Abstract
The objective of this meta-analysis is to explore the correlation between the apparent diffusion coefficient (ADC) on diffusion-weighted MR and the standard uptake value (SUV) of 18F-FDG on PET/CT in patients with cancer. Databases such as PubMed (MEDLINE included), EMBASE, and Cochrane Database of Systematic Review were searched for relevant original articles that explored the correlation between SUV and ADC in English. After applying Fisher's r-to-z transformation, correlation coefficient (r) values were extracted from each study and 95% confidence intervals (CIs) were calculated. Sensitivity and subgroup analyses based on tumor type were performed to investigate the potential heterogeneity. Forty-nine studies were eligible for the meta-analysis, comprising 1927 patients. Pooled r for all studies was −0.35 (95% CI: −0.42–0.28) and exhibited a notable heterogeneity (I2 = 78.4%; P < 0.01). In terms of the cancer type subgroup analysis, combined correlation coefficients of ADC/SUV range from −0.12 (lymphoma, n = 5) to −0.59 (pancreatic cancer, n = 2). We concluded that there is an average negative correlation between ADC and SUV in patients with cancer. Higher correlations were found in the brain tumor, cervix carcinoma, and pancreas cancer. However, a larger, prospective study is warranted to validate these findings in different cancer types.
1. Introduction
At present, various imaging modalities play an important role in diagnosis, staging, follow-up, and therapeutic evaluation of patients with cancer. Positron emission tomography/computed tomography with F-18 based fluorodeoxyglucose (18F-FDG PET/CT) is considered as an accurate method for characterizing tumor lesions due to the availability of anatomic and glucose metabolic information of tumor [1]. The standardized uptake value (SUV) is the most frequently used parameter derived from 18F-FDG PET, which has been used for assessing tumor aggressiveness, differentiating benign from malignant tumors, and monitoring treatment [2, 3].
Magnetic resonance imaging (MRI) is another important tool to detect and characterize tumors. Specifically, diffusion-weighted imaging (DWI) provides an additional promising dimension to the conventional anatomical MRI. Apparent diffusion coefficient (ADC) is a parameter obtained by MR-DWI, reflecting the Brownian movement of water molecules. The ADC value has been shown to link with the cell density, microvascular circulation, and membrane integrity of a tumor tissue [4].
Although glucose metabolism and cell density represent two different facets of tumor biology, many researchers tried to find the relationship between ADC and SUV. However, there is a controversy in this relationship. Some data demonstrated that there was no significant correlation observed between SUV and ADC [5], while other studies reported that SUV was inversely correlated with ADC [6, 7]. Given the conflicting evidence on this issue, we conducted this meta-analysis to explore the correlation between ADC and SUV in cancer patients.
2. Methods
2.1. Literature Search
Two observers independently searched the PubMed (MEDLINE included), EMBASE, and Cochrane Library databases for published studies. The search was limited to publications written in English. The databases were searched using the terms ((positron emission tomography) OR (PET) OR (positron emission tomography/computed tomography) OR (PET/CT) OR (PET-CT) OR (positron emission tomography-computed tomography)) AND ((18F-FDG) OR (fluorodeoxyglucose) OR (FDG) OR (18FDG) OR (FDG-F18)) AND ((apparent diffusion coefficient) OR (ADC)) AND ((Diffusion Magnetic Resonance Imaging) OR (Diffusion MRI) OR (Diffusion Weighted MRI) OR (DWI) OR (diffusion-weighted magnetic resonance imaging) OR (MRI DWI) OR (diffusion-weighted imaging) OR (diffusion-weighted MRI)).
2.2. Study Identification and Selection
Two independent reviewers evaluated the potentially relevant articles on the basis of the inclusion and exclusion criteria. Articles were included if they met the following criteria:
Investigation of the relationship between ADC measured by MR and SUV measured with PET or PET/CT scanning
Studies focusing on patients with malignant tumors, which may include patients with benign conditions as long as the vast majority of patients (>50%) in the study had cancer
Research article published in the peer-reviewed journals
The exclusion criteria included the following:
Data or part of data presented in more than one article (in this case, the article containing the latest and/or the most complete data was chosen)
Animal studies, reviews, case report, letters, editorials, abstracts, comments, and in vitro studies
Studies including less than 10 patients or 10 lesions
Articles without sufficient information for calculation of correlation coefficient
If there was discordance among the 2 independent researchers for one study, its eligibility was decided by the 3rd investigator.
2.3. Data Extraction
The data were extracted from the included literatures by two investigators (Shengming Deng and Bin Zhang) independently, and the extracted contents included the following:
Overall characteristics of studies, including authors, year of publication, number of patients and lesions, and tumor type
Technical characteristics of PET or PET/CT measurement of 18F-FDG, including characteristics of the scanner, 18F-FDG dose, uptake time of the tracer, emission scan time, delineation of the tumor, and indexes of uptake (SUVmax, SUVmean, or others)
Technical characteristics of MR or PET/MR measurement ADC covered imaging equipment, b value, MRI field strength, and the index used to characterize the ADC (average, minimum, or others)
The degree of correlation between ADC and SUV, including Spearman's correlation coefficient (SCC), Pearson's correlation coefficient (PCS), and r2. If the article did not report the value of correlation coefficient r directly, r value was calculated based on the raw data or scatter plot using the free software Engauge Digitizer (free software downloaded from https://sourceforge.net) and the SPSS 18.0 software. SCC was used for this meta-analysis. Since the SCC has already been processed by logarithmic conversion, it does not need to undergo the conversion again. The published PCSs were converted to SCCs for further analysis [57]. The sampling of SCC is not normally distributed. Because its confidence interval (CI) depends on the value of correlation coefficient, we converted the SCC by Fisher transformation to obtain z value with an approximately normal distribution. z value was then converted by inverse Fisher transformation to obtain the SCC and the corresponding CI.
If more than one correlation coefficient value calculated according to various SUV indexes or ADC indexes was reported in the article, the lowest value was chosen.
When disagreements occurred between the two reviewers, a third investigator joined to vote for a decision.
2.4. Methodology of Quality Assessment
Two investigators (Shengming Deng and Bin Zhang) assessed the quality of the articles independently according to the QUADAS-2 [58], which consists of 2 parts of contents: “risk assessment” and “practical application.” The former was assessed from 4 key domains as patient selection, index test, reference standard, and flow and timing, and the latter included 3 aspects as patient selection, index test, and reference standard.
To make sure that the QUADAS-2 tool is applicable to the present study, we designated SUV measurement as the “reference test” and ADC measurement as the “index test.” In this study, we chose one month as the threshold interval between PET or PET/CT examination and DWI-MRI detection in case tumor biology will change much. A third reviewer was introduced when there were assessing differences between the two observers.
2.5. Meta-Analysis
The pooled correlation coefficient between SUV and ADC was calculated according to the values of correlation coefficients obtained in each individual study. Correlation coefficient values were converted by Fisher's r-to-z transformation to obtain approximately normally distributed z values to further calculate 95% CIs. The random-effects model was used for the pooled analysis in this study. Correlations were classified as poor (correlation coefficient r < 0.20), average (r = 0.20–0.39), moderate (r = 0.40–0.59), significant (r = 0.60–0.79), and strong (r > 0.80) [59]. Publication bias was assessed by means of Begg's funnel plots and Begg's statistical test.
The heterogeneity of r values between studies was tested by calculating Q statistic and the inconsistency index (I2). p < 0.05 or I2 > 50% indicated the presence of heterogeneity. In case of the existence of heterogeneity, a sensitivity analysis was performed for all studies to further investigate the study heterogeneity. In a subgroup analysis, studies were stratified according to tumor type and correlation coefficient value (SUVmean/ADCmean, SUVmax/ADCmin, SUVmax/ADCmean, etc.).
Statistical analysis was performed using STATA 11 software package (Stata Corporation, College Station, TX, USA). p < 0.05 was considered statistically significant.
3. Results
3.1. Literature Search and Selection of Studies
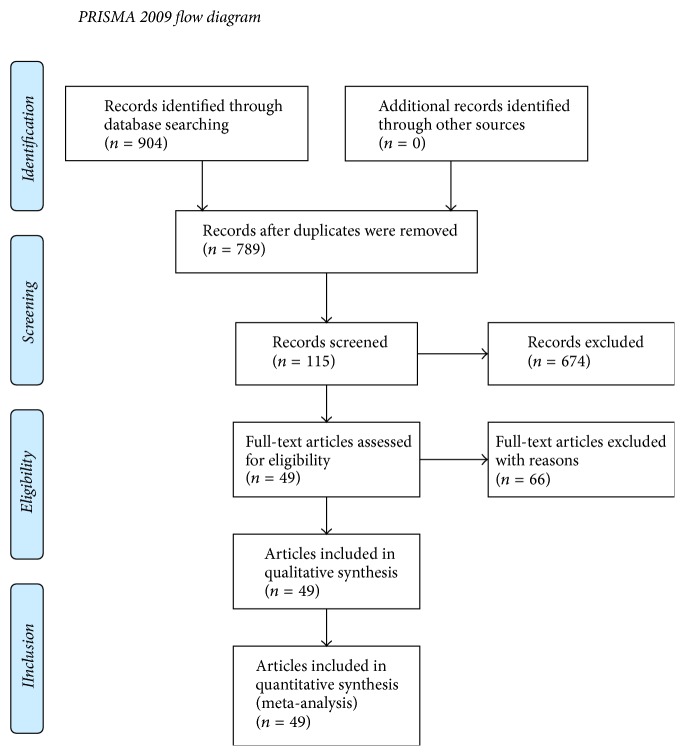
The original search identified 145 articles in PubMed and 759 articles in EMBASE. After removing duplicates, 789 abstracts were screened according to the evaluation criteria, and 115 in total were selected to be read in full as potentially eligible. After reading the full texts, 66 studies were excluded for the following reasons: (1) the article did not involve the evaluation of the relationship between ADC value and 18F-FDG uptake (n = 38); (2) the number of cases or tumor sites studied was fewer than 10 (n = 13); (3) the raw data in the article failed to generate the correlation coefficient values (n = 10); (4) part of the data in the study appeared in other articles (n = 3); (5) parameters measured by two individual reviewers were presented in the article which was difficult to choose (n = 1); and (6) most of the cases studied were benign tumors (n = 1). Figure 1 describes the study selection process and results according to the PRISMA guidelines. Finally, 49 published articles were included in the present study [8–56].
Figure 1.
Flow diagram of study selection.
3.2. Study Characteristics
The selected studies were published between 2008 and 2015. The median number of patients per study was 32 (range: 7–131) with a total number of 1927 patients. In some studies, more than one tumor site was analyzed on several patients; therefore, a total of 2356 samples were assessed in the meta-analysis. Studies covering a range of cancer sites are summarized in Table 1.
Table 1.
18F-FDG PET scan characteristics and MRI scanner.
| Author | Year | Scanner | FDG dose (MBq) | Uptake period (min) | Emission time (min) | SUV index | Delineation |
|---|---|---|---|---|---|---|---|
| Mori et al. [8] | 2008 | GE Discovery ST PET/CT + Philips Intera Achieva Nova Dual 1.5 T MR | 3.7/kg | 60 | 3 | SUV-CR | Manual |
| Ho et al. [9] | 2009 | GE Discovery ST16 PET/CT + Siemens Tim Trio 3.0 T MR | 333–407 | 50 | 3 | SUVmax/SUVmean | Automatic |
| Palumbo et al. [10] | 2009 | GE Advance PET + GE 1.5 T MR | 444–555 | 45 | 6–10 | SUV-CR | Semiautomatic |
| Aoyagi et al. [11] | 2010 | PET + Philips Intera Achieva Nova Dual 1.5 T MR | N | N | N | SUVmax | N |
| Nakajo et al. [12] | 2010 | GE Discovery STE PET/CT + Philips Intera Achieva 1.5 T MR | 3.7/kg | 60 | 2.5 | SUVmax | Automatic |
| Punwani et al. [13] | 2010 | GE Discovery LS PET/CT + Siemens Avanto 1.5 T MR | 370 | 60 | N | SUVmax | Manual |
| Tanimoto et al. [14] | 2010 | Toshiba Aquiduo PET/CT + GE Signa HDx 3.0 T MR | 217.8–372.5 | 60 | 3 | SUV | Automatic |
| Choi et al. [15] | 2011 | Philips Gemini or Siemens Biograph 40 PET/CT + GE Signa HDx or HDxt 1.5 T MR | 5.2/kg | 60 | 2 | SUVmean | Manual |
| Fruehwald-Pallamar et al. [16] | 2011 | Siemens Biograph 64 PET/CT + Philips Achieva 3.0 T MR | 300 | 50 | 3 | SUVmax | Automatic |
| Gu et al. [17] | 2011 | GE Discovery VCT PET/CT + Philips Achieva 3.0 T MR | 4.8/kg | 60 | 4 | SUVmax | Automatic |
| Ohba et al. [18] | 2011 | GE Discovery ST PET/CT + Philips Achieva 3.0 T or Philips Gyroscan Intera Achieva Nova Dual 1.5 T MR | 3.7/kg | 60 | 3 | SUV-CR | N |
| Usuda et al. [19] | 2011 | Siemens Biography Sensation 16 PET/CT + Siemens Magnetom Avanto 1.5 T MR | 185 | 60 | 3 | SUVmax | Automatic |
| Wu et al. [20] | 2011 | GE Discovery STE 16 PET/CT + Siemens Trio-Tim 3.0 T MR | 370 | 60 | 3 | SUVmax | Manual |
| Cafagna et al. [21] | 2012 | GE Discovery STE 16 PET/CT + Philips Achieva 1.5 T MR | 3.7/kg | 60 | 3 | SUVmax | N |
| Choi et al. [22] | 2012 | Siemens Biograph Duo or Biograph Truepoint PET/CT + Philips Achieva 1.5 T or Siemens Magnetom Verio 3.0 T MR | N | N | 2-3 | SUVmax | N |
| Matsushima et al. [23] | 2012 | Toshiba Aquiduo PCA-7000B PET/CT + GE Signa Excite HDxt 1.5 T MR | 3.7/kg | 60 | 6 | SUV-CR | Manual |
| Nakajo et al. [24] | 2012 | GE Discovery STE PET/CT + Siemens Magnetom Avanto 1.5 T MR | 3.7/kg | 60 | N | SUVmax | Automatic |
| Nakamatsu et al. [25] | 2012 | Toshiba Aquiduo 16 PET/CT + Siemens Magnetom Symphony 1.5 T MR | 166.7–320.8 | 60 | 2 | SUVmean | Manual |
| Nakamura et al. [26] | 2012 | Siemens Biograph LS/Sensation 16 PET/CT + Siemens Magnetom Avanto 1.5 T MR | 3.7/kg | 90 | 2.4 | SUVmax | Manual |
| Regier et al. [27] | 2012 | Philips Gemini GXL 10 PET/CT + Philips Achieva 1.5 T MR | 5/kg | 60 | 1–1.5 | SUVmax | N |
| Ahn et al. [28] | 2013 | Siemens Biograph Truepoint 40 PET/CT + Siemens Magnetom Tim Trio 3.0 T MR | 5.5/kg | 45 | N | SUVmax | N |
| Byun et al. [29] | 2013 | Siemens Biograph 6 PET/CT + Siemens Magnetom TrioA Tim 3.0 T MR | 7.4/kg | 60 | 3.5 | SUVmax | Automatic or manual |
| Gong et al. [30] | 2013 | GE Discovery VCT PET/CT + Philips Achieva 3.0 T MR | 4.8/kg | 60 | 4 | SUVmax | Manual |
| Nakamura et al. [31] | 2013 | Siemens Biograph LS/Sensation 16 PET/CT + Siemens Magnetom Avanto 1.5 T MR | 3.7/kg | 90 | 2.4 | SUVmax | Manual |
| Rakheja et al. [32] | 2013 | Siemens Biograph mCT PET/CT + Siemens Biograph mMR PET/MR | 555 | 45 | 2 | SUVmax | Manual |
| Schmidt et al. [33] | 2013 | Siemens HI-REZ Biograph 16 or Siemens Biograph mCT PET/CT + Siemens Biograph mMR PET/MR | 317–381 | 55–61 | 2-3 | SUVmax | N |
| Tsuchida et al. [34] | 2013 | GE Discovery LS4 PET/CT + GE Signa Excite 1.5 T MR | 185 | 50 | 2 | SUVmean | N |
| Varoquaux et al. [35] | 2013 | Siemens Biograph 16-slice PET/CT + Siemens Espree 1.5 T or Trio 3.0 T MR | 370 | 60 | 3 | SUV | Manual |
| Baba et al. [36] | 2014 | GE Advance NXi PET/CT + Philips Intera Achieva 1.5 T MR | 3.7/kg | 60 | 2 | SUVmax | Manual |
| de Jong et al. [37] | 2014 | Siemens Biograph 40 True Point or Philips Gemini TOF PET/CT + Philips Achieva or Siemens Magnetom Avanto 1.5 T MR | 2.0–3.7/kg | 60–75 | 2-3 | SUVmax | Manual |
| Er et al. [38] | 2014 | GE Discovery ST PET/CT + Siemens Magnetom Verio 3.0 T MR | 5.55/kg | 50–60 | N | SUVmax | Manual |
| Giganti et al. [39] | 2014 | GE Discovery ST, Discovery STE, Discovery-690, or Philips Gemini GXL PET/CT + Philips Achieva 1.5 T MR | 3.7/kg | 60 | 2.5 | PVC-SUVmean | Automatic |
| Grueneisen et al. [40] | 2014 | Siemens Biograph mMR PET/MR | 201 ± 69 | 102 ± 39 | 8 | SUVmax | Manual |
| Iizuka et al. [41] | 2014 | GE Discovery ST Elite PET/CT + Siemens Avanto 1.5 T MR | 3.7/kg | 60 | 2-3 | SUVmax | N |
| Sakane et al. [42] | 2015 | Philips Gemini GXL PET/CT + GE Signa HDxt 3.0 T MR | 3.7/kg | 60 | 2 | SUVmean | Manual |
| Schwenzer et al. [43] | 2014 | PET/CT + Siemens Biograph mMR PET/MR | 294–386 | 62 ± 4 | 6 | SUVmean | Manual |
| Sun et al. [44] | 2014 | Philips Ingenuity TF PET/MR | 240–350 | 60 ± 12 | 4 | SUVmean | Automatic |
| Yu et al. [45] | 2014 | GE Discovery VCT PET/CT + Philips Achieva 3.0 T MR | 4.8/kg | 60 | 2.5 | SUVmean | Manual |
| Zhang et al. [46] | 2014 | Siemens Biograph 40 PET/CT + Siemens Trio-Tim 3.0 T MR | 5.55/kg | 60 | N | SUVmax | N |
| Zukotynski et al. [47] | 2014 | GE Advance NXi, Discovery LS, and Discovery STE; Philips G-PET; Siemens HR1 and HI-REZ Bioscan PET + 1.5 T MR | 5.55/kg | 40–60 | 10 | SUVmean/WM | Manual |
| Brandmaier et al. [48] | 2015 | Siemens Biograph mMR 3.0 T PET/MR | 309 ± 70.32 | 130 | 5 | SUVmax | Manual |
| Covello et al. [49] | 2015 | Philips Gemini TF PET/CT + Siemens Biograph mMR 3.0 T MR | 406 ± 40 | 81 ± 15 | 3 | SUV | Automatic |
| Han et al. [50] | 2015 | GE Discovery STE PET/CT + GE Signa HDxt 1.5 T MR | 5/kg | 60 | 2.5 | SUVmean | Manual |
| Heacock et al. [51] | 2015 | Siemens Biograph mCT PET/CT + Siemens Biograph mMR 3.0 T PET/MR | 506.9–566.1 | 45 | 2-3 | SUVmax | Manual |
| Karan et al. [52] | 2016 | GE Discovery STE 8 PET/CT + Siemens Avanto 1.5 T MR | 296–370 | 60 | 2.5 | SUVmax | Automatic |
| Littooij et al. [53] | 2015 | Siemens Biograph 16 or Biograph 40 Truepoint, Philips Gemini TOF or Allegro PET-CT + Philips Achieva, Siemens Avanto or GE Signa 1.5 T MR | 2–3.7/kg | 60 | N | SUVmax | N |
| Liu et al. [54] | 2015 | Siemens Biograph 40 PET/CT + GE Signa HDE 1.5 T MR | 5.55/kg | 60 | N | SUVmax | N |
| Metz et al. [55] | 2015 | Siemens Biograph Sensation 16 PET/CT + Siemens Magnetom Avanto 1.5 T MR | 456 ± 25 | 64 ± 3 | 2 | SUVmean | Manual |
| Schaarschmidt et al. [56] | 2015 | Siemens mCT™ PET/CT + Siemens Biograph mMR PET/MR | 280 ± 50 | 58 ± 11 | 2 | SUVmean | Manual |
N: not reported.
The most studied tumor location was the lung with 10 studies. The second tumor type was head and neck cancer, for which there were 6 studies. Five groups studied breast cancer, lymphoma, and cervical cancer. Other tumor types include metastatic gastrointestinal stromal tumors (GIST), brain cancer, hepatocellular cancer, esophageal cancer, peritoneal carcinomatosis, pancreatic cancer, and gastric, rectal, uterus, hepatocellular, and various types.
For MR-DWI examination, forty-one studies used a stand-alone MR scanner, while 8 studies used a PET/MR scanner. For MRI field strength, twenty-four studies used 1.5 T, twenty-three studies used 3.0 T, and 2 studies used both. For the index of ADC, twenty-six studies used ADCmean, fifteen studies used ADCmin, and 8 studies used other indexes. For 18F-FDG PET scan, SUVmax, SUVmean, and other SUV were used to calculate r values in 29, 11, and 9 studies, respectively (Table 2).
Table 2.
MRI characteristics, cancer types, and r values.
| Author | Year | Nation | Number of patients | Number of tumors | Tumor | Age | Design | Field | Index | b value (s/m2) | r |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mori et al. [8] | 2008 | Japan | 104 | 140 | Lung (various) | Adult | Prospective | 1.5 T | ADCmin | 1000 | −0.504 |
| Ho et al. [9] | 2009 | Taiwan | 33 | 33 | Cervix (various) | Adult | Prospective | 3.0 T | ADCmin/ADCmean | 0 and 1000 | −0.526 |
| Palumbo et al. [10] | 2009 | USA | 15 | 18 | Brain (metastases) | Adult | N | 1.5 T | ADC-CR | N | −0.524 |
| Aoyagi et al. [11] | 2010 | Japan | 123 | 123 | Esophageal (SCC) | Adult | N | 1.5 T | ADC | 0 and 1000 | −0.398 |
| Nakajo et al. [12] | 2010 | Japan | 44 | 44 | Breast (ductal carcinoma) | Adult | Retrospective | 1.5 T | ADCmean | 0 and 1000 | −0.486 |
| Punwani et al. [13] | 2010 | UK | 16 | 53 | Lymphoma (MCL) | Children | N | 1.5 T | ADCmean | 500 | −0.38 |
| Tanimoto et al. [14] | 2010 | Japan | 16 | 16 | Pancreas (various) | Adult | N | 3.0 T | ADC | 400, 800, and 1200 | −0.48 |
| Choi et al. [15] | 2011 | Korea | 47 | 47 | Head and neck (SCC) | Adult | Retrospective | 1.5 T | ADCmean | 1000 | −0.222 |
| Fruehwald-Pallamar et al. [16] | 2011 | Austria | 31 | 31 | Head and neck (SCC) | Adult | Prospective | 3.0 T | ADCmean | 0 and 800 | −0.238 |
| Gu et al. [17] | 2011 | China | 33 | 33 | Rectum (adenocarcinoma) | Adult | N | 3.0 T | ADCmin | 0 and 1000 | −0.45 |
| Ohba et al. [18] | 2011 | Japan | 58 | 76 | Lung (various) | N | Prospective | 1.5 T | ADCmin | 1000 | −0.31 |
| Usuda et al. [19] | 2011 | Japan | 63 | 63 | Lung (various) | Adult | N | 1.5 T | ADCmean | 0 and 800 | −0.286 |
| Wu et al. [20] | 2011 | Finland | 15 | 15 | Lymphoma (DLBCL) | Adult | Prospective | 3.0 T | ADCmean | 0 and 800 | 0.215 |
| Cafagna et al. [21] | 2012 | Italy | 38 | 88 | Various | N | Retrospective | 1.5 T | ADC | 500 and 1000 | 0.238 |
| Choi et al. [22] | 2012 | Korea | 118 | 118 | Breast (IDC) | Adult | N | 1.5 and 3.0 T | ADCmean | 0, 750, and 1000 | −0.025 |
| Matsushima et al. [23] | 2012 | Japan | 36 | 36 | Glioma and lymphoma | Children and adult | Retrospective | 1.5 T | ADCmin | 1000 | −0.68 |
| Nakajo et al. [24] | 2012 | Japan | 26 | 26 | Head and neck (SCC) | Adult | Retrospective | 1.5 T | ADCmean | 0 and 800 | −0.566 |
| Nakamatsu et al. [25] | 2012 | Japan | 24 | 41 | Metastatic neck lymph nodes of head and neck (SCC) | Adult | Retrospective | 1.5 T | ADCmin | 0 and 1000 | −0.489 |
| Nakamura et al. [26] | 2012 | Japan | 66 | 66 | Cervix (various) | Adult | N | 1.5 T | ADCmin | 0, 50, and 1000 | −0.529 |
| Regier et al. [27] | 2012 | Germany | 41 | 41 | Lung (NSCLC) | N | Prospective | 1.5 T | ADCmin | 0 and 500 | −0.46 |
| Ahn et al. [28] | 2013 | Korea | 21 | 21 | Liver (HCC) | Adult | Retrospective | 3.0 T | ADCmax | 50, 400, and 800 | 0.369 |
| Byun et al. [29] | 2013 | Korea | 75 | 75 | Breast (IDC) | Adult | Retrospective | 3.0 T | ADCmin | 0 and 800 | −0.267 |
| Gong et al. [30] | 2013 | China | 7 | 21 | Metastatic GIST | Adult | Retrospective | 3.0 T | ADCmean | 0, 150, and 1000 | −0.843 |
| Nakamura et al. [31] | 2013 | Japan | 131 | 131 | Endometria | Adult | Prospective | 1.5 T | ADCmin | 0, 50, and 1000 | −0.677 |
| Rakheja et al. [32] | 2013 | USA | 24 | 69 | Various | Adult | N | 3.0 T | ADCmin | 0, 350, and 750 | −0.29 |
| Schmidt et al. [33] | 2013 | Germany | 14 | 14 | Lung (various) | Adult | N | 3.0 T | ADCmin | 0 and 800 | −0.8 |
| Tsuchida et al. [34] | 2013 | Japan | 28 | 28 | Lung (various) | Adult | N | 1.5 T | ADC | 0 and 800 | 0.043 |
| Varoquaux et al. [35] | 2013 | Switzerland | 33 | 34 | Head and neck (SCC) | Children and adult | Retrospective | 1.5 and 3.0 T | ADC | 0 and 1000 | −0.103 |
| Baba et al. [36] | 2014 | Japan | 79 | 83 | Breast (various) | Adult | Retrospective | 1.5 T | ADCmean | 1000 | −0.36 |
| de Jong et al. [37] | 2014 | Netherlands | 21 | 21 | Lymphoma (DLBCL) | Adult | Prospective | 1.5 T | ADCmean | 0 and 1000 | −0.103 |
| Er et al. [38] | 2014 | Turkey | 41 | 41 | Rectum (adenocarcinoma) | Adult | Retrospective | 3.0 T | ADCmin | 50, 400, and 1000 | −0.347 |
| Giganti et al. [39] | 2014 | Italy | 17 | 17 | Gastric (adenocarcinoma) | Adult | Prospective | 1.5 T | ADCmean | 0 and 600 | −0.01 |
| Grueneisen et al. [40] | 2014 | Germany | 15 | 54 | Cervix (various) | Adult | Prospective | 3.0 T | ADCmin | 0, 500, and 1000 | −0.342 |
| Iizuka et al. [41] | 2014 | Japan | 15 | 15 | Lung (NSCLC) | Adult | N | 1.5 T | ADCmean | 0, 500, and 1000 | 0.046 |
| Sakane et al. [42] | 2015 | Japan | 20 | 20 | Pancreas (adenocarcinoma) | Adult | Retrospective | 3.0 T | ADCmean | 0 and 800 | −0.66 |
| Schwenzer et al. [43] | 2014 | Germany | 20 | 52 | Peritoneal carcinomatosis | Adult | Prospective | 3.0 T | ADCmean | 50 and 800 | −0.58 |
| Sun et al. [44] | 2014 | China | 35 | 35 | Cervix (various) | Adult | Prospective | 3.0 T | ADCmean | 0, 200, 500, and 1000 | −0.505 |
| Yu et al. [45] | 2014 | China | 8 | 34 | Peritoneal metastases | Adult | Prospective | 3.0 T | ADCmean | 0, 400, and 800 | −0.548 |
| Zhang et al. [46] | 2014 | China | 113 | 113 | Lung (various) | Adult | N | 3.0 T | ADCmean | 1000 | −0.37 |
| Zukotynski et al. [47] | 2014 | USA | 36 | 36 | Brain (BSG) | Children | Retrospective | 1.5 T | ADCmean | 5 and 1000 | −0.54 |
| Brandmaier et al. [48] | 2015 | Germany | 14 | 14 | Cervix (various) | Adult | Prospective | 3.0 T | ADCmin | 0 and 800 | −0.532 |
| Covello et al. [49] | 2015 | Italy | 44 | 44 | Head and neck (various) | Adult | N | 3.0 T | ADCmean | 0, 500, and 800 | −0.36 |
| Han et al. [50] | 2015 | Korea | 34 | 34 | Head and neck (SCC) | Adult | Retrospective | 1.5 T | ADCmin | 0 and 1000 | −0.333 |
| Heacock et al. [51] | 2015 | USA | 13 | 51 | Lymphoma (various) | Adult | Prospective | 3.0 T | ADCmean | 0, 350, and 750 | 0.06 |
| Karan et al. [52] | 2016 | Turkey | 70 | 70 | Breast (IDC) | Adult | Retrospective | 1.5 T | ADCmean | 0, 200, 600, and 800 | −0.112 |
| Littooij et al. [53] | 2015 | Netherlands | 11 | 19 | Lymphoma (various) | Children and adult | Prospective | 1.5 T | ADCmean | 0 and 1000 | −0.24 |
| Liu et al. [54] | 2015 | China | 11 | 11 | Lung (various) | Adult | N | 1.5 T | ADCmean | 1000 | −0.55 |
| Metz et al. [55] | 2015 | Germany | 12 | 12 | Lung (metastatic NSCLC) | Adult | Prospective | 1.5 T | ADCmean | 50, 300, and 600 | 0.3 |
| Schaarschmidt et al. [56] | 2015 | Germany | 25 | 100 | Lymph node metastases of NSCLC | Adult | Retrospective | 3.0 T | ADCmean | 0, 500, and 1000 | −0.36 |
SCC: squamous cell carcinoma; NSCLC: non-small cell lung cancer; MCL: mantle cell lymphoma; DLBCL: diffuse large B cell lymphoma; HCC: hepatocellular carcinoma; BSG: brain stem glioma; IDC: invasive ductal carcinoma; N: no report.
3.3. The Results of QUADAS-2 Assessing the Quality of the Included Articles
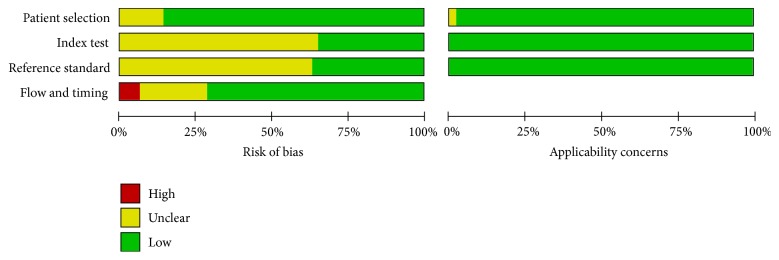
As shown in Figure 2, the results of QUADAS-2 assessing the quality of the included articles indicated that the results of 10 studies adequately addressed all risk of bias domains. Among all the 49 studies, risk of bias was high or unclear with regard to patient selection for 7 studies, the index text for 32 studies, the reference standard for 31 studies, and flow and timing for 14 studies.
Figure 2.
Methodological quality of all eligible studies.
Interpretation of ADC or SUV in a blinded fashion was an item which most studies did not report. Seventeen studies clearly stated that the index test was assessed without knowledge of the results of the reference standard, while this was unclear in 32 studies. Similarly, in 18 studies, the interpretation of reference standard was clearly stated as under unknown index test, while the other 31 studies did not state the interpretation of reference standard clearly.
Acceptable delay between reference and index tests was the item which many studies did not report. Eleven studies provided no information about the time interval between the execution of MR-DWI and the 18F-FDG PET/CT scan. In 3 studies documented, the interval was more than 4 weeks.
In addition, patients enrolled in 1 study were investigated on residual tumors after completion of therapy. In these patients, whether the relationship between 18F-FDG uptake and ADC value differs from that in patients with pretherapeutic tumor is unclear; therefore, the risk of case selection bias in this study was considered unclear in the present analysis.
3.4. The Results of a Meta-Analysis
The data provided by the finally chosen studies all met the standard of meta-analysis. r values for 3 studies were calculated from provided r2, and r values for 2 other studies were determined from the provided scatter plot. For 3 other studies, r values were calculated based on the provided raw data of corresponding ADC and SUV.
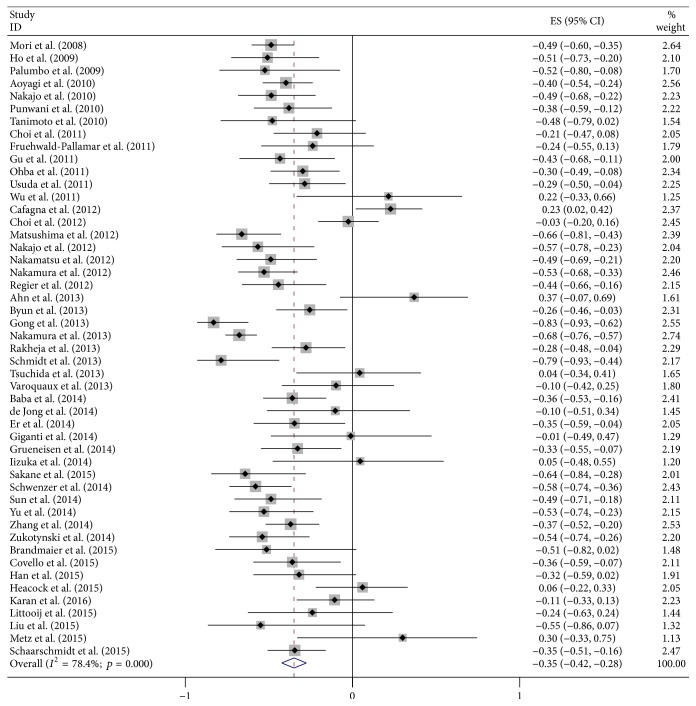
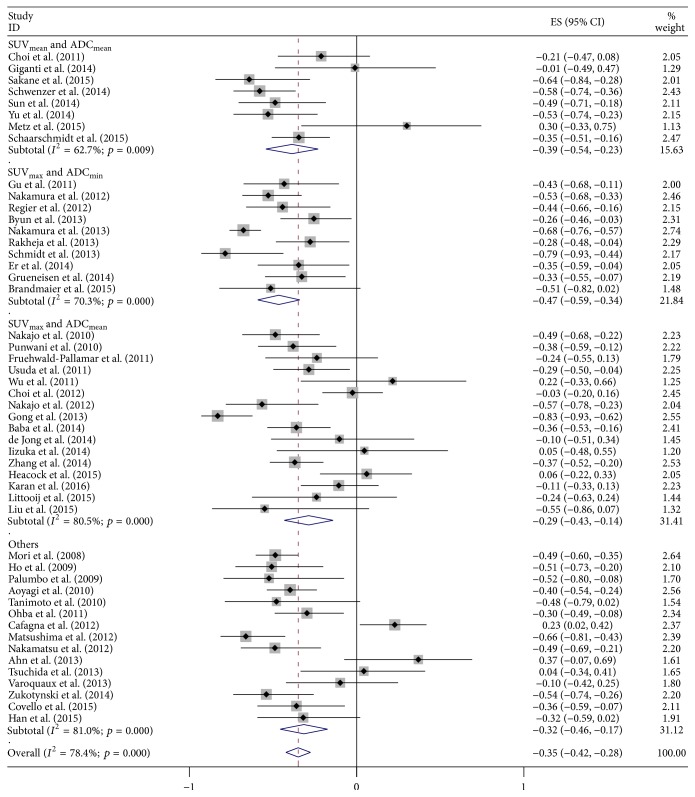
Final combined r value calculated from all the included articles was −0.35 (95% CI: −0.42–−0.28), but the results of heterogeneity test indicated the presence of marked heterogeneity among studies (I2 = 78.4%; p < 0.01; Figure 3). We then conducted a sensitivity analysis by excluding each article at a time to observe its effect on the final outcome, but the results showed that no individual study contributed more greatly to the total heterogeneity. The results of Begg's test indicated no significant publication deviation among the included articles (p > 0.05; Figure 4).
Figure 3.
Forest plots of the summary correlation coefficient (r) with corresponding 95% CIs for the correlation between SUV and ADC in all eligible studies.
Figure 4.
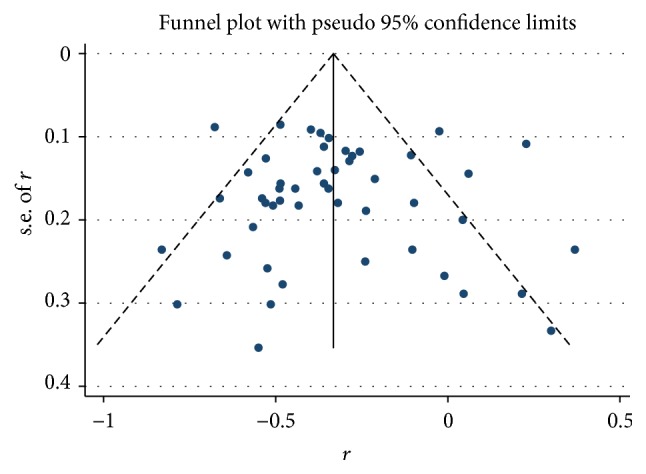
The funnel plot of the publication bias.
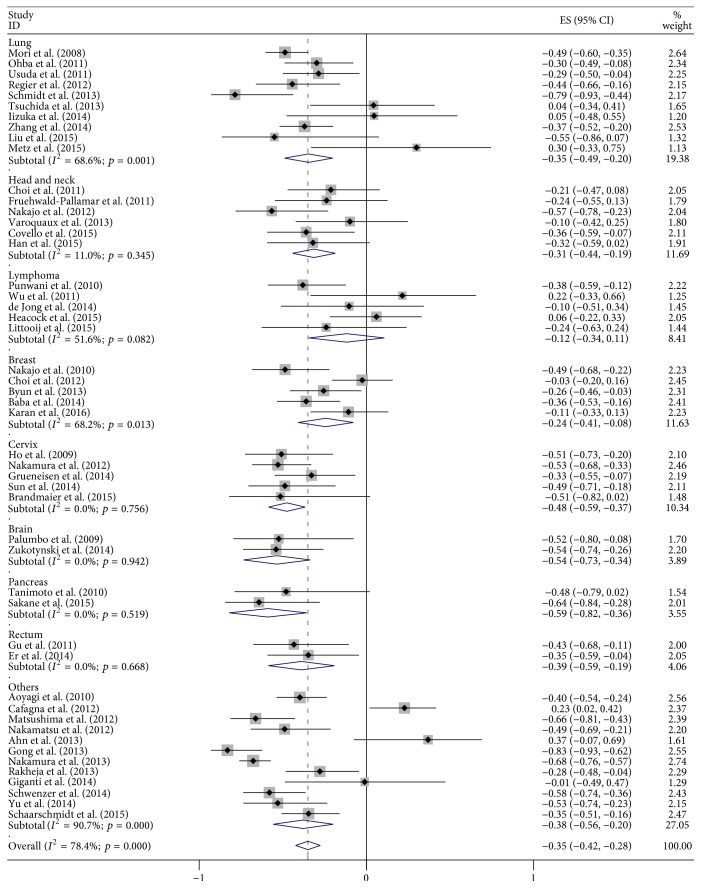
As shown in Figure 5, the subgroup analysis for tumor types showed that combined r for the 10 studies of lung cancer was −0.35 (95% CI: −0.49–−0.20), and there was significant heterogeneity among the included studies (I2 = 68.6%; p < 0.01). Combined r value for the 6 studies on head and neck cancer was −0.31 (95% CI: −0.44–−0.19; I2 = 11.0%; p > 0.05) which displayed no heterogeneity. Combined r value for the subgroup of 5 studies on lymphoma and cervical cancer was −0.12 (95% CI: −0.34–0.11) and −0.48 (95% CI: −0.59–−0.37), respectively, without significant heterogeneity ((I2 = 51.6%; p > 0.05) and (I2 = 0.0%; p > 0.05)). Combined r value for the 5 studies on breast cancer was −0.24 (95% CI: −0.41–−0.08; I2 = 68.2%; p < 0.01).
Figure 5.
Forest plot of subgroup analysis based on cancer type.
Results for the subgroup analysis based on correlation coefficient value are shown in Figure 6. Eight studies in SUVmean/ADCmean resulted in r = −0.39 (95% CI: −0.54–−0.23), with I2 = 62.7% (p < 0.01). Pooled r for ten studies in SUVmax/ADCmin was −0.47 (95% CI: −0.59–−0.34), with I2 = 70.3% (p < 0.01). In SUVmax/ADCmean, sixteen studies provided r = −0.29 (95% CI: −0.43–−0.14) with I2 = 80.5% (p < 0.01).
Figure 6.
Forest plot of subgroup analysis based on correlation coefficient value.
4. Discussion
In the recent years, the correlation between ADC and SUV has been increasingly studied. In the present study, we investigated the relationship between 18F-FDG uptake and ADC value using meta-analysis methods. Our meta-analysis showed that, in cancer patients, there was an average negative correlation between the SUV and ADC. Subgroup analysis on different tumor types indicated that degrees of correlation among different tumor types varied and heterogeneity of some subgroups changed significantly. The subgroup analysis on various correlation coefficient values indicated that combined r values of subgroups did not show significant changes, and there were no significant changes in heterogeneity.
In this study, we used QUADAS-2 as an evidence-based quality assessment tool. In the present analysis, the vast majority of the articles did not mention whether the test results of DWI-MRI and 18F-FDG PET (or PET/CT) are interpreted blindly. In most studies, the time interval between 18F-FDG PET (or PET/CT) imaging and the acquirement of ADC was not clearly stated. In addition, some studies did not address the inclusion criteria of patients adequately. The above problems may increase the bias of study.
DWI provides an excellent tissue contrast through detection of differences in the Brownian motion of water molecules in tissues. ADC is a parameter calculated from DWI and altered by any architectural changes in the proportion of extracellular to intracellular water molecules because the diffusion of water molecules is disturbed by intracellular organelles and macromolecules [60]. Malignant tumors usually show decreased ADC values because they are characterized by increased cellularity, larger nuclear/cytoplasmic ratio, and less extracellular space relative to normal tissues which restrict the diffusion of water molecules [61]. Currently, 18F-FDG PET/CT has been considered as the standard of care in various cancers. 18F-FDG uptake is correlated with the number of viable tumor cells and their metabolic activity. Glucose utilization in tumors is increased due to the Warburg effect [62]. Recently, the introduction of simultaneous PET/MRI makes it possible to combine functional and metabolic studies in malignancies in one examination. It was postulated that there is a correlation between ADC and SUV. The present study showed that the pooled correlation coefficient between SUV and ADC was −0.35, indicating an average negative correlation. A possible explanation of this result might be that although there is a certain overlap of the information provided by 18F-FDG PET and DW-MRI, the two parameters (SUV and ADC) reflect different tumor biology. For example, except for cellularity, ADC is correlated directly with tumor necrosis because of increased presence of free water in the necrotic area [63]. However, 18F-FDG PET demonstrates tumor necrosis as photopenic defects. In addition, although the ADC measurement is derived from DWI which is an MR sequence that is known for a high detection rate of lesions, it is not always very specific [64]. Our result suggested that 18F-FDG PET and DWI-MRI might complement each other on the clinical diagnosis.
We conducted a subgroup analysis based on different tumor types. The meta-analysis about ADC and tumor cellularity correlation revealed no notable variation between the subgroups based on cancer type [65]. In this study, our results showed that the correlation between 18F-FDG SUV and ADC differed between histological types. Combined correlation coefficients range from −0.12 (lymphoma, n = 5) to −0.59 (pancreatic cancer, n = 2). Correlation was moderate in brain, cervix, and pancreas, average in lung, head and neck, breast, and rectum, and poor in lymphoma. However, this issue needs to be further explored with more experiments.
The present study has some potential limitations. First, although the number of patients included in this study was large, they were relatively limited to a certain type of tumors. This may cause limitations in our inference based on the results of subgroup analysis on different histological types. Second, our meta-analysis was based only on published studies which provided r values or raw data which can be used to calculate r values. Other articles which only report positive or negative results without specific data were excluded from this analysis. In addition, this study was restricted to articles published in English, which would cause publication bias. However, the results of Begg's test showed no evidence of publication bias. We also used the random-effects model to reduce heterogeneity. Therefore, the results of the present study are reliable.
In short, although there are limitations in this study, our meta-analysis demonstrated an average negative correlation between the SUV and ADC values in patients with cancer. Sufficient data support a moderate correlation for brain, cervix, and pancreas, average correction for lung, head and neck, breast, and rectum, and poor for lymphoma. However, a prospective study with a larger population is warranted to validate these findings in different cancer types.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81671775 and 81601522).
Disclosure
Shengming Deng and Zhifang Wu are co-first authors.
Competing Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Authors' Contributions
Shengming Deng and Zhifang Wu contributed equally to this article.
References
- 1.Wang X.-Y., Yang F., Jin C., Fu D.-L. Utility of PET/CT in diagnosis, staging, assessment of resectability and metabolic response of pancreatic cancer. World Journal of Gastroenterology. 2014;20(42):15580–15589. doi: 10.3748/wjg.v20.i42.15580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu S. L., Yang Z. Y., Zhou Z. R., Yu X. J., Ping B., Zhang Y. J. Role of SUV(max) obtained by 18F-FDG PET/CT in patients with a solitary pancreatic lesion: predicting malignant potential and proliferation. Nuclear Medicine Communications. 2013;34(6):533–539. doi: 10.1097/MNM.0b013e328360668a. [DOI] [PubMed] [Google Scholar]
- 3.Tomasi G., Rosso L. PET imaging: implications for the future of therapy monitoring with PET/CT in oncology. Current Opinion in Pharmacology. 2012;12(5):569–575. doi: 10.1016/j.coph.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 4.Le Bihan D., Breton E., Lallemand D., Grenier P., Cabanis E., Laval-Jeantet M. MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology. 1986;161(2):401–407. doi: 10.1148/radiology.161.2.3763909. [DOI] [PubMed] [Google Scholar]
- 5.Mosavi F., Wassberg C., Selling J., Molin D., Ahlström H. Whole-body diffusion-weighted MRI and 18F-FDG PET/CT can discriminate between different lymphoma subtypes. Clinical Radiology. 2015;70(11):1229–1236. doi: 10.1016/j.crad.2015.06.087. [DOI] [PubMed] [Google Scholar]
- 6.Wu X., Kellokumpu-Lehtinen P.-L., Pertovaara H., et al. Diffusion-weighted MRI in early chemotherapy response evaluation of patients with diffuse large B-cell lymphoma—a pilot study: comparison with 2-deoxy-2-fluoro-D-glucose-positron emission tomography/computed tomography. NMR in Biomedicine. 2011;24(10):1181–1190. doi: 10.1002/nbm.1689. [DOI] [PubMed] [Google Scholar]
- 7.Heusch P., Köhler J., Wittsack H.-J., et al. Hybrid [18F]-FDG PET/MRI including non-Gaussian diffusion-weighted imaging (DWI): preliminary results in non-small cell lung cancer (NSCLC) European Journal of Radiology. 2013;82(11):2055–2060. doi: 10.1016/j.ejrad.2013.05.027. [DOI] [PubMed] [Google Scholar]
- 8.Mori T., Nomori H., Ikeda K., et al. Diffusion-weighted magnetic resonance imaging for diagnosing malignant pulmonary nodules/masses: comparison with positron emission tomography. Journal of Thoracic Oncology. 2008;3(4):358–364. doi: 10.1097/jto.0b013e318168d9ed. [DOI] [PubMed] [Google Scholar]
- 9.Ho K.-C., Lin G., Wang J.-J., Lai C.-H., Chang C.-J., Yen T.-C. Correlation of apparent diffusion coefficients measured by 3T diffusion-weighted MRI and SUV from FDG PET/CT in primary cervical cancer. European Journal of Nuclear Medicine and Molecular Imaging. 2009;36(2):200–208. doi: 10.1007/s00259-008-0936-5. [DOI] [PubMed] [Google Scholar]
- 10.Palumbo B., Angotti F., Marano G. D. Relationship between PET-FDG and MRI apparent diffusion coefficients in brain tumors. Quarterly Journal of Nuclear Medicine and Molecular Imaging. 2009;53(1):17–22. [PubMed] [Google Scholar]
- 11.Aoyagi T., Shuto K., Okazumi S., et al. Evaluation of the clinical staging of esophageal cancer by using diffusion-weighted imaging. Experimental and Therapeutic Medicine. 2010;1(5):847–851. doi: 10.3892/etm.2010.112. [DOI] [Google Scholar]
- 12.Nakajo M., Kajiya Y., Kaneko T., et al. FDG PET/CT and diffusion-weighted imaging for breast cancer: prognostic value of maximum standardized uptake values and apparent diffusion coefficient values of the primary lesion. European Journal of Nuclear Medicine and Molecular Imaging. 2010;37(11):2011–2020. doi: 10.1007/s00259-010-1529-7. [DOI] [PubMed] [Google Scholar]
- 13.Punwani S., Prakash V., Bainbridge A., et al. Quantitative diffusion weighted MRI: a functional biomarker of nodal disease in Hodgkin lymphoma? Cancer Biomarkers. 2010;7(4):249–259. doi: 10.3233/cbm-2010-0197. [DOI] [PubMed] [Google Scholar]
- 14.Tanimoto K., Yoshikawa K., Obata T., et al. Role of glucose metabolism and cellularity for tumor malignancy evaluation using FDG-PET/CT and MRI. Nuclear Medicine Communications. 2010;31(6):604–609. doi: 10.1097/MNM.0b013e328339350c. [DOI] [PubMed] [Google Scholar]
- 15.Choi S. H., Paeng J. C., Sohn C.-H., et al. Correlation of 18F-FDG uptake with apparent diffusion coefficient ratio measured on standard and high b value diffusion MRI in head and neck cancer. Journal of Nuclear Medicine. 2011;52(7):1056–1062. doi: 10.2967/jnumed.111.089334. [DOI] [PubMed] [Google Scholar]
- 16.Fruehwald-Pallamar J., Czerny C., Mayerhoefer M. E., et al. Functional imaging in head and neck squamous cell carcinoma: correlation of PET/CT and diffusion-weighted imaging at 3 Tesla. European Journal of Nuclear Medicine and Molecular Imaging. 2011;38(6):1009–1019. doi: 10.1007/s00259-010-1718-4. [DOI] [PubMed] [Google Scholar]
- 17.Gu J., Khong P.-L., Wang S., Chan Q., Law W., Zhang J. Quantitative assessment of diffusion-weighted MR imaging in patients with primary rectal cancer: correlation with FDG-PET/CT. Molecular Imaging and Biology. 2011;13(5):1020–1028. doi: 10.1007/s11307-010-0433-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohba Y., Nomori H., Mori T., Shiraishi K., Namimoto T., Katahira K. Diffusion-weighted magnetic resonance for pulmonary nodules: 1.5 vs. 3 Tesla. Asian Cardiovascular and Thoracic Annals. 2011;19(2):108–114. doi: 10.1177/0218492310385152. [DOI] [PubMed] [Google Scholar]
- 19.Usuda K., Zhao X.-T., Sagawa M., et al. Diffusion-weighted imaging is superior to positron emission tomography in the detection and nodal assessment of lung cancers. Annals of Thoracic Surgery. 2011;91(6):1689–1695. doi: 10.1016/j.athoracsur.2011.02.037. [DOI] [PubMed] [Google Scholar]
- 20.Wu X., Korkola P., Pertovaara H., Eskola H., Järvenpää R., Kellokumpu-Lehtinen P.-L. No correlation between glucose metabolism and apparent diffusion coefficient in diffuse large B-cell lymphoma: a PET/CT and DW-MRI study. European Journal of Radiology. 2011;79(2):e117–e121. doi: 10.1016/j.ejrad.2011.04.062. [DOI] [PubMed] [Google Scholar]
- 21.Cafagna D., Rubini G., Iuele F., et al. Whole-body MR-DWIBS vs. [18F]-FDG-PET/CT in the study of malignant tumors: a retrospective study. Radiologia Medica. 2012;117(2):293–311. doi: 10.1007/s11547-011-0708-3. [DOI] [PubMed] [Google Scholar]
- 22.Choi B. B., Kim S. H., Kang B. J., et al. Diffusion-weighted imaging and FDG PET/CT: predicting the prognoses with apparent diffusion coefficient values and maximum standardized uptake values in patients with invasive ductal carcinoma. World Journal of Surgical Oncology. 2012;10, article 126 doi: 10.1186/1477-7819-10-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsushima N., Maeda M., Umino M., Suzawa N., Yamada T., Takeda K. Relation between FDG uptake and apparent diffusion coefficients in glioma and malignant lymphoma. Annals of Nuclear Medicine. 2012;26(3):262–271. doi: 10.1007/s12149-012-0570-y. [DOI] [PubMed] [Google Scholar]
- 24.Nakajo M., Nakajo M., Kajiya Y., et al. FDG PET/CT and diffusion-weighted imaging of head and neck squamous cell carcinoma: comparison of prognostic significance between primary tumor standardized uptake value and apparent diffusion coefficient. Clinical Nuclear Medicine. 2012;37(5):475–480. doi: 10.1097/rlu.0b013e318248524a. [DOI] [PubMed] [Google Scholar]
- 25.Nakamatsu S., Matsusue E., Miyoshi H., Kakite S., Kaminou T., Ogawa T. Correlation of apparent diffusion coefficients measured by diffusion-weighted MR imaging and standardized uptake values from FDG PET/CT in metastatic neck lymph nodes of head and neck squamous cell carcinomas. Clinical Imaging. 2012;36(2):90–97. doi: 10.1016/j.clinimag.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 26.Nakamura K., Joja I., Kodama J., Hongo A., Hiramatsu Y. Measurement of SUVmax plus ADCmin of the primary tumour is a predictor of prognosis in patients with cervical cancer. European Journal of Nuclear Medicine and Molecular Imaging. 2012;39(2):283–290. doi: 10.1007/s00259-011-1978-7. [DOI] [PubMed] [Google Scholar]
- 27.Regier M., Derlin T., Schwarz D., et al. Diffusion weighted MRI and 18F-FDG PET/CT in non-small cell lung cancer (NSCLC): does the apparent diffusion coefficient (ADC) correlate with tracer uptake (SUV)? European Journal of Radiology. 2012;81(10):2913–2918. doi: 10.1016/j.ejrad.2011.11.050. [DOI] [PubMed] [Google Scholar]
- 28.Ahn S. J., Park M.-S., Kim K. A., et al. 18F-FDG PET metabolic parameters and MRI perfusion and diffusion parameters in hepatocellular carcinoma: a preliminary study. PLoS ONE. 2013;8(8) doi: 10.1371/journal.pone.0071571.e71571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Byun B. H., Noh W. C., Lim I., et al. A new method for apparent diffusion coefficient measurement using sequential 18F-FDG PET and MRI: correlation with histological grade of invasive ductal carcinoma of the breast. Annals of Nuclear Medicine. 2013;27(8):720–728. doi: 10.1007/s12149-013-0737-1. [DOI] [PubMed] [Google Scholar]
- 30.Gong N.-J., Wong C.-S., Chu Y.-C., Guo H., Huang B., Chan Q. Increasing the accuracy of volume and ADC delineation for heterogeneous tumor on diffusion-weighted MRI: correlation with PET/CT. International Journal of Radiation Oncology, Biology, Physics. 2013;87(2):407–413. doi: 10.1016/j.ijrobp.2013.05.026. [DOI] [PubMed] [Google Scholar]
- 31.Nakamura K., Joja I., Fukushima C., et al. The preoperative SUVmax is superior to ADCmin of the primary tumour as a predictor of disease recurrence and survival in patients with endometrial cancer. European Journal of Nuclear Medicine and Molecular Imaging. 2013;40(1):52–60. doi: 10.1007/s00259-012-2240-7. [DOI] [PubMed] [Google Scholar]
- 32.Rakheja R., Chandarana H., DeMello L., et al. Correlation between standardized uptake value and apparent diffusion coefficient of neoplastic lesions evaluated with whole-body simultaneous hybrid PET/MRI. American Journal of Roentgenology. 2013;201(5):1115–1119. doi: 10.2214/AJR.13.11304. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt H., Brendle C., Schraml C., et al. Correlation of simultaneously acquired diffusion-weighted imaging and 2-deoxy-[18F] fluoro-2-D-glucose positron emission tomography of pulmonary lesions in a dedicated whole-body magnetic resonance/positron emission tomography system. Investigative Radiology. 2013;48(5):247–255. doi: 10.1097/rli.0b013e31828d56a1. [DOI] [PubMed] [Google Scholar]
- 34.Tsuchida T., Morikawa M., Demura Y., Umeda Y., Okazawa H., Kimura H. Imaging the early response to chemotherapy in advanced lung cancer with diffusion-weighted magnetic resonance imaging compared to fluorine-18 fluorodeoxyglucose positron emission tomography and computed tomography. Journal of Magnetic Resonance Imaging. 2013;38(1):80–88. doi: 10.1002/jmri.23959. [DOI] [PubMed] [Google Scholar]
- 35.Varoquaux A., Rager O., Lovblad K.-O., et al. Functional imaging of head and neck squamous cell carcinoma with diffusion-weighted MRI and FDG PET/CT: quantitative analysis of ADC and SUV. European Journal of Nuclear Medicine and Molecular Imaging. 2013;40(6):842–852. doi: 10.1007/s00259-013-2351-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baba S., Isoda T., Maruoka Y., et al. Diagnostic and prognostic value of pretreatment SUV in 18F-FDG/PET in breast cancer: comparison with apparent diffusion coefficient from diffusion-weighted MR imaging. Journal of Nuclear Medicine. 2014;55(5):736–742. doi: 10.2967/jnumed.113.129395. [DOI] [PubMed] [Google Scholar]
- 37.de Jong A., Kwee T. C., de Klerk J. M., et al. Relationship between pretreatment FDG-PET and diffusion-weighted MRI biomarkers in diffuse large B-cell lymphoma. American Journal of Nuclear Medicine and Molecular Imaging. 2014;4(3):231–238. [PMC free article] [PubMed] [Google Scholar]
- 38.Er H. Ç., Erden A., Küçük N. Ö., Geçim E. Correlation of minimum apparent diffusion coefficient with maximum standardized uptake on fluorodeoxyglucose PET-CT in patients with rectal adenocarcinoma. Diagnostic and Interventional Radiology. 2014;20(2):105–109. doi: 10.5152/dir.2013.13275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giganti F., De Cobelli F., Canevari C., et al. Response to chemotherapy in gastric adenocarcinoma with diffusion-weighted MRI and 18F-FDG-PET/CT: correlation of apparent diffusion coefficient and partial volume corrected standardized uptake value with histological tumor regression grade. Journal of Magnetic Resonance Imaging. 2014;40(5):1147–1157. doi: 10.1002/jmri.24464. [DOI] [PubMed] [Google Scholar]
- 40.Grueneisen J., Beiderwellen K., Heusch P., et al. Correlation of standardized uptake value and apparent diffusion coefficient in integrated whole-body PET/MRI of primary and recurrent cervical cancer. PLoS ONE. 2014;9(5) doi: 10.1371/journal.pone.0096751.e96751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iizuka Y., Matsuo Y., Umeoka S., et al. Prediction of clinical outcome after stereotactic body radiotherapy for non-small cell lung cancer using diffusion-weighted MRI and 18F-FDG PET. European Journal of Radiology. 2014;83(11):2087–2092. doi: 10.1016/j.ejrad.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 42.Sakane M., Tatsumi M., Kim T., et al. Correlation between apparent diffusion coefficients on diffusion-weighted MRI and standardized uptake value on FDG-PET/CT in pancreatic adenocarcinoma. Acta Radiologica. 2015;56(9):1034–1041. doi: 10.1177/0284185114549825. [DOI] [PubMed] [Google Scholar]
- 43.Schwenzer N. F., Schmidt H., Gatidis S., et al. Measurement of apparent diffusion coefficient with simultaneous MR/positron emission tomography in patients with peritoneal carcinomatosis: comparison with 18F-FDG-PET. Journal of Magnetic Resonance Imaging. 2014;40(5):1121–1128. doi: 10.1002/jmri.24497. [DOI] [PubMed] [Google Scholar]
- 44.Sun H., Xin J., Zhang S., et al. Anatomical and functional volume concordance between FDG PET, and T2 and diffusion-weighted MRI for cervical cancer: a hybrid PET/MR study. European Journal of Nuclear Medicine and Molecular Imaging. 2014;41(5):898–905. doi: 10.1007/s00259-013-2668-4. [DOI] [PubMed] [Google Scholar]
- 45.Yu X., Lee E. Y. P., Lai V., Chan Q. Correlation between tissue metabolism and cellularity assessed by standardized uptake value and apparent diffusion coefficient in peritoneal metastasis. Journal of Magnetic Resonance Imaging. 2014;40(1):99–105. doi: 10.1002/jmri.24361. [DOI] [PubMed] [Google Scholar]
- 46.Zhang J., Cui L.-B., Tang X., et al. DW MRI at 3.0 T versus FDG PET/CT for detection of malignant pulmonary tumors. International Journal of Cancer. 2014;134(3):606–611. doi: 10.1002/ijc.28394. [DOI] [PubMed] [Google Scholar]
- 47.Zukotynski K., Fahey F., Kocak M., et al. 18F-FDG PET and MR imaging associations across a spectrum of pediatric brain tumors: a report from the pediatric brain tumor consortium. Journal of Nuclear Medicine. 2014;55(9):1473–1480. doi: 10.2967/jnumed.114.139626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brandmaier P., Purz S., Bremicker K., et al. Simultaneous [18F]FDG-PET/MRI: correlation of apparent diffusion coefficient (ADC) and standardized uptake value (SUV) in primary and recurrent cervical cancer. PLoS ONE. 2015;10(11) doi: 10.1371/journal.pone.0141684.e0141684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Covello M., Cavaliere C., Aiello M., et al. Simultaneous PET/MR head-neck cancer imaging: preliminary clinical experience and multiparametric evaluation. European Journal of Radiology. 2015;84(7):1269–1276. doi: 10.1016/j.ejrad.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 50.Han M., Kim S. Y. O., Lee S. J. I., Choi J. W. O. The correlations between mri perfusion, diffusion parameters, and 18F-FDG PET metabolic parameters in primary head-and-neck cancer: a cross-sectional analysis in single institute. Medicine. 2015;94(47) doi: 10.1097/md.0000000000002141.e2141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heacock L., Weissbrot J., Raad R., et al. PET/MRI for the evaluation of patients with Lymphoma: initial observations. American Journal of Roentgenology. 2015;204(4):842–848. doi: 10.2214/ajr.14.13181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Karan B., Pourbagher A., Torun N. Diffusion-weighted imaging and 18F-fluorodeoxyglucose positron emission tomography/computed tomography in breast cancer: correlation of the apparent diffusion coefficient and maximum standardized uptake values with prognostic factors. Journal of Magnetic Resonance Imaging. 2016;43(6):1434–1444. doi: 10.1002/jmri.25112. [DOI] [PubMed] [Google Scholar]
- 53.Littooij A. S., Kwee T. C., de Keizer B., et al. Whole-body MRI-DWI for assessment of residual disease after completion of therapy in lymphoma: a prospective multicenter study. Journal of Magnetic Resonance Imaging. 2015;42(6):1646–1655. doi: 10.1002/jmri.24938. [DOI] [PubMed] [Google Scholar]
- 54.Liu L.-P., Zhang X.-X., Cui L.-B., et al. Preliminary comparison of diffusion-weighted MRI and PET/CT in predicting histological type and malignancy of lung cancer. The Clinical Respiratory Journal. 2015 doi: 10.1111/crj.12316. [DOI] [PubMed] [Google Scholar]
- 55.Metz S., Ganter C., Lorenzen S., et al. Multiparametric MR and PET imaging of intratumoral biological heterogeneity in patients with metastatic lung cancer using voxel-by-voxel analysis. PLoS ONE. 2015;10(7) doi: 10.1371/journal.pone.0132386.e0132386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schaarschmidt B. M., Buchbender C., Nensa F., et al. Correlation of the apparent diffusion coefficient (ADC) with the standardized uptake value (SUV) in lymph node metastases of non-small cell lung cancer (NSCLC) patients using hybrid 18F-FDG PET/MRI. PLoS ONE. 2015;10(1) doi: 10.1371/journal.pone.0116277.e0116277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rupinski M. T., Dunlap W. P. Approximating Pearson product-moment correlations from Kendall's tau and Spearman's rho. Educational and Psychological Measurement. 1996;56(3):419–429. doi: 10.1177/0013164496056003004. [DOI] [Google Scholar]
- 58.Whiting P. F., Rutjes A. W. S., Westwood M. E., et al. Quadas-2: a revised tool for the quality assessment of diagnostic accuracy studies. Annals of Internal Medicine. 2011;155(8):529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 59.Landis J. R., Koch G. G. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. doi: 10.2307/2529310. [DOI] [PubMed] [Google Scholar]
- 60.Ohba Y., Nomori H., Mori T., et al. Is diffusion-weighted magnetic resonance imaging superior to positron emission tomography with fludeoxyglucose F 18 in imaging non-small cell lung cancer? Journal of Thoracic and Cardiovascular Surgery. 2009;138(2):439–445. doi: 10.1016/j.jtcvs.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 61.Murakami R., Sugahara T., Nakamura H., et al. Malignant supratentorial astrocytoma treated with postoperative radiation therapy: prognostic value of pretreatment quantitative diffusion-weighted MR imaging. Radiology. 2007;243(2):493–499. doi: 10.1148/radiol.2432060450. [DOI] [PubMed] [Google Scholar]
- 62.Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 63.Bajpai J., Gamnagatti S., Kumar R., et al. Role of MRI in osteosarcoma for evaluation and prediction of chemotherapy response: correlation with histological necrosis. Pediatric Radiology. 2011;41(4):441–450. doi: 10.1007/s00247-010-1876-3. [DOI] [PubMed] [Google Scholar]
- 64.Lövblad K.-O., Laubach H.-J., Baird A. E., et al. Clinical experience with diffusion-weighted MR in patients with acute stroke. American Journal of Neuroradiology. 1998;19(6):1061–1066. [PMC free article] [PubMed] [Google Scholar]
- 65.Chen L., Liu M., Bao J., et al. The correlation between apparent diffusion coefficient and tumor cellularity in patients: a meta-analysis. PLoS ONE. 2013;8(11) doi: 10.1371/journal.pone.0079008.e79008 [DOI] [PMC free article] [PubMed] [Google Scholar]