Behavioral differences of invasive container-dwelling mosquitoes to a native predator. Journal of Medical Entomology. 2011;48:526–532. doi: 10.1603/me10200. [DOI] [PubMed] [Google Scholar]
- Laporta GZ, Anice M, Sallum M. Coexistence mechanisms at multiple scales in mosquito assemblages. BioMed Central Ecology. 2014;14:1–10. doi: 10.1186/s12898-014-0030-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurila A, Kujasalo J. Habitat duration, predation risk, and phenotypic plasticity in common frog (Rana temporaria) tadpoles. Journal of Animal Ecology. 1999;68:1123–1132. [Google Scholar]
- Leisnham PT, LaDeau SL, Juliano SA. Spatial and temporal habitat segregation of mosquitoes in Urban Florida. PLoS One. 2014;9:e91655. doi: 10.1371/journal.pone.0091655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livdahl TP. Interspecific interactions and the r-K continuum: laboratory comparisons of geographic strains of Aedes triseriatus. Oikos. 1984;42:193–202. [Google Scholar]
- Lounibos LP. Interactions influencing production of treehole mosquitoes in south Florida. In: Lounibos LP, Rey JR, Frank JH, editors. Ecology of Mosquitoes: Proceeding of a workshop. Florida Medical Entomology Laboratory; Vero Beach, FL: 1985. pp. 65–77. [Google Scholar]
- Merritt RW, Dadd RH, Walker ED. Feeding behavior, natural food, and nutritional relationships of larval mosquitoes. Annual Review of Entomology. 1992;37:349–376. doi: 10.1146/annurev.en.37.010192.002025. [DOI] [PubMed] [Google Scholar]
- Munga S, Vulule J, Kweka EJ. Response of Anopheles gambiae s.l. (Diptera: Culicidae) to larval habitat age in western Kenya highlands. Parasites and Vectors. 2013;6:13. doi: 10.1186/1756-3305-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrell EG, Juliano SA. Competitive abilities in experimental microcosms are accurately predicted by a demographic index for R*. PLoS ONE. 2012;7:e43458. doi: 10.1371/journal.pone.0043458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrell EG, Juliano SA. Predation resistance does not trade off with competitive ability in early-colonizing mosquitoes. Oecologia. 2013;173:1033–1042. doi: 10.1007/s00442-013-2674-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak RJ, Peloquin J, Rohrer W. Vertical distribution of adult mosquitoes (Diptera: Culicidae) in a Northern deciduous forest in Indiana. Journal of Medical Entomology. 1981;18:116–122. [Google Scholar]
- O’Rourke N, Hatcher L, Stepanski EJ. A step-by-step approach to Using SAS®for Univariate and Multivariate Statistics. 2nd. SAS Institute Inc; Cary NC USA: 2005. [Google Scholar]
- Petermann JS, Farjalla VF, Jocque M, Kratina P, MacDonald AM, Marino NC, de Omena PM, Piccoli GC, Richardson BA, Richardson MJ, Romero GQ, Videla M, Srivastava DS. Dominant predators mediate the impact of habitat size on trophic structure in bromeliad invertebrate communities. Ecology. 2015;96:428–439. doi: 10.1890/14-0304.1. [DOI] [PubMed] [Google Scholar]
- Smith CD, Freed TZ, Leisnham PT. Prior hydrologic disturbance affects competition between Aedes mosquitoes via changes in leaf litter. PLoS One. 2015;10:e0128956. doi: 10.1371/journal.pone.0128956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunahara T, Ishizaka K, Mogi M. Habitat size: a factor determining the opportunity for encounters between mosquito larvae and aquatic predators. Journal of Vector Ecology. 2002;27:8–20. [PubMed] [Google Scholar]
- Turner AM, Montgomery SL. Hydroperiod, predators and the distribution of physid snails across the freshwater habitat gradient. Freshwater Bioliogy. 2009;54:1189–1201. [Google Scholar]
- Wellborn GA, Skelly DK, Werner EE. Mechanisms creating community structure across a freshwater habitat gradient. Annual Review of Ecological Systems. 1996;27:337–363. [Google Scholar]
- Wormington JD, Juliano SA. Hunger-dependent and sex-specific antipredator behaviour of larvae of a size-dimorphic mosquito. Ecological Entomology. 2014;39:548–555. doi: 10.1111/een.12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanoviak SP. Effects of leaf litter species on macroinvertebrate properties community and mosquito tree hole microcosms yield in Neotropical tree hole microcosms. Oecologia. 1999;120:147–155. doi: 10.1007/s004420050843. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. A. Schematic representation of container arrangement along transects. B. Schematic representation of drying regime. Volumes shown in the figure are for the small barrels though all container sizes were dried on the same schedule.
Table S1. Summary of abundances of nine larval mosquito species (excluding Toxorhynchites rutilus) across samples from late May through September. Absolute abundances are expressed as larvae/liter. Relative abundances express larvae/liter for a species as a proportion of total mosquito larvae/liter per sample. Sample sizes (N) differ because relative abundances cannot be calculated in samples with 0 total mosquito larvae.


