Abstract
Enuresis, or “bedwetting,” in children is associated with obstructive sleep apnea (OSA), and often resolves with treatment of OSA. However, it is poorly understood whether a similar relationship exists in adults. We describe a case series of 5 adult patients in whom OSA was diagnosed by laboratory polysomnography, who presented with enuresis that resolved after treatment with continuous positive airway pressure (CPAP). All cases occurred in the setting of obesity, in addition to other known risk factors for urinary incontinence and enuresis. OSA was diagnosed as severe in all but one case, which was mild. One patient noted recurrence of enuresis that coincided with malfunction of his CPAP machine. There is growing evidence that CPAP therapy may alleviate OSA and enuresis in adults with both conditions. Clinicians should routinely ask about enuresis in patients suspected of having OSA. A systematic study of the association between enuresis and OSA in adults is warranted.
Citation:
McInnis RP, Dodds EB, Johnsen J, Auerbach S, Pyatkevich Y. CPAP treats enuresis in adults with obstructive sleep apnea. J Clin Sleep Med. 2017;13(10):1209–1212.
Keywords: bedwetting, CPAP, enuresis, obstructive sleep apnea, quality of life, sleep-disordered breathing
INTRODUCTION
Although an association between enuresis and obstructive sleep apnea (OSA) in children has been demonstrated repeatedly in large population-based studies, whether this association exists within the adult population remains poorly understood.1–4 In children, enuresis has a higher prevalence among those who have OSA, correlates with disease severity on polysomnography (PSG), and often resolves with treatment of OSA by adenotonsillectomy.5,6
The reported prevalence of enuresis in adults varies widely, depending on how enuresis is clinically defined and on the population studied. In a large cohort of randomly sampled adults aged 18 to 64 years old, 0.5% reported nocturnal enuresis, defined as any bedwetting in the prior 4 weeks.7 Among postmenopausal women and women referred to a urogyneco-logic outpatient clinic, the prevalence of enuresis was 1.7% and 23%, respectively.8,9 In a large cohort of male and female patients referred for urologic evaluation of lower urinary tract symptoms, only 0.02%, all male, reported isolated enuresis, when excluding those who also reported daytime urinary incontinence (UI).10
To date, there is a paucity of research examining the association between enuresis and OSA in adults, and existing evidence is largely isolated to case reports and case series. Most previously reported cases noted enuresis in the setting of weight gain and severe OSA, and that enuresis resolved upon treatment of OSA with continuous positive airway pressure (CPAP).11–14 To our knowledge, the prevalence of enuresis among adults with sleep-disordered breathing has not been reported. Here, we describe 5 adult patients with OSA and enuresis, who experienced resolution of both conditions after initiating treatment with CPAP. Although enuresis can broadly refer to any UI, for our purposes, all references to enuresis here will refer specifically to “nocturnal enuresis,” also known as “bedwetting.”
REPORT OF CASES
Case 1
A morbidly obese 27-year-old woman with a body mass index (BMI) of 60.3 kg/m2, with medical history notable for type 2 diabetes mellitus and idiopathic lower leg edema, was referred to the sleep clinic by her primary care provider to evaluate years of progressively worsening daytime somnolence. At presentation, she reported that her daytime somnolence had become so severe that she had begun falling asleep standing up, and it was limiting her ability to perform daily activities. Her somnolence had led to several falls at home. During the interview, the patient was markedly somnolent, and repeatedly dozed off. She reported frequent morning headaches, as well as weekly episodes of enuresis, in addition to occasional episodes of daytime UI. She reported sleep paralysis, but denied other symptoms of narcolepsy, including cataplexy or hypnagogic and hypnopompic hallucinations. At night, her husband had observed periodic apneic episodes, severe snoring, and strange behaviors in bed, including full-body convulsive episodes during which the patient could not speak but had retained consciousness, allowing her to corroborate the episodes herself. Enuresis was not associated with these convulsive events. At presentation, the patient's medications included metformin, furosemide for lower leg edema, and cyclobenzaprine.
The patient was evaluated with a laboratory split-night PSG with an extended electroencephalographic montage. The baseline diagnostic portion of the study revealed severe OSA (severe OSA defined as an apnea-hypopnea index greater than 30, expressed as the rate of apneas and hypopneas per hour), as well as episodes of hypoxemia with oxygen desaturations to as low as 50% during obstructive events. During the CPAP titration portion, she was titrated to an optimal CPAP of 16 cm H2O. The patient's sleep architecture was notable only for the REM sleep rebound effect upon initiation of CPAP. No epileptiform activity was observed on electroencephalogram.
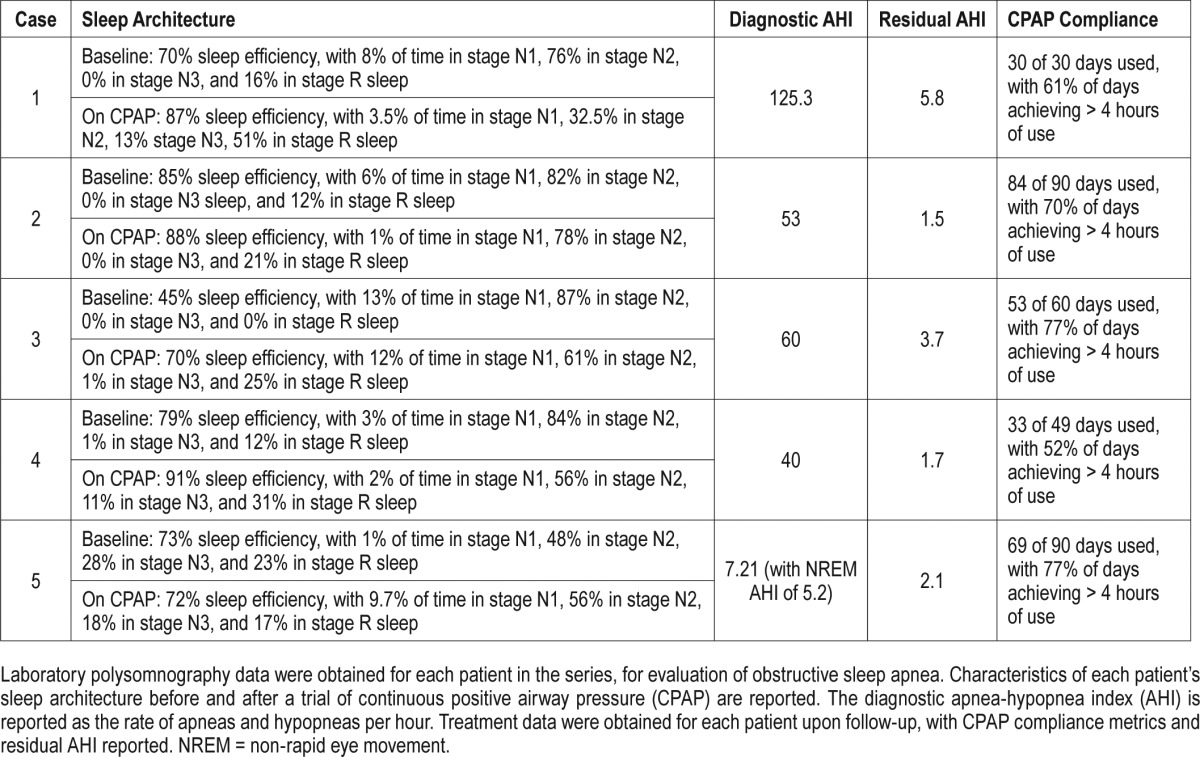
The patient was initiated that day on CPAP, and was seen in clinic 1 month later. At this visit, the patient was markedly more alert, and she was elated about the improvement in symptoms she noticed on CPAP. With CPAP, she felt rested upon waking, and remained alert throughout the day. Her enuresis resolved, her headaches improved, and her convulsive episodes during sleep had also ceased. Her ability to function during the day was dramatically improved, and she felt she was able to return to work. Additional diagnostic, treatment, and compliance data are reported in Table 1.
Table 1.
Summary diagnostic, treatment, and compliance data from each patient in the series.
Case 2
An obese 44-year-old man (BMI 31 kg/m2) with severe OSA on CPAP, and medical history notable for anxiety, depression, and opioid dependence in recovery on sublingual buprenorphinenaloxone, presented to the clinic with recurrence of previously well-controlled OSA symptoms. The patient received a diagnosis of severe OSA with a split-night PSG performed 5 years prior to presentation. He started and remained on CPAP until his re-presentation to the sleep clinic.
At re-presentation, the patient complained of daytime fatigue and nighttime awakenings due to intermittent apneic episodes and kicking in his sleep. He reported new symptoms of nocturia, polyuria, and occasional enuresis; evaluation for diabetes mellitus and benign prostatic hyperplasia (BPH) was unrevealing. The patient reported poor mask fit and CPAP malfunction directly preceding the onset of these symptoms, as well as weight gain of approximately 4.5 kg over the previous year.
The patient underwent repeat CPAP titration. On CPAP, repeat PSG showed stability with respect to the patient's sleep architecture, when compared to his original presentation, but he was found to have REM sleep without atonia, not observed on prior testing. He was titrated to an optimal CPAP of 12 cm H2O. Afterward, he reported occasional episodes of enuresis while waiting to receive the new CPAP machine. Once he resumed use of CPAP, the patient experienced improvement in polyuria with resolution of enuresis and restlessness during sleep. Additional diagnostic, treatment, and compliance data are reported in Table 1.
Case 3
An obese 59-year-old man (BMI 38.3 kg/m2) with a medical history notable for hypertension, hyperlipidemia, type 2 diabetes mellitus on insulin, BPH, peripheral neuropathy, and depression presented to clinic for initial evaluation of poor sleep. He reported recent weight gain and several symptoms of poor sleep, including daytime sleepiness, nighttime awakenings, snoring, and nocturia. The patient also reported episodes of enuresis that coincided with dreams about wetting the bed. At the time of presentation, his medications included metformin, insulin, pravastatin, terazosin, fluoxetine, nabumetone, and albuterol, as needed.
The patient underwent a split-night laboratory PSG, which yielded a diagnosis of severe OSA. During the CPAP titration, he was titrated to optimal CPAP of 14 cm H2O.
After 2 months of treatment with CPAP, the patient reported resolution of enuresis and nocturia, as well as improvement in daytime sleepiness, sleep latency, and nighttime awakenings. Additional diagnostic, treatment, and compliance data are reported in Table 1. Later, the patient lost insurance coverage for his CPAP machine, because he failed to meet compliance standards mandated by his health insurance company. The patient subsequently experienced recurrence of nocturia, with several “close calls” when asked about enuresis events.
Case 4
A morbidly obese 29-year-old woman (BMI 46 kg/m2) with a medical history notable for Crohn disease, and cervical intraepithelial neoplasia, grade 2 was referred to the sleep clinic for enuresis. The patient reported a history of secondary childhood enuresis, with onset occurring at approximately 12 years of age. The patient underwent tonsillectomy with subsequent resolution of enuresis. At 15 years of age, however, she began experiencing recurrent episodes of enuresis. Enuresis occurred once every other month at most, usually in the context of dreaming that she was urinating. She also reported acting out her dreams and shouting during sleep, which was often followed by a headache the following morning. Her medications included adalimumab, methotrexate, and supplemental folic acid for Crohn colitis.
She underwent a split-night laboratory PSG, which revealed severe OSA. During the CPAP titration, she was titrated to an optimal CPAP of 14 cm H2O.
After 7 weeks of treatment with CPAP, her enuresis had resolved, as well as her headaches. Her intense dreams became much less frequent, and she no longer acted out her dreams. She also noted improved daytime sleepiness. Additional diagnostic, treatment, and compliance data are reported in Table 1.
Case 5
A morbidly obese 52-year-old woman (BMI 41.8 kg/m2) with a medical history notable for hypertension, hyperlipidemia, migraines, systemic lupus erythematosus, and 3 prior ischemic strokes secondary to cocaine use, was referred to the sleep clinic by her primary care physician because of daytime somnolence and refractory headaches. On review of systems, she also reported snoring, nocturia, and significant daytime somnolence. Her medications at presentation included simvastatin, lisinopril, clopidogrel, bupropion, ranitidine, hydroxychloroquine, valproate (which she had been prescribed for headaches), and prednisone, as well as albuterol and fluticasone inhalers.
She completed a baseline diagnostic laboratory PSG, which revealed mild OSA (mild OSA defined as AHI between 5 and 15). At a later date, she underwent a laboratory CPAP titration study, during which she was titrated to an optimal CPAP of 10 cm H2O. In the 2 months before delivery of her CPAP machine, nightly enuresis developed in the patient. She completed a trial of oxybutynin, without effect.
After initiating CPAP, her daytime somnolence and snoring improved, nocturia became less frequent, and enuresis completely resolved. Additional diagnostic, treatment, and compliance data are reported in Table 1.
DISCUSSION
In children, OSA is a well-documented risk factor for enuresis. Adenotonsillectomy is often the first-line therapy for severe OSA in this population, and resolution of enuresis after successful treatment of OSA with adenotonsillectomy has been reported.5,6 Here, we present a case series describing a clear temporal relationship among adult patients with enuresis, who experienced resolution of enuresis upon diagnosis of OSA and treatment with CPAP. Our observation suggests that OSA may be causally related to enuresis in some adults, and that both conditions can be simultaneously treated with CPAP.
There is a wide range of structural and nonstructural risk factors for enuresis and UI in adults. Proposed mechanisms for the association between OSA and enuresis include direct mechanical pressure against the bladder that occurs with breathing against an obstructed airway, as well as indirect influence of hormones, such as natriuretic peptides, which are released in response to hemodynamic changes occurring in OSA.3,4 Obesity is another important risk factor, with studies demonstrating an association with UI in both men and women, and improvement in UI after weight loss.15 Obesity is also a strong risk factor for OSA, but likely contributes independently of OSA toward UI and enuresis by increasing intra-abdominal pressure and pressure on the bladder.9,15
Many additional factors are known to contribute to UI. Those relevant to this series include advanced age, diabetes mellitus, stroke, BPH, and depression, as well as medications, including diuretics, antidepressants, muscle relaxants, central nervous system depressants, and anti-histamines.16 These factors likely also contribute to enuresis; however, further study is necessary. Of particular interest in this case series is the fact that each patient possessed multiple risk factors, but in spite of this, enuresis resolved primarily with management of OSA using CPAP.
In addition to a focus on risk factors, considerable attention has been given to the changes in sleep architecture that occur in association with enuresis, particularly in children. Although a review of what is known about sleep architecture in childhood enuresis is beyond the scope of this case series, it is relevant to consider sleep architecture in the context of the patient in case 4, who had a history of secondary childhood enuresis that resolved initially after adenotonsillectomy, but recurred several years later and persisted into adulthood. Her enuresis remitted again as an adult, when OSA was diagnosed and treated with CPAP. This history invites inquiry into whether any features in sleep architecture in childhood enuresis are present in those with adult enuresis.
Examining the sleep architecture in the patients in our series, we did not note any remarkable patterns before or after initiating CPAP. The patient in case 1 exhibited a REM sleep rebound effect, which refers to a proportional increase in REM sleep that is sometimes observed upon initiation of CPAP in individuals with OSA. It is thought to occur as an initial response to a restored sleep cycle after chronic sleep fragmentation.17 The patient in case 2 was discovered to have REM atonia after a recurrence of previously well-treated OSA. It is unknown whether the onset of REM atonia occurred before or after the onset of enuresis, though it is possible that this condition predisposed him to enuresis. Dedicated study of sleep architecture in adults with enuresis is worthy of pursuit, as it has potential to further characterize the condition, and guide treatment strategies.
In light of current evidence, we postulate that OSA is an important risk factor for enuresis in adults, and can be the immediate cause in predisposed individuals. When an adult presents with enuresis, untreated OSA should be considered in the differential diagnosis. Routine screening for enuresis in patients referred for PSG, and for OSA in those reporting enuresis, may aid in diagnosis and treatment of both burdensome conditions. Systematic study of the association between enuresis and OSA in adults is warranted.
DISCLOSURE STATEMENT
All authors have reviewed and approved this manuscript. This was not an industry-supported study. The authors report no financial conflicts of interest.
ACKNOWLEDGMENTS
Author Contributions: Drs. McInnis, Dodds, Johnsen, Auerbach, and Pyatkevich had full access to all of the data in the manuscript and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: Drs. Johnsen, Auerbach, and Pyatkevich; Acquisition of data: Drs. Johnsen, Auerbach and Pyatkevich; Analysis and interpretation of data: Drs. McInnis, Dodds, Johnsen, Auerbach, and Pyatkevich; Drafting of the manuscript: Drs. McInnis, Dodds, Johnsen, and Pyatkevich; Critical revision of the manuscript for important intellectual content: Drs. Johnsen, Auerbach, and Pyatkevich; Supervision: Dr. Pyatkevich.
ABBREVIATIONS:
- AHI
apnea-hypopnea index
- BMI
body mass index
- BPH
benign prostatic hyperplasia
- CPAP
continuous positive airway pressure
- EEG
electroencephalogram
- OSA
obstructive sleep apnea
- PSG
polysomnography
- REM
rapid eye movement
- UI
urinary incontinence
REFERENCES
- 1.Brooks LJ, Topol HI. Enuresis in children with sleep apnea. J Pediatr. 2003;142(5):515–518. doi: 10.1067/mpd.2003.158. [DOI] [PubMed] [Google Scholar]
- 2.Barone JG, Hanson C, DaJusta DG, Gioia K, England SJ, Schneider D. Nocturnal enuresis and overweight are associated with obstructive sleep apnea. Pediatrics. 2009;124(1):e53–e59. doi: 10.1542/peds.2008-2805. [DOI] [PubMed] [Google Scholar]
- 3.Jeyakumar A, Rahman SI, Armbrecht ES, Mitchell R. The association between sleep-disordered breathing and enuresis in children. Laryngoscope. 2012;122(8):1873–1877. doi: 10.1002/lary.23323. [DOI] [PubMed] [Google Scholar]
- 4.Alexopoulous E, Malakasioti G, Varlami V, Miligkos M, Gourgoulianis K, Kaditis A. Nocturnal enuresis as risk factor for moderate-to-severe OSA in children with snoring. Pediatr Res. 2014;76(6):555–559. doi: 10.1038/pr.2014.137. [DOI] [PubMed] [Google Scholar]
- 5.Weissbach A, Leiberman A, Tarasiuk A, Goldbart A, Tal A. Adenotonsilectomy improves enuresis in children with obstructive sleep apnea syndrome. Int J Pediatr Otorhinolaryngol. 2006;70(8):1351–1356. doi: 10.1016/j.ijporl.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 6.Bascom A, Penney T, Metcalfe M, Knox A, Witmans M, Uweira T, Metcalfe PD. High risk of sleep disordered breathing in the enuresis population. J Urol. 2011;186(4):1710–1713. doi: 10.1016/j.juro.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 7.Hirasing RA, van Leerdam FJM, Bolk-Bennink L, Jannegt RA. Enuresis nocturna in adults. Scand J Urol Nephrol. 1997;31(6):533–536. doi: 10.3109/00365599709030657. [DOI] [PubMed] [Google Scholar]
- 8.Campbell P, Weiguang L, Money-Taylor J, Davies J, Gray T, Radley S. Nocturnal enuresis: prevalence and associated LUTS in adult women attending a urogynaecology clinic. Int Urogynecol J. 2017;28(2):315–320. doi: 10.1007/s00192-016-3099-0. [DOI] [PubMed] [Google Scholar]
- 9.Koo P, McCool FD, Hale L, Stone K, Eaton CB. Association of obstructive sleep apnea risk factors with nocturnal enuresis in postmenopausal women. Menopause. 2016;23(2):175–182. doi: 10.1097/GME.0000000000000517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakamoto K, Blaivas JG. Adult onset nocturnal enuresis. J Urol. 2001;165(6):1914–1917. doi: 10.1097/00005392-200106000-00017. [DOI] [PubMed] [Google Scholar]
- 11.Brown MA, Jacobs MB, Pelayo R. Adult obstructive sleep apnea with secondary enuresis. West J Med. 1995;163(5):478–480. [PMC free article] [PubMed] [Google Scholar]
- 12.Everaert K, Pevernagie D, Oosterlinck W. Nocturnal enuresis provoked by an obstructive sleep apnea syndrome. J Urol. 1995;153(4):1236. [PubMed] [Google Scholar]
- 13.Yokoyama O, Lee SW, Ohkawa M, Amano T, Ishiura Y, Furuta H. Enuresis in an adult female with obstructive sleep apnea. Urology. 1995;45(1):150–154. doi: 10.1016/s0090-4295(95)97686-8. [DOI] [PubMed] [Google Scholar]
- 14.Kramer NR, Bonitati AE. Millman RP. Enuresis and obstructive sleep apnea in adults. Chest. 1998;114(2):634–637. doi: 10.1378/chest.114.2.634. [DOI] [PubMed] [Google Scholar]
- 15.Markland AD, Richter HE, Fwu Chyng-Wen, Eggers P, Kusek JW. Prevalence and trends of urinary incontinence in adults in the United States, 2001 to 2008. J Urol. 2011;186(2):589–593. doi: 10.1016/j.juro.2011.03.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khandelwal C, Kistler C. Diagnosis of urinary incontinence. Am Fam Physician. 2013;37(8):543–550. [PubMed] [Google Scholar]
- 17.Verma A, Radtke RA, VanLandingham KE, King JH, Husain AM. Slow wave sleep rebound and REM rebound following the first night of treatment with CPAP for sleep apnea: correlation with subjective improvement in sleep quality. Sleep Med. 2001;2(3):215–223. doi: 10.1016/s1389-9457(00)00069-1. [DOI] [PubMed] [Google Scholar]


