Abstract
Study Objectives:
Obstructive sleep apnea (OSA) and posttraumatic stress disorder (PTSD) are common in United States veterans. These conditions often coexist and symptoms overlap. Previous studies reported improvement in PTSD symptoms with continuous positive airway pressure (CPAP) therapy for comorbid OSA but its effect has not been assessed in a non-PTSD cohort. We have prospectively assessed the effect of CPAP therapy on clinical symptom improvement as a function of CPAP compliance levels among PTSD and non-PTSD veterans.
Methods:
Veterans in whom OSA was newly diagnosed were enrolled in our study (n = 192). Assignment to PTSD and non-PTSD cohorts was determined by chart review. Each patient completed the military version of the PTSD Checklist (PCL), Epworth Sleepiness Scale (ESS), and reported nightmare frequency (NMF) at baseline and 6 months after CPAP therapy. CPAP adherence was objectively documented from machine compliance data.
Results:
We had complete data for 177 veterans (PTSD n = 59, non-PTSD n = 118) for analysis. The mean ages were 51.24 years in the PTSD cohort and 52.36 years in the non-PTSD cohort (P = .30). In the PTSD cohort, the mean total PCL score (baseline = 66.06, post-CPAP = 61.27, P = .004, d = −0.34) and NMF (baseline = 4.61, post-CPAP = 1.49, P = .0001, d = −0.51) decreased after 6 months of CPAP treatment. Linear regression analysis showed that the CPAP compliance was the only significant predictor for these changes among veterans with PTSD (PCL score: P = .033, R2 = .65; NMF; P = .03, R2 = .61). Further analysis by CPAP compliance quartiles in this cohort (Q1 = 0% to 25%, Q2 = 26% to 50%, Q3 = 51% to 75%, Q4 > 75%) revealed that mean total PCL score declined in Q2 (change = −3.91, P = .045, d = 0.43), Q3 (change = −6.6, P = .002, d = 0.59), and Q4 (change = −7.94, P = .037, d = 0.49). In the non-PTSD cohort, the PCL score increased despite CPAP therapy in lower CPAP compliance quartiles Q1 (change = 8.71, P = .0001, d = 0.46) and Q2 (change = 4.51, P = .046, d = 0.27). With higher CPAP compliance (in Q3 and Q4) in this cohort, the mean total PCL scores slightly improved with CPAP but they were not statistically significant (P > .05).
Conclusions:
CPAP treatment reduces total PCL score and NMF in veterans with PTSD and OSA. Those with overt PTSD respond to even lower CPAP compliance, whereas non-PTSD patients require higher compliance to achieve any symptom improvement. Poor CPAP compliance results in increased PCL score in non-PTSD veterans and may lead to overt PTSD if the OSA remains undertreated.
Commentary:
A commentary on this article appears in this issue on page 1121.
Citation:
Ullah MI, Campbell DG, Bhagat R, Lyons JA, Tamanna S. Improving PTSD symptoms and preventing progression of subclinical PTSD to an overt disorder by treating comorbid OSA with CPAP. J Clin Sleep Med. 2017;13(10):1191–1198.
Keywords: CPAP, OSA, PTSD, veterans
INTRODUCTION
Sleep is one of the physiologic systems of the body that is affected early when someone undergoes any physical or mental stress.1,2 Sleep disruption is considered an important feature of posttraumatic stress disorder (PTSD),3 which is a psychiatric disorder characterized by intrusive recollections, avoidance behavior, and hyperarousal state following experience of a traumatic event.4 A diagnosis of PTSD is made if the characteristic symptoms are present for at least 1 month after exposure to a traumatic event. Symptoms typically begin within 3 months of a traumatic exposure but it may be delayed for months or even years before the diagnostic criteria are met. If the full diagnostic criteria are not met until at least 6 months after the event, it is labeled as “PTSD with delayed expression.”5 The veterans who have some symptoms of PTSD but failed to qualify for a formal diagnosis within 6 months of traumatic exposure have been referred to as having “subclinical,” “subthreshold” or “partial” PTSD.6 The lifetime prevalence of PTSD among all adult Americans is approximately 8.7%,7 but it is variable among United States veterans who participated in different wars. It is estimated that approximately 30% of Vietnam veterans will experience PTSD in their lifetime,8 whereas 10% to 20% of Afghanistan and Iraq war veterans9 experience PTSD in a given year. PTSD with delayed expression has also been commonly referred to as “delayed-onset PTSD” in the existing literature. This condition is not uncommon and symptoms may manifest several years after returning home from war.4 A study done in the United Kingdom on Iraq war veterans revealed that deployed soldiers remained at higher risk of the development of PTSD with delayed expression even 5 years after returning home.10 Previous studies suggested that sleep-disordered breathing and PTSD exhibit significant overlaps in symptoms and may exacerbate each other and these two conditions frequently coexist.11,12 Obstructive sleep apnea (OSA) affects approximately 1 in 5 adults in the general population.13 However, its prevalence is about 2 to 3 times higher (50% to 67%) among active-duty soldiers who also suffer from deployment-related PTSD.14,15
BRIEF SUMMARY
Current Knowledge/Study Rationale: Obstructive sleep apnea (OSA) and posttraumatic stress disorder (PTSD) are common among United States veterans, and these two conditions often coexist with overlapping clinical symptoms. CPAP therapy has been shown to improve PTSD symptoms but its effect on subclinical PTSD is unknown.
Study Impact: Our study shows that veterans with OSA and overt PTSD respond readily to CPAP therapy with symptom improvement proportionate to the CPAP compliance level. However, those with OSA and subclinical PTSD demonstrate worsening clinical symptoms with poor CPAP compliance. This may suggest that adequate treatment of veterans with OSA by CPAP may prevent development of overt PTSD.
Treatment of PTSD continues to be a major challenge for both veterans and health professionals. PTSD has been diagnosed in approximately 25% of combat veterans evaluated at United States Veterans Health Administration Hospitals from 2004 to 2009, costing approximately $1.4 billion to the United States government for their treatment.16 Conventional treatment of PTSD comprises medications and cognitive behavior therapy, but treatment failure is very common. Veterans with PTSD are 4 times more likely to have suicidal ideation than veterans without PTSD who are receiving current treatment.17 A few previous studies suggested improvement in nightmares and PTSD symptoms with continuous positive airway pressure (CPAP) therapy, but they were either retrospective or did not have a non-PTSD comparison cohort.18–21 In addition, poor sleep quality has been found to be associated with subclinical PTSD symptoms in healthy young individuals without a known history of PTSD, and interventions directed at sleep quality may relieve symptoms in persons with PTSD.22 In our prospective study, we hypothesized that treating coexisting OSA with CPAP therapy will improve overall symptoms of PTSD and untreated OSA may increase the severity of PTSD symptoms or change sub-clinical symptoms to overt PTSD over time.
METHODS
Participant Selection
After receiving approval from the Institutional Review Board of G.V. (Sonny) Montgomery VA Medical Center at Jackson, Mississippi, 200 consecutive patients in whom OSA was diagnosed between May 2013 and June 2015 were selected. These patients were approached when they came to the sleep clinic for their follow-up appointment to discuss the sleep study result and receive the CPAP equipment after their overnight polysomnography (PSG). By reviewing their electronic medical record, we assigned the veterans into the PTSD cohort if they met all of the following criteria: (1) a prior diagnosis of PTSD made by a certified mental health care provider at least 1 year prior to the sleep clinic visit, and (2) on a stable medication regimen for PTSD for at least 6 months. Those in whom PTSD was never diagnosed were assigned to the non-PTSD cohort. Veterans with marked cognitive impairment and active alcohol or drug abuse were excluded from the study. If a subject needed any change in medication regimen for PTSD during the study period, that individual would also be dropped from the study.
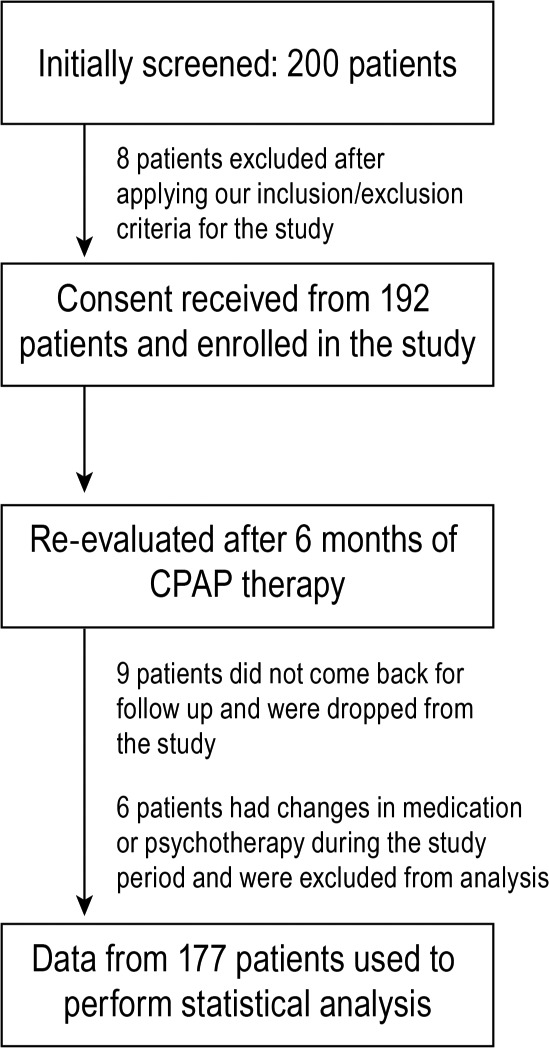
After applying our inclusion and exclusion criteria, 8 patients were excluded from the study because they did not meet these criteria. The remaining 192 subjects were enrolled in the study, and each individual's written informed consent was obtained. Six of the 192 patients needed one or more changes in their medication and/or psychotherapy by their mental health providers during the study and were excluded from the study analysis. Nine of the participants did not report for their visit at the sleep clinic after 6 months and they were also excluded from the study. Complete data for 177 patients were available for analysis (Figure 1).
Figure 1. Flow diagram of study design and patient distribution.
Criteria for OSA Diagnosis
OSA was diagnosed with full-night PSG with standard protocol approved by American Academy of Sleep Medicine using the following criteria: apnea-hypopnea index (AHI) ≥ 15 events/h, or AHI ≥ 5 events/h but < 15 events/h with one of the following: excessive daytime sleepiness, insomnia, mood disorder, impaired cognition, essential hypertension, ischemic heart disease, or history of stroke.23 The therapeutic CPAP pressure was determined from the split-night study with CPAP titration in most patients. A few patients whose therapeutic pressure could not be determined on the first night of PSG had to come back for a full night of PSG with CPAP titration.
Procedures and Measures
During their initial encounter, each participant received a CPAP machine equipped with a modem for monitoring their CPAP compliance data. CPAP compliance was defined as the percentage of nights a patient used the CPAP therapy for at least 4 hours during the study period (the standard Medicare criteria). For example, a CPAP compliance of 70% indicates that the patient used CPAP in 70% of the nights for ≥ 4 hours in the previous 6 months. The patients also filled out a questionnaire to assess their PTSD Checklist (PCL) score, Epworth Sleepiness Scale (ESS) score, and average number of nightmares experienced per week in the previous month. The PSG reports of the subjects were reviewed and data about the following variables were collected: age, body mass index (BMI), total AHI and AHI while in stage R sleep, stage R sleep percentage, total sleep time (TST), and sleep efficiency (SE). These questionnaires were repeated at the follow-up visit after 6 months in the sleep clinic. The patients' CPAP compliance data were also downloaded during this time and the following information was extracted: average hours of use/night and percentage of nights used for ≥ 4 h/night.
Questionnaires Used
PTSD Checklist
The PCL is a 17-item self-report measure reflecting Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) symptoms of PTSD asking, “how much you have been bothered by that problem in the last month.” The subjects answer on a scale of 1 to 5, (1 = not at all, 2 = a little bit, 3 = moderately, 4 = quite a bit, 5 = extremely).24,25 The PCL has a variety of purposes, including (1) screening individuals for PTSD, (2) aiding in diagnostic assessment of PTSD, and (3) monitoring change in PTSD symptoms.26 There are 3 versions of the PCL questionnaire available for DSM-IV and we have used the PCL-M, which is more specific for assessing stressful military experience. The 17 questions may be subdivided into 3 subscales or clusters: Items 1 to 5 represent stressful recaps of the military experiences (intrusions subscale), items 6 to 12 represent active efforts to avoid specific situations that may bring back those stressful memories (avoidance subscale), and items 13 to 17 represent the tendency to be hypersensitive to ordinary stimuli, leading to difficulty in concentrating or sleeping (hyperarousal subscale). The total possible scores range from 17 to 85. When the instrument is used as a continuous measure, a cutoff score of 50 is optimal for making the diagnosis of combat-related PTSD.27 Despite being a self-reported score, PCL has been found to have no differences in percentage of agreement between clinicians and patients in clinical symptom changes.28 Evidence suggest that a 5- to 10-point change represents a reliable change (ie, change not due to chance) and a greater than 10-point change represents a clinically significant change.28
Epworth Sleepiness Scale
The ESS determines the severity of one's daytime sleepiness through self-rating on 8 items, asking “How likely are you to doze off or fall asleep in the following situations, in contrast to feeling just tired?” Each item scores from 0 to 3 (0 = never, 1 = slight chance, 2 = moderate chance and 3 = high chance). Scores may range from 0 to 24 after adding up all the items. Available over the last 25 years, this scale has shown good sensitivity for measuring daytime sleepiness in a variety of clinical settings and research paradigms.29
Statistical Analysis
The patients were categorized into cohorts of PTSD (n = 59) and non-PTSD (n = 118). The demographic and polysomno-graphic characteristics of both groups were compared using chi-square tests. Changes in mean total PCL scores and nightmare frequency per week before and after the CPAP therapy were assessed by repeated-measure t tests. Effect sizes have been calculated (Cohen d) that expresses the mean difference between 2 groups in standard deviation units. This provides additional information regarding the magnitude of changes between “means” as the P value may indicate statistical significance of a change that may not be clinically meaningful. Generally, the Cohen d is interpreted as: .8 = large effect (8/10 of a standard deviation unit), .5 = moderate effect (1/2 of a standard deviation) and .2 = small effect (1/5 of a standard deviation). A one-way analysis of variance was performed to check for statistically significant differences in changes of mean total PCL score across quartiles of CPAP compliance. A multiple linear regression analysis was performed to identify factors that may predict change in total PCL score with CPAP therapy. Independent variables tested in the initial model included age, BMI, TST, total AHI, AHI while in stage R sleep, CPAP compliance, baseline total PCL score, and number of average weekly nightmares. Stepwise regressions eliminated the variables that were statistically insignificant (P > .05). A logistic regression analysis (using a dichotomous outcome variable of symptom reduction) was also performed to estimate the odds of decline in total PCL score by at least 10 points after CPAP therapy, adjusting for similar potential confounders. Data were analyzed with STATA software, version 14.1 (StataCorp, College Station, Texas, United States).
RESULTS
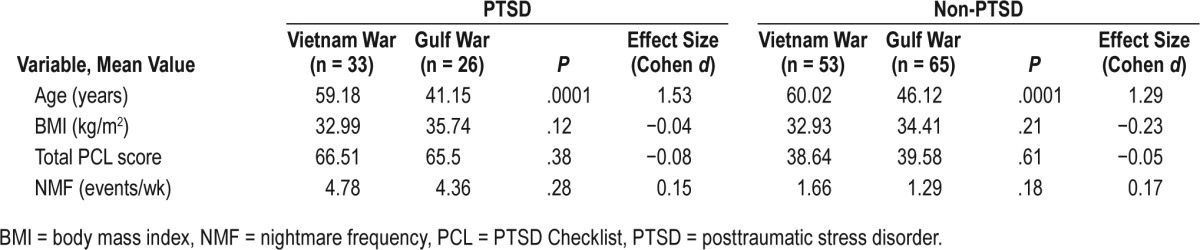
Demographic and polysomnographic characteristics of patients with PTSD (n = 59) and non-PTSD (n = 118) are described in Table 1. There were no significant differences in demographic characteristics (age, BMI) and baseline polysomnographic characteristics including total AHI, AHI while in stage R sleep AHI, stage R sleep percentage, TST, SE, ESS, or CPAP compliance among these two groups. However, the mean total PCL score (P = .0001) and weekly nightmare frequency (NMF) (P = .0001) were higher in the PTSD group than the non-PTSD group before starting CPAP. In the PTSD group, 26 veterans served in the Gulf War and 33 in the Vietnam War. In the non-PTSD cohort, 65 veterans served in the Gulf War and 53 in the Vietnam War. The Vietnam War veterans (mean age = 59.7 years) were approximately 15 years older than the Gulf War veterans (mean age = 44.7 years). There was no significant difference in BMI, baseline mean total PCL score, or weekly NMF between these 2 groups of veterans (Table 2).
Table 1.
Demographic and polysomnographic features before CPAP.
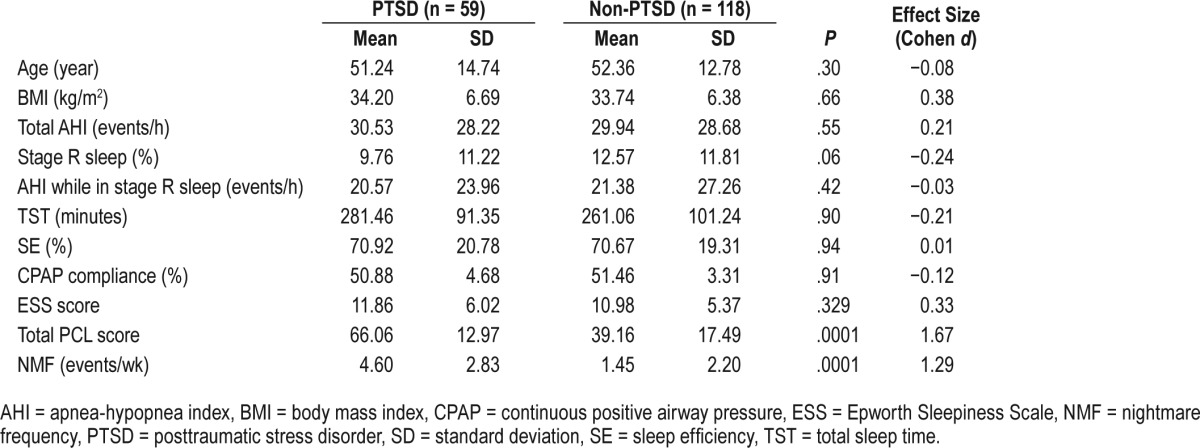
Table 2.
Comparison of mean age, BMI, total PCL score, and NMF among Vietnam and Gulf War veterans at baseline.
After 6 months of CPAP therapy, changes in ESS, mean total PCL score, and NMF show variable changes based on their cohort assignment (Table 3). Daytime sleepiness (ESS) improved significantly in all patients. In the PTSD cohort, mean total PCL score (P = .004, d = −0.34) and NMF (P = .0001, d = −0.51) decreased with CPAP therapy. The PCL intrusions subscale (P = .002, d = −0.34) and hyperarousal subscale (P = .003, d = −0.49) also showed lower mean scores among the PTSD cohort. But in the non-PTSD cohort, there were no significant changes in NMF (P = .164, d = −0.05), overall mean total PCL score (P = .061, d = 0.14), or subscales of PCL after CPAP therapy. In a stepwise regression analysis, changes in total PCL score (t = −4.91, df = 2, P = .033, R2 = .65) and NMF (t = −4.42, df = 2, P = 0.03, R2 = 0.61) after 6 months of CPAP therapy were predicted only by CPAP compliance. There was a positive correlation between the CPAP compliance percentage and the degree of decline in mean total PCL score after CPAP in both the PTSD (r = .38) and the non-PTSD group (r = .24).
Table 3.
Comparison of mean total and subscale PCL scores, NMF, and ESS before and after CPAP therapy.
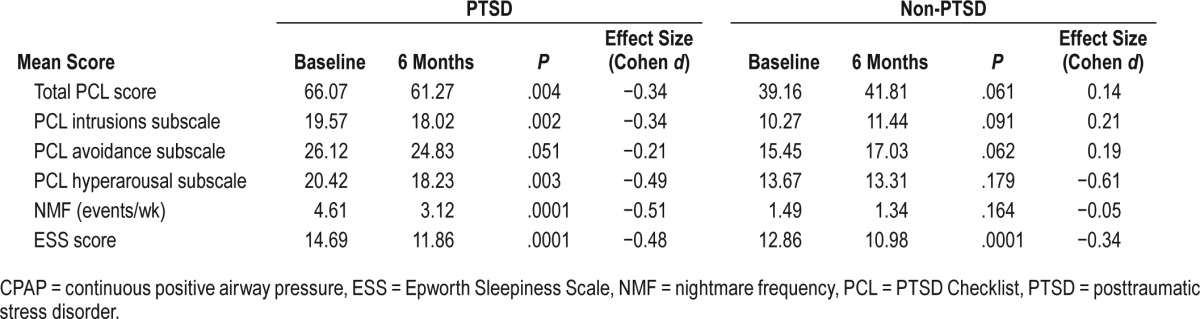
We also investigated the changes in mean total PCL score (contrast = mean total PCL score at baseline – PCL score after 6 months) further across various CPAP compliance levels and the compliance was divided into 4 quartiles: Q1 = 0% to 25%, Q2 = 26% to 50%, Q3 = 51% to 75%, Q4 > 75%. A one-way analysis of variance confirmed statistically significant differences in mean total PCL score changes across various quartiles of CPAP use among patients with PTSD (F = 1.08, P = .0481) and without PTSD (F = 4.78, P = .0036). In the PTSD cohort, there was a steady decline in mean total PCL score after 6 months in each quartile of CPAP compliance, with the highest decline in Q4. The contrasts were statistically significant in all quartiles (Q2 contrast = 3.91, P = .045, d = 0.43; Q3 contrast = 6.6, P = .002, d = 0.59; Q4 contrast = 7.94, P = .037, d = 0.48) except in Q1 (contrast = 1.12, P = .641, d = 0.08). However, in the non-PTSD cohort, mean total PCL score increased after 6 months in Q1 (contrast = −8.71, P = .0001, d = −0.46) and Q2 (contrast = −4.51, P = .046, d = −0.27). In higher quartiles, the mean total PCL score started to decline but was not statistically significant (Q3 contrast = 1.58, P = .68, d = 0.08, Q4 contrast = 2.27, P = .93, d = 0.14) (Figure 2).
Figure 2. Change in mean PCL score by four quartiles of CPAP compliance.
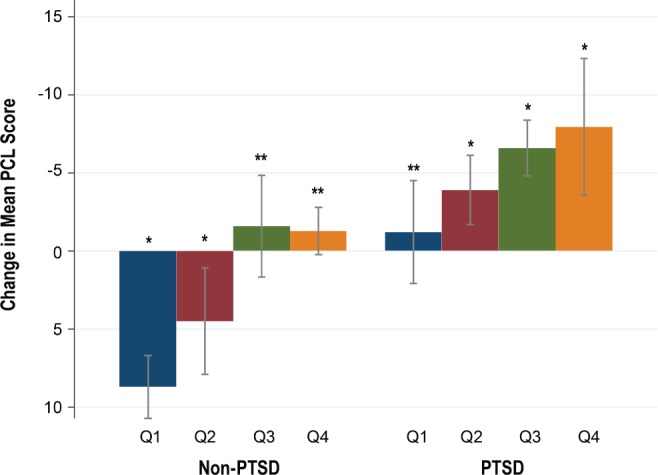
* = P < .05. ** = P > .05. Error bars denote 95% confidence interval. Negative change in mean PCL score means worsening of PTSD symptom, positive change means symptom improvement. CPAP = continuous positive airway pressure, PCL = PTSD Checklist, PTSD = posttraumatic stress disorder, Q = quartile.
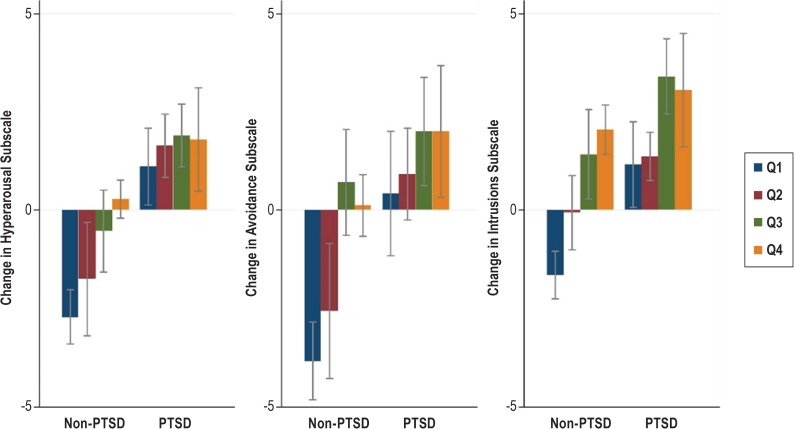
When comparing the change in scores for subscales of PCL by CPAP compliance quartiles, significant progressive decline in mean scores (P < .01) was seen in all subscales after 6 months in all quartiles in the PTSD cohort. In the non-PTSD cohort, the mean scores increased in all subscales (P < .003) when the CPAP compliance was in Q1 and Q2. The scores declined in higher quartiles but the changes were not statistically significant (Figure 3).
Figure 3. Comparison of changes in mean PCL subscale scores by CPAP compliance quartiles.
Negative change in total score means worsening of PTSD symptom, positive change means symptom improvement. Error bars denote 95% confidence interval. CPAP = continuous positive airway pressure, PCL = PTSD Checklist, PTSD = posttraumatic stress disorder, Q = quartile.
We also looked at the proportion of subjects who had changes in mean total PCL score by at least 10 points after CPAP therapy (the accepted threshold denoting a clinically significant change).28 In the PTSD cohort, 27% (n = 16) had a decline in score ≥ 10 points (improvement of PTSD) with CPAP therapy. In the non-PTSD cohort, 11% (n = 13) had a decline in score with CPAP therapy after 6 months. For every 10% increase in CPAP compliance, the odds of clinically meaningful decline in mean total PCL score increases by 1.77-fold (P = .02) in the PTSD cohort and 1.09-fold (P = .043) in the non-PTSD cohort.
DISCUSSION
Consistent with previous study findings, our study demonstrated that CPAP treatment helps improve symptoms of PTSD and reduces the frequency of nightmares. Additionally, it showed the beneficial effect of PCL score improvement in non-PTSD subjects. It has been reported previously that veterans with PTSD tend to have lower CPAP compliance compared to the non-PTSD group (PTSD versus non-PTSD: 30.2% versus 55.1%, P = .02;12 25.2% versus 58.3%, P = .01;30 and 41% versus 70%, P < .00131). PTSD itself was thought to be a barrier for effective CPAP use from different factors including higher insomnia prevalence, polypharmacy, and causation of flash-backs (eg, for veterans whose trauma occurred while wearing a gas mask or scuba gear). However, our study population had similar CPAP compliance in both PTSD and non-PTSD groups (50.88% versus 51.46%, P = .91). This finding may not be generalized to other settings because various factors may influence CPAP compliance. For our referral center, it may be partly explained by the fact that we had a robust follow-up policy for those who have been newly initiated on CPAP. Our sleep laboratory staff proactively call the patients to troubleshoot any problem with the machine or the mask and encourage them to keep using it nightly. Common mask-related issues are dealt with aggressively, resulting in better CPAP adherence. It is also possible that the PTSD group in our study might have been somehow better treated prior to starting CPAP compared to the PTSD subjects in previous studies, resulting in better sleep continuity and improved CPAP compliance. After CPAP therapy for 6 months, the PTSD group demonstrated a significant drop in mean total PCL score (baseline score 66.06, post-CPAP score = 61.27, P = .004, d = −0.34), whereas the non-PTSD group had an increase in mean total PCL score (baseline score 39.16, post-CPAP score = 41.81, P = .061, d = 0.14). Although this increase was not statistically significant, it clearly showed an increasing trend in total PCL score. Further analysis of change in total PCL score scores by CPAP compliance quartiles helped explain this finding (Figure 2). In the PTSD cohort, the mean total PCL score started to decline in the second CPAP compliance quartile and progressive increase of CPAP compliance in each higher quartile resulted in more substantial decrease in total PCL score. However, in the non-PTSD cohort, low CPAP compliance (in Q1 and Q2) failed to decrease the mean total PCL score at all. In fact, the total PCL score increased after 6 months in these lower two quartiles. Their mean total PCL score scores were 37.45 in Q1 and 46.87 in Q2 at baseline and their score increased to 46.17 (P = .0001) in Q1 and 51.37 (P = .041) in Q2 after 6 months. From this finding, we may postulate that the patients with overt PTSD respond readily even with minimum CPAP usage, whereas non-PTSD patients require a compliance of more than 50% to reduce the risk of the development of overt PTSD.
PTSD with delayed expression is a known phenomenon that has been reported widely, especially among veterans. It has been commonly referred to as “delayed-onset PTSD” in the existing literature. We are using the term “PTSD with delayed expression” to conform to the DSM-5 terminology. A systematic meta-analysis of 39 prospective studies reported that 24.5% (95% confidence interval 19.5% to 30.3%) of all PTSD cases were PTSD with delayed expression.4 Another study using data from the National Health and Resilience in Veterans Study (NHRVS) revealed that approximately 1 in 10 older United States veterans experiences a clinically significant exacerbation of PTSD symptoms in late life. A comparison of immediate and PTSD with delayed expression showed that both were similar in the number and type of symptoms reported at onset, but the delayed-onset group differed in showing a gradual accumulation of symptoms that began earlier and continued throughout their military career.32 Because sleep disruption is an important feature of PTSD, the presence of untreated OSA may serve as a continued stressor to trigger PTSD with delayed expression by sleep fragmentation and sympathetic over-activity.33,34 All our study subjects served either in the Vietnam War (ended in 1975) or the Gulf War (ended in 1991), yet the non-PTSD cohort with CPAP noncompliance demonstrated a trend of increasing PCL score in only 6 months. A future study with a larger sample size and a longer follow-up duration may further strengthen (or refute) the causal association between untreated OSA and PTSD with delayed expression.
As mentioned before, a cutoff total DSM-IV PCL score of 50 may be considered optimal for making the diagnosis of combat-related PTSD.27 In our study, we found that the mean total PCL score already increased to PTSD range for non-PTSD patients in Q2 after 6 months. The patients in Q1 had a much lower baseline total PCL score (37.45) and it went up to 46.17 after 6 months. With its upward trend, it may be presumed that their score may eventually reach the “50” cutoff mark soon if their CPAP compliance remains low. Only the CPAP compliance of greater than 50% (in Q3 and Q4) had any meaningful effect to decrease the total PCL score in the non-PTSD cohort. This finding may indicate that, if OSA remains untreated or undertreated, veterans with OSA who have been exposed to combat experience in the past but still had lower total PCL scores may have a gradual increase in their total PCL score, leading to overt PTSD.
The CPAP therapy (or the lack of it) also showed an effect on both cohorts in terms of clinically significant change (at least 10 points) in mean PCL score. One-fourth of all PTSD patients (n = 16) experienced clinically significant decline in PCL score after CPAP, whereas only one-tenth of non-PTSD patients (n = 13) had such decline. This may again be explained by the better responsiveness of the PTSD patients to any level of CPAP compliance.
Chronic sleep deprivation from any cause (including OSA) has significant detrimental effect on memory, and the role of sleep in memory processing and retention is a well-known phenomenon.35 Encoding and retention of memory is adversely affected by lack of sleep, and memories associated with negative emotions are better retained with chronic sleep deprivation.36–38 It is believed that a memory has two components: an emotional component and an informational core. Memories with positive emotions easily lose their emotional components over time despite sleep deprivation, whereas the negative emotions are resistant from decoupling from their informational core. This may partly explain the progressive increase in PCL scores when sleep continuity is disrupted from OSA.
Research on neurobiological findings of PTSD showed evidence of volumetric changes in the hippocampus, amygdala, and multiple frontal-limbic system structures of the brain in individuals with PTSD. Germain et al.39 propose that stage R sleep amplifies activation of the amygdala and reduces medial frontal cortex activation in PTSD patients, causing worsening nightmares. It also causes increased activity in arousal-promoting centers and reduced activity in sleep-promoting centers, resulting in fragmentation of sleep and insomnia.39 Restoration of stage R and non-stage R sleep help consolidate both fear and extinction memory.40 Because PTSD is characterized by impairments of both sleep and extinction memory, improving sleep quality may improve these symptoms by strengthening naturally acquired extinction. It is also known that the hippocampus is extremely sensitive to hypoxia41 and reduction in hippocampal activity correlates with the severity of PTSD symptoms.42 Nocturnal hypoxia is common in untreated OSA, which may be a trigger for PTSD symptoms. CPAP therapy has been reported to make significant improvements in memory, attention, and executive function and paralleled gray-matter volume increases in hippocampal and frontal structures.43 Thus, CPAP therapy may help improve PTSD symptoms by restoring sleep continuity and correcting nocturnal hypoxia.
Our study had some advantages compared to previous studies. We had a unique combination of a prospective study, presence of a non-PTSD comparison cohort, availability of PCL scores before and after CPAP treatment for both cohorts, and objective documentation of CPAP compliance. The United States Department of Veteran Affairs requires PCL administration to veterans with PTSD in active treatment as part of an effort to establish national PTSD outcome measures.44 As a psychometric measure, PCL shows good temporal stability, internal consistency, test-retest reliability and convergence validity.45 Use of downloaded CPAP compliance data eliminated patient recall bias (common in self-reported compliance data) and helped accurately measure usage data.46
We also had some limitations in our study. The patients in the PTSD cohort were already under treatment by their psychiatrists on a stable regimen before initiating CPAP. Therefore, it is not clear if there was any interaction between the treatment effect of medications and CPAP. Most psychiatric medications have variable effects on sleep including altering the sleep architecture, particularly causing change in sleep stages, which may result in change in severity of sleep apnea. These medications obviously also cause improvement in PTSD symptoms. A future study may be done recruiting patients from psychiatric clinics in whom PTSD has just been diagnosed and who are randomized to either psychotherapy or pharmacotherapy. They may be screened for OSA symptoms and then referred to the sleep clinic for PSG if indicated. These patients may then be prescribed CPAP and be compared to a control group of non-PTSD patients with OSA. This may be helpful to tease apart the true effect of CPAP without any interaction with medication. Another study could be done where recent combat veterans are recruited and specifically screened for subclinical PTSD. Those who meet a specific range of PCL criteria may then be screened for OSA. Thereafter, the veterans with OSA are followed up, looking for change in PCL score for a given period of time to test if higher CPAP compliance can prevent development of overt PTSD. Chronicity of PTSD may also influence treatment-response to both medication and CPAP therapy. We have not collected this information and were unable to control for time since PTSD diagnosis.
In summary, CPAP treatment improves total PCL scores and reduces nightmare frequency in veterans with PTSD and OSA. CPAP compliance is the most important predictor for symptom improvement and those with PTSD readily respond to even low CPAP compliance. However, veterans with sub-clinical PTSD require high CPAP compliance to achieve any improvement in PCL score and poor compliance leads to rapid increase in symptoms, potentially unmasking the delayed expression of PTSD over time. Early screening of combat veterans with OSA for PTSD with optimum comprehensive therapy may help reduce the social and financial burden of this disease.
DISCLOSURE STATEMENT
Work for this study was performed at G.V. (Sonny) Montgomery VA Medical Center, Jackson, Mississippi. All authors have reviewed and approved the manuscript. No off-label or investigational use of medication has been done in this study. Financial support was received by Dr. Sadeka Tamanna from a pilot grant by South Central VA Network (VISN 16) to conduct this study. The authors report no conflicts of interest.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- ANOVA
analysis of variance
- BMI
body mass index
- CPAP
continuous airway positive pressure
- ESS
Epworth Sleepiness Scale
- NMF
nightmare frequency
- OSA
obstructive sleep apnea
- PCL
PTSD Checklist
- PTSD
posttraumatic stress disorder
- Q
quartile
- SE
sleep efficiency
- TST
total sleep time
REFERENCES
- 1.Haynes SN, Adams A, Franzen M. The effects of presleep stress on sleep-onset insomnia. J Abnorm Psychol. 1981;90(6):601–606. doi: 10.1037//0021-843x.90.6.601. [DOI] [PubMed] [Google Scholar]
- 2.Morin CM, Rodrigue S, Ivers H. Role of stress, arousal, and coping skills in primary insomnia. Psychosom Med. 2003;65(2):259–267. doi: 10.1097/01.psy.0000030391.09558.a3. [DOI] [PubMed] [Google Scholar]
- 3.Germain A. Sleep disturbances as the hallmark of PTSD: where are we now? Am J Psychiatry. 2013;170(4):372–382. doi: 10.1176/appi.ajp.2012.12040432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Utzon-Frank N, Breinegaard N, Bertelsen M, et al. Occurrence of delayed-onset post-traumatic stress disorder: a systematic review and meta-analysis of prospective studies. Scand J Work Environ Health. 2014;40(3):215–229. doi: 10.5271/sjweh.3420. [DOI] [PubMed] [Google Scholar]
- 5.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 6.Schnurr PP. A Guide to the Literature on Partial PTSD. PTSD Research Quarterly. [Accessed August 15, 2017]. https://www.ptsd.va.gov/professional/newsletters/research-quarterly/v25n1.pdf. Volume 25, Number 1. Published 2014.
- 7.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 8.Kilpatrick DG, Ruggiero KJ, Acierno R, Saunders BE, Resnick HS, Best CL. Violence and risk of PTSD, major depression, substance abuse/dependence, and comorbidity: results from the National Survey of Adolescents. J Consult Clin Psychol. 2003;71(4):692–700. doi: 10.1037/0022-006x.71.4.692. [DOI] [PubMed] [Google Scholar]
- 9.Tanielian T, Jaycox L, editors. Invisible Wounds of War: Psychological and Cognitive Injuries, Their Consequences, and Services to Assist Recovery. Santa Monica, CA: RAND Corporation; 2008. [Google Scholar]
- 10.Harvey SB, Hatch SL, Jones M, et al. The long-term consequences of military deployment: A 5-year cohort study of United Kingdom reservists deployed to Iraq in 2003. Am J Epidemiol. 2012;176(12):1177–1184. doi: 10.1093/aje/kws248. [DOI] [PubMed] [Google Scholar]
- 11.Sripada RK, Pfeiffer PN, Rampton J, et al. Predictors of PTSD symptom change among outpatients in the U.S. Department of Veterans Affairs Health Care System. J Trauma Stress. 2017;30(1):45–53. doi: 10.1002/jts.22156. [DOI] [PubMed] [Google Scholar]
- 12.Lettieri CJ, Williams SG, Collen JF. OSA syndrome and posttraumatic stress disorder. Chest. 2016;149(2):483–490. doi: 10.1378/chest.15-0693. [DOI] [PubMed] [Google Scholar]
- 13.Caples SM, Gami AS, Somers VK. Obstructive sleep apnea. Ann Intern Med. 2005;142(3):187–197. doi: 10.7326/0003-4819-142-3-200502010-00010. [DOI] [PubMed] [Google Scholar]
- 14.Mysliwiec V, McGraw L, Pierce R, Smith P, Trapp B, Roth BJ. Sleep disorders and associated medical comorbidities in active duty military personnel. Sleep. 2013;36(2):167–174. doi: 10.5665/sleep.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams SG, Collen J, Orr N, Holley AB, Lettieri CJ. Sleep disorders in combat-related PTSD. Sleep Breath. 2015;19(1):175–182. doi: 10.1007/s11325-014-0984-y. [DOI] [PubMed] [Google Scholar]
- 16.The Veterans Health Administration's Treatment of PTSD and Traumatic Brain Injury Among Recent Combat Veterans. Congressional Budget Office website. [Accessed August 15, 2017]. https://www.cbo.gov/publication/42969. Published February 9, 2012.
- 17.Jakupcak M, Cook J, Imel Z, Fontana A, Rosenheck R, McFall M. Posttraumatic stress disorder as a risk factor for suicidal ideation in Iraq and Afghanistan War veterans. J Trauma Stress. 2009;22(4):303–306. doi: 10.1002/jts.20423. [DOI] [PubMed] [Google Scholar]
- 18.Tamanna S, Parker J, Lyons J, Ullah MI. The effect of continuous positive air pressure (CPAP) on nightmares in patients with posttraumatic stress disorder (PTSD) and obstructive sleep apnea (OSA) J Clin Sleep Med. 2014;10(6):631–636. doi: 10.5664/jcsm.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krakow B, Lowry C, Germain A, et al. A retrospective study on improvements in nightmares and post-traumatic stress disorder following treatment for co-morbid sleep-disordered breathing. J Psychosom Res. 2000;49(5):291–298. doi: 10.1016/s0022-3999(00)00147-1. [DOI] [PubMed] [Google Scholar]
- 20.Orr JE, Smales C, Alexander TH, et al. Treatment of OSA with CPAP is associated with improvement in PTSD symptoms among Veterans. J Clin Sleep Med. 2017;13(1):57–63. doi: 10.5664/jcsm.6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.El-Solh AA, Vermont L, Homish GG, Kufel T. The effect of continuous positive airway pressure on post-traumatic stress disorder symptoms in veterans with post-traumatic stress disorder and obstructive sleep apnea: a prospective study. Sleep Med. 2017;33:145–150. doi: 10.1016/j.sleep.2016.12.025. [DOI] [PubMed] [Google Scholar]
- 22.McCubbin JA, Zinzow HM, Hibdon MA, et al. Subclinical posttraumatic stress disorder symptoms: Relationships with blood pressure, hostility, and sleep. Cardiovasc Psychiatry Neurol. 2016;2016:4720941. doi: 10.1155/2016/4720941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. p. 53. [Google Scholar]
- 24.Weathers F, Huska J, Keane T. The PTSD checklist military version (PCL-M) Boston, MA: National Center for PTSD; 1991. [Google Scholar]
- 25.Blanchard EBEB, Jones-Alexander J, Buckley TC, Forneris CA. Psychometric properties of the PTSD Checklist (PCL) Behav Res Ther. 1996;34(8):669–673. doi: 10.1016/0005-7967(96)00033-2. [DOI] [PubMed] [Google Scholar]
- 26.Using the PTSD Checklist for DSM-IV (PCL) National Center for PTSD website. [Accessed August 18, 2017]. https://www.ptsd.va.gov/professional/assessment/adult-sr/ptsd-checklist.asp.
- 27.Forbes D, Creamer M, Biddle D. The validity of the PTSD checklist as a measure of symptomatic change in combat-related PTSD. Behav Res Ther. 2001;39(8):977–986. doi: 10.1016/s0005-7967(00)00084-x. [DOI] [PubMed] [Google Scholar]
- 28.Monson CM, Gradus JL, Young-Xu Y, Schnurr PP, Price JL, Schumm JA. Change in posttraumatic stress disorder symptoms: do clinicians and patients agree? Psychol Assess. 2008;20(2):131–138. doi: 10.1037/1040-3590.20.2.131. [DOI] [PubMed] [Google Scholar]
- 29.Johns MW. Daytime sleepiness, snoring, and obstructive sleep apnea. The Epworth Sleepiness Scale. Chest. 1993;103(1):30–36. doi: 10.1378/chest.103.1.30. [DOI] [PubMed] [Google Scholar]
- 30.Collen JF, Lettieri CJ, Hoffman M. The impact of posttraumatic stress disorder on CPAP adherence in patients with obstructive sleep apnea. J Clin Sleep Med. 2012;8(6):667–672. doi: 10.5664/jcsm.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El-Solh AA, Ayyar L, Akinnusi M, Relia S, Akinnusi O. Positive airway pressure adherence in veterans with posttraumatic stress disorder. Sleep. 2010;33(11):1495–1500. doi: 10.1093/sleep/33.11.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andrews B, Brewin CR, Stewart L, Philpott R, Hejdenberg J. Comparison of immediate-onset and delayed-onset posttraumatic stress disorder in military veterans. J Abnorm Psychol. 2009;118(4):767–777. doi: 10.1037/a0017203. [DOI] [PubMed] [Google Scholar]
- 33.Cahan C, Arafah B, Decker MJ, Arnold JL, Strohl KP. Adrenal steroids in sleep apnea before and after nCPAP treatment. Am Rev Respir Dis. 1991;143:A382. [Google Scholar]
- 34.Schmoller A, Eberhardt F, Jauch-Chara K, et al. Continuous positive airway pressure therapy decreases evening cortisol concentrations in patients with severe obstructive sleep apnea. Metabolism. 2009;58(6):848–853. doi: 10.1016/j.metabol.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 35.Tempesta D, Socci V, Coppo M, et al. The effect of sleep deprivation on the encoding of contextual and non-contextual aspects of emotional memory. Neurobiol Learn Mem. 2016;131:9–17. doi: 10.1016/j.nlm.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 36.Walker MP, van der Helm E. Overnight therapy? The role of sleep in emotional brain processing. Psychol Bull. 2009;135(5):731–748. doi: 10.1037/a0016570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tempesta D, Mazza M, Iaria G, De Gennaro L, Ferrara M. A specific deficit in spatial memory acquisition in post-traumatic stress disorder and the role of sleep in its consolidation. Hippocampus. 2012;22(5):1154–1163. doi: 10.1002/hipo.20961. [DOI] [PubMed] [Google Scholar]
- 38.Walker MP. The role of sleep in cognition and emotion. Ann N Y Acad Sci. 2009;1156:168–197. doi: 10.1111/j.1749-6632.2009.04416.x. [DOI] [PubMed] [Google Scholar]
- 39.Germain A, Buysse DJ, Nofzinger E. Sleep-specific mechanisms underlying posttraumatic stress disorder: Integrative review and neurobiological hypotheses. Sleep Med Rev. 2008;12(3):185–195. doi: 10.1016/j.smrv.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pace-Schott EF, Germain A, Milad MR. Effects of sleep on memory for conditioned fear and fear extinction. Psychol Bull. 2015;141(4):835–857. doi: 10.1037/bul0000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ferini-Strambi L, Lombardi GE, Marelli S, Galbiati A. Neurological deficits in obstructive sleep apnea. Curr Treat Options Neurol. 2017;19(4):16. doi: 10.1007/s11940-017-0451-8. [DOI] [PubMed] [Google Scholar]
- 42.Astur RS, St. Germain SA, Tolin D, Ford J, Russell D, Stevens M. Hippocampus function predicts severity of post-traumatic stress disorder. Cyberpsychol Behav. 2006;9(2):234–240. doi: 10.1089/cpb.2006.9.234. [DOI] [PubMed] [Google Scholar]
- 43.Canessa N, Castronovo V, Cappa SF, et al. Obstructive sleep apnea: Brain structural changes and neurocognitive function before and after treatment. Am J Respir Crit Care Med. 2011;183(10):1419–1426. doi: 10.1164/rccm.201005-0693OC. [DOI] [PubMed] [Google Scholar]
- 44.Elhai JD, Gray MJ, Kashdan TB, Franklin CL. Which instruments are most commonly used to assess traumatic event exposure and posttraumatic effects?: A survey of traumatic stress professionals. J Trauma Stress. 2005;18(5):541–545. doi: 10.1002/jts.20062. [DOI] [PubMed] [Google Scholar]
- 45.Wilkins KC, Lang AJ, Norman SB. Synthesis of the psychometric properties of the PTSD checklist (PCL) military, civilian, and specific versions. Depress Anxiety. 2011;28(7):596–606. doi: 10.1002/da.20837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rauscher H, Formanek D, Popp W, Zwick H. Self-reported vs measured compliance with nasal CPAP for obstructive sleep apnea. Chest. 1993;103(6):1675–1680. doi: 10.1378/chest.103.6.1675. [DOI] [PubMed] [Google Scholar]