Abstract
Spinal cord ischemia is a fatal complication following thoracoabdominal aortic aneurysm surgery. Researchers can investigate the strategies for preventing and treating this complication using experimental models of spinal cord ischemia. The model described here demonstrates varying degrees of paraplegia that relate to the length of occlusion following thoracic aortic occlusion in a rat spinal cord ischemia model.
A 2-Fr. balloon-tipped catheter was advanced through the femoral artery into the descending thoracic aorta until the catheter tip was placed at the left subclavian artery in anesthetized male Sprague-Dawley rats. Spinal cord ischemia was induced by inflating the catheter balloon. After a set period of occlusion (9, 10, or 11 min), the balloon was deflated. Neurologic assessment was performed using the motor deficit index at 24 h after surgery, and the spinal cord was harvested for histopathological examination.
Rats that underwent 9 min of aortic occlusion showed mild and reversible motor impairment in the hind limb. Rats subjected to 10 min of aortic occlusion presented with moderate but reversible motor impairment. Rats subjected to 11 min of aortic occlusion displayed complete and persistent paralysis. The motor neurons in the spinal cord sections were more preserved in rats subjected to shorter duration of aortic occlusion.
Researchers can achieve a reproducible hind limb motor deficit following thoracic aortic occlusion using this spinal cord ischemia model.
Keywords: Medicine, Issue 125, Spinal cord, thoracic aorta, rat, ischemia, paraplegia, occlusion time
Introduction
Paraplegia is a fatal complication of thoracoabdominal aortic aneurysm surgery. It results from spinal cord ischemia-reperfusion injury that occurs during cross-clamping and unclamping of the aorta.1 Several strategies including systemic hypothermia and cerebrospinal drainage have been introduced to protect the spinal cord,2,3,4 but many patients remain affected by the injury.
Several animal spinal cord ischemia models have been introduced to investigate its pathogenesis and devise protective strategies against the injury. In the current study, we outline a rat model of spinal cord ischemia based on Taira and Marsala's method.5 The spinal circulation system in rats is very similar to the spinal cord vascular and collateral system in humans, although there are some differences in the size and location.6,7 Thus, a rat is an anatomically suitable animal to utilize for an experimental model investigating the pathogenesis, complications, and treatment of spinal cord ischemia. Moreover, this spinal cord ischemia model produces reliable aortic occlusion with minimal intervention by utilizing an intravascular balloon occlusion of the thoracic aorta.
In this study, we demonstrated that this rat model of spinal cord ischemia induces reproducible motor deficits in the hind limbs that vary in severity depending on the aortic occlusion time.
Protocol
This protocol was approved by the Institutional Animal Care and Use Committee of Seoul National University Bundang Hospital. Animal care and experiments were conducted according to the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals.
1. Surgical Preparation
Prior to surgery, flush the catheters with sterile saline to ensure patency.
Put a heating blanket on the operating table, and cover the table with a sterile drape.
Place Male Sprague−Dawley rats (270-330 g) in an acrylic box with 3.0%-4.0% of isoflurane in 100% oxygen.
Apply lubricant to the rat's eyes.
Place the rat on the operating table in the supine position, and maintain anesthesia using a facial mask with continuous administration of inhaled isoflurane (1.0%-2.5 vol.%).
Place a rectal probe to monitor and maintain body temperature between 37.0-38.0 °C.
2. Femoral Artery Catheterization
Gently scrub the right inguinal area using Betadine and 70% ethanol.
Make a 2-cm horizontal skin incision using scissors on the right inguinal area.
Using a retractor, expose the surgical field.
Dissect the femoral artery from the surrounding vein and nerve. Isolate a 1-cm section of the artery using curved forceps and blunted forceps.
Using a 4.0 black silk suture, place a loose tie on both the proximal and distal ends of the artery to maximize the exposure.
Make an incision on the femoral artery using micro-scissors.
Insert a 2-Fr. balloon-tipped catheter into the femoral artery using micro-forceps. Secure the catheter to the vessel about 1 cm from the catheter head with the proximal ligature, and then tie the distal ligature.
3. Carotid Artery Catheterization
Scrub the right anterior neck using betadine and 70% ethanol, and then make a skin incision.
Using a retractor, expose the surgical field. Isolate a 1-cm section of the artery, and tie it loosely using a silk suture on both the proximal and distal ends of the artery to maximize the exposure.
Puncture the carotid artery using a 24 G intravenous catheter, and advance the proximal 1 cm of the catheter towards the heart.
Secure the catheter with the proximal ligature, and then tie the distal ligature.
Using a 3-way stopcock, connect the distal end of the catheter to the saline-filled micro-tube and external reservoir.
4. Tail Artery Catheterization
Scrub the ventral area of the tail using betadine and 70% ethanol, and make a 2-cm skin incision.
Dissect the tail artery from the surrounding structures and isolate a 1 cm section of the artery using curved forceps and blunted forceps.
Using a 4.0 black silk suture, place a loose tie on both the proximal and distal ends of the artery to maximize the exposure.
Puncture the tail artery using a 24 G intravenous catheter, and advance the catheter into the artery.
Secure the catheter to the vessel (about 1 cm from the catheter head) with the proximal ligature, and tie the distal ligature.
Connect the distal part of the catheter to the arterial pressure monitoring device.
5. Induction of Spinal Cord Ischemia
After catheterization is complete, advance the 2 Fr. balloon-tipped catheter into the descending thoracic aorta so that the tip of the catheter reaches the left subclavian artery.
Insert to a depth of 10 cm from the insertion site.
Inject 150 units of heparin at a concentration of 100 units/mL into the carotid catheter.
Inflate the catheter balloon with 0.05 mL of saline.
Simultaneously, drain the blood into the external blood container from the carotid artery to regulate the proximal arterial pressure to 80 mmHg.
Connect the arterial pressure monitoring system to the remaining lumen of the 3-way stop cock, and control the amount of blood drainage with monitoring the arterial pressure.
Confirm the success of aortic occlusion by an abrupt decrease and continuous loss of distal arterial pressure.
After aortic occlusion of 9, 10, or 11 min, deflate the Fogarty catheter balloon, and reinfuse the drained blood.
6. Post-surgical Care and Neurologic Assessment
While monitoring the arterial pressure through the tail artery, remove the catheters from the femoral and carotid arteries and close the wounds with silk sutures.
After confirming that the arterial pressure is recovered to the normal range, remove the catheter from the tail artery and close the wound.
After recovery from the anesthesia, return the rat to its cage.
Assess hind limb motor function at 24 h after surgery using the motor deficit index (Table 1). The motor deficit index is defined as the sum of the ambulation score and the placing/stepping reflex score. A motor deficit index of 6 indicates the maximum deficit.
7. Histopathologic Evaluation
After the neurologic assessment, anesthetize rats with mask-delivered isoflurane, sacrifice them by transcardial perfusion with 100 mL of heparinized saline under anesthesia, and extract the spinal cord.8
Embed the cord sections from the level of lumbar vertebrae 3-5 in paraffin.
Stain transverse sections with hematoxylin and eosin (H & E).
Observe the motor neuron injury under a microscope.9
8. Statistical Analysis
Perform statistical analysis using Statistical Package for the Social Sciences version 20. The motor deficit index of the three groups was compared using the Kruskal-Wallis test, followed by a Mann-Whitney U-test. A P-value <0.05 was considered statistically significant.
Representative Results
During a period of spinal cord ischemia, aortic occlusion was performed for 9 min (n=3), 10 min (n=3), or 11 min (n=3). The motor deficit index in rats is presented in Table 2. Rats that underwent 9 min of aortic occlusion showed a mild and reversible motor impairment in the hind-limb. Rats subjected to 10 min of aortic occlusion presented with moderate motor deficit, but not complete paralysis. Rats that underwent 11 min of occlusion time displayed complete and persistent paralysis.
Representative photographs of spinal cord sections stained with H & E are shown in Figure 1. The motor neurons in the spinal cord sections were more preserved in rats subjected to a shorter duration of aortic occlusion.
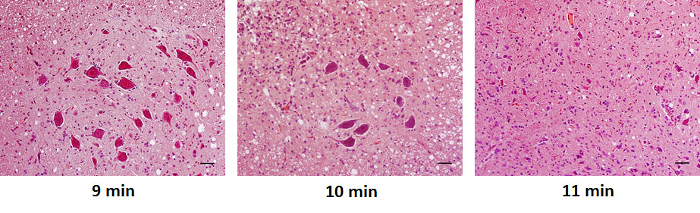
Figure 1. Histologic Examination of Spinal Cord Sections The motor neurons were more preserved in rats subjected to a shorter duration of aortic occlusion (original magnification, 200X). Scale bar in all images = 50 µm. Please click here to view a larger version of this figure.
| Ambulation (walking with lower extremities) | Placing/stepping reflex |
| 0: normal (symmetrical and coordinated ambulation) | 0: normal |
| 1: toes flat under body when walking but ataxia present | 1: weak |
| 2: knuckle-walking | 2: no stepping |
| 3: movement in lower extremities but unable to knuckle-walk | |
| 4: no movement, drags lower extremities |
Table 1. Evaluation of Ambulation and the Placing/stepping Reflex.
| Aortic occlusion time | 24 h after surgery |
| 9 min (n = 3) | 2 (2 − 3) |
| 10 min (n = 3) | 4 (4 − 4) |
| 11 min (n = 3) | 5 (5 − 6) |
Table 2. Motor Deficit Index. Values are presented as median (interquartile range).
Discussion
In the current study, we demonstrated a rat model of spinal cord ischemia based on Taira and Marsala's method5 that induces variable degrees of motor deficit in the hind limb depending on the aortic occlusion time.
The length of aortic occlusion can affect the degree of motor deficit. If the aortic occlusion time is longer, the motor deficit becomes more severe. Thus, researchers can achieve a certain degree of motor deficit by controlling the aortic occlusion time in this model.
Our model involves the ligation of the common carotid artery and the femoral artery, and the possibility of neurologic deficits resulting from the ligation of these arteries is a potential concern. However, rats have efficient collateral network systems. Thus, when the carotid artery or femoral artery is ligated, sufficient blood flow can be provided by the extensive collateral network. Unilateral carotid artery occlusion is reported to produce only minor effects on cerebral blood flow.10,11 Although unilateral carotid artery occlusion can produce stroke in rats, this occurs only when in combination with severe systemic hypoxia.12 During our experiment, no systemic hypoxia occurred and none of the rats presented with neurological deficits suggestive of cerebral infarction. Furthermore, when the femoral artery of rats is ligated, the collateral circulation provides sufficient blood flow to the hind limb muscles.13 This collateral circulation provides sufficient blood flow to the hind limb muscles when they are at rest, but does not provide sufficient flow during exercise.14
Furthermore, the proximal arterial pressure during spinal cord ischemia affects the development of motor deficits. According to a previous study,5 the collateral blood supply almost disappeared at the proximal arterial pressure of 40 mmHg during aortic clamping in a rat spinal ischemia model. Thus, subsequent studies maintained the proximal arterial pressure at 40 mmHg during spinal cord ischemia in their models of rat spinal cord ischemia.9,15,16 However, in this protocol, we maintained the proximal arterial pressure at 80 mmHg during aortic occlusion because it is recommended to maintain the mean arterial pressure at 80 mmHg or greater for preserving adequate spinal cord perfusion during spinal cord ischemia in the clinical practice,17 although there might be a difference in what constitutes an adequate proximal arterial pressure between humans and rodents.
The spinal cord vasculature and collateral system of rats and humans are similar,6,7 which makes rats an appropriate choice for an experimental spinal cord ischemia model. However, it should not be discounted that the results may be different according to the species in which spinal cord ischemia occurs.
In conclusion, researchers can easily adopt this rat model of spinal cord ischemia and achieve highly reproducible findings. Furthermore, they can modify the aortic occlusion time to vary the degree of motor deficit produced. As such, this model can facilitate further studies examining the underlying pathophysiology of neurologic complications following thoracoabdominal aortic aneurysm, and allow the development of neuroprotective strategies against these complications.
Disclosures
The authors have no competing financial interests. This work was supported by grant 2012R1A1A3014010 from the National Research Foundation of Korean Government.
Acknowledgments
The authors have no acknowledgements.
References
- Greenberg RK, et al. Contemporary analysis of descending thoracic and thoracoabdominal aneurysm repair: a comparison of endovascular and open techniques. Circulation. 2008;118(8):808–817. doi: 10.1161/CIRCULATIONAHA.108.769695. [DOI] [PubMed] [Google Scholar]
- Okita Y. Fighting spinal cord complication during surgery for thoracoabdominal aortic disease. Gen Thorac Cardiovasc Surg. 2011;59(2):79–90. doi: 10.1007/s11748-010-0668-x. [DOI] [PubMed] [Google Scholar]
- Fleck TM, et al. Improved outcome in thoracoabdominal aortic aneurysm repair: the role of cerebrospinal fluid drainage. Neurocrit Care. 2005;2(1):11–16. doi: 10.1385/NCC:2:1:011. [DOI] [PubMed] [Google Scholar]
- Kouchoukos NT, et al. Hypothermic bypass and circulatory arrest for operations on the descending thoracic and thoracoabdominal aorta. Ann Thorac Surg. 1995;60(1):67–76. [PubMed] [Google Scholar]
- Taira Y, Marsala M. Effect of proximal arterial perfusion pressure on function, spinal cord blood flow, and histopathologic changes after increasing intervals of aortic occlusion in the rat. Stroke. 1996;27(10):1850–1858. doi: 10.1161/01.str.27.10.1850. [DOI] [PubMed] [Google Scholar]
- Tveten L. Spinal cord vascularity. III. The spinal cord arteries in man. Acta Radiol Diagn (Stockh) 1976;17(3):257–273. doi: 10.1177/028418517601700301. [DOI] [PubMed] [Google Scholar]
- Woollam DH, Millen JW. The arterial supply of the spinal cord and its significance. J Neurol Neurosurg Psychiatry. 1955;18(2):97–102. doi: 10.1136/jnnp.18.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy HS, Puth F, Van Hoy M, Le Pichon C. A method for removing the brain and spinal cord as one unit from adult mice and rats. Lab Anim (NY) 2011;40(2):53–57. doi: 10.1038/laban0211-53. [DOI] [PubMed] [Google Scholar]
- Umehara S, Goyagi T, Nishikawa T, Tobe Y, Masaki Y. Esmolol and landiolol, selective β1 adrenoreceptor antagonists, provide neuroprotection against spinal cord ischemia and reperfusion in rats. Anesth Analg. 2010;110(4):1133–1137. doi: 10.1213/ANE.0b013e3181cdb06b. [DOI] [PubMed] [Google Scholar]
- De Ley G, Nshimyumuremyi JB, Leusen I. Hemispheric blood flow in the rat after unilateral common carotid occlusion: evolution with time. Stroke. 1985;16(1):69–73. doi: 10.1161/01.str.16.1.69. [DOI] [PubMed] [Google Scholar]
- Coyle P, Panzenbeck MJ. Collateral development after carotid artery occlusion in Fischer 344 rats. Stroke. 1990;21(2):316–321. doi: 10.1161/01.str.21.2.316. [DOI] [PubMed] [Google Scholar]
- Levine S. Anoxic-ischemic encephalopathy in rats. Am J Pathol. 1960;36:1–17. [PMC free article] [PubMed] [Google Scholar]
- Prior BM, et al. Time course of changes in collateral blood flow and isolated vessel size and gene expression after femoral artery occlusion in rats. Am J Physiol Heart Circ Physiol. 2004;287(6):H2434–H2447. doi: 10.1152/ajpheart.00398.2004. [DOI] [PubMed] [Google Scholar]
- Yang HT, Feng Y, Allen LA, Protter A, Terjung RL. Efficacy and specificity of bFGFincreased collateral flow in experimental peripheral arterial insufficiency. Am J Physiol Heart Circ Physiol. 2000;278(6):H1966–H1973. doi: 10.1152/ajpheart.2000.278.6.H1966. [DOI] [PubMed] [Google Scholar]
- Kakinohana M, Fuchigami T, Nakamura S, Sasara T, Kawabata T, Sugahara K. Intrathecal administration of morphine, but not small dose, induced spastic paraparesis after a noninjurious interval of aortic occlusion in rats. Anesth Analg. 2003;96(3):769–775. doi: 10.1213/01.ANE.0000048855.24190.5F. [DOI] [PubMed] [Google Scholar]
- Horiuchi T, et al. The effects of the delta-opioid agonist SNC80 on hind-limb motor function and neuronal injury after spinal cord ischemia in rats. Anesth Analg. 2004;99(1):235–240. doi: 10.1213/01.ANE.0000130389.77859.1C. [DOI] [PubMed] [Google Scholar]
- Griepp RB, Griepp EB. Spinal cord perfusion and protection during descending thoracic and thoracoabdominal aortic surgery: the collateral network concept. Ann Thorac Surg. 2007;83(2):S865–S869. doi: 10.1016/j.athoracsur.2006.10.092. [DOI] [PubMed] [Google Scholar]


