Abstract
The human Respiratory Syncytial Virus (hRSV) causes lower respiratory tract infections including pneumonia and bronchiolitis. Such infections also cause a large number of hospitalizations and affects mainly newborns, young children and the elderly worldwide. Symptoms associated with hRSV infection are due to an exacerbated immune response characterized by low levels of IFN-γ, recruitment of neutrophils and eosinophils to the site of infection and lung damage. Although hRSV is a major health problem, no vaccines are currently available. Different immunization approaches have been developed to achieve a vaccine that activates the immune system, without triggering an unbalanced inflammation. These approaches include live attenuated vaccine, DNA or proteins technologies, and the use of vectors to express proteins of the virus. In this review, we discuss the host immune response to hRSV and the immunological mechanisms underlying an effective and safe BCG vectored vaccine against hRSV.
Keywords: BCG, hRSV immune response, hRSV vaccine
Introduction
The human Respiratory Syncytial Virus (hRSV) causes lower respiratory tract infections and a large number of hospitalizations of newborns, young children and elderly worldwide.1 hRSV can be responsible from mild symptoms, such as cough and rhinitis, to severe symptoms, such as alveolitis, bronchiolitis and pneumonia. Interestingly, although such a disease mainly affects the respiratory system, extrapulmonary manifestations such as encephalitis have been reported.2 Recruitment of immune cells to the lungs, impaired production of IFN-γ and lung damage characterize the hRSV infection. Importantly, hRSV-associated pathology is mainly caused by an exacerbation of the host immune response. Further, after hRSV infection an inefficient adaptive response is established, resulting in frequent reinfection of the host throughout life.3,4 Different virus immune modulatory strategies, such as inhibition of the Type I IFN signaling cascade and interference with T cell activation, among others, have been shown to contribute to this inefficient host response. Up to date, no licensed vaccine to prevent the disease caused by hRSV infection is available for the population. Although several formulations are currently on preclinical development, only a minor fraction of them are under clinical evaluation.5 Only an expensive and multi-dose treatment, consisting on injections of a humanized monoclonal antibody, Palivizumab, is available to treat severe cases of hRSV-associated bronchitis or pneumonia. In this review, we discuss the host immune response during hRSV infection and a proposed new vaccine approach that protects against it.
hRSV evasion from host immune system
hRSV infects primary airway epithelial cells and causes an inflammatory cells recruitment to the site of infection. Upon hRSV infection, distal bronchial airway inflammation occurs, thereby prompting to lung damage. Such an inflammatory response fails to achieve a memory response since reinfections in young children and adults are frequent during winter period.6 Further, an impairment of the innate response machinery has been reported during hRSV infection, since Interferon (IFN) α/β signaling has been shown to be blocked by both viral non-structural proteins NS1 and NS2 of the hRSV.7,8 Upon hRSV recognition, the NLRP3 inflammosome is activated through the viral viporin SH, triggering the production of Interleukine (IL) 1β and IL-18.9,10 Also, it is thought that NS1 and NS2 proteins impair dendritic cell (DC) maturation, which in turn inhibits T cell activation.11 Further, it has been found that hRSV impairs the capacity of DCs to induce T cell activation, making them defective for IL-2 secretion and also up-regulates the expression of surface activation markers.12,13 Interestingly, a decrease in the polarization of Golgi apparatus in T cells toward hRSV infected-DCs takes place, herein impairing the immunological synapse (IS). The Nucleoprotein (N) of the hRSV seems to be the hRSV protein responsible of such an inhibition of the IS, which in turns causes an impaired T cell activation.14,15 The synapse is crucial to trigger an effective and specific adaptive response against hRSV. Impairment of T cell activation by hRSV has been described by various researchers is consistent with the reduced production of IFN-γ found in patients with hRSV-induced bronchiolitis and in infected animals.16-18 Moreover, the low production of IFN-γ causes a weak T helper (TH) 1 response, which is key for viral clearance. As a result, hRSV infection induces a biased TH-2 like response characterized by the production of pro-inflammatory cytokines, such as IL-4 and IL-13 (Fig. 1). Further, a direct correlation between the production of IL-13, IL-12p40 and recurrent wheezing in infants with hRSV bronchiolitis has been reported.19 Such a cytokine production is accompanied by the infiltration of abundant immune cells to the airways, generating inflammation and lung damage. Similarly, upregulation of IL-17 in the lungs was reported upon hRSV infection in mice and it is thought that this cytokine induces mucus production20 and downmodulation of the CD8+ T cell response.21 Further, in vitro studies suggested that IL-17 could be suppressing T-bet expression impairing IFN-γ secretion.20 IL-17 upregulation, along with an increase of IL-6 and IL-23p19, can lead to a TH-17 cell differentiation profile.20 Furthermore, Foxp3+ Treg cells have been found to be recruited to the lungs upon hRSV infection.22 The importance of this subset of T cells in the response to hRSV infection has been supported by experiments consisting of CD4+Foxp3+CD25+depleted-mice, which displayed an exacerbation of inflammation after an hRSV challenge.23,24 Further, the balance between both Treg and TH-17 cells has been reported to be associated with the severity of the hRSV infection.25 Along these lines, it has been reported that IL-27 plays an important role at defining the TH phenotype by suppressing the IL-17-induced polarization.26 Importantly, one of the potentially detrimental host responses during hRSV infection is the induction of IgG1 and IgG3 antibody isotypes, which fail to efficiently neutralize the virus. The hRSV reinfection that occurs throughout the life further underscores the detrimental response of the host to a second exposure. Along these lines, although memory CD8+ T cells can be detected upon hRSV challenge, this response seems insufficient to protect against subsequent reinfections.
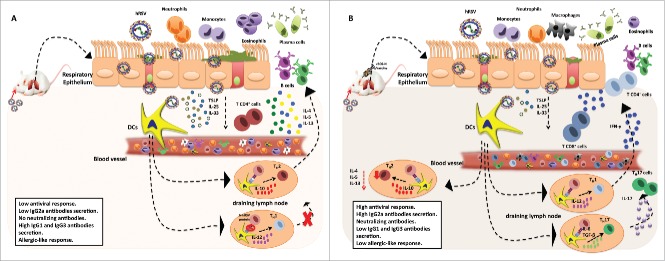
Figure 1.
Immune response induced by hRSV in absence or presence of the recombinant rBCG-N-hRSV vaccine. hRSV induce an aberrant immune response due to the erratic T cell polarization, producing a high pro-inflammatory and allergic-like response (A). On the other hand, the rBCG-N-hRSV induces an effective immune response that could control the infection through the polarization of CD4+ and CD8+ T cells and neutralizing antibodies decreasing the recruitment of immune cells such as neutrophils, eosinophils and the mucus secretion (B).
Development of a vaccine against hRSV
In the 1960s, one of the first vaccines candidate for hRSV was the formalin-inactivated vaccine (FI-hRSV). Unfortunately, when the vaccine recipients were exposed to hRSV, they showed increased rates of severe disease, and up to 80% of vaccinated children required hospitalization.27,28 This immune-mediated enhanced disease was characterized by an excessive eosinophil infiltration into the airways of vaccinated children. This finding of an enhanced disease in the hRSV seronegative infants warned about a vaccine that promotes an enhancement of the pulmonary disease. Therefore, an efficient vaccine against this pathogen should avoid exacerbation of the hRSV-associated inflammation and induce an efficient TH-1 response characterized by 1) IFN-γ production, 2) hRSV-specific memory CD4+ and CD8+ T cell response, 3) efficient and neutralizing specific antibodies, preferably of the IgG2a isotype. After FI-hRSV failure, several vaccine strategies have been developed, such as monoclonal antibodies against proteins of hRSV,29-32 live attenuated hRSV vaccines,33,34 virus like particles (VLPs),35,36 and recombinant vaccines using as a vector parainfluenza virus,37 adenovirus38,39 or Mycobacterium bovis Calmette-Guerin (BCG).40,41
Mycobacterium bovis BCG as a safe and efficient vector vaccine for hRSV that could be applied to newborns.
Mycobacterium bovis Calmette-Guerin (BCG) is a live, attenuated vaccine that protects against Tuberculosis (TB) and has been used since 1920. Currently, although several TB vaccines approaches are undergoing, BCG is the only licensed vaccine available to protect against TB and is given to newborns in most countries. Several clinical studies provide support to the notion that BCG is a safe and immunogenic vaccine to prevent TB meningitis and miliary disease.42 Importantly, BCG induces a CD4+ TH-1 polarized response in neonates by producing adult-like levels of IL-12p70,43,44 thereby being an attractive approach for the development of vaccines against several pathogens. The use of BCG as a vaccine vector has been previously reported against human immunodeficiency virus (HIV), rotavirus, Plasmodium yoelii, Bortedella pertussis, measles virus and hepatitis B virus.45 Our group has designed and developed a recombinant BCG that expresses the nucleoprotein (N) of hRSV (rBCG-N-hRSV). Importantly, immunization with this rBCG-N-hRSV protects of hRSV associated-lung damage, decreases the infiltration of inflammatory immune cells to the lungs and reduces the virus in the lung tissues when mice were infected with hRSV (Fig. 2).40,46,47 Further, rBCG-N-hRSV induces an early CD4+ and CD8+ T cell recruitment to the lungs and IFN-γ and IL-17 secretion in response to specific hRSV antigens.41 Also, no significant IL-4 production was found after rBCG-N-hRSV immunization, herein supporting a TH-1 polarization.40 The lack of protection found in RAG-mice and in recipient mice with IFN-γ blocked,41 suggested that the vaccine requires an IFN-γ producer T cell repertoire. Further, CD8+IFN-γ+ T cells were found in the lungs of rBCG-N-hRSV and hRSV-infected mice,47 indicating a memory response induced by the vaccine. Interestingly, the nucleoprotein of hRSV in this vectored vaccine is being delivered from APCs to T cells without impairing the IS, as previously shown in the hRSV-infected DCs. Moreover, it has been found that rBCG-N-hRSV elicits long-lasting immune memory in mice.47 Vaccination with rBCG-N-hRSV, along with its subsequent infection, not only induces cellular immunity but also a humoral response against the virus with an antibody secretion against several hRSV proteins, including N, F and G (unpublished results). Thus, although the rBCG-N-hRSV is only expressing the nucleoprotein of the virus, antibodies against other proteins of the virus are produced, a phenomenon known as Linked Recognition. Further, hRSV vaccine triggers an IgG isotype switching from IgG1 to IgG2a, which is key for viral clearance (unpublished results). The importance of this humoral response in the immunity against hRSV is supported by a reduced pathology reported in naïve mice transferred with antibodies of vaccinated mice. Also, the rBCG-N-hRSV antibodies generated displayed neutralizing properties, being able to reduce hRSV plaque forming units in infected cells in vitro.
Figure 2.
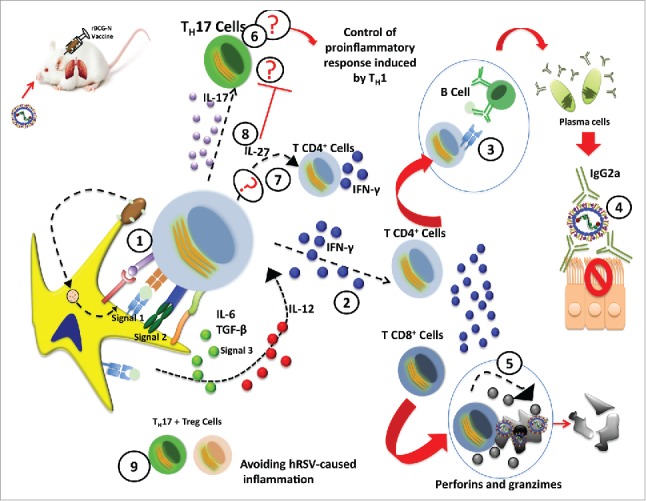
Proposed mechanism of the rBCG-N-hRSV vaccine that controls the hRSV-infection. Subsequent to the rBCG-N-hRSV infection and the viral infection, the events goes as follows. (1) Immunological synapsis between the APC (Dendritic cells) and T cells. (2) TH-1 response polarization and secretion of IFN-γ by CD4+ and CD8+ T cells. (3) Activation of B cells by the T cells. (4) Antibodies secretion by plasma cells with the proper neutralizing isotype. (5) Cytotoxic activity of the CD8+ T cell and depletion of infected-cells. (6) A TH-17 profile induction suggests a possible way of control for the exacerbated TH-1 response. (7) Possible IL-27 secretion and proliferation of CD4+ T cells and induction of IFN-γ secretion. (8) IL-27 secretion could inhibit the TH-17 response. (9) Relation between the TH-17+ Treg that might decrease the hRSV-pathology.
The rBCG-N-hRSV is currently the only hRSV vaccine under development that is intended for use in newborns, which is crucial since most of the hospitalized hRSV-infected children are younger than 6 months old. This vaccine could be an useful tool to prevent TB meningitis, miliary disease and hRSV infection in a single immunization event for this risk population. Along these lines, the rBCG-N-hRSV has also shown to be able to produce mycobacterial antigen-specific response as the wild type BCG counterpart.47 Besides the capacity to protect from lung pathology, the rBCG-N-hRSV is able to avoid the long-term behavioral impairment in hRSV-infected mice.47 These are promising results of an efficient and safe vaccine against the disease caused by 2 major respiratory pathogens, TB and hRSV.
Immune mechanisms accounting for the protection conferred by the rBCG-N-hRSV vaccine
Taking in account all the previously mentioned results observed in the rBCG-N-hRSV immunized mice, we propose the following model as the mechanism for the protection induced by the vaccine against hRSV. Upon rBCG-N-hRSV immunization, nucleoprotein expressed on the BCG vector is processed by DCs and presented to T cells (Fig. 2). Thereafter, proliferation and expansion of CD4+ and CD8+IFN-γ+ T cells specific for the N protein is triggered. After hRSV challenge, these T cells are activated and produce high levels of IL-12 and low levels of IL-10, inducing the differentiation into a TH-1 profile. We found high levels of IL-17 in vaccinated and infected mice but no inflammation or hypersensitivity. This result could be explained by the TH-17 cells repertoire that are counteracted by the production of IL-27, which plays a key role in promoting the IFN-γ secretion by CD4+ TH-1 cells and is characterized by inducing the inhibition of the TH-17 pathology associated with autoimmunity.48 Other possible explanation is that IL-17+ Treg cells are diminishing the hRSV-caused inflammation. The CD8+IFN-γ+ T cells found in vaccinated mice probably display cytotoxicity activity that depletes hRSV-infected cells through the perforin and granzime system. On the other hand, the CD4+ T cells present the N antigen and other proteins to B cells, which promotes their differentiation into plasma cells that secrete antibodies against several proteins of the virus with an IgG2a isotype. We have found high levels of antibodies against the nucleoprotein in vaccinated mice before hRSV challenge (unpublished data). Such result indicates that rBCG-N-hRSV could induce memory B cells, which rapidly proliferate and differentiate into plasma cells and after repetitively hRSV exposure, results in high a frequency of hRSV-specific memory B cells. Further, these antibodies neutralize the virus, resulting in a decrease of the viral replication in the lungs. This IgG2a isotype along with the TH-1 response reduces the recruitment of inflammatory cells to the lungs. In conclusion, the hRSV-associated immunopathology is prevented by rBCG-N-hRSV immunization in mice.
Disclosure of potential conflicts of interest
The authors declare no conflict of interest.
Funding
This work was supported by the Millennium Institute on Immunology and Immunotherapy from Chile (P09/016-F for AMK), CONICYT/FONDECYT POSTDOCTORADO No. 3160249, CONICYT DOCTORADO N°21151028, FONDECYT grants number: 1150862, 1070352, 1050979, 1040349, 1100926, 1110397, 1131012, 1140010, 1140011, 3140455. Biomedical Research Consortium (BMRC 13CTI-21526 for AMK). FONDEF grant D11I1080.
References
- [1].Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, O'Brien KL, Roca A, Wright PF, Bruce N, et al. . Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: A systematic review and meta-analysis. Lancet 2010; 375:1545–55; PMID:20399493; https://doi.org/ 10.1016/S0140-6736(10)60206-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Sweetman LL, Ng YT, Butler IJ, Bodensteiner JB. Neurologic complications associated with respiratory syncytial virus. Pediatr Neurol 2005; 32:307-10; PMID:15866430; https://doi.org/ 10.1016/j.pediatrneurol.2005.01.010 [DOI] [PubMed] [Google Scholar]
- [3].Bont L, Versteegh J, Swelsen WT, Heijnen CJ, Kavelaars A, Brus F, Draaisma JM, Pekelharing-Berghuis M, van Diemen-Steenvoorde RA, Kimpen JL. Natural reinfection with respiratory syncytial virus does not boost virus-specific t-cell immunity. Pediatr Res 2002; 52:363-7; PMID:12193668; https://doi.org/ 10.1203/00006450-200209000-00009 [DOI] [PubMed] [Google Scholar]
- [4].Hall CB. The burgeoning burden of respiratory syncytial virus among children. Infect Disord Drug Targets 2012; 12:92-7; PMID:22335498 [DOI] [PubMed] [Google Scholar]
- [5].PATH Respiratory syncytial virus. Vaccine development against a major cause of childhood respiratory illness Http://sites.Path.Org/vaccinedevelopment/respiratory-syncytial-virus-rsv. Accesed 17 febreary 2017 2016 [Google Scholar]
- [6].Hall CB, Walsh EE, Long CE, Schnabel KC. Immunity to and frequency of reinfection with respiratory syncytial virus. J Infect Dis 1991; 163:4; https://doi.org/ 10.1093/infdis/163.4.693 [DOI] [PubMed] [Google Scholar]
- [7].Spann KM, Tran KC, Collins PL. Effects of nonstructural proteins ns1 and ns2 of human respiratory syncytial virus on interferon regulatory factor 3, nf-kappab, and proinflammatory cytokines. J Virol 2005; 79:5353-62; PMID:15827150; https://doi.org/ 10.1128/JVI.79.9.5353-5362.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wright PF, Karron RA, Madhi SA, Treanor JJ, King JC, O'Shea A, Ikizler MR, Zhu Y, Collins PL, Cutland C, et al. . The interferon antagonist ns2 protein of respiratory syncytial virus is an important virulence determinant for humans. J Infect Dis 2006; 193:573-81; PMID:16425137; https://doi.org/ 10.1086/499600 [DOI] [PubMed] [Google Scholar]
- [9].Segovia J, Sabbah A, Mgbemena V, Tsai SY, Chang TH, Berton MT, Morris IR, Allen IC, Ting JP, Bose S. Tlr2/myd88/nf-κb pathway, reactive oxygen species, potassium efflux activates nlrp3/asc inflammasome during respiratory syncytial virus infection. PLoS One 2012; 7:e29695; PMID:22295065; https://doi.org/ 10.1371/journal.pone.0029695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Triantafilou K, Kar S, Vakakis E, Kotecha S, Triantafilou M. Human respiratory syncytial virus viroporin sh: A viral recognition pathway used by the host to signal inflammasome activation. Thorax 2013; 68:66-75; PMID:23229815; https://doi.org/ 10.1136/thoraxjnl-2012-202182 [DOI] [PubMed] [Google Scholar]
- [11].Munir S, Hillyer P, Le Nouen C, Buchholz UJ, Rabin RL, Collins PL, Bukreyev A. Respiratory syncytial virus interferon antagonist ns1 protein suppresses and skews the human t lymphocyte response. PLoS Pathog 2011; 7:e1001336; PMID:21533073; https://doi.org/ 10.1371/journal.ppat.1001336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Graaff P. Respiratory syncytial virus infection of monocyte-derived dendritic cells decreases their capacity to activate cd4 t cells. J Immunol 2005; 175:5904-11; PMID:16237083; https://doi.org/ 10.4049/jimmunol.175.9.5904 [DOI] [PubMed] [Google Scholar]
- [13].Gonzalez PA, Prado CE, Leiva ED, Carreno LJ, Bueno SM, Riedel CA, Kalergis AM. Respiratory syncytial virus impairs t cell activation by preventing synapse assembly with dendritic cells. Proc Natl Acad Sci U S A 2008; 105:14999-5004; PMID:18818306; https://doi.org/ 10.1073/pnas.0802555105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gonzalez PA, Bueno SM, Riedel CA, Kalergis AM. Impairment of t cell immunity by the respiratory syncytial virus: Targeting virulence mechanisms for therapy and prophylaxis. Curr Med Chem 2009; 16:4609-25; PMID:19903147; https://doi.org/ 10.2174/092986709789760724 [DOI] [PubMed] [Google Scholar]
- [15].Cespedes PF, Bueno SM, Ramirez BA, Gomez RS, Riquelme SA, Palavecino CE, Mackern-Oberti JP, Mora JE, Depoil D, Sacristan C, et al. . Surface expression of the hrsv nucleoprotein impairs immunological synapse formation with t cells. Proc Natl Acad Sci U S A 2014; 111:E3214-23; PMID:25056968; https://doi.org/ 10.1073/pnas.1400760111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bendelja K, Gagro A, Bace A, Lokar-Kolbas R, Krsulovic-Hresic V, Drazenovic V, Mlinaric-Galinovic G, Rabatic S. Predominant type-2 response in infants with respiratory syncytial virus (rsv) infection demonstrated by cytokine flow cytometry. Clin Exp Immunol 2000; 121:332-8; PMID:10931150; https://doi.org/ 10.1046/j.1365-2249.2000.01297.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Aoyagi M, Shimojo N, Sekine K, Nishimuta T, Kohno Y. Respiratory syncytial virus infection suppresses ifn-γ production of γδ t cells. Clin Exp Immunol 2003; 131:312-7; PMID:12562394; https://doi.org/ 10.1046/j.1365-2249.2003.02062.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zeng S, Wu J, Liu J, Qi F, Liu B. Il-33 receptor (st2) signalling is important for regulation of th2-mediated airway inflammation in a murine model of acute respiratory syncytial virus infection. Scand J Immunol 2015; 81:494-501; PMID:25721734; https://doi.org/ 10.1111/sji.12284 [DOI] [PubMed] [Google Scholar]
- [19].Bertrand P, Lay MK, Piedimonte G, Brockmann PE, Palavecino CE, Hernández J, León MA, Kalergis AM, Bueno SM. Elevated il-3 and il-12p40 levels in the lower airway of infants with rsv-induced bronchiolitis correlate with recurrent wheezing. Cytokine 2015; 76:417-23; PMID:26299549; https://doi.org/ 10.1016/j.cyto.2015.07.017 [DOI] [PubMed] [Google Scholar]
- [20].Mukherjee S, Lindell DM, Berlin AA, Morris SB, Shanley TP, Hershenson MB, Lukacs NW. Il-17-induced pulmonary pathogenesis during respiratory viral infection and exacerbation of allergic disease. Am J Pathol 2011; 179:248-58; PMID:21703407; https://doi.org/ 10.1016/j.ajpath.2011.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bystrom J, Al-Adhoubi N, Al-Bogami M, Jawad AS, Mageed RA. Th17 lymphocytes in respiratory syncytial virus infection. Viruses 2013; 5:777-91; PMID:23462708; https://doi.org/ 10.3390/v5030777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Fulton RB, Meyerholz DK, Varga SM. Foxp3+ cd4 regulatory t cells limit pulmonary immunopathology by modulating the cd8 t cell response during respiratory syncytial virus infection. J Immunol 2010; 185:2382-92; PMID:20639494; https://doi.org/ 10.4049/jimmunol.1000423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lee DC, Harker JA, Tregoning JS, Atabani SF, Johansson C, Schwarze J, Openshaw PJ. Cd25+ natural regulatory t cells are critical in limiting innate and adaptive immunity and resolving disease following respiratory syncytial virus infection. J Virol 2010; 84:8790-8; PMID:20573822; https://doi.org/ 10.1128/JVI.00796-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Durant LR, Makris S, Voorburg CM, Loebbermann J, Johansson C, Openshaw PJ. Regulatory t cells prevent th2 immune responses and pulmonary eosinophilia during respiratory syncytial virus infection in mice. J Virol 2013; 87:10946-54; PMID:23926350; https://doi.org/ 10.1128/JVI.01295-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lu X, McCoy KS, Xu J. Galectin-9 ameliorates respiratory syncytial virus-induced pulmonary immunopathology through regulating the balance between th17 and regulatory t cells. Virus Res 2015; 195:162-71; PMID:25451068; https://doi.org/ 10.1016/j.virusres.2014.10.011 [DOI] [PubMed] [Google Scholar]
- [26].de Almeida Nagata DE, Demoor T, Ptaschinski C, Ting HA, Jang S, Reed M, Mukherjee S, Lukacs NW. Il-27r-mediated regulation of il-17 controls the development of respiratory syncytial virus-associated pathogenesis. Am J Pathol 2014; 184:1807-18; PMID:24726498; https://doi.org/ 10.1016/j.ajpath.2014.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kim HW, Canchola JG, Brandt CD. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol 1969; 89:422-34; PMID:4305198; https://doi.org/ 10.1093/oxfordjournals.aje.a120955 [DOI] [PubMed] [Google Scholar]
- [28].Kruijsen D, Schijf MA, Lukens MV, van Uden NO, Kimpen JL, Coenjaerts FE, van Bleek GM. Local innate and adaptive immune responses regulate inflammatory cell influx into the lungs after vaccination with formalin inactivated rsv. Vaccine 2011; 29:2730-41; PMID:21316502; https://doi.org/ 10.1016/j.vaccine.2011.01.087 [DOI] [PubMed] [Google Scholar]
- [29].Carbonell-Estrany X, Simões EA, Dagan R, Hall CB, Harris B, Hultquist M, Connor EM, Losonsky GA; Motavizumab Study Group Motavizumab for prophylaxis of respiratory syncytial virus in high-risk children: A noninferiority trial. Pediatrics 2010; 125:e35-51; PMID:20008423; https://doi.org/ 10.1542/peds.2008-1036 [DOI] [PubMed] [Google Scholar]
- [30].Fernández P, Trenholme A, Abarca K, Griffin MP, Hultquist M, Harris B, Losonsky GA; Motavizumab Study Group A phase 2, randomized, double-blind safety and pharmacokinetic assessment of respiratory syncytial virus (rsv) prophylaxis with motavizumab and palivizumab administered in the same season. BMC Pediatr 2010; 10:38; PMID:20525274; https://doi.org/ 10.1186/1471-2431-10-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Robbie GJ, Criste R, Dall'acqua WF, Jensen K, Patel NK, Losonsky GA, Griffin MP. A novel investigational fc-modified humanized monoclonal antibody, motavizumab-yte, has an extended half-life in healthy adults. Antimicrob Agents Chemother 2013; 57:6147-53; PMID:24080653; https://doi.org/ 10.1128/AAC.01285-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Griffin MP, Khan AA, Esser MT, Jensen K, Takas T, Kankam MK, Villafana T, Dubovsky F. Safety, tolerability, and pharmacokinetics of the respiratory syncytial virus-prefusion f-targeting monoclonal antibody with an extended half-life, medi8897, in healthy adults. Antimicrob Agents Chemother 2017; 61(3):e01714-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Boyce TG, Mellen BG, Mitchel EF, Jr, Wright PF, Griffin MR. Rates of hospitalization for respiratory syncytial virus infection among children in medicaid. J Pediatr 2000; 137:865-70; PMID:11113845; https://doi.org/ 10.1067/mpd.2000.110531 [DOI] [PubMed] [Google Scholar]
- [34].Krarup A, Truan D, Furmanova-Hollenstein P, Bogaert L, Bouchier P, Bisschop IJ, Widjojoatmodjo MN, Zahn R, Schuitemaker H, McLellan JS, et al. . A highly stable prefusion rsv f vaccine derived from structural analysis of the fusion mechanism. Nat Commun 2015; 3:8143; https://doi.org/ 10.1038/ncomms9143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Smith G, Raghunandan R, Wu Y, Liu Y, Massare M, Nathan M. Respiratory syncytial virus fusion glycoprotein expressed in insect cells form protein nanoparticles that induce protective immunity in cotton rats. PLoS One 2012; 7(11):e50852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Cimica V, Boigard H, Bhatia B, Fallon JT, Alimova A, Gottlieb P, Galarza JM. Novel respiratory syncytial virus-like particle vaccine composed of the postfusion and prefusion conformations of the f glycoprotein. Clin Vaccine Immunol 2016; 23:451-9; PMID:27030590; https://doi.org/ 10.1128/CVI.00720-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Liang B, Munir S, Amaro-Carambot E, Surman S, Mackow N, Yang L, Buchholz UJ, Collins PL, Schaap-Nutt A. Chimeric bovine/human parainfluenza virus type 3 expressing respiratory syncytial virus (rsv) f glycoprotein: Effect of insert position on expression, replication, immunogenicity, stability, and protection against rsv infection. J Virol 2014; 88:4237-50; PMID:24478424; https://doi.org/ 10.1128/JVI.03481-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Xu J, Ding Y, Yang Y. Enhancement of mucosal and cellular immune response in mice by vaccination with respiratory syncytial virus DNA encapsulated with transfersome. Viral Immunol 2008; 21:483-90; PMID:19115938; https://doi.org/ 10.1089/vim.2008.0044 [DOI] [PubMed] [Google Scholar]
- [39].Hwang HS, Lee YT, Kim KH, Park S, Kwon YM, Lee Y, Ko EJ, Jung YJ, Lee JS, Kim YJ, et al. . Combined virus-like particle and fusion protein-encoding DNA vaccination of cotton rats induces protection against respiratory syncytial virus without causing vaccine-enhanced disease. Virology 2016; 494:215-24; PMID:27123586; https://doi.org/ 10.1016/j.virol.2016.04.014 [DOI] [PubMed] [Google Scholar]
- [40].Bueno SM, Gonzalez PA, Cautivo KM, Mora JE, Leiva ED, Tobar HE. Protective t cell immunity against respiratory syncytial virus is efficiently induced by recombinant bcg. Proc Natl Acad Sci USA 2008; 105:20822-7; PMID:19075247; https://doi.org/ 10.1073/pnas.0806244105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Cautivo KM, Bueno SM, Cortes CM, Wozniak A, Riedel CA, Kalergis AM. Efficient lung recruitment of respiratory syncytial virus-specific th1 cells induced by recombinant bacillus calmette-guerin promotes virus clearance and protects from infection. J Immunol 2010; 185:7633-45; PMID:21084664; https://doi.org/ 10.4049/jimmunol.0903452 [DOI] [PubMed] [Google Scholar]
- [42].Abubakar I, Pimpin L, Ariti C, Beynon R, Mangtani P, Sterne JA, Fine PE, Smith PG, Lipman M, Elliman D, et al. . Systematic review and meta-analysis of the current evidence on the duration of protection by bacillus calmette-guérin vaccination against tuberculosis. Health Technol Assess 2013; 17:1-372; PMID:24021245; https://doi.org/ 10.3310/hta17370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Marchant A, Goetghebuer T, Ota MO, Wolfe I, Ceesay SJ, De Groote D, et al. . Newborns develop a th1-type immune response to mycobacterium bovis bacillus calmette-guerin vaccination. J Immunol 1999; 163:2249-55; PMID:10438968 [PubMed] [Google Scholar]
- [44].Kativhu CL, Libraty DH. A model to explain how the bacille calmette guérin (bcg) vaccine drives interleukin-12 production in neonates. PLoS One 2016; 11:e0162148; PMID:27571272; https://doi.org/ 10.1371/journal.pone.0162148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Mahant A, Saubi N, Eto Y, Guitart N, Gatell JM, Hanke T, Joseph J. Preclinical development of bcg.Hiva2auxo.Int, harboring an integrative expression vector, for a hiv-tb pediatric vaccine. Enhancement of stability and specific hiv-1 t-cell immunity. Hum Vaccin Immunother 2017; [epub ahead of print]; PMID:28426273;doi: 10.1080/21645515.2017.1316911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Cautivo KM, Bueno SM, Cortes CM, Wozniak A, Riedel CA, Kalergis AM. Efficient lung recruitment of respiratory syncytial virusspecific th1 cells induced by recombinant bacillus calmette-guerin promotes virus clearance and protects from infection. J Immunol 2010; 185:7633-45; PMID:21084664;https://doi.org/ 10.4049/jimmunol.0903452 [DOI] [PubMed] [Google Scholar]
- [47].Céspedes PC, Rey-Jurado E, Espinoza JA, Rivera CA, Canedo-Marroquín G, Bueno SM, Kalergis A. A single, low dose of a cGMP recombinant BCG vaccine elicits protective T cell immunity against the human respiratory syncytial virus infection and prevents lung pathology in mice. Vaccine 2016; 35(5):757-66 [DOI] [PubMed] [Google Scholar]
- [48].Zhang J, Qian J, Ning H, Eickhoff CS, Hoft DF, Liu J. Transcriptional suppression of il-27 production by mycobacterium tuberculosis-activated p38 mapk via inhibition of ap-1 binding. J Immunol 2011; 186:5885-95; PMID:21482740; https://doi.org/ 10.4049/jimmunol.1003447 [DOI] [PMC free article] [PubMed] [Google Scholar]