Abstract
Study Objectives:
Although drug-induced sleep endoscopy (DISE) is an accepted method to localize upper airway obstruction, it is not known whether all sites identified by DISE must be treated to achieve sufficient apnea-hypopnea index (AHI) improvement. The aim of this study was to compare outcomes for unilevel (upper) versus multilevel DISE collapse patterns in a patient cohort that only underwent modern palatopharyngoplasty. Our hypothesis was that not all tongue base-level obstructions on DISE must be treated.
Methods:
Thirty-eight patients with mean AHI of 45 events/h underwent DISE followed by palatopharyngoplasty. Outcome was measured by polysomnography or home sleep apnea testing.
Results:
Eleven patients (29%) had multilevel, complete tongue base obstruction and nineteen (50%) had no obstruction. These two groups were similar in age, body mass index, and AHI; the complete group had smaller tonsils and higher tongue position. The postoperative success rate and AHI in the group without tongue base obstruction were not significantly different from those of the complete group (68%; 17.4 ± 11.0 versus 73%; 15.4 ± 20.5, P > .99). Seventeen patients (45%) had circumferential collapse of velum. The postoperative AHI was higher for patients with circumferential collapse (23.6 ± 15.8 from 55.3 ± 22.1 versus 10.5 ± 9.94 from 36.4 ± 16.7, P < .0001), but both groups had clinically and statistically significant AHI reductions.
Conclusions:
Patients with multilevel obstruction on DISE, treated with palatopharyngoplasty alone, had similar AHI outcome as those with unilevel obstruction. Multilevel surgery may not be needed in some patients with a multilevel obstruction pattern. Circumferential collapse of velum, however, was associated with a higher residual AHI.
Citation:
Hsu YS, Jacobowitz O. Does sleep endoscopy staging pattern correlate with outcome of advanced palatopharyngoplasty for moderate to severe obstructive sleep apnea? J Clin Sleep Med. 2017;13(10):1137–1144.
Keywords: drug-induced sleep endoscopy, expansion sphincter pharyngoplasty, lateral pharyngoplasty, obstructive sleep apnea, transpalatal advancement pharyngoplasty
INTRODUCTION
Obstructive sleep apnea (OSA) is a prevalent disorder1 that is associated with excessive daytime sleepiness2 and with an increased risk for sudden cardiac death,3 hypertension,4 cerebrovascular incidents,5 and type 2 diabetes.6 Continuous positive airway pressure (CPAP) is often used as the first-line treatment for OSA.7 However, long-term adherence to CPAP treatment is often suboptimal,8,9 prompting a substantial proportion of patients with OSA to seek alternatives such as upper airway surgery.
To be effective, upper airway surgery should target the sites and structures that mediate obstruction. Multiple sites may often be involved; thus, a multilevel surgery approach has been used successfully by many, including the corresponding author,10 and advocated in literature reviews.11,12 Patient selection and choice of procedure for multilevel surgery has traditionally relied on static and dynamic examination of the patient while awake, and thus may not accurately predict sites of obstruction during the state of sleep.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Drug-induced sleep endoscopy (DISE) is an accepted method to localize the site of upper airway obstruction, but there are few data regarding correlation with surgical outcome. It is not known whether all sites and collapse zones identified by DISE must always be treated to achieve sufficient apnea-hypopnea index (AHI) improvement. This study assessed AHI outcome in patients who underwent palatopharyngoplasty alone regardless of unilevel or multilevel obstruction on DISE.
Study Impact: This study showed that for both unilevel or multilevel obstruction patterns on DISE, significant and similar AHI improvement can be achieved using palatopharyngoplasty techniques alone. This may indicate that some DISE obstructions are secondary and could be treated, if needed, in a staged manner for some patients.
To better identify the loci of obstruction, drug-induced sleep endoscopy (DISE) has been used for more than two decades to help direct surgery and oral appliance treatment for patients with OSA.13,14 Though it is presumed that by using DISE one would be able to better select surgical procedures for specific collapse sites, outcome data for surgeries predicated on DISE are sparse.15,16 In most studies, the patient population underwent multilevel surgery and thus the effect of individual procedures on collapse patterns is not known. In addition, without postoperative DISE examinations, it is likely that some unsuccessful outcomes of palatopharyngoplasty may have resulted from incomplete treatment of palatal level obstruction17 and are not necessarily due to tongue base or epiglottic obstruction noted on DISE.
The more recent palatopharyngoplasty procedures, including lateral pharyngoplasty (LP), expansion sphincter pharyngoplasty (ESP), and transpalatal advancement pharyngoplasty (TAP) appear to be more effective18–20 than traditional uvulopalatopharyngoplasty (UPPP). The lateral pharyngeal wall palatopharyngoplasty procedures, LP and ESP, differ from UPPP in that they are not soft palatal shortening techniques, but rather repositioning procedures for the lateral wall muscles and result in enlargement of the transverse pharyngeal airway. The TAP procedure also does not shorten the soft palate as does UPPP, but advances the soft palate anteriorly and expands the retropalatal space via trimming of the posterior hard palate.
We selected to analyze AHI outcome of these modern upper pharyngeal procedures as it relates to DISE findings. A patient cohort underwent DISE immediately prior to palatopharyngoplasty procedures, and regardless of unilevel (upper) or multilevel (upper and lower) pharyngeal obstruction pattern, only underwent palatopharyngoplasty. We retrospectively assessed the DISE collapse pattern as it related to AHI improvement outcome.
Our assumption was that for some patients, despite lower pharyngeal collapse pattern on DISE, upper palatopharyngoplasty alone may suffice, possibly via a postoperative reduction in the pressure gradient for collapse of downstream sites.21
METHODS
Study Subjects
The study assessed a group of patients with OSA who underwent propofol-induced sleep endoscopy and palatopharyngoplasty by a single surgeon (Dr. Ofer Jacobowitz) from 2008 to 2016 at Orange Regional Medical Center (Middletown, New York, United States) and New York Eye & Ear Infirmary (New York, New York, United States). Inclusion criteria were: (1) AHI or respiratory event index on baseline attended polysomnogram (PSG) or home sleep apnea testing study > 15 events/h. (2) Follow-up sleep study performed at least 2 months after upper airway surgery. (3) No prior upper airway surgery other than nasal or tonsillectomy. (4) Patients were nonadherent to CPAP therapy under the care of a board-certified sleep medicine specialist. (5) Clinically significant nasal obstruction was not present or was already treated. The protocol was approved by the hospital's institutional review board.
The overnight standard PSG or unattended home sleep apnea testing study was performed and scored in accordance with American Academy of Sleep Medicine's 2007 or 2012 definitions,22,23 using the same scoring criteria for given patients' preoperative and postoperative studies. Studies were interpreted by a board-certified sleep physician.
Drug-Induced Sleep Endoscopy
All patients underwent DISE in the supine position in the surgical theatre immediately prior to palatopharyngoplasty. Any hypopharyngeal surgery was to be performed at a later stage, if needed, and thus DISE was performed for informational purposes only. A topical vasoconstrictor (oxymetazoline) was applied to both nostrils. Propofol was administered by the anesthetist as the sole agent to achieve a target level of anesthesia of absent arousal to loud verbal stimulation.24 In brief, an initial bolus of propofol 1 mg/kg was given, followed by infusion at 50 to 75 μg/kg/min, and the rate was adjusted and/ or small boluses were given to meet the target level of anesthesia. When achieved, a flexible endoscope was introduced into the nasal cavity and the examination video was recorded. The nasal passage, nasopharynx, velum, oropharynx, tongue base, epiglottis, and larynx were observed, and the level of obstruction during inspiration was assessed.
VOTE Classification and Staging Level Definitions
DISE findings was scored using the VOTE classification system.25 Accordingly, three parameters were reported: (1) site of obstruction (velum, oropharynx, tongue base, and/or epiglottis), (2) degree of obstruction (< 50% of narrowing corresponds to no/mild obstruction, 50% to 75% of narrowing corresponds to partial obstruction, > 75% of narrowing corresponds to complete obstruction), and (3) configuration of obstruction (anteroposterior, circumferential, or lateral). Scoring was performed by the authors by consensus, retrospectively, in a manner blinded to outcome.
We defined the unilevel obstruction group as having no tongue base obstruction or < 50% obstruction pattern. The multilevel group was defined as having complete tongue base or epiglottic obstruction.
Palatopharyngoplasty Surgery and Success Definition
All patients underwent palatopharyngoplasty for OSA, which included one or a combination of the following procedures: ESP,18 LP,19 and TAP.26 TAP was performed for proximal retropalatal airway anteroposterior narrowing as assessed by fiber-optic endoscopy while awake20 and confirmed by posterior or circumferential collapse on DISE. ESP and LP were performed for lateral retropalatal narrowing as assessed by fiberoptic endoscopy while awake, confirmed by lateral to medial collapse on DISE. LP replaced ESP in 2015–2016 as a preferred lateral pharyngeal wall technique.
ESP was performed beginning with coblation-assisted tonsillectomy or if no tonsils were present, via coblation-assisted exposure of the palatopharyngeus in the tonsillar fossae. The palatopharyngeus muscle was partially divided in the lower third of the tonsillar fossa and separated from the superior constrictor using coblation. Medial fibers of the palatopharyngeus were preserved to maintain attachment to the palatal pillar mucosa. The palatopharyngeus was then rotated laterally-superiorly and suture-suspended to the perihamular dense connective tissue. Uvular shortening to 1 cm length was performed as needed. Myomucosal rotation flaps from the periuvular area were often used for closure of any mucosal defects. LP was performed as described by Cahali et al.,19 except that coblation was used for muscle division. Briefly, the palatopharyngeus muscle was exposed and separated using coblation from the superior constrictor muscle. The palatopharyngeus was partially lysed inferiorly and not rotated upward. Only a limited 1-cm superior constrictor muscle division was performed, followed by layered suture closure. Uvular shortening to 1-cm length was performed as needed. Vertical posterior wall-releasing incisions were performed.
Surgical AHI “success” was defined as a postoperative AHI of < 20 events/h along with at least 50% decrease from the baseline AHI.
Statistical Analysis
Statistical analysis was performed using commercially available software (IBM SPSS statistics 22 for Windows; SPSS Inc., Chicago, Illinois, United States). Quantitative data are reported as mean ± standard deviation. Due to small sample size, we used nonparametric tests to compare data both between groups or before and after surgery. The significance of the changes after the surgical procedures, in each group, was verified with the nonparametric Wilcoxon signed-rank test. Comparisons between groups were made with the Mann-Whitney U test. Differences in categorical values were assessed by Fisher exact test. A P value of .05 or below was set to indicate significance.
RESULTS
Demographics and Procedures
Of the 38 patients who met the inclusion criteria from July 2008 to July 2016, 20 patients (53%) underwent ESP, 8 patients (21%) underwent LP, 1 patient (3%) underwent TAP, and 9 patients (24%) underwent both ESP and TAP based on awake examination upper airway morphology as noted. Patients were 29 males and 9 females, with an average age of 47.0 ± 12.5 years, a baseline severely elevated AHI of 44.9 ± 21.3 events/h, a body mass index (BMI) of 32.3 ± 4.9 kg/m2, a Friedman tongue position score of 2.4 ± 0.6, and a tonsil size of 1.5 ± 0.9. The follow-up sleep study took place at a mean 13.8 ± 19.9 months after upper airway surgery.
Procedures and Outcome
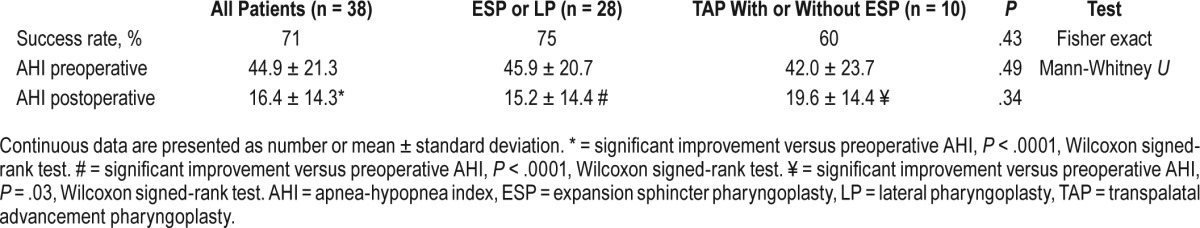
The overall AHI success rate of all 38 patients was 71%. The AHI success rate of 28 patients who underwent ESP or LP was 75%, and 10 patients underwent TAP only or combined with ESP was 60%. There was no statistical difference of surgical success rate between the groups that underwent LP versus TAP (P = .43, Fisher exact test). Postoperative AHI for all patients was 16.4 ± 14.3 events/h, which is significantly improved from baseline (P < .0001, Wilcoxon signed-rank test) (Table 1).
Table 1.
Procedures and outcome.
AHI Outcome for Multilevel Versus Unilevel DISE Patterns
We stratified our patients into 3 groups according to the severity of tongue base obstruction on DISE. Group 1 had no obstruction (unilevel), defined by < 50% DISE obstruction (19 patients, 50%). Group 2 had partial or no obstruction, defined by < 75% obstruction (27 patients, 71%). Group 3 had complete obstruction (multilevel), defined by 75% to 100% obstruction (11 patients, 29%) (Table 2). All groups had additionally velopharyngeal/oropharyngeal obstruction.
Table 2.
Patient features and AHI outcome after palatopharyngoplasty stratified by extent of tongue base obstruction on DISE.
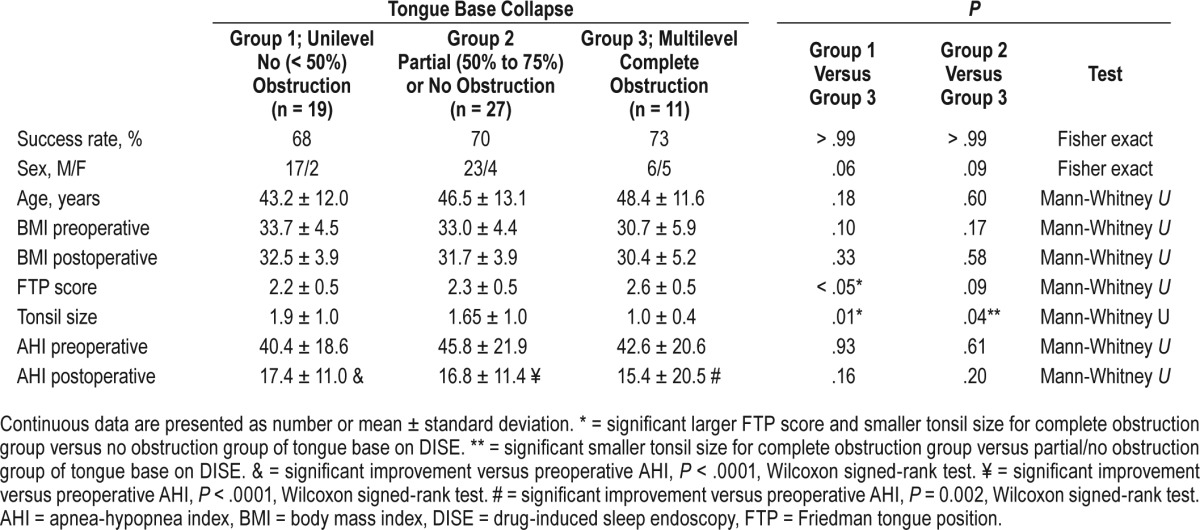
Compared to group 3, groups 1 and 2 had no statistically significant differences in age, sex, preoperative and postoperative BMI, or preoperative and postoperative AHI. The Friedman tongue position was significantly higher in group 3 versus group 1 (2.6 ± 0.5 versus 2.2 ± 0.5, P < .05). Tonsil size was significantly smaller in group 3 versus groups 1 and 2 (1.1 ± 0.4 versus 1.9 ± 1.0 and 1.7 ± 1.0, P < .01).
The postoperative AHI means were significantly lower than the preoperative AHI means for group 3 (15.4 ± 20.5 versus 42.6 ± 20.6, P = .0002), group 2 (16.8 ± 11.4 versus 45.8 ± 21.9, P < .0001), and group 1 (17.4 ± 11.0 versus 40.4 ± 18.6, P < .0001). The preoperative and postoperative AHI means for all groups were not significantly different from each other (P = .9, analysis of variance). Boxplots of AHI medians, 25th to 75th percentiles are shown in Figure 1.
Figure 1. AHI outcome after palatopharyngoplasty stratified by no obstruction, partial/no obstruction, and complete obstruction of tongue base on DISE.
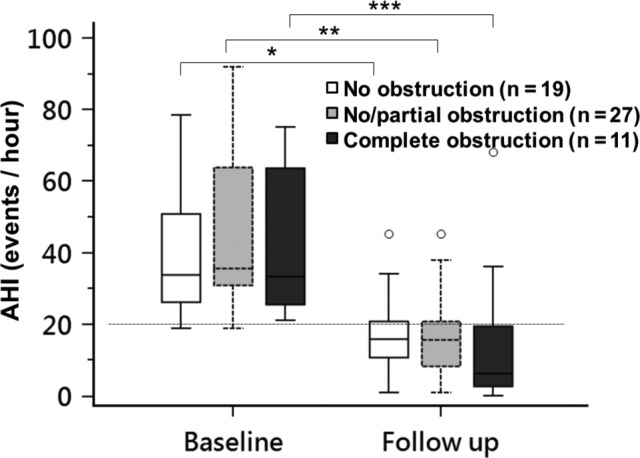
AHI in patients with no tongue base collapse (white color), partial/no tongue base collapse (gray color) and complete tongue base collapse (black color) on DISE before and after palatopharyngoplasty. Boxplots showing the 75th and 25th percentiles by the upper and lower margins, and the median values by the horizontal line. Whiskers represent the maximum value (top) and the minimum value (bottom) of the dataset; this range includes all data except the outliers. Outliers are represented by circles. * = P < .0001 versus baseline. ** = P < .001 versus baseline. *** = P = .002 versus baseline. AHI = apnea-hypopnea index, DISE = drug-induced sleep endoscopy.
Circumferential Collapse of Velum
Patients were also stratified into complete circumferential collapse of velum (17 patients, 45%) and noncircumferential collapse (21 patients, 55%) on DISE (Table 3). There was no difference in age, sex, preoperative and postoperative BMI, Friedman tongue position score, and tonsil size between these 2 groups, but there was significant statistical difference between preoperative AHI and postoperative AHI, both higher in the circumferential collapse group. Although the circumferential velopharyngeal collapse group's AHI mean improved significantly (preoperative: 55.3 ± 22.1 versus postoperative: 23.6 ± 15.8, P < .0001), the noncircumferential collapse group's AHI mean improved to a greater extent (preoperative: 36.4 ± 16.7 versus postoperative: 10.5 ± 9.94, P < .0001) (Figure 2). The odds ratio for patients without circumferential palatal collapse to “success” was: [9/2] / [8/19]: 10.7 (95% confidence interval: 1.87–60.9; P < .01).
Table 3.
Patient demographics and AHI outcome after palatopharyngoplasty stratified by presence or absence of circumferential obstruction on DISE.
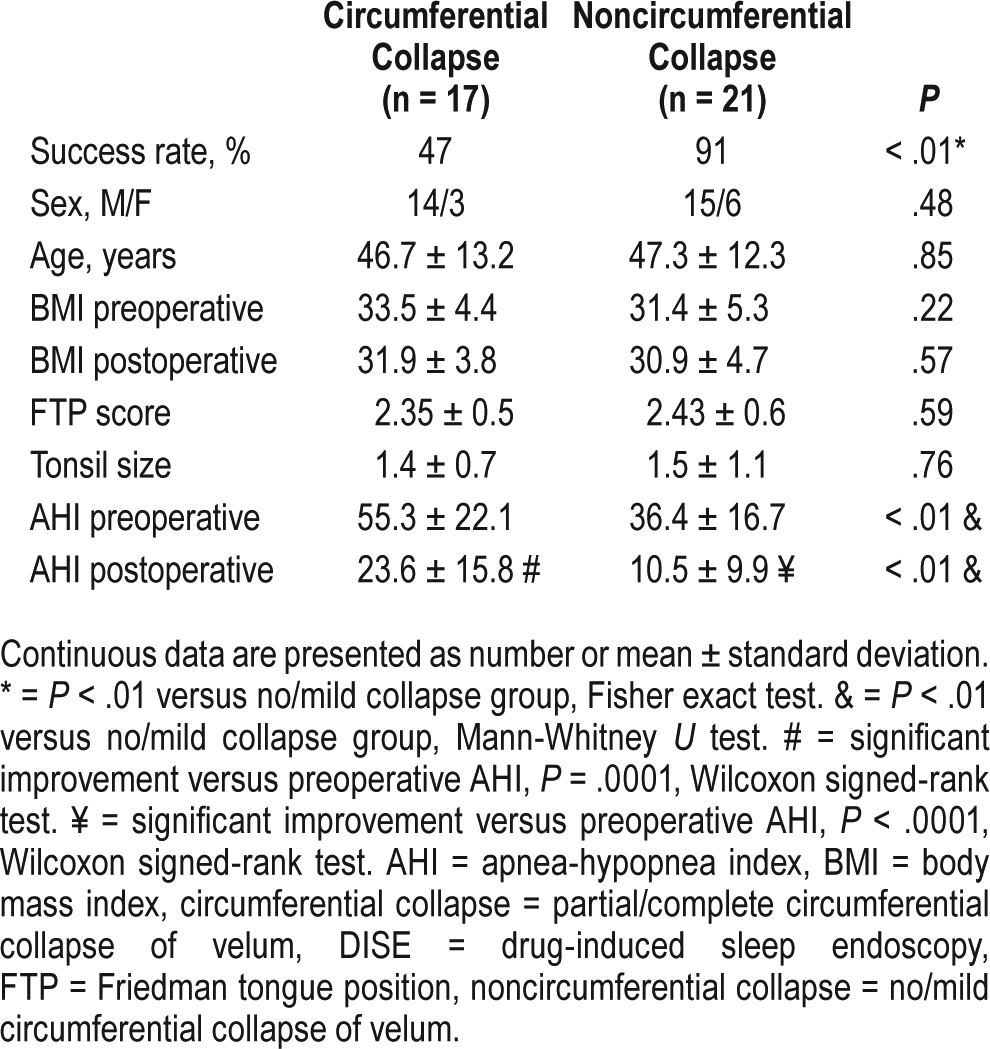
Figure 2. AHI outcome after palatopharyngoplasty stratified by presence or absence of circumferential obstruction on DISE.
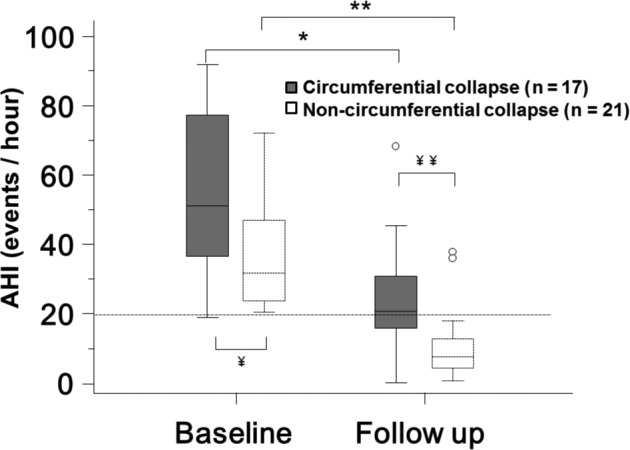
AHI in patients with (gray color) and without (white color) circumferential velopharyngeal on DISE before and after palatopharyngoplasty. Boxplots showing the 75th and 25th percentiles by the upper and lower margins, and the median values by the horizontal line. Whiskers represent the maximum value (top) and the minimum value (bottom) of the dataset; this range includes all data except the outliers. Outliers are represented by circles. * = P = .0001 versus baseline. ** = P < .0001 versus baseline. ¥ = P < .01 between groups in baseline. ¥¥ = P < .01 between groups in follow-up. AHI = apnea-hypopnea index, DISE = drug-induced sleep endoscopy.
DISCUSSION
Synopsis of Key/New Findings
Our study revealed that even in obese patients with moderate to severe OSA and multilevel collapse on DISE, modern palatopharyngoplasty procedures such as ESP, LP, and TAP can significantly improve patients' AHI without treatment of the tongue base or epiglottis. More surprisingly, the AHI mean and success rate after palatopharyngoplasty alone in patients with multilevel collapse with complete tongue base obstruction was not different than that in patients with only upper pharyngeal collapse.
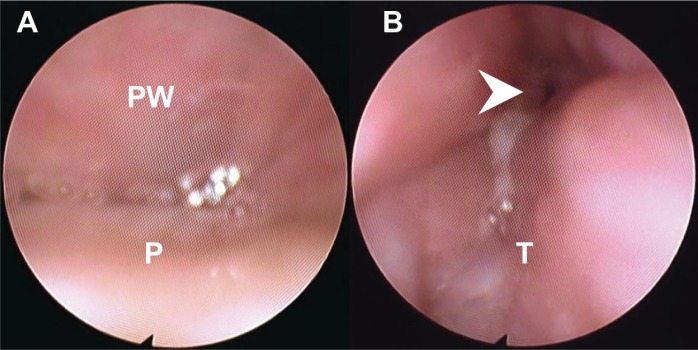
Figure 3 includes images from the DISE study for a patient with preoperative AHI of 25 events/h where complete anterior-posterior palatal obstruction and complete tongue base obstruction were noted. After ESP, the follow-up AHI at 26 months dropped to 6 events/h. The postoperative awake fiberoptic pharyngoscopy showed a larger transverse retropalatal dimension as compared with the preoperative appearance, whereas the tongue base appearance remained the same (Figure 4). It appears that the hypopharyngeal obstruction seen on DISE did not contribute significantly to the patient's AHI.
Figure 3. Patterns of airway collapse of a patient during drug-induced sleep endoscopy.
(A) Complete anteroposterior collapse at velum on inspiration (the pattern was the same on expiration). PW = posterior pharyngeal wall, P = soft palate. (B) Complete tongue base and epiglottic collapse on inspiration. Arrow head: epiglottis. T = tongue base.
Figure 4. Awake fiberoptic pharyngoscopy of velum and tongue base during inspiration of a patient before and after palatopharyngoplasty.
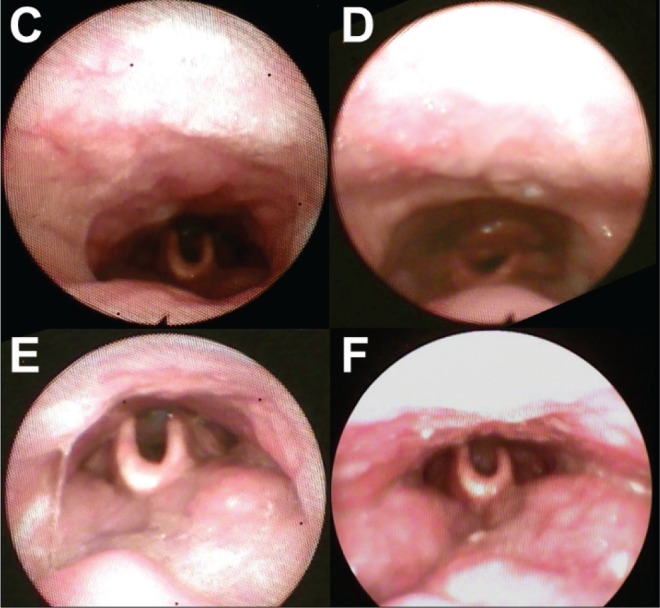
Velopharynx (C) before surgery and (D) after surgery. Hypopharynx (E) before surgery and (F) after surgery
Our study did not exclude patients with larger tonsils. Instead, we included patients with larger tonsils in the unilevel group for comparison, and they had similar age, sex, and AHI severity as the multilevel group, with a higher BMI, although not meeting statistical significance (Table 2). The patients in the multilevel group had significantly smaller tonsils and larger tongues than those in the unilevel group. Based on the aforementioned findings, one would think that the patients with multilevel collapse would not fare as well with only palatopharyngoplasty, but in this study the AHI means were not significantly or clinically different. In fact, more than 70% of patients with multilevel collapse benefited from palatopharyngoplasty alone with AHI reduction to levels of low medical risk. Thus, we would propose that one option that could reduce patient morbidity and possibly surgical risk27 is to reserve hypopharyngeal surgery for some to a second stage if after palatopharyngoplasty the AHI or clinical outcome was suboptimal. The results do not argue against multilevel surgery as the study is small and retrospective, with outcome assessment limited to the AHI. Even in terms of the AHI, potentially a greater improvement may be obtained with additional pharyngeal procedures. In addition, for some patients, resource availability and other personal factors may require all surgery to be performed as multilevel in one stage.
Circumferential collapse on DISE is most likely a marker of greater collapsibility of the pharynx and more severe disease.28 We found that circumferential collapse of the velum in DISE was associated with a higher preoperative and postoperative AHI, and the surgical “success” rate for those patients was significantly lower, although one may argue that a decrease of the AHI from 55 to 22 events/h for the group was clinically beneficial. There was no difference in Friedman tongue position and tonsil grading between these 2 groups, and they had similar age, sex, and BMI. Thus, performing DISE prior to OSA surgery may have some utility for sleep surgery when circumferential collapse in identified. For this group, one may consider performing concurrent or subsequent upper or hypopharyngeal procedures, maxillomandibular advancement, or implement medical treatments such positional appliances, oral appliances, or positive airway pressure that may be more viable as options postoperatively.
Comparisons With Other Studies
There are several studies where based on DISE the surgical procedure was changed from palatopharyngoplasty to multilevel surgery,29,30 but the effect on AHI outcome was not significantly better or was not determined. Others showed that certain collapse patterns were associated with inferior AHI outcome. Soares et al.31 described that the presence of severe lateral pharyngeal wall and/or supraglottic collapse on preoperative DISE is associated with OSA surgical failure. Koutsourelakis et al.15 described that circumferential collapse at the velar level, and complete anterior-posterior collapse of tongue base or epiglottis were independent predictors of failure of upper airway surgery failure. In our study, we only found an association of reduced AHI response with circumferential collapse at the velar level. In these two studies15,31 the investigators performed multilevel surgery and treated the upper pharynx and palate using UPPP or Z-plasty, whereas in our study unilevel surgery only was performed using ESP, LP, and or TAP and tongue base collapse did not predict outcome. There could be multiple explanations for the differences with our study including patient population differences, variations in DISE, and perhaps also that the lateral wall procedures we used were more effective at stabilizing and/or widening the retropalatal airway and reduced the gradient for collapse at the tongue base level. Osnes and colleagues21 showed that after UPPP alone, pharyngeal collapse, as measured by manometry in sleep, decreased in the lower pharyngeal segment. Victores and colleagues32 showed that nasopharyngeal stenting during sleep endoscopy was associated with reduction in lower pharyngeal and lateral wall collapse.
Two studies support the notion that tongue base obstruction on DISE may not always need treatment. Zhang et al.33 treated 43 patients with revised H-UPPP and TAP and AHI success rates of patients with tongue base partial/complete obstruction was 55% (17/31), as compared to 75% (9/12) in those without obstruction, but the rates were not significantly different (P = .31). In the study, many patients benefited from UPPP and TAP even with tongue base obstruction noted on DISE, but the unilevel obstruction alone versus multilevel groups were not specifically compared. The success rate for patients with circumferential collapse of velum was 57% (17/30), compared to 69% (9/13) without circumferential obstruction. Our treatment success rate was similarly lower for circumferential collapse of velum (47% versus 57%, P = .56), and is somewhat similar for patients without circumferential collapse of velum (91% versus 69%, P = .17). Blumen and colleagues also studied DISE findings as they relate to outcome of upper airway surgery consisting of mostly UPPP and some lingual tonsillectomies.16 All patients volunteered for DISE that was not used for surgical decision making. In some patients in the AHI success group, DISE showed obstruction sites that were not treated by surgery, with overall 42% sensitivity and 40% specificity. In their study both the success and failure groups had ∼90% prevalence of multilevel collapse on DISE. In the current study, one half of the patients had unilevel collapse and their mean palatopharyngoplasty success rates and AHIs were not significantly different from those of the multilevel group. Thus, it is possible that in this study's DISE and surgical protocol, lower pharyngeal collapse on DISE was mostly secondary to proximal obstruction.
Limitations of the Study
There are some limitations of DISE as used in the study. During DISE there is stage R sleep suppression, decreased N1 sleep, and increased N3 sleep.34 The degree of upper airway narrowing can be aggravated with the depth of sedation35 and can vary over the sedation period. Perhaps a longer period of DISE would have yielded a different pattern of obstruction. The scoring of DISE is subjective, and there are concerns about interrater reliability despite expert reviewers. The different protocols used for DISE may also cause variability. Propofol may be associated with greater incidence of tongue base collapse as compared with use of dexmedetomidine36; target-controlled infusion could find more multiple anatomic site obstruction than conventional bolus technique.37 Those limitations could increase the variability in the reporting of tongue base obstruction in DISE and ability to apply conclusions from this and other studies. DISE was not performed postoperatively in this study due to practical and economic reasons, but had it been performed it would better allow comparison between residual collapse or stabilization of airway sites and the AHI outcome.
DISE examination only provides a limited view of “what's in the box,” and does not include the view of skeletal framework that could be an important prognostic feature as well. It also misses the view of the oral side in sleep, where the tongue may abut the palate and produce retropalatal obstruction.
The study is retrospective and is prone to surgical selection bias of the patients and surgical maneuvers. The number of subjects is small and several palatopharyngeal techniques were used, although all were upper pharyngeal and none directly treated the tongue base or epiglottis. AHI scoring criteria varied for the group, although they were kept consistent for each patient's sleep studies. Only AHI outcome was reported as oxygen desaturation index and quality of life data were not available for a sufficient number of patients in the study groups. Clinical measures such as sleepiness and quality of life are not usually reflected by the AHI38 but arguably reduction of severely elevated AHI to the level of low medical risk is a reasonable success metric.
Finally, given the study's limitations, one does not conclude that tongue base and hypopharyngeal procedures are not needed or are not of benefit. It is not known whether the patients would have obtained a greater AHI improvement with hypopharyngeal surgery. Future prospective or larger retrospective studies are needed.
DISCLOSURE STATEMENT
The work for this study was performed at ENT and Allergy Associates. All authors have seen and approved the manuscript. The authors have no funding, financial relationships, or conflicts of interest to disclose.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- BMI
body mass index
- CPAP
continuous positive airway pressure
- DISE
drug-induced sleep endoscopy
- ESP
expansion sphincter pharyngoplasty
- FTP
Friedman tongue position
- LP
lateral pharyngoplasty
- OSA
obstructive sleep apnea
- PSG
polysomnogram
- TAP
transpalatal advancement pharyngoplasty
- UPPP
uvulopalatopharyngoplasty
- VOTE
velum, oropharynx, tongue base, epiglottis
REFERENCES
- 1.Peppard PE, Young T, Barnet JH, et al. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Engleman HM, Douglas NJ. Sleep 4: sleepiness, cognitive function, and quality of life in obstructive sleep apnoea/hypopnoea syndrome. Thorax. 2004;59(7):618–622. doi: 10.1136/thx.2003.015867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gami AS, Olson EJ, Shen WK, et al. Obstructive sleep apnea and the risk of sudden cardiac death. J Am Coll Cardiol. 2013;62(7):610–616. doi: 10.1016/j.jacc.2013.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 5.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353(19):2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 6.Reichmuth KJ, Austin D, Skatrud JB, Young T. Association of sleep apnea and type II diabetes: a population-based study. Am J Respir Crit Care Med. 2005;172(12):1590–1595. doi: 10.1164/rccm.200504-637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kushida CA, Littner MR, Hirshkowitz M, et al. Practice parameters for the use of continuous and bilevel positive airway pressure devices to treat adult patients with sleep related breathing disorders. Sleep. 2006;29(3):375–380. doi: 10.1093/sleep/29.3.375. [DOI] [PubMed] [Google Scholar]
- 8.Kohler M, Smith D, Tippett V, Stradling JR. Predictors of long-term compliance with continuous positive airway pressure. Thorax. 2010;65(9):829–832. doi: 10.1136/thx.2010.135848. [DOI] [PubMed] [Google Scholar]
- 9.McEvoy RD, Antic NA, Heeley E, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375(10):919–931. doi: 10.1056/NEJMoa1606599. [DOI] [PubMed] [Google Scholar]
- 10.Jacobowitz O. Palatal and tongue base surgery for surgical treatment of obstructive sleep apnea: A prospective study. Otolaryngol Head Neck Surg. 2006;135(2):258–264. doi: 10.1016/j.otohns.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 11.Aurora RN, Casey KR, Kristo D, et al. Practice parameters for the surgical modifications of the upper airway for obstructive sleep apnea in adults. Sleep. 2010;33(10):1408–1413. doi: 10.1093/sleep/33.10.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin HC, Friedman M, Chang HW, Gurpinar B. The efficacy of multilevel surgery of the upper airway in adults with obstructive sleep apnea/hypopnea syndrome. Laryngoscope. 2008;118(5):902–908. doi: 10.1097/MLG.0b013e31816422ea. [DOI] [PubMed] [Google Scholar]
- 13.Croft CB, Pringle M. Sleep nasendoscopy: a technique of assessment in snoring and obstructive sleep apnoea. Clin Otolaryngol Allied Sci. 1991;16(5):504–509. doi: 10.1111/j.1365-2273.1991.tb01050.x. [DOI] [PubMed] [Google Scholar]
- 14.Johal A, Battagel JM, Kotecha BT. Sleep nasendoscopy: a diagnostic tool for predicting treatment success with mandibular advancement splints in obstructive sleep apnoea. Eur J Orthod. 2005;27(6):607–614. doi: 10.1093/ejo/cji063. [DOI] [PubMed] [Google Scholar]
- 15.Koutsourelakis I, Safiruddin F, Ravesloot M, Zakynthinos S, de Vries N. Surgery for obstructive sleep apnea: Sleep endoscopy determinants of outcome. Laryngoscope. 2012;122(11):2587–2591. doi: 10.1002/lary.23462. [DOI] [PubMed] [Google Scholar]
- 16.Blumen MB, Latournerie V, Bequignon E, Guillere L, Chabolle F. Are the obstruction sites visualized on drug-induced sleep endoscopy reliable? Sleep Breath. 2015;19(3):1021–1026. doi: 10.1007/s11325-014-1107-5. [DOI] [PubMed] [Google Scholar]
- 17.Shepard JW, Thawley SE. Evaluation of the upper airway by computerized tomography in patients undergoing uvulopalatopharyngoplasty for obstructive sleep apnea. Am Rev Respir Dis. 1989;140(3):711–716. doi: 10.1164/ajrccm/140.3.711. [DOI] [PubMed] [Google Scholar]
- 18.Pang K, Woodson B. Expansion sphincter pharyngoplasty: a new technique for the treatment of obstructive sleep apnea. Otolaryngol Head Neck Surg. 2007;137(1):110–114. doi: 10.1016/j.otohns.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 19.Cahali MB, Formigoni GGS, Gebrim EMMS, Miziara ID. Lateral pharyngoplasty versus uvulopalatopharyngoplasty: a clinical, polysomnographic and computed tomography measurement comparison. Sleep. 2004;27(5):942–950. doi: 10.1093/sleep/27.5.942. [DOI] [PubMed] [Google Scholar]
- 20.Woodson B, Robinson S, Lim H. Transpalatal advancement pharyngoplasty outcomes compared with uvulopalatopharyngoplasty. Otolaryngol Head Neck Surg. 2005;133(2):211–217. doi: 10.1016/j.otohns.2005.03.061. [DOI] [PubMed] [Google Scholar]
- 21.Osnes T, Rollheim J, Hartmann E. Effect of UPPP with respect to site of pharyngeal obstruction in sleep apnoea: follow-up at 18 months by overnight recording of airway pressure and flow. Clin Otolaryngol Allied Sci. 2002;27(1):38–43. doi: 10.1046/j.0307-7772.2001.00520.x. [DOI] [PubMed] [Google Scholar]
- 22.Iber C, Ancoli-Israel S, Chesson AL, Quan SF for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 23.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. J Clin Sleep Med. 2012;8(5):597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kezirian EJ. Drug-induced sleep endoscopy. Oper Tech Otolaryngol Head Neck Surg. 2006;17(4):230–232. doi: 10.1001/archoto.2010.26. [DOI] [PubMed] [Google Scholar]
- 25.Hohenhorst W, Ravesloot MJL, Kezirian EJ, de Vries N. Drug-induced sleep endoscopy in adults with sleep-disordered breathing: technique and the VOTE classification system. Oper Tech Otolaryngol Head Neck Surg. 2012;23(1):11–18. [Google Scholar]
- 26.Woodson BT, Sitton M, Jacobowitz O. Expansion sphincter pharyngoplasty and palatal advancement pharyngoplasty: airway evaluation and surgical techniques. Oper Tech Otolaryngol Head Neck Surg. 2012;23(1):3–10. [Google Scholar]
- 27.Kezirian EJ, Weaver EM, Yueh B, Khuri SF, Daley J, Henderson WG. Risk factors for serious complication after uvulopalatopharyngoplasty. Arch Otolaryngol Neck Surg. 2006;132(10):1091–1098. doi: 10.1001/archotol.132.10.1091. [DOI] [PubMed] [Google Scholar]
- 28.Lan MC, Liu SYC, Lan MY, Modi R, Capasso R. Lateral pharyngeal wall collapse associated with hypoxemia in obstructive sleep apnea: pharyngeal wall collapse and hypoxemia. Laryngoscope. 2015;125(10):2408–2412. doi: 10.1002/lary.25126. [DOI] [PubMed] [Google Scholar]
- 29.Eichler C, Sommer JU, Stuck BA, Hörmann K, Maurer JT. Does drug-induced sleep endoscopy change the treatment concept of patients with snoring and obstructive sleep apnea? Sleep Breath. 2013;17(1):63–68. doi: 10.1007/s11325-012-0647-9. [DOI] [PubMed] [Google Scholar]
- 30.Certal VF, Pratas R, Guimarães L, et al. Awake examination versus DISE for surgical decision making in patients with OSA: a systematic review: the importance of DISE for surgical decision making. Laryngoscope. 2016;126(3):768–774. doi: 10.1002/lary.25722. [DOI] [PubMed] [Google Scholar]
- 31.Soares D, Sinawe H, Folbe AJ, et al. Lateral oropharyngeal wall and supraglottic airway collapse associated with failure in sleep apnea surgery. Laryngoscope. 2012;122(2):473–479. doi: 10.1002/lary.22474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Victores AJ, Olson K, Takashima M. Interventional drug-induced sleep endoscopy: a novel technique to guide surgical planning for obstructive sleep apnea. J Clin Sleep Med. 2017;13(2):169–174. doi: 10.5664/jcsm.6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang P, Ye J, Pan C, Sun N, Kang D. The role of obstruction length and height in predicting outcome of velopharyngeal surgery. Otolaryngol Head Neck Surg. 2015;153(1):144–149. doi: 10.1177/0194599815576719. [DOI] [PubMed] [Google Scholar]
- 34.Rabelo FAW, Küpper DS, Sander HH, Fernandes RMF, Valera FCP. Polysomnographic evaluation of propofol-induced sleep in patients with respiratory sleep disorders and controls. Laryngoscope. 2013;123(9):2300–2305. doi: 10.1002/lary.23664. [DOI] [PubMed] [Google Scholar]
- 35.Hong SD, Dhong HJ, Kim HY, et al. Change of obstruction level during drug-induced sleep endoscopy according to sedation depth in obstructive sleep apnea. Laryngoscope. 2013;123(11):2896–2899. doi: 10.1002/lary.24045. [DOI] [PubMed] [Google Scholar]
- 36.Capasso R, Rosa T, Tsou DYA, et al. Variable findings for drug-induced sleep endoscopy in obstructive sleep apnea with propofol versus dexmedetomidine. Otolaryngol Head Neck Surg. 2016;154(4):765–770. doi: 10.1177/0194599815625972. [DOI] [PubMed] [Google Scholar]
- 37.De Vito A, Agnoletti V, Berrettini S, et al. Drug-induced sleep endoscopy: conventional versus target controlled infusion techniques—a randomized controlled study. Eur Arch Otorhinolaryngol. 2011;268(3):457–462. doi: 10.1007/s00405-010-1376-y. [DOI] [PubMed] [Google Scholar]
- 38.Weaver E, Woodson B, Steward D. Polysomnography indexes are discordant with quality of life, symptoms, and reaction times in sleep apnea patients. Otolaryngol Head Neck Surg. 2005;132(2):255–262. doi: 10.1016/j.otohns.2004.11.001. [DOI] [PubMed] [Google Scholar]