Abstract
The predictive value of aortic arch pulse wave velocity (PWV) assessed by magnetic resonance imaging (MRI) for cardiovascular disease (CVD) events has not been fully established. The aim of the present study was to evaluate the association of arch PWV with incident CVD events in MESA. Aortic arch PWV was measured using MRI at baseline in 3,527 MESA participants (mean age = 62 ± 10 years at baseline, 47% male) free of overt CVD. Cox regression was used to evaluate the risk of incident CVD (coronary heart disease, stroke, transient ischemic attack, or heart failure) in relation to arch PWV adjusted for age, gender, race, and CVD risk factors. The median value of arch PWV was 7.4 (IQR; 5.6 to 10.2) m/s. There was significant interaction between arch PWV and age for outcomes, so analysis was stratified by age categories (45–54 and over 54 years). There were 456 CVD events over the 10-year follow-up. 45–54 year-old participants had significant association of arch PWV with incident CVD independent of CVD risk factors (HR, 1.44; 95% confidence interval, 1.07 to 1.95; p=0.018; per 1SD increase for logarithmically transformed PWV), whereas over 54 years group did not (p=0.93). Aortic arch PWV assessed by MRI is a significant predictor of CVD events among middle-aged (45 to 54 years old) individuals, whereas arch PWV is not associated with CVD among an elderly in a large multi-ethnic population.
Keywords: aortic stiffness, magnetic resonance imaging, pulse wave velocity, cardiovascular disease, multi-ethnic study of atherosclerosis
Introduction
Pulse wave velocity (PWV)—a non-invasive marker of arterial stiffness—predicts the risk of future cardiovascular disease (CVD) events and total mortality 1–4. Arterial PWV is commonly measured using arterial applanation tonometry, an indirect method which divides the surface distance between the carotid artery and the femoral artery by the time delay of the arterial pulse between two sites. Cf-PWV has been regarded as the gold standard method because of its relative ease in determination and its perceived reliability 5. However, cf-PWV measurements by arterial tonometry may be affected by measurement error related to the surface distance of the aortic path (for instance, it does not take into account the torturous vessel routes) and related to inaccurate pulse wave detection, especially in the femoral artery 2, 6, 7. Cf-PWV also has a relative blind spot for the aortic arch that normally provides nearly half of total arterial compliance 8.
Magnetic resonance imaging (MRI) assessment for aortic PWV is a novel method that has substantially reduced measurement error in PWV measurements by using accurate aortic length and transit times between flow waves 9–11. MRI also allows the measurement of the aortic arch PWV that is ignored in cf-PWV assessed by tonometry. The structure of the aorta is regionally heterogeneous, so different regional PWV may have different prognostic values corresponding to differences in structural properties of the aortic wall; however, the independent association of aortic arch PWV assessed by MRI with CVD outcomes has not been established in the general population, and it is unclear whether arch PWV may improve prediction of incident CVD outcomes beyond the information provided by conventional CVD risk factors.
The aim of the present study is to assess the prospective association of aortic arch PWV from MRI with the risk of incident CVD outcomes in a large multi-ethnic population, as well as the predictive value of arch PWV to identify individuals who will develop CVD outcomes beyond the information provided by conventional CVD risk factors.
Method
Study population
The Multi-Ethnic Study of Atherosclerosis (MESA) is a prospective study designed to evaluate risk factors and mechanisms that underlie the development and progression of subclinical cardiovascular diseases among asymptomatic individuals across the population 12. A total of 6,814 men and women 45 to 84 years of age without clinical CVD who identified themselves as White, African-American, Hispanic, or Chinese from 6 US field centers (Wake Forest University, Winston-Salem, NC; Columbia University, New York, NY; Johns Hopkins Hospital, Baltimore, MD; University of Minnesota, Minneapolis, MN; Northwestern University, Chicago, IL; UCLA, Los Angeles, CA) were recruited between 2000 and 2002. Out of 5,098 participants who took part in the CMR imaging examination, 3,527 underwent suitable PWV measurements for analysis (173 were excluded due to poor image quality). All participants gave informed consent for the study protocol, which was approved by the institutional review boards of all MESA field centers and the CMR reading center.
MRI imaging
MRI images were acquired with 1.5T whole-body scanners 13. Gradient echo phase-contrast cine MRI (PC-CMR) with electrocardiographic gating was performed to evaluate aortic flow and aortic area. Images of the ascending and descending aorta were obtained in the transverse plane perpendicular to the aortic lumen at the level of the right pulmonary artery. Imaging parameters were as follows: repetition time: 10ms, echo time: 1.9 ms, flip angle 20 degree, field of view: 340mm, slice thickness: 8mm, matrix 256 × 256, number of images: 20 for one cardiac cycle, encoding velocity: 150cm/s, bandwidth: 245Hz/pixel.
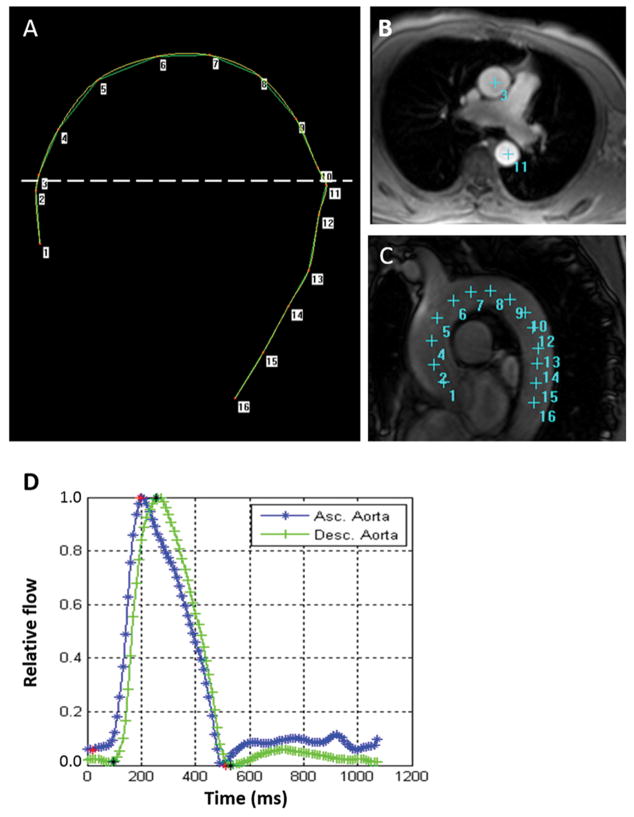
By providing an automated segmentation of the modulus and velocity images acquired by PC-CMR, ARTFUN software (INSERM U678) allowed us to obtain the flow wave transit time between the ascending to descending aorta 14, 15. The transit time was calculated as the average time difference using the least squares estimate between all data points on the systolic upslope of the ascending and descending aortic flow curves after peak flow normalization. Using oblique sagittal images with a bright-blood sequence through the thoracic aorta, the distance between ascending and descending aorta were measured at precise locations where the through-plane velocities were measured (Figure 1). Aortic arch PWV was then calculated as follows:
Figure 1.
Measurement for aortic arch PWV. A: Phase contrast cine transverse view. B: Aortic arch view with SSFP sequence. C: Measurement of the transit distance in the aortic arch. Numbers correspond to those in A and B. Arch length is measured as the distance from 3 to 11 in this case. D: Flow wave curves of ascending (Asc.) aorta and descending (Desc.) aorta after peak flow normalization. Transit time is measured as the average time difference using the least squares estimate between all data points on the systolic upslope of the ascending and descending aortic flow curves. PWV is calculated as transit distance divided by transit time.
Ascending and descending aortic area at diastole were measured using the modulus images of PC-CMR.
Cardiac magnetic resonance (CMR) imaging was also performed with measures of left ventricular (LV) mass as previously described 16.
CVD risk factors and measures of subclinical atherosclerosis
During baseline examination, all participants completed standardized questionnaires to provide information about demographic variables, smoking history, and medication use. Resting systolic (SBP) and diastolic blood pressures (DBP) were measured in the seated position using an automated oscillometric sphygmomanometer. Pulse pressure (PP) was calculated as (SBP – DBP). Glucose and lipids were measured after a 12-hour fast. Diabetes mellitus was defined as fasting glucose ≥126 mg/dl or use of insulin or oral hypoglycemic medication. Hypertension was defined as SBP ≥140 mmHg, DBP ≥90 mmHg, or current use of antihypertensive medications. The ankle-brachial pressure index (ABI) was calculated by dividing SBP at the ankle by brachial SBP. Carotid artery intima-media thickness (IMT) was measured on near and far walls of the common and internal carotid artery using B-mode echocardiography. A composite Z-score for overall maximal IMT was calculated by summing the 2 carotid IMT sites after normalization by the SD of each measure and divided by the SD of the sum 17. Coronary artery calcium (CAC) score was measured by computed tomography as previously described 17. The sex-specific global CVD Framingham 10-year Risk Score was calculated on the basis of the following risk factors: SBP, antihypertensive medication, total cholesterol, HDL cholesterol, smoking, and diabetes 18.
Outcomes
Participants were followed for an average 10.3 years from their baseline examinations. In addition to MESA follow-up examinations every 2 years, a telephone interviewer contacted each participant (or representative) every 6 to 9 months to inquire about all interim hospital admissions, cardiovascular outpatient diagnoses, or deaths. Two physicians reviewed all records for independent end-point classification and assignment of every date. Adjudication of events has been previously described 16. CVD events in the present study were defined as composite events including myocardial infarction (MI), resuscitated cardiac arrest, definite or probable angina followed by coronary revascularization, and definite angina not followed by coronary revascularization, death due to coronary heart disease (CHD), stroke, death due to stroke, transient ischemic attack, and congestive heart failure (CHF).
Statistical analysis
Continuous variables are shown as mean ± SD unless otherwise specified and categorical variables are shown as percentages. Normality was evaluated by the Shapiro-Wilk W test, histogram and quantile-quantile (Q-Q) plot. Comparisons between participants with or without CVD events were performed using student’s t test and Mann-Whitney U test for normally and non-normally distributed data, respectively. Categorical variables are presented as frequencies and percentages and analyzed using χ2 tests. Arch PWV were logarithmically transformed for linear and COX regression models due to its right-skewed distribution (logPWV).
We used Cox proportional hazards regression to analyze the associations between PWV and CVD events. We show the hazard ratio (HR) for a 1-SD increase (z score) of logPWV levels. Cox regression models using PWV tertile instead of logPWV was also performed. Model 1 adjusted for demographic factors (age, gender, race, height, and weight) and Model 2 adjusted for conventional cardiovascular risk factors (systolic blood pressure, use of anti-hypertensive medication, diabetes mellitus, smoking status, total cholesterol, high-density lipoprotein) adding to Model 1. After Model 2, further adjustment for measures of subclinical markers—including carotid IMT, ABI, CAC, and LV mass individually and all together—was performed. Further adjustment for ascending or descending aortic area was also performed after Model2. Models that alternatively included DBP or MBP, and PP instead of SBP were also evaluated. We examined the interaction of arch PWV with the age decade (45–54, 55–64, 65–74, 75–84 years, with 75–84 chosen as the reference group) in its association with outcome using multiplicative interaction terms. This result showed that there was significant interaction between arch PWV and 45–54 years group (HR for interaction: 1.55; 95% CI: 1.13 to 2.13; p = 0.007), while the interactions between arch PWV and the other groups are not significant (Table S1). Because of the significant interaction between arch PWV and 45–54 years group, analysis was repeated in age categories (45–54 and over 54 years).
Unadjusted Kaplan-Meier survival curves illustrated the association between arch PWV and CVD events. The log-rank test assessed statistical significance. Receiver-operating characteristic (ROC) curve analysis was used to determine optimal cut-off value for incident CVD event with arch PWV. The best cut-off point value was defined as the point with the highest sum of the sensitivity and specificity. The incremental predictive value of arch PWV above the Framingham Risk Score was assessed by comparing the global χ2 values for each model and differences in Harrell’s C-statistic. We also evaluated the added predictive ability of arch PWV for the distributions of time to CVD events using net reclassification improvement (NRI) 19. NRI was calculated from the Framingham predicted risk cut points of 6% and 20% at 10 years 20.
The cross-sectional association of arch PWV with age was assessed by the piecewise linear regression model.
A 2-tailed p value of <0.05 was considered statistically significant. All statistical analyses were performed using Stata version 12.0 (Stata Corp LP, College Station, TX). NRI and IDI were calculated with the help of a STAT add-on from the Uppsala Clinical Research Center (http://www.ucr.uu.se/en/index.php/ucr-statistics/program-code/306-nri-and-idi).
Results
MESA Participant Characteristics
Demographic and clinical parameters at baseline for participants is presented in Table 1. The study population was 47% male, 36% Caucasian, 15% Chinese American, 29% African American, and 20% Hispanic, with mean age of 62 ± 10 years. MESA participants with aortic data more often tended to be White or Hispanic and overall had a mildly lower risk profile than participants without aortic MRI data (Table S2). The median value of arch PWV was 7.4 (IQR; 5.6 to 10.2) m/s. A total of 456 participants (13%) experienced CVD events over an average of 10.3 years follow-up. Participants with CVD were older and more likely to be hypertensive, diabetic, and active smokers, and more likely to have increased weight, body mass index, and blood pressures, and decreased HDL and increased Framingham CVD Risk Score compared to participants without events. Arch PWV and aortic area were greater in participants with CVD events. Carotid IMT, CAC, and LV mass were greater; ABI was smaller in participants with events.
Table 1.
Baseline Characteristics Stratified by Age Categories.
| Characteristics | All participants (n=3,527) | No events (n=3,071) | CVD events (n=456) | p value |
|---|---|---|---|---|
| Age, y | 62 ± 10 | 61 ± 10 | 67.3 ± 9.4 | <0.001 |
| Men, % | 47 | 45 | 59 | <0.001 |
| Ethnicity, % | <0.001 | |||
| White | 36 | 35 | 44 | |
| Chinese | 15 | 16 | 9 | |
| Black | 29 | 29 | 27 | |
| Hispanic | 20 | 20 | 20 | |
| Height, cm | 166 ± 10 | 166 ± 10 | 167 ± 10 | 0.19 |
| Weight, kg | 77 ± 16 | 76 ± 16 | 79 ± 16 | 0.001 |
| BMI, kg.m-2 | 27.5 ± 4.9 | 27.5 ± 4.9 | 28.3 ± 4.9 | 0.001 |
| Hypertension, % | 45 | 42 | 66 | <0.001 |
| Antihypertensive medication, % | 38 | 35 | 53 | <0.001 |
| Diabetes mellitus, % | 12 | 11 | 21 | <0.001 |
| Current Smoking status, % | 13 | 12 | 18 | 0.001 |
| Total cholesterol, mg/dl | 194 ± 34 | 194 ± 34 | 194 ± 36 | 0.99 |
| HDL cholesterol, mg/dl | 52 ± 15 | 52 ± 15 | 49 ± 14 | <0.001 |
| Blood pressures | ||||
| SBP, mmHg | 126 ± 22 | 125 ± 21 | 137 ± 23 | <0.001 |
| DBP, mmHg | 72.0 ± 11 | 71.7 ± 10 | 74 ± 11 | <0.001 |
| PP, mmHg | 54 ± 17 | 53 ± 17 | 63 ± 18 | <0.001 |
| Heart rate, bpm | 63 ± 10 | 63 ± 9 | 64 ± 10 | 0.01 |
| PWV, m/s, median (IQR) | 7.4 (5.6–10.2) | 7.2 (5.5–10.1) | 8.0 (6.1–11.6) | <0.001 |
| Ascending aortic area, cm2 | 7.9 ± 2.0 | 7.8 ± 1.9 | 8.6 ± 2.2 | <0.001 |
| Descending aortic area, cm2 | 4.5 ± 1.3 | 4.5 ± 1.3 | 5.0 ± 1.3 | <0.001 |
| Framingham risk score | 14.3 ± 9.6 | 13.3 ± 9.3 | 20.8 ± 8.6 | <0.001 |
| Maximum internal carotic IMT, mm | 1.05 ± 0.59 | 1.02 ± 0.56 | 1.30 ± 0.71 | <0.001 |
| ABI | 1.11 ± 0.12 | 1.11 ± 0.11 | 1.08 ± 0.15 | <0.001 |
| CAC score, median (IQR) | 0 (0–82) | 0 (0–54) | 92 (5–369) | <0.001 |
| LV mass, g | 120 ± 30 | 118 ± 28 | 131 ± 36 | <0.001 |
Values are mean (SD) or %. BMI indicates body mass index; HDL, high density lipoprotein; SBP, systolic blood pressure; DBP, diastolic blood pressure; PP, pulse pressure; PWV, pulse wave velocity; IMT, intima media thickness; ABI, ankle-brachial index; CAC, coronary calcium score; LV left ventricular; CVD, cardiovascular; SD, standard deviation.
Age-related change in arch PWV
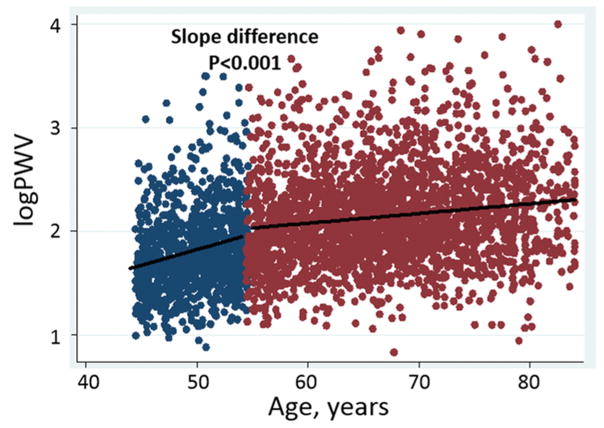
Arch PWV was greater with increasing age; however, the association of arch PWV with age was nonlinear (Figure S1). In the piecewise linear regression model, B coefficients for logPWV per 10 years of age were 0.36 (standard error, 0.03) at 45–54 years of age and 0.10 (0.01) above 55 years. The difference in slope was significant between 45–54 and above 54 years (p<0.001) (Figure 2). This association and slope difference remained significant even after adjusting for cardiovascular risk factors.
Figure 2.
Association of arch PWV with age (blue dots, <50 years of age; red dots, ≥55 years of age).
Relationship of Arch PWV with CVD Events
Cox proportional hazard models for all participants and for each age category are presented in Table 2. In univariate analysis, arch PWV was associated with incident CVD among all participants; however, this association was not present after adjustment for demographic variables. In age group, only 45–54-year-old participants had significant association of arch PWV with incident CVD in both univariate analysis (HR, 1.59; 95% CI, 1.23 to 2.06; p<0.001; per 1SD increase for logPWV) and multivariate analysis adjusted for CVD risk factors (HR, 1.47; 95% CI, 1.10 to 1.97; p=0.009), whereas participants over 54 years of age did not (p=0.93). Hazard ratio for highest tertile group for PWV was 2.37 (95%CI; 1.13–4.96) compared with lowest tertile in 45–54 year-old participants, (Table S3). This association of arch PWV with incident CVD in 45–54 year-old participants was maintained after further adjusting for measures of other subclinical markers individually and together (carotid IMT, IBI, CAC, and LV mass) (Table S4). This association was maintained in models further adjusted for ascending or descending aortic area as potential confounders. Similar results were obtained in models using diastolic blood pressure, or pulse pressure instead of systolic blood pressure (data not shown).
Table 2.
Hazard Ratios of the logPWV for Cardiovascular Events Stratified by Age Groups
| Participants group | no.of events | Unadjusted | Model 1 | Model 2 | |||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | ||
| All Participants (n=3,527) | 456 | 1.27 (1.16–1.38) | <0.001 | 1.07 (0.98–1.18) | 0.14 | 1.03 (0.94–1.14) | 0.5 |
| Age categories | |||||||
| 45–54 years old (n=1,027) | 54 | 1.56 (1.20–2.02) | <0.001 | 1.52 (1.15–2.00) | 0.003 | 1.44 (1.07–1.95) | 0.018 |
| 55–84 years old (n=2,500) | 402 | 1.08 (0.98–1.19) | 0.11 | 1.02 (0.92–1.13) | 0.64 | 0.99 (0.90–1.10) | 0.93 |
Hazard ratios are indicated per 1SD increase for logPWV. Adjustment was performed for the following risk factors: model 1 = adjusted for age, gender, race, height, and weight; model 2 = model 1 + systolic blood pressure, antihypertensive medication use, diabetes, smoking, total cholesterol, HDL cholesterol.
HR indicates hazard ratio; other abbreviation as in Table 1.
Goodness-of-fit and discriminatory values for arch PWV in the age 45–54 year group are shown in Table 3. When arch PWV was added to the Framingham Risk Score-adjusted model in the age 45–54 group, arch PWV had an incremental predictive value, as indicated by an increase in global χ2 values (57.9 to 62.7, p=0.03; Table 3); however, the C statistic was unchanged (0.788 to 0.799; p=0.42; Table 3). Addition of arch PWV to the Framingham Risk Score-adjusted model resulted in overall estimated net reclassification improvement of 0.154 (p=0.005; Table 3). Together, these findings indicate that the addition of arch PWV to the Framingham Risk Score resulted in improved risk discrimination and risk reclassification in the age 45–54 group.
Table 3.
Measures of Model Fit and Discrimination for Models with and without PWV in Age 45–54 Years
| Models | Estimate | P-value | |
|---|---|---|---|
| Model without PWV | C-statistic | 0.788 (95%CI 0.723 to 0.853) | <0.001 |
| LR chi2 | 57.9 | ||
|
|
|||
| Model with PWV | C-statistic | 0.799 (95%CI 0.738 to 0.860) | <0.001 |
| LR chi2 | 62.7 | ||
|
| |||
| Comparing models with or without logPWV | |||
|
| |||
| Difference between C-statics | 0.42 | ||
| LR test | 4.8 | 0.03 | |
| NRI | 0.154 | 0.006 | |
Model without PWV was adjusted for Framingham Risk Score (FRS).
Model with PWV was adjusted for FRS and logPWV.
CI indicates confidence interval; LR, likelihood ratio; IDI, integrated discrimination improvement; NRI, net reclassification in improvement.
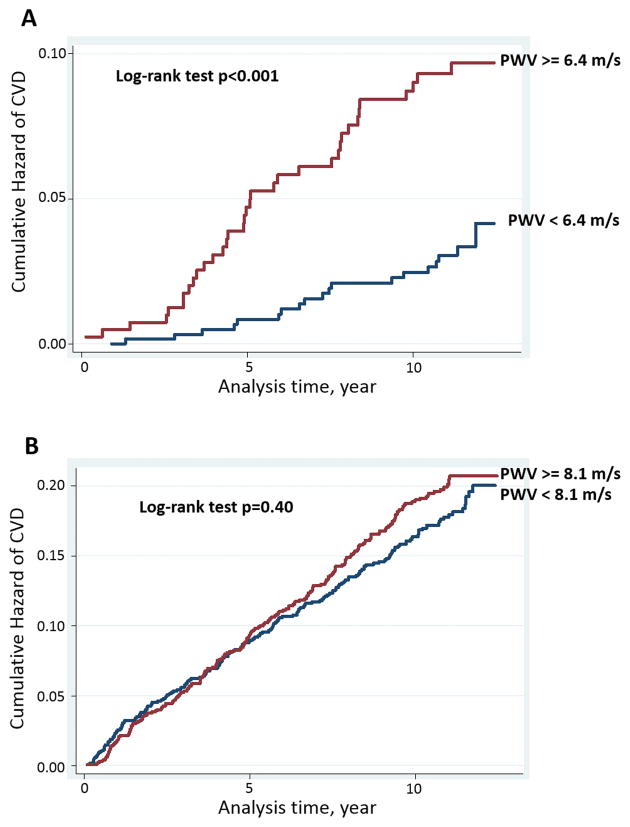
The area under the ROC curve (AUC) was 0.643 for logPWV to CVD events (95%CI, 0.574 to 0.711; p=0.001) in the age 45–54 group. The optimal cut-off value of arch PWV for the prediction of CVD was 6.4 m/s (sensitivity of 67% and specificity of 61%). The cumulative hazard of CVD was significantly higher in participants with arch PWV higher than 6.4m/s by the log-rank test in this age group (p<0.001) (Figure 3A). In participants over 54 years of age, the cumulative incidence of CVD events were similar between two groups divided by median value (PWV=8.1m/s) (p=0.40) (Figure 3B).
Figure 3.
Kaplan-Meier curve for CVD event according to pulse wave velocity (PWV) in participants aged 45–54 years (A) and above 55 years (B).
Discussion
The present study evaluates the association of arch PWV assessed by MRI with incident CVD event in a large multi-ethnic population with middle age to elderly participants. Arch PWV had incremental predictive value for incident CVD above traditional CVD risk factors in participants 45–54 years of age. Arch PWV was not associated with incident CVD events in participants over 54 years of age.
The aorta is not a simple conduit for blood distribution. The structure of aorta is regionally heterogeneous; the proximal aorta has more elastic fiber, and the distal aorta has more smooth muscle cells. The viscoelastic property of the proximal aorta provides a cushioning function that absorbs the energy of left ventricular ejection and dampens the pulsatile flow 21. Cf-PWV assessed by tonometry—the standard marker for arterial stiffness—omits the aortic arch 8, so aortic arch PWV assessed by MRI may have a unique value as central aortic stiffness and could provide complementary information regarding aortic stiffness.
Maroules et al. reported that arch PWV assessed by MRI was not associated with CVD events in the Dallas Heart Study 22. This result is consistent with the present study that shows no significant association of arch PWV with CVD events in the overall MESA population. However, the present study showed that there was a strong age interaction for arch PWV with CVD. In the present study, arch PWV predicted future incidence of CVD events in 45–54-year-old participants, but not in those above 54 years in the general population. For individuals 45–54 years of age, arch PWV is an independent predictor even after adjustment for CVD risk factors and other subclinical markers such as ABI, CAC, carotid IMT, and LV mass, and had incremental predictive value above Framingham Risk Scores. These results suggest that arch PWV may be useful for risk stratification for CVD in middle age. Along with this association of arch PWV with incident CVD, the increase in arch PWV was particularly marked in participants 45–54 years of age compared to those over 54 years of age. This lower increase of arch PWV with advancing age is not consistent with a study that indicated a steep increase in arch PWV in the elderly compared to young adults 14, but might in part contribute to results in the present study.
Cf-PWV assessed by tonometry is associated with incident CVD events in the general population within a broad range of age, especially the elderly 1, 4. On the other hand, in the present study, arch PWV can predict future incident CVD events only in middle age. This finding is partially consistent with the result of meta-analysis that demonstrated cf-PWV was a stronger risk factor among younger population, although it was still predictive in older population4. Cf-PWV has information on aortic stiffness including distal aorta; therefore, including additional information in cf-PWV might contribute to the different impact on CVD event seen for cf- and arch PWV. Hickson et al. reported that the greater age-related increase in aortic PWV occurred in the abdominal aorta compared to the ascending aorta 23. Stiffening in the distal segment might be mainly caused by localized calcification, which is strongly correlated with increasing PWV 24. This may also be important as abdominal aortic calcium deposits have been linked with independently predicting cardiovascular morbidity and mortality 25. In addition, PWV in the proximal segment of the aorta showed the smallest association with older age, whereas this segment also showed the greatest increase in diameter with increased age, which may help offset an increase in PWV 23.
Redheuil et al. reported that ascending aorta distensiblity (AAD) as a measure of proximal aortic stiffness is a strong predictive marker for CVD events in the MESA population 13. Although both arch PWV and AAD represents proximal aortic stiffness, PWV indicates increased regional aortic wall stiffness, whereas distensibility, which is calculated using cross-sectional aortic area change through the cardiac cycle, represents local aortic function and is more sensitive to load. In this regard, aortic area changes across the cardiac cycle may better reflect the specific role of the proximal aorta, dampening the effect of pulsatile flow from the heart on the arterial system as a whole 26. PWV reflects more advanced alterations of material properties involving the regional vessel. These differences might contribute to the differences in predictive power between arch PWV and AAD.
Our study has limitations. Because our sample group was a relatively elderly cohort (mean age 60 years at baseline), it is unsuitable to assess how arch PWV is associated with CVD in younger populations. We had only a modest number of events; therefore, we lacked power to examine threshold models or to analyze specific types of CVD events. We might have inflated type I error due to multiple testing. Because MESA participants had no overt cardiovascular disease at baseline, the participants at baseline represent a relatively healthy sample of the population. Thus, generalization of the study results is limited by selection and survival bias. Because we did not have the cf-PWV in MESA, we could not compare arch PWV with cf-PWV regarding an association of CVD events to elucidate the impact of aortic stiffness in a differential segment on future events. We assessed only aortic arch PWV, and PWV of other segments such as abdominal aortic PWV was not measured in MESA. We did not assess the other variables that relate to aortic stiffness, such as augmentation index or reflection magnitude that represents wave reflections, and characteristic impedance that is the highly recommended measurement in assessment of arterial stiffness 27, 28. Future study should be conducted to assess whether other variables will have impact on future cardiac events and how other variables relate to arch PWV. The increased wave reflection with greater age might have differential influence on ascending and descending flow wave forms29, resulting in increased difficulty to reliably assess short transit times. However, the impact of reflected waves on global flow curves is usually seen after systolic peak flow 30 and minimally alters the upslopes that are used to calculate transit time in the present study. We acknowledge moderate temporal resolution to be a potential limitation in the present study. The strength of our study is a large sample size and the assessment for arch PWV by a sophisticated methodology using MRI.
Perspective
Previous studies demonstrated a steep increase in cf-PWV in the elderly compared to young adults. Whereas the present study showed that the increase in arch PWV was particularly marked in a middle-aged population compared to an elderly population. Along with this association of age with arch PWV, arch PWV was associated with incident CVD only in participants 45–54 years of age in the present study. Other arterial stiffness markers, such as cf-PWV, are associated with incident CVD in the general population. These findings imply that proximal aortic stiffness would occur earlier compared to distal and arch PWV assessed by MRI would be more helpful to stratify the risk for incident CVD among middle-aged population compared to other arterial stiffness markers. Moreover, measurement for arch PWV by MRI has high inter-study reproducibility 31, 32, so arch PWV would be used as an surrogate measure for CVD in the evaluation of pharmacological treatment.
Conclusion
The present study demonstrates that arch PWV assessed by MRI is a significant predictor of CVD events in a middle-aged population, but is not associated with CVD among an elderly population. The incremental value of arch PWV was seen in a 45–54-year-old population. The differential segment of aortic stiffness may have a different impact on outcomes among a different population. Future studies need to assess the association of arch PWV with CVD in younger adults.
Supplementary Material
Novelty and Significance.
1) What Is New?
We measured arch PWV by a sophisticated methodology using MRI in a large multi-ethnic cohort. We showed significant interaction of arch PWV with the age in its association.
2) What Is Relevant?
Arch PWV increased steeply in a middle-aged population compared to an elderly population. Along with this finding, arch PWV was associated with incident CVD events only in a middle-aged population.
3) Summary
Aortic arch PWV assessed by MRI is a significant predictor of CVD events among middle-aged (45 to 54 years old) individuals, whereas arch PWV is not associated with CVD among an elderly in a large multi-ethnic population.
Acknowledgments
We thank the other investigators, staff, and participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Sources of Funding
This research was supported by contracts N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, and N01-HC-95168 from the National Heart, Lung, and Blood Institute.
Abbreviations
- CVD
Cardiovascular Disease
- PWV
Pulse Wave Velocity
- MESA
Multiethnic Study of Atherosclerosis
- MRI
Magnetic Resonance Imaging
- SBP,DBP
Systolic and Diastolic Blood Pressure
- PP
Pulse pressure
Footnotes
Conflict of Interest
None declared.
References
- 1.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: A systematic review and meta-analysis. Journal of the American College of Cardiology. 2010;55:1318–1327. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: The framingham heart study. Circulation. 2010;121:505–511. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, Ducimetiere P, Benetos A. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241. doi: 10.1161/01.hyp.37.5.1236. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Shlomo Y, Spears M, Boustred C, et al. Aortic pulse wave velocity improves cardiovascular event prediction: An individual participant meta-analysis of prospective observational data from 17,635 subjects. Journal of the American College of Cardiology. 2014;63:636–646. doi: 10.1016/j.jacc.2013.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mancia G, De Backer G, Dominiczak A, et al. 2007 guidelines for the management of arterial hypertension: The task force for the management of arterial hypertension of the european society of hypertension (esh) and of the european society of cardiology (esc) European heart journal. 2007;28:1462–1536. doi: 10.1093/eurheartj/ehm236. [DOI] [PubMed] [Google Scholar]
- 6.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H European Network for Non-invasive Investigation of Large A. Expert consensus document on arterial stiffness: Methodological issues and clinical applications. European heart journal. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 7.Chiu YC, Arand PW, Shroff SG, Feldman T, Carroll JD. Determination of pulse wave velocities with computerized algorithms. American heart journal. 1991;121:1460–1470. doi: 10.1016/0002-8703(91)90153-9. [DOI] [PubMed] [Google Scholar]
- 8.Mitchell GF. Arterial stiffness and wave reflection: Biomarkers of cardiovascular risk. Artery Res. 2009;3:56–64. doi: 10.1016/j.artres.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grotenhuis HB, Westenberg JJ, Steendijk P, van der Geest RJ, Ottenkamp J, Bax JJ, Jukema JW, de Roos A. Validation and reproducibility of aortic pulse wave velocity as assessed with velocity-encoded mri. Journal of magnetic resonance imaging : JMRI. 2009;30:521–526. doi: 10.1002/jmri.21886. [DOI] [PubMed] [Google Scholar]
- 10.Fielden SW, Fornwalt BK, Jerosch-Herold M, Eisner RL, Stillman AE, Oshinski JN. A new method for the determination of aortic pulse wave velocity using cross-correlation on 2d pcmr velocity data. Journal of magnetic resonance imaging : JMRI. 2008;27:1382–1387. doi: 10.1002/jmri.21387. [DOI] [PubMed] [Google Scholar]
- 11.Cavalcante JL, Lima JA, Redheuil A, Al-Mallah MH. Aortic stiffness: Current understanding and future directions. Journal of the American College of Cardiology. 2011;57:1511–1522. doi: 10.1016/j.jacc.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 12.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: Objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 13.Redheuil A, Wu CO, Kachenoura N, et al. Proximal aortic distensibility is an independent predictor of all-cause mortality and incident cv events: The mesa study. Journal of the American College of Cardiology. 2014;64:2619–2629. doi: 10.1016/j.jacc.2014.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Redheuil A, Yu WC, Wu CO, Mousseaux E, de Cesare A, Yan R, Kachenoura N, Bluemke D, Lima JA. Reduced ascending aortic strain and distensibility: Earliest manifestations of vascular aging in humans. Hypertension. 2010;55:319–326. doi: 10.1161/HYPERTENSIONAHA.109.141275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dogui A, Redheuil A, Lefort M, DeCesare A, Kachenoura N, Herment A, Mousseaux E. Measurement of aortic arch pulse wave velocity in cardiovascular mr: Comparison of transit time estimators and description of a new approach. Journal of magnetic resonance imaging : JMRI. 2011;33:1321–1329. doi: 10.1002/jmri.22570. [DOI] [PubMed] [Google Scholar]
- 16.Bluemke DA, Kronmal RA, Lima JA, Liu K, Olson J, Burke GL, Folsom AR. The relationship of left ventricular mass and geometry to incident cardiovascular events: The mesa (multi-ethnic study of atherosclerosis) study. Journal of the American College of Cardiology. 2008;52:2148–2155. doi: 10.1016/j.jacc.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Folsom AR, Kronmal RA, Detrano RC, O’Leary DH, Bild DE, Bluemke DA, Budoff MJ, Liu K, Shea S, Szklo M, Tracy RP, Watson KE, Burke GL. Coronary artery calcification compared with carotid intima-media thickness in the prediction of cardiovascular disease incidence: The multi-ethnic study of atherosclerosis (mesa) Archives of internal medicine. 2008;168:1333–1339. doi: 10.1001/archinte.168.12.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D’Agostino RB, Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: The framingham heart study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 19.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: From area under the roc curve to reclassification and beyond. Statistics in medicine. 2008;27:157–172. doi: 10.1002/sim.2929. discussion 207–112. [DOI] [PubMed] [Google Scholar]
- 20.Polak JF, Pencina MJ, Pencina KM, O’Donnell CJ, Wolf PA, D’Agostino RB., Sr Carotid-wall intima-media thickness and cardiovascular events. The New England journal of medicine. 2011;365:213–221. doi: 10.1056/NEJMoa1012592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Rourke MF, Hashimoto J. Mechanical factors in arterial aging: A clinical perspective. Journal of the American College of Cardiology. 2007;50:1–13. doi: 10.1016/j.jacc.2006.12.050. [DOI] [PubMed] [Google Scholar]
- 22.Maroules CD, Khera A, Ayers C, Goel A, Peshock RM, Abbara S, King KS. Cardiovascular outcome associations among cardiovascular magnetic resonance measures of arterial stiffness: The dallas heart study. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance. 2014;16:33. doi: 10.1186/1532-429X-16-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hickson SS, Butlin M, Graves M, Taviani V, Avolio AP, McEniery CM, Wilkinson IB. The relationship of age with regional aortic stiffness and diameter. JACC. Cardiovascular imaging. 2010;3:1247–1255. doi: 10.1016/j.jcmg.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 24.McEniery CM, McDonnell BJ, So A, Aitken S, Bolton CE, Munnery M, Hickson SS, Yasmin, Maki-Petaja KM, Cockcroft JR, Dixon AK, Wilkinson IB. Aortic calcification is associated with aortic stiffness and isolated systolic hypertension in healthy individuals. Hypertension. 2009;53:524–531. doi: 10.1161/HYPERTENSIONAHA.108.126615. [DOI] [PubMed] [Google Scholar]
- 25.Wilson PW, Kauppila LI, O’Donnell CJ, Kiel DP, Hannan M, Polak JM, Cupples LA. Abdominal aortic calcific deposits are an important predictor of vascular morbidity and mortality. Circulation. 2001;103:1529–1534. doi: 10.1161/01.cir.103.11.1529. [DOI] [PubMed] [Google Scholar]
- 26.Redheuil A. Cardiovascular aging: Insights from local and regional measures of aortic stiffness using magnetic resonance imaging. Artery Research. 2014;8:66–72. [Google Scholar]
- 27.Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, Heffernan KS, Lakatta EG, McEniery CM, Mitchell GF, Najjar SS, Nichols WW, Urbina EM, Weber T American Heart Association Council on H. Recommendations for improving and standardizing vascular research on arterial stiffness: A scientific statement from the american heart association. Hypertension. 2015;66:698–722. doi: 10.1161/HYP.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adji A, Kachenoura N, Bollache E, Avolio AP, O’Rourke MF, Mousseaux E. Magnetic resonance and applanation tonometry for noninvasive determination of left ventricular load and ventricular vascular coupling in the time and frequency domain. Journal of hypertension. 2016;34:1099–1108. doi: 10.1097/HJH.0000000000000925. [DOI] [PubMed] [Google Scholar]
- 29.Miyashita H, Ikeda U, Tsuruya Y, Sekiguchi H, Shimada K, Yaginuma T. Noninvasive evaluation of the influence of aortic wave reflection on left ventricular ejection during auxotonic contraction. Heart and Vessels. 1994;9:30–39. doi: 10.1007/BF01744493. [DOI] [PubMed] [Google Scholar]
- 30.Bensalah MZ, Bollache E, Kachenoura N, Giron A, De Cesare A, Macron L, Lefort M, Redheuil A, Mousseaux E. Geometry is a major determinant of flow reversal in proximal aorta. Am J Physiol Heart Circ Physiol. 2014;306:H1408–1416. doi: 10.1152/ajpheart.00647.2013. [DOI] [PubMed] [Google Scholar]
- 31.Noda C, Ambale Venkatesh B, Ohyama Y, Liu CY, Chamera E, Redheuil A, Teixido-Tura G, Chugh AR, Wu CO, Hundley GW, Bluemke DA, Lima JA. Reproducibility of functional aortic analysis using magnetic resonance imaging: The mesa. European heart journal cardiovascular Imaging. 2016;17:909–917. doi: 10.1093/ehjci/jev215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohyama Y, Teixido-Tura G, Ambale-Venkatesh B, et al. Ten-year longitudinal change in aortic stiffness assessed by cardiac mri in the second half of the human lifespan: The multi-ethnic study of atherosclerosis. European heart journal cardiovascular Imaging. 2016;17:1044–1053. doi: 10.1093/ehjci/jev332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.