Figure 1.
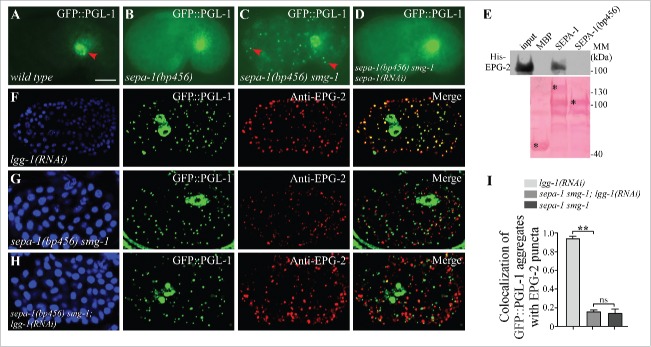
Direct interaction of EPG-2 with SEPA-1 is essential for the degradation of PGL granules. (A) In wild-type embryos, GFP::PGL-1 is absent in somatic cells and is only observed in germline precursor cells (arrowhead). (B) In sepa-1(bp456) mutant embryos, GFP::PGL-1 is diffusely localized in somatic cells. (C) In sepa-1(bp456) smg-1 mutant embryos, GFP::PGL-1 aggregates are formed in somatic cells (examples are indicated by arrowheads). (D) Depletion of sepa-1 by RNAi in sepa-1(b456) smg-1 mutant embryos results in diffuse localization of GFP::PGL-1 in somatic cells. The phenotypes shown in (B to D) are fully penetrant. (E) MBP-SEPA-1(bp456), the truncated protein in sepa-1(bp456), fails to interact with His-EPG-2 in an affinity isolation assay. *indicates corresponding fusion proteins. Other bands may result from partial degradation of purified proteins. (F to I) GFP::PGL-1 granules are colocalized with EPG-2 in lgg-1(RNAi) mutant embryos (F), but are separable from EPG-2 aggregates in sepa-1 smg-1 mutant embryos (G) and in sepa-1 smg-1; lgg-1(RNAi) mutant embryos (H). Quantification of the colocalization of GFP::PGL-1 aggregates and EPG-2 puncta in lgg-1(RNAi), sepa-1 smg-1 and sepa-1 smg-1; lgg-1(RNAi) embryos at the ∼100 cell stage is shown in (I), n = 3 focal planes from 3 embryos, data are shown as mean ± SD, **P < 0.01, ns, no significant difference. DAPI images of the embryos are show in the left panels. Scale bar: 10 μm (A to D, F to H).