Abstract
Primary chronic cold agglutinin disease (CAD) is an autoimmune haemolytic anaemia in which a specific bone marrow lymphoproliferative disorder causes production of cold agglutinins (CA). Binding of CA to erythrocyte surface antigens results in a predominantly extravascular haemolysis that is entirely complement dependent. Because of complement activation, exacerbations are common during febrile infections, trauma or major surgery. Involvement of the terminal complement pathway with C5-mediated intravascular haemolysis is probably not prominent in stable disease but is supposed to be of importance in exacerbations following acute phase reaction.
We report on a patient with CAD prone to exacerbation of haemolysis during acute phase reactions who was scheduled for cardiac surgery. To prevent her having an exacerbation of haemolysis, we chose to treat her prophylactically with eculizumab along with the usual perioperative precautions. Aortic valve replacement was undertaken with full cardiopulmonary bypass at normothermia. The procedure was successful; no exacerbation of haemolysis was observed, and transfusion requirements did not exceed what could be expected.
Keywords: Drug therapy related to surgery, Haematology (drugs and medicines), Haematology (incl blood transfusion)
Background
Cold agglutinins (CA) are antibodies that bind to erythrocyte surface antigens at low temperatures, causing agglutination and complement-mediated haemolysis.1 CA can be assessed by their titre, which reflects the ability to agglutinate erythrocytes at 4°C and the thermal amplitude (TA), defined as the highest temperature at which the CA reacts with the antigen. In general, the TA is more important than the titre with respect to pathogenicity.
The terms cold agglutinin disease (CAD) and cold agglutinin syndrome (CAS) are often used interchangeably but should be distinguished. CAD is a well-defined clinicopathological entity, whereas CAS should be used for secondary CA-mediated syndrome occasionally complicating specific infections or malignant diseases.1 In typical CAD, a low-grade lymphoproliferative bone marrow disorder (LPD) termed ‘CAD-associated LPD’ causes production of monoclonal CA with anti-I specificity.2 3 The underlying lymphoproliferative disorder has been studied by Randen et al 2 and a typical immunophenotype has been identified; CD20+, IgMs+, IgDs+, CD27+, CD5−/+, CD11c−, CD23−, CD38−. Our patient had the following immunophenotype; CD5+, CD10-, CD11c-, CD19+, CD20+, CD23+, CD43-, CD45+, IgM-kappa+.
All patients with CAD have haemolysis, but they are not always anaemic because haemolysis may be compensated, especially in stable disease. However, half of the patients will be transfusion dependent in periods. Other clinical features are cold-induced acrocyanosis and Raynaud phenomena (90%), and seasonal variations are characteristic in cool or temperate climates. Exacerbations of haemolysis are common (70%) during infections, and after trauma or major surgery.4–6
Cooling of blood during flow through acral parts of the body allows CA to bind to erythrocytes and agglutinate them. Being a strong complement activator, antigen-bound IgM-CA on the cell surface binds complement protein 1 (C1) initiating the classical complement pathway.1 7 The subsequent steps in the complement cascade explain why direct antiglobulin test (DAT) is strongly positive for C3d in patients with CA-mediated haemolysis and, in most cases, negative for IgM and IgG (figure 1). Complement activation may proceed beyond the C3b formation, resulting in C5 activation, formation of membrane attack complex (MAC) and intravascular haemolysis. This is usually controlled by CD55 and CD59 on the erythrocyte surface, and the intravascular haemolysis is usually limited. The major mechanism of haemolysis in stable disease, therefore, is the extravascular destruction of C3b-coated erythrocytes in the liver. During acute phase reactions, however, C5-mediated intravascular haemolysis may become significant.1 2 6 8 9
Figure 1.
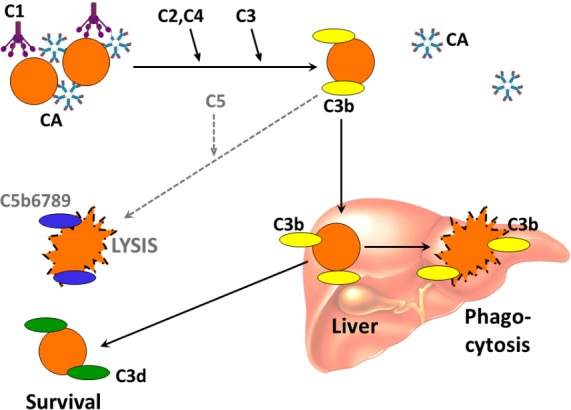
Complement-mediated haemolysis in chronic cold agglutinin disease and cold agglutinin syndrome. Black arrows indicate major pathway; dotted arrows indicate minor pathway. C, complement; CA, cold agglutinin. Previously published in Biomed Res Int (Berentsen S, Sundic T. Red blood cell destruction in autoimmune hemolytic anaemia: role of complement and potential new targets for therapy. Biomed Res Int 2015; doi10.1155/2015/363278). Reproduced with permission. Copyright: S Berentsen and T Sundic.
It has been suggested that the complement C5 inhibitor eculizumab may be used in CAD; and a small, prospective uncontrolled study demonstrated favourable effect, in particular by significantly reducing transfusion requirements among transfusion-dependent patients.10 Based on the relative importance of the classical complement pathway, C3 opsonisation with extravascular haemolysis and terminal pathway activation with intravascular haemolysis, as described above, one may hypothesise that eculizumab may be particularly useful during acute exacerbation related to infections, trauma and surgery.1 10
CA are antibodies that bind to erythrocyte surface antigens at low temperatures, causing agglutination and complement-mediated haemolysis.1 CA can be assessed by their titre, which reflects the ability to agglutinate erythrocytes at 4°C and the TA, defined as the highest temperature at which the CA reacts with the antigen. In general, the TA is more important than the titre with respect to pathogenicity.
The terms CAD and CAS are often used interchangeably but should be distinguished. CAD is a well-defined clinicopathological entity, whereas CAS should be used for secondary CA-mediated syndrome occasionally complicating specific infections or malignant diseases.1 In typical CAD, an LPD termed ‘CAD-associated LPD’ causes production of monoclonal CA with anti-I specificity.2 3
All patients with CAD have haemolysis, but they are not always anaemic because haemolysis may be compensated, especially in stable disease. However, half of the patients will be transfusion dependent in periods. Other clinical features are cold-induced acrocyanosis and Raynaud phenomena (90%), and seasonal variations are characteristic in cool or temperate climates. Exacerbations of haemolysis are common (70%) during infections, and after trauma or major surgery.4–6
Cooling of blood during flow through acral parts of the body allows CA to bind to erythrocytes and agglutinate them. Being a strong complement activator, antigen-bound IgM-CA on the cell surface binds complement protein 1 (C1) initiating the classical complement pathway.1 7 The subsequent steps in the complement cascade explain why DAT is strongly positive for C3d in patients with CA-mediated haemolysis and, in most cases, negative for IgM and IgG (figure 1). Complement activation may proceed beyond the C3b formation, resulting in C5 activation, formation of MAC and intravascular haemolysis. This is usually controlled by CD55 and CD59 on the erythrocyte surface, and the intravascular haemolysis is usually limited. The major mechanism of haemolysis in stable disease, therefore, is the extravascular destruction of C3b-coated erythrocytes in the liver. During acute phase reactions, however, C5-mediated intravascular haemolysis may become significant.1 2 6 8 9
It has been suggested that the complement C5 inhibitor eculizumab may be used in CAD; and a small, prospective uncontrolled study demonstrated favourable effect, in particular by significantly reducing transfusion requirements among transfusion-dependent patients.10 Based on the relative importance of the classical complement pathway, C3 opsonisation with extravascular haemolysis, and terminal pathway activation with intravascular haemolysis, as described above, one may hypothesise that eculizumab may be particularly useful during acute exacerbation related to infections, trauma and surgery.1 10
Case presentation
We report on the prophylactic use of eculizumab in a 76-year-old woman with CAD undergoing cardiac surgery. She was diagnosed with CAD in 1995 based on chronic haemolytic anaemia with a strongly positive DAT for C3d, negative for IgG and CA titre 16 384. Her pretreatment laboratory data were typically (February 2013) haemoglobin (hgb) 9.3 g/dL, reticulocytes 233×109/L, bilirubin 53 μmol/L, lactate dehydrogenase (LDH) 368 U/L and undetectable haptoglobin. Serum capillary electrophoresis revealed a monoclonal band of IgMk estimated to 4.5 g/L; total IgM 8.8 g/L. In February 2013, a bone marrow biopsy showed clonal lymphoproliferation classified as CAD-associated LPD with about 15% infiltration.2
In 1995–2015, she was hospitalised many times because of severe haemolytic anaemia triggered by febrile infections, requiring transfusions on every occasion. From March to June 2013 she received four cycles of bendamustine and rituximab without any immediate response. Two years later, however, she was transfusion independent, and following substitution treatment for secondary hypogammaglobulinaemia, she did not suffer severe recurrent infections. Apart from CAD, she had been diagnosed with eosinophilic pneumonia, for which she received low-dose prednisolone and aortic valve stenosis. Her haemolytic was non-responsive to prednisolone.
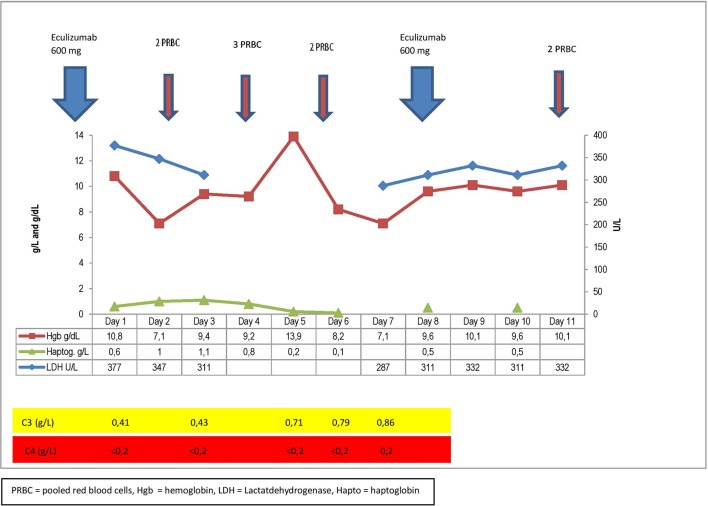
In the spring of 2015, her physical condition was severely impaired due to her heart condition, and aortic valve replacement was indicated. At that time, her hgb was 9.6 g/dL and LDH 345 U/L, bilirubin 51 μmol/L, haptoglobin <0.2 g/L and IgM 3.6 g/L (figure 2). Her previous medical history of severe of haemolysis during acute phase reactions indicated that she might suffer severe haemolysis postoperatively. Based on the critical role of complement activation in this setting, we considered eculizumab as a measure to prevent or reduce exacerbation of haemolysis following surgery.1 10
Figure 2.
Timeline showing the most important blood samples and actions during the time of operation.
She received 600 mg of eculizumab the day before the operation and a second dose (600 mg) 1 week later. The operation, which is usually done at hypothermia, was performed at normothermia due to her CAD and went successful. The postoperative course was uneventful apart from a short episode of seizures and reduced strength in her left arm. This was thought to be caused by cerebral ischaemia related to anaemia and resulted in extra transfusion of red blood cells. Her total transfusion requirements were nine units of packed red cells (PRC), which is well within the usual requirements for PRC following aortic valve replacement surgery in a patient being anaemic prior to surgery.11 Eculizumab controlled haemolysis and during the postoperative period haptoglobin was measurable and all parameters related to haemolysis were stable (figure 2). Prior to surgery she received 7.5 mg prednisolone daily which was increased to 15 mg daily from the day of surgery and the following 6 days.
Treatment
She received 600 mg of eculizumab the day before the operation and a second dose (600 mg) 1 week later. The operation, which is usually done at hypothermia, was performed at normothermia due to her CAD and went successful.
Outcome and follow-up
The postoperative course was uneventful apart from a short episode of seizures and reduced strength in her left arm. This was thought to be caused by cerebral ischaemia related to anaemia and resulted in extra transfusion of red cells. Her total transfusion requirements were nine units of PRC which is well within the usual requirements for PRC following aortic valve replacement surgery in a patient being anaemic prior to surgery.11 Eculizumab-controlled haemolysis and during the postoperative period, haptoglobin was measurable and all parameters were stable (figure 2). During the time from heart surgery until March 2017, the patient continued to have exacerbation of haemolysis associated with acute phase reactions.
Discussion
The diagnosis of primary CAD was well established by chronic haemolytic anaemia combined with a typical DAT pattern, high CA titre, monoclonal IgMκ in serum and the finding of CAD-associated LPD in a bone marrow biopsy.1 2 Determination of the TA was not done and might possibly have been of interest. Given the disease features, however, the TA was obviously high and clinically relevant.1
Seventy per cent of patients with CAD experience exacerbations of haemolytic anaemia during infections and following major trauma or surgery.1 8 9 These paradoxical exacerbations may be explained as follows: During steady state CAD, patients are complement depleted with low levels of C3 and particularly C4 as in our case, but during acute phase reactions, C3 and C4 are replete and complement-induced intravascular haemolysis increases by activation of C5. It is also important to notice that due to activation of the alternative pathway, the heart–lung machine activates the alternative complement pathway through ‘foreign body’ reaction when plastic comes in contact with blood. For our patient, this would complicate and further increase the complement activation and haemolysis and is another argument for prophylactic complement inhibition.
In this case, eculizumab was used prophylactically to prevent an exacerbation of haemolysis following major surgery. Despite the observation that C3 was replete, haemolysis was well controlled, as evidenced by measurable levels of haptoglobin throughout the postoperative period, compared with the unmeasurable haptoglobin levels prior to surgery. Although haptoglobin concentrations may, in principle, have been influenced by acute phase reaction, the levels of LDH and bilirubin even showed a partial normalisation. It may be argued that performing surgery at normothermia might be a sufficient measure to prevent exacerbation of CAD. However, exacerbations have been observed following major surgery per se9; the patient was without any doubt prone to acute phase-associated exacerbations; and the rise in C3 levels document an acute phase-induced complement activation. The fact that the patient received prednisolone for an unrelated disease is also worth noticing. Corticosteroids are generally ineffective in CAD, showing very low response rates, at least at low to moderate doses.1 6 Our patient was a non-responder to corticosteroids. She had several previous admissions to hospital due to exacerbation of her haemolytic anaemia during acute phase reactions, usually infections, with no effect of a modest increase in the dose of her corticosteroids. She had also been unresponsive to high-dose corticosteroids which she received as the initial treatment when she was diagnosed with haemolytic anaemia.
Therefore, prednisolone is very unlikely to have prevented postoperative exacerbation of CAD.
In conclusion, complement inhibition may be a valuable tool in the treatment of patients with CAD suffering paradoxical exacerbations during infections and following trauma or surgery. This report, however, does not permit the conclusion that eculizumab is the ideal drug for this purpose. In the future, complement modulation at a proximal level may prove still more efficient.7
To our knowledge, any similar cases have not been reported in the literature.
Learning points.
Haemolysis in chronic cold agglutinin disease is complement dependent.
Exacerbation with intravascular haemolysis occurs in a majority of patients during acute phase reaction.
Exacerbation of haemolysis during acute phase reaction may be prevented by blocking complement activation.
Footnotes
Contributors: ET planned, wrote the manuscript, provided figure 2 and put everything together. ØAV contributed on the surgical part of case with normal procedure. ØAV collected the data and summarised the report. SB was involved in planning, acquisition and design of figure and reporting and interpretation of data as expert on haemolysis. SB reviewed the paper. GET was also involved in planning, reporting, conception and design of the study. GET was also responsible in acquiring patients and case, and reviewing and interpreting clinical and paraclinical data.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Berentsen S, Randen U, Tjønnfjord GE. Cold agglutinin-mediated autoimmune hemolytic anemia. Hematol Oncol Clin North Am 2015;29:455–71. 10.1016/j.hoc.2015.01.002 [DOI] [PubMed] [Google Scholar]
- 2. Randen U, Trøen G, Tierens A, et al. . Primary cold agglutinin-associated lymphoproliferative disease: a B-cell lymphoma of the bone marrow distinct from lymphoplasmacytic lymphoma. Haematologica 2014;99:497–504. 10.3324/haematol.2013.091702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Małecka A, Trøen G, Tierens A, et al. . Immunoglobulin heavy and light chain gene features are correlated with primary cold agglutinin disease onset and activity. Haematologica 2016;101 10.3324/haematol.2016.146126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schubothe H. The cold hemagglutinin disease. Semin Hematol 1966;3:27–47. [PubMed] [Google Scholar]
- 5. Swiecicki PL, Hegerova LT, Gertz MA. Cold agglutinin disease. Blood 2013;122:1114–21. 10.1182/blood-2013-02-474437 [DOI] [PubMed] [Google Scholar]
- 6. Berentsen S, Ulvestad E, Langholm R, et al. . Primary chronic cold agglutinin disease: a population based clinical study of 86 patients. Haematologica 2006;91:460–6. [PubMed] [Google Scholar]
- 7. Shi J, Rose EL, Singh A, et al. . TNT003, an inhibitor of the serine protease C1s, prevents complement activation induced by cold agglutinins. Blood 2014;123:4015–22. 10.1182/blood-2014-02-556027 [DOI] [PubMed] [Google Scholar]
- 8. Ulvestad E. Paradoxical haemolysis in a patient with cold agglutinin disease. Eur J Haematol 1998;60:93–100. 10.1111/j.1600-0609.1998.tb01004.x [DOI] [PubMed] [Google Scholar]
- 9. Ulvestad E, Berentsen S, Mollnes TE. Acute phase haemolysis in chronic cold agglutinin disease. Scand J Immunol 2001;54:239–42. 10.1046/j.1365-3083.2001.00960.x [DOI] [PubMed] [Google Scholar]
- 10. Röth A, Bommer M, Hüttmann A, et al. . Complement inhibition with Eculizumab in patients with Cold Agglutinin Disease (CAD): Results from a prospective phase II trial (DECADE trial). 57th annual meeting of the American Society of Hematology, Orlando, FL, USA: dec 5, 2015. Blood 2015;126:1–274.26138534 [Google Scholar]
- 11. Barbara DW, Mauermann WJ, Neal JR, et al. . Cold agglutinins in patients undergoing cardiac surgery requiring cardiopulmonary bypass. J Thorac Cardiovasc Surg 2013;146:668–80. 10.1016/j.jtcvs.2013.03.009 [DOI] [PubMed] [Google Scholar]