Abstract
Introduction:
Sleep-disordered breathing (SDB) is highly prevalent in hospitalized patients with congestive heart failure (CHF) and the condition is diagnosed and treated in only a minority of these patients. Portable monitoring (PM) is a screening option, but due to costs and the expertise required, many hospitals may find it impractical to implement. We sought to test the utility of an alternative approach for screening hospitalized CHF patients for SDB, high-resolution pulse oximetry (HRPO).
Methods:
We conducted a prospective controlled trial of 125 consecutive patients admitted to the hospital with CHF. Simultaneous PM and HRPO for a single night was performed. All but one patient were monitored on breathing room air. The HRPO-derived ODI (oxygen desaturation index) was compared with PM-derived respiratory event index (REI) using both receiver operator characteristic (ROC) curve analysis and a Bland-Altman plot.
Results:
Of 105 consecutive CHF patients with analyzable data, 61 (58%) were males with mean age of 64.9 ± 15.1 years and mean body mass index of 30.3 ± 8.3 kg/m2. Of the 105 patients, 10 (9.5%) had predominantly central sleep apnea (central events > 50% of the total events), although central events were noted in 42 (40%) of the patients. The ROC analysis showed an area under the curve of 0.89 for REI > 5 events/h. The Bland-Altman plot showed acceptable agreement with 95% limits of agreement between −28.5 to 33.7 events/h and little bias.
Conclusions:
We conclude that high-resolution pulse oximetry is a simple and cost-effective screening tool for SDB in CHF patients admitted to the hospital. Such screening approaches may be valuable for large-scale implementation and for the optimal design of interventional trials.
Citation:
Sharma S, Mather PJ, Chowdhury A, Gupta S, Mukhtar U, Willes L, Whellan DJ, Malhotra A, Quan SF. Sleep overnight monitoring for apnea in patients hospitalized with heart failure (SOMA-HF Study). J Clin Sleep Med. 2017;13(10):1185–1190.
Keywords: decompensated congestive heart failure, early diagnosis, high-resolution pulse oximetry, hospitalized patients, lung, portable monitoring, sleep-disordered breathing
INTRODUCTION
Congestive heart failure (CHF) is the most common cause of hospital admissions and readmissions in the United States.1 Despite identifying and mitigating several risk factors for CHF decompensation, readmissions for decompensated heart failure remain unacceptably high.2 As a result, efforts are ongoing to find ways to reduce readmissions related to CHF.
Studies have shown that patients with untreated sleep-disordered breathing (SDB) are at increased risk of heart failure, and untreated SDB in CHF is independently associated with higher mortality.3,4 The implications of these findings are considerable, because SDB is highly prevalent5,6 and also highly underrecognized and under-treated in patients with CHF.6,7 Recent data suggest that early recognition of SDB in CHF patients and subsequent intervention can reduce hospital readmissions.8,9
Although hospitalized patients with CHF present a unique opportunity for screening and diagnosing SDB, they are rarely screened or evaluated. This is partly due to low awareness, lack of expertise for evaluation, and major costs associated with performing traditional polysomnography (PSG) in hospitalized settings.10 Furthermore, although portable monitoring (PM) is simpler to perform than PSG, it is associated with additional expense for equipment and personnel. In a prior study, we showed that a cost-effective screening strategy using high-resolution pulse oximetry (HRPO) can be very practical and effective in identifying SDB in hospitalized patients.7 HRPO differs from standard oximetry in that the former utilizes a much shorter averaging time of 3 seconds or less. In this study, we sought to test the hypothesis that a HRPO screening test is an accurate method of identifying SDB in the hospital environment in patients with CHF.
BRIEF SUMMARY
Current Knowledge/Study Rationale: This study evaluates whether high-resolution pulse oximetry (HRPO) is an effective screening tool for early detection of sleep apnea in patients hospitalized with decompensated heart failure (CHF).
Study Impact: To our knowledge, it is the first study that validates the use of HRPO as a cost-effective and objective screening tool to help guide clinical management of acute CHF. It also may be a useful tool in the design of subsequent clinical trials in patients with CHF.
METHODS
This prospective trial was conducted at Thomas Jefferson University Hospital from October 2014 to May 2015. Inclusion criteria were: age older than 18 years, admittance to the hospital and a diagnosis of CHF (systolic or diastolic). Exclusion criteria included presence of a left ventricular assist device, heart transplant, hemodynamic instability, high oxygen requirement (> 2 L oxygen/min or inspiratory fraction of inspired oxygen, FiO2 > 28%), major psychiatric conditions, or language barriers that might affect the ability to consent for the trial. Patients with known SDB and adherent with positive airway pressure therapy were also excluded. Patients admitted and discharged over the weekend and holidays were excluded as well. A total of 258 patients were admitted to the advanced heart failure center during this period.
After informed consent was obtained, sleep monitoring was scheduled after participants were stabilized. All patients underwent testing on room air except for one patient who was on chronic supplemental oxygen delivered at FiO2) of 28%. To minimize any night-to-night variability of the patients' sleep studies, patients underwent PM and an overnight HRPO on the same night.
A Masimo RAD-57 (Irvine, California, United States) was used to obtain HRPO. Its sampling frequency is 1 Hz and an averaging time of 3 seconds with a resolution of 0.1% oxygen saturation (SpO2). The data were downloaded onto a personal computer the following morning and were processed with specifically designed software, Profox (Profox Associates, Escon-dido, California, United States). We then extracted the oxygen desaturation index (ODI) (the hourly average number of de-saturation episodes defined as a ≥ 4% decrease in saturation from the average saturation in the preceding 120 seconds), the cumulative percentage time with oxygen saturations < 90% (CT90) and lowest and average SpO2.
Portable monitoring11 was performed by using the Apnea-Link Plus (ResMed, San Diego, California, United States) and was simultaneous with the HRPO test. The pulse oximeter used in ApneaLink was an OEM device (XPOD 3012 Nonin, Nonin Medical, Inc. Plymouth, Minnesota, United States). Apnea was defined as a reduction of inspiratory airflow by 80% to 100% over 10 seconds; hypopnea was defined as a reduction of tidal breathing of 30% from baseline tidal breathing lasting 10 seconds or more and accompanied by a 4% desaturation in oxygen. The data were autoscored. The respiratory event index (REI) was calculated as the number of apneas plus hypopneas divided by the recording time.
The HRPO and PM data were then interpreted by a board-certified physician who was blinded to the clinical background of the patient and demographic information of the subjects. A minimum of 2 hours of recording time for all channels was considered adequate for interpretation. Lack of recording by any channel for a minimum of 2 hours was considered a failed test.
Statistical Analysis
Baseline characteristics were summarized using descriptive statistics. For continuous variables, the mean, standard deviation, median, minimum, and maximum were presented. For categorical parameters, the number with the characteristic and the proportion were presented.
The performance of ODI (measured by HRPO) was compared to REI (measured by PM) using several methods. The REI was considered the true value for all comparisons, and 5 different REI thresholds (REI ≥ 5, 10, 15, 20, and 30 events/h) for diagnosing SDB were considered. Estimates of sensitivity and specificity were calculated for each threshold for SDB along with the associated 95% confidence intervals (CI) for the proportions. Additionally, estimates of accuracy, positive and negative predictive values, positive and negative likelihood ratios, and d-squared were presented (where d2 = [1 − sensitivity]2 + [1 − specificity]2). Receiver operating characteristic (ROC) curves were generated for diagnosing SDB at 5 different PM-REI thresholds (REI ≥ 5, 10, 15, 20, and 30 events/h) using the ODI. The area under the curve was presented for each REI threshold along with the associated 95% CI. A Bland-Altman plot was utilized in order to depict agreement visually between REI and ODI. The correlation between ODI was calculated using the Spearman correlation coefficient. In addition to REI and ODI, characteristics such as recording time, cannula time, and saturation time were compared between PM and HRPO using paired t tests. All statistical analyses were generated and quality checked using SAS, version 9.3 or higher (SAS Software, SAS Institute Inc., Cary, North Carolina, United States) and R statistical software (R Software, The R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
A total of 125 consecutive patients who consented to participate were included in the study, but only 105 were included in the final analysis due to incomplete data on 20 patients. The PM data were insufficient due to inadequate time or nonfunctioning leads (with the nonfunctioning lead almost entirely secondary to low cannula time) in 19 patients and the HRPO not recording data in 1 additional patient.
Of the 105 CHF patients enrolled, 61 (58%) were males with mean age of 64.9 ± 15.1 years and mean body mass index of 30.3 ± 8.3 kg/m2. Comorbid conditions included hypertension (92%), atrial fibrillation (32%), chronic obstructive pulmonary disease (10%), and type 2 diabetes (50%). A history of obstructive sleep apnea was noted in 18 of the patients (17%) although the patients were either not on therapy or currently nonadherent. Table 1 summarizes patient characteristics. Using ApneaLink as a reference standard, 91 of the 105 patients (87%) had SDB based on an REI ≥ 5 events/h criteria. Of the 105 patients, 10 (9.5%) had predominantly central sleep apnea (central events > 50% of the total events) and 42 (40%) had mixed apneas (central plus obstructive) although central events were less than 50% of the total events. The distribution of patients based on REI severity was: no OSA 17 (16%), mild OSA 26 (25%), moderate OSA 20 (19%) and severe OSA 42 (40%).
Table 1.
Baseline characteristics of patients.
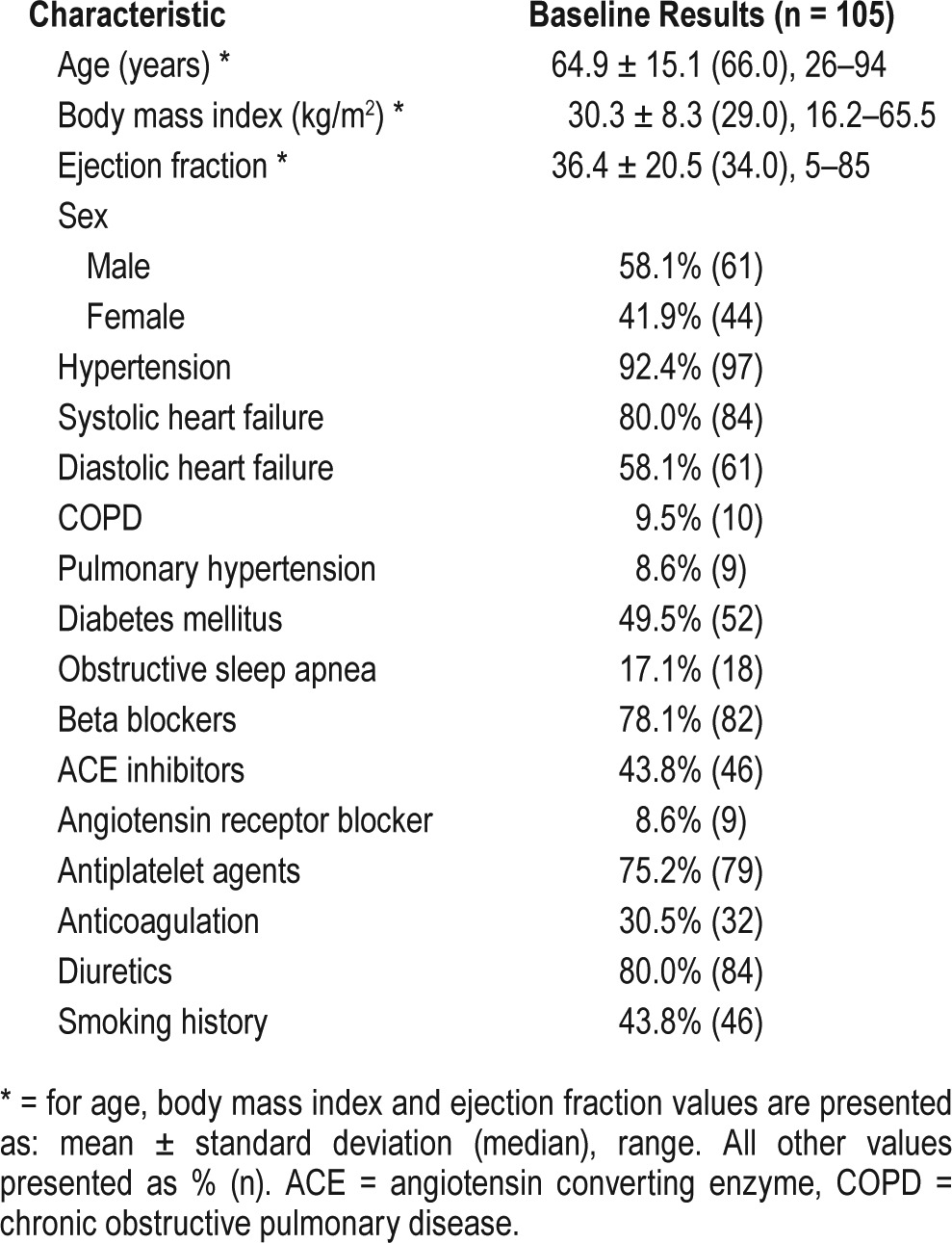
Displayed in Table 2 is the comparison of analysis parameters between PM and HRPO. As previously observed, PM was responsible for all but 1 case of insufficient data. In most instances, it was the result of inadequate recording from the airflow cannula. Furthermore, the length of cannula recording was significantly less than the HRPO recording time (5.1 ± 1.9 versus 6.5 ± 1.4 hours; P < .001). Duration of time spent with oxygen saturations below 88% was significantly higher in PM compared to HRPO (69.6 ± 87.5 versus 34.7 ± 87.2 minutes, P < .001). Also, the mean oxygen desaturation recorded by PM was significantly lower than the HRPO (91.5 ± 2.9% versus 95.0 ± 3.2%).
Table 2.
Comparison of analysis parameters between HRPO and PM.
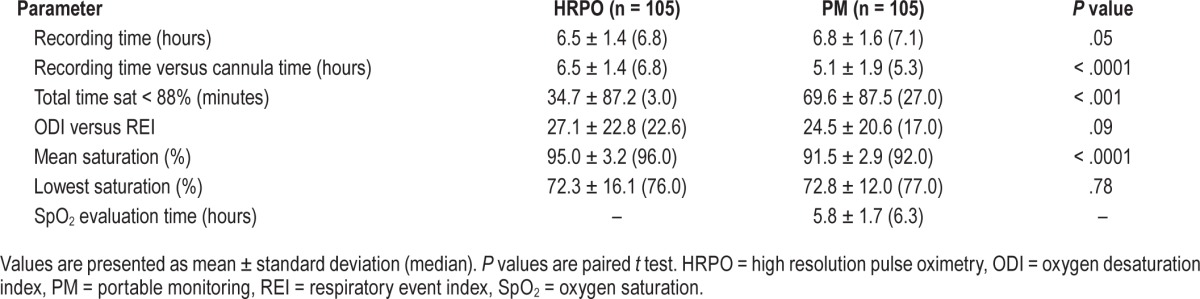
ROC curves for the various thresholds of REI are shown in Figure 1. For all REI thresholds, the area under the curve ranged from 0.84 (REI ≤ 10 events/h) to 0.89 (REI ≤ 5 events/h and REI ≤ 20 events/h).
Figure 1. ROC curves for diagnosing SDB using ODI measured by HRPO (true diagnosis of SDB is defined by 5 REI thresholds [ ≥ 5, ≥ 10, ≥ 15, ≥ 20, ≥ 30 events/h]).
HRPO = high resolution pulse oximetry, ODI = oxygen desaturation index, REI = repiratory event index, ROC = receiver operating characteristic, SDB = sleep-disordered breathing.
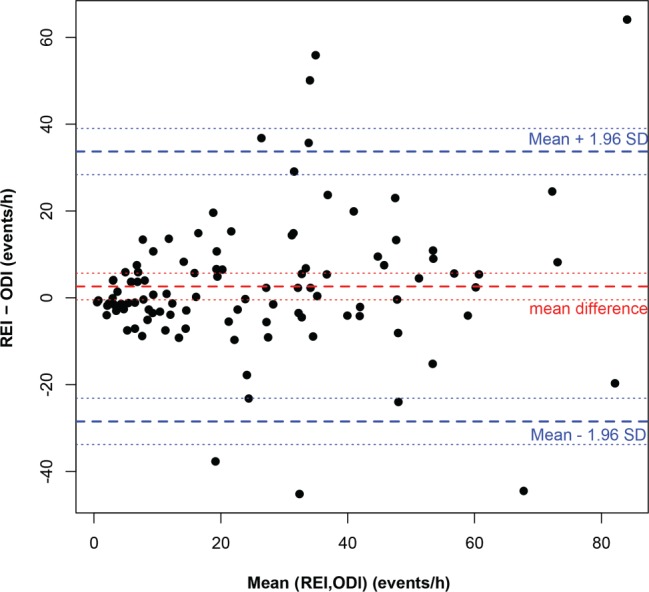
The Bland-Altman plot displayed in Figure 2 indicates adequate agreement between the REI and the ODI with 95% CI between −28.5 to 33.7 events/h. The correlation between ODI measured by HRPO and REI measured by PM also was good with a Spearman correlation coefficient = 0.76. The likelihood ratio at REI of 15 versus ODI of 15 was over 4 (Table 3).
Figure 2. Bland-Altman plot illustrating the agreement between ODI measured by HRPO and REI by PM.
HRPO = high-resolution pulse oximetry, ODI = oxygen desaturation index, PM = portable monitoring, REI = repiratory event index, SD = standard deviation.
Table 3.
Accuracy of HRPO-ODI in diagnosing SDB.
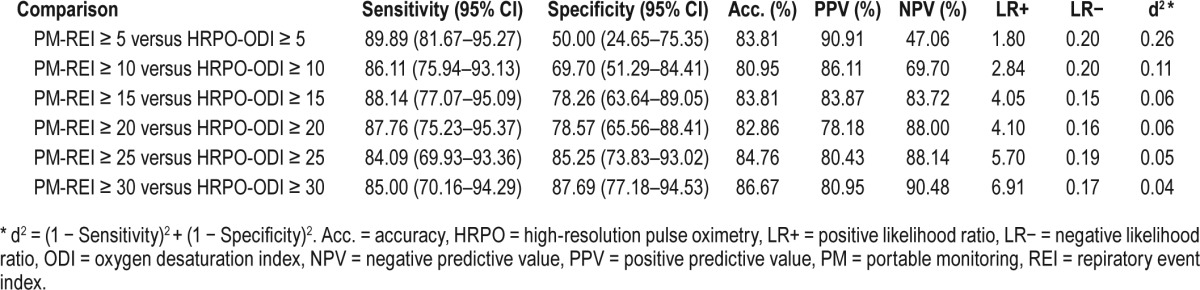
To study the degree of impact on different categories of the disease state (normal, REI < 5; mild, REI 5–14; moderate-severe, REI > 14), REI obtained from PM was compared with ODI obtained from HRPO. The total agreement is 69% and the agreement ± 1 category is 94%. More specifically, 88% of the REI in the moderate-severe category was correctly classified by ODI (Table 4, Figure 3).
Table 4.
Agreement between HRPO and PM in different categories of REI severity.
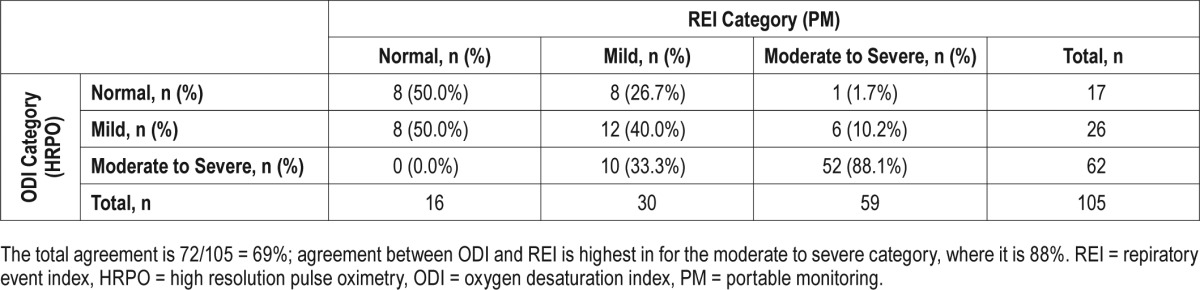
Figure 3. Agreement of REI and ODI.
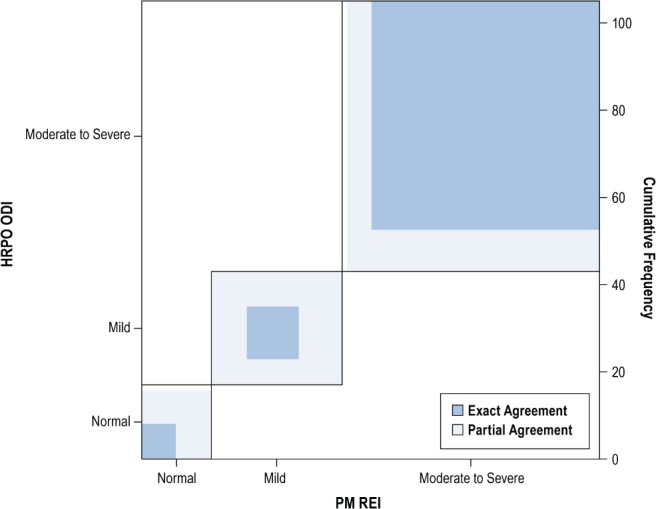
The percentage of patients in each (REI or ODI) category is represented by the length of the box (on the x-axis or y-axis, respectively). The percentage of patients with exact agreement on REI and ODI is represented by the blue shading in each category; the percentage of patients with partial agreement (ie. off by one category) is represented by the light blue shading. REI = respiratory event index, ODI = oxygen desaturation index.
DISCUSSION
Our prior research suggested that no matter how SDB was detected, early diagnosis and treatment of SDB in hospitalized patients reduced readmissions in patients adherent with positive airway pressure therapy.12 Now our new findings show that HRPO provides an effective low-cost tool to screen CHF patients, potentially leading to successful treatment. The results add considerably to the existing literature because they provide a substrate for the implementation of large-scale screening programs and may help guide subsequent interventional studies.
To our knowledge, this is the first study of HRPO validity in hospitalized CHF patients. Previous studies have evaluated overnight pulse oximetry in patients with CHF in the outpatient setting and found that it had a high predictive value for the presence of SDB and subsequent morbidity. In particular, Ohmura and colleagues showed that predischarge overnight pulse oximetry in CHF patients was an independent predictor of combined readmission and mortality.13 Additionally, our prior data showed that HRPO had a high predictive value for identifying SDB in hospitalized patients who underwent PSG within 2 weeks of discharge.7,14
Our current prospective study of hospitalized CHF patients shows that HRPO-measured ODI correlates strongly with the severity of SDB as measured by PM in hospitalized patients with CHF. In addition, we observed that HRPO was a more reliable technique with only 1 patient having inadequate data. Thus, the current study confirms our prior data that screening with HRPO performed in acute settings after initial stabilization accurately identifies underlying SDB.7,14
Greater oxygen desaturation was noted with PM. However, it is likely that this reflects spurious desaturations caused by motion artifact as has been reported in previous comparisons of conventional pulse oximeters to HRPO. Different pulse oximetry devices in the market have varying proprietary algorithms, which creates differences among them. The pulse oximeter used by ApneaLink (Nonin XPOD 3012) is different from the HRPO used in our study (Masimo Rad-7). Studies have shown that the signal processing algorithms (Masimo SET) used by Masimo new-generation pulse oximeters reduce motion artifact and false alarms. Motion artifact adds pulsatility to the venous blood component, which results in falsely low SpO2 values. In addition, an OEM device may result in some alteration of signal when used in the ApneaLink platform. This may also explain why the average oxygen saturations and time below 88% were lower in PM compared to HRPO.15–17
To reduce the future risk for previously unidentified SDB patients hospitalized with CHF, screening and diagnosis should ideally occur while the patient is admitted to the hospital. This approach ensures that patients can be adequately screened while they are a “captive audience,”14,18 and that they can also be provided education and implementation of therapy (eg, a trial of positive airway pressure therapy). A previous study has noted the value of PM in hospitalized patients.19 However, PM can be expensive due to logistic and technical support requirements. Many smaller facilities in the community may not have these resources.
Like PM, HRPO testing can occur in the hospital. As demonstrated in this study, it offers an attractive alternative to portable PM. because of its low cost and minimal training required for acquisition of reliable data. As such, the HRPO approach may be easily implemented on a wide scale. The results of HRPO could then be used to stratify the need for further testing or to guide interventions. Nevertheless, further studies will be required to determine how best to position this screening test. For instance, establishing criteria to maximize sensitivity may reduce the number of falsely negative studies, but also may lead to excessive confirmatory testing. However, using a stricter criterion may reduce the sensitivity of HRPO, which may be acceptable if the most severely affected patients requiring intervention were being identified. Because moderate to severe SDB can have an effect on comorbid conditions, the goal during hospitalization is to not miss its detection. Thus, a high specificity is more valuable than high sensitivity. Our study reveals that the agreement between HRPO and PM is highest in this category, making it an ideal hospital screening tool. Randomized clinical trials will be helpful in determining the appropriate ODI thresholds that ultimately result in improvement in cardiac function, reduction in readmissions, and ultimately mortality.
There are, however, some limitations to using HRPO for SDB diagnosis. In particular, HRPO is unable to differentiate reliably between central and obstructive respiratory events. We also acknowledge this is a single- center study in a tertiary care academic program and further studies are required to confirm its validity in a more generalized setting. In addition, our patient population was limited to those persons with CHF. Despite these caveats, however, our findings indicate that HRPO is potentially a low-cost, highly informative technique to identify hospitalized patients with SDB.
In conclusion, hospital admissions for CHF place a major burden on the health care system. Although diagnosis and treatment of sleep disorders may be a viable therapeutic target for these patients, logistical and economic challenges limit widespread implementation strategies. HRPO is a simple, inexpensive screening test that may be used to guide clinical management of acute CHF and may be useful in the design of subsequent clinical trials.
DISCLOSURE STATEMENT
This study was funded by an unrestricted research grant from ResMed Inc. ApneaLink Plus equipment was provided by ResMed Inc.
ACKNOWLEDGMENTS
The authors are grateful to our respiratory therapists, Ashley Adam and Katrina Flemming, for performing portable monitoring and HRPO on all patients participating in the SOMA-HF trial. Author contributions: Conceived and designed the experiment: SS PJM AC SG UM LW AM, DJW; Analyzed the data: SS, PJM, AC, UM, LW, AM, DJW, SQ; Wrote the paper: SS, PJM, AC, UM, LW, AM, DJW, SQ.
ABBREVIATIONS
- CHF
congestive heart failure
- HRPO
high-resolution pulse oximetry
- ODI
oxygen desaturation index
- OSA
obstructive sleep apnea
- PM
portable monitoring
- PSG
polysomnography
- REI
respiratory event index
- ROC
receiver operating curves
- SDB
sleep-disordered breathing
REFERENCES
- 1.Pfuntner A, Wier LM, Stocks C. Most Frequent Conditions in U.S. Hospitals, 2010. HCUP Statistical Brief #148. Healthcare Cost and Utilization Project website. [Accessed July 31, 2017]. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb148.pdf. Published January 2013.
- 2.Heidenreich PA, Trogdon JG, Khavjou OA, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123(8):933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 3.Gottlieb DJ, Yenokyan G, Newman AB, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the Sleep Heart Health Study. Circulation. 2010;122(4):352–360. doi: 10.1161/CIRCULATIONAHA.109.901801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang H, Parker JD, Newton GE, et al. Influence of obstructive sleep apnea on mortality in patients with heart failure. J Am Coll Cardiol. 2007;17;49(15):1625–1631. doi: 10.1016/j.jacc.2006.12.046. [DOI] [PubMed] [Google Scholar]
- 5.Sin DD, Fitzgerald F, Parker JD, Newton G, Floras JS, Bradley TD. Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med. 1999;160(4):1101–1106. doi: 10.1164/ajrccm.160.4.9903020. [DOI] [PubMed] [Google Scholar]
- 6.Javaheri S, Caref EB, Chen E, Tong KB, Abraham WT. Sleep apnea testing and outcomes in a large cohort of Medicare beneficiaries with newly diagnosed heart failure. Am J Respir Crit Care Med. 2011;183(4):539–546. doi: 10.1164/rccm.201003-0406OC. [DOI] [PubMed] [Google Scholar]
- 7.Sharma S, Mather P, Efird JT, et al. Photoplethysmographic signal to screen sleep-disordered breathing in hospitalized heart failure patients: feasibility of a prospective clinical pathway. JACC Heart Fail. 2015;3(9):725–731. doi: 10.1016/j.jchf.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 8.Sharma S, Mather P, Gupta A, et al. Effect of early intervention with positive airway pressure therapy for sleep disordered breathing on six-month readmission rates in hospitalized patients with heart failure. Am J Cardiol. 2016;117(6):940–945. doi: 10.1016/j.amjcard.2015.12.032. [DOI] [PubMed] [Google Scholar]
- 9.Kauta SR, Keenan BT, Goldberg L, Schwab RJ. Diagnosis and treatment of sleep disordered breathing in hospitalized cardiac patients: a reduction in 30-day hospital readmission rates. J Clin Sleep Med. 2014;10(10):1051–1059. doi: 10.5664/jcsm.4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma S. Hospital sleep medicine: the elephant in the room? J Clin Sleep Med. 2014;10(10):1067–1068. doi: 10.5664/jcsm.4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collop NA, Anderson WM, Boehlecke B, et al. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. J Clin Sleep Med. 2007;3(7):737–747. [PMC free article] [PubMed] [Google Scholar]
- 12.Sharma S, Mather P, Gupta A, et al. Effect of early intervention with positive airway pressure therapy for sleep disordered breathing on six-month readmission rates in hospitalized patients with heart failure. Am J Cardiol. 2016;117(6):940–945. doi: 10.1016/j.amjcard.2015.12.032. [DOI] [PubMed] [Google Scholar]
- 13.Ohmura T, Iwama Y, Kasai T, et al. Impact of predischarge nocturnal pulse oximetry (sleep-disordered breathing) on postdischarge clinical outcomes in hospitalized patients with left ventricular systolic dysfunction after acute decompensated heart failure. Am J Cardiol. 2014;113(4):697–700. doi: 10.1016/j.amjcard.2013.10.048. [DOI] [PubMed] [Google Scholar]
- 14.Sharma S, Mather PJ, Efird JT, et al. Obstructive sleep apnea in obese hospitalized patients: a single center experience. J Clin Sleep Med. 2015;11(7):717–723. doi: 10.5664/jcsm.4842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dumas C, Wahr JA, Tremper KK. Clinical evaluation of a prototype motion artifact resistant pulse oximeter in the recovery room. Anesth Analg. 1996;83(2):269–272. doi: 10.1097/00000539-199608000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Brouillette RT, Lavergne J, Leimanis A, Nixon GM, Ladan S, McGregor CD. Differences in pulse oximetry technology can affect detection of sleep-disordered breathing in children. Anesth Analg. 2002;94(1 Suppl):S47–S53. [PubMed] [Google Scholar]
- 17.Hay WW, Jr, Rodden DJ, Collins SM, Melara DL, Hale KA, Fashaw LM. Reliability of conventional and new pulse oximetry in neonatal patients. J Perinatol. 2002;22(5):360–366. doi: 10.1038/sj.jp.7210740. [DOI] [PubMed] [Google Scholar]
- 18.Oldenburg O, Teerlink JR. Screening for sleep-disordered breathing in patients hospitalized for heart failure. JACC Heart Fail. 2015;3(9):732–733. doi: 10.1016/j.jchf.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 19.Khayat RN, Abraham WT, Patt B, Pu M, Jarjoura D. In-hospital treatment of obstructive sleep apnea during decompensation of heart failure. Chest. 2009;136(4):991–997. doi: 10.1378/chest.09-0597. [DOI] [PMC free article] [PubMed] [Google Scholar]


