Abstract
Background and purpose
Early neurologic deterioration (END) during the acute stage of stroke is clinically important because of its association with poor outcomes. The purpose of this study was (1) to investigate variables associated with END, (2) to determine the distribution of atherosclerotic stenosis associated with END, and (3) to clarify the relationship between END and clinical outcomes.
Methods
516 patients with acute ischemic stroke were included. The median follow-up period was 31.7 months. END was defined as a ≥2 point increase in the National Institutes of Health Stroke Scale (NIHSS), ≥1 point increase in level of consciousness or motor item of the NIHSS, or the development of any new neurological deficits during the first 72 hours of hospitalization. A signal loss on 1.5-T magnetic resonance angiography exceeding 50% was considered to be significant for the categorization of stenosis pattern.
Results
The prevalence of END was 19.0%. END was associated with intracranial atherosclerotic stenosis (IAS) together with large artery atherosclerosis (LAA) subtype. In particular, stenosis of basilar artery or posterior cerebral artery was independently associated with END. Lesion growth or hypoperfusion was more accountable for END in patients with IAS, whereas intracerebral hemorrhage or edema/herniation was more frequently observed in END patients without IAS. Patients with END had a higher rate of mortality, but a similar rate of further vascular events compared to patients without END.
Conclusion
Pre-stroke IAS and LAA subtype could determine the development of END during the acute stage of ischemic stroke.
Introduction
Neurologic worsening during the early period of stroke, which is referred to as early neurologic deterioration (END), is clinically crucial because it is frequently encountered in real-world stroke practice and is associated with poor clinical outcomes [1, 2]. Many studies have investigated the factors associated with END. Reported factors include advanced age [3], initial stroke severity [1–3], high blood glucose values or history of diabetes mellitus [1, 3, 4], blood pressure variability [5], occlusion of internal carotid (ICA) or middle cerebral artery (MCA) [6, 7], symptomatic steno-occlusive arterial disease [8], and ICA territory infarct [9]. However, only one study has examined the distribution of atherosclerotic stenosis (intracranial versus extracranial); the study included only symptomatic stenotic lesion [8].
Intracranial atherosclerotic stenosis (IAS) is important in stroke management, especially in Asian countries where IAS comprises 30–50% of strokes [10]. Patients with IAS have higher recurrence rates of stroke and death than those without [11]. Considering its anatomical location, intracranial collateral channels may be more limited with IAS compared to extracranial atherosclerotic stenosis (EAS), leading to stagnated flow in relevant arterial territories and thus resulting in decreased washout of emboli [12, 13].
Accordingly, we hypothesized that IAS interrupts early recovery of perfusion into the peri-infarct area during the acute period of stroke, which causes END. This study explored the hypothesis by investigating clinical variables associated with END, determining if the distribution of atherosclerotic stenosis is associated with END, and clarifying the relationship between END and long-term clinical outcomes (functional outcome, long-term survival, and further vascular events).
Methods
Ethics statement
The Institutional Review Board of Sejong General Hospital approved this study with informed consent waived.
Patients
A prospective stroke registry database was used for the retrospective investigation of 661 acute ischemic stroke patients who were consecutively admitted to Sejong General Hospital within 7 days after symptom onset between January 2011 and September 2016. Patients were excluded if they presented > 48 hours after being last seen as normal (n = 124) or had no data of brain vessel or 6-month outcome (n = 21). Finally, 516 patients were included for the analysis.
All survivors were principally followed-up by outpatient clinic attendance. However, 104 patients were not followed-up by our clinic at the time of this study. Of these, the conditions of 81 patients were ascertained by telephone contact with the patients or their relatives. The other 23 patients were censored at the last clinic visit. Modified Rankin scale (mRS) score at 6 months was determined using a structured interview for accurate grading according to the Korean Clinical Research Center for Stroke [14].
We monitored mortality and major vascular events (stroke, acute coronary syndrome, or peripheral artery occlusion) after index stroke. The nature of the vascular event was principally based on medical records from the treating physician at Sejong General Hospital. In the absence of records, medical information was acquired from treating physicians at other institutions. Uncertain information was excluded from the study.
Definitions
Ischemic stroke was defined as a focal neurologic deficit of an abrupt onset lasting > 24 hours with evidence of new infarct lesions on brain imaging. All the strokes were classified as large artery atherosclerosis (LAA), cardioembolism, lacune, two or more mechanisms, cryptogenic stroke, and other causes according to the Trial of Org 10172 in Acute Stroke Treatment subtype classification system [15]. Branch atheromatous disease (BAD) was defined as an elongated infarct lesion extending from the origin of penetrating artery territory with any discernible stenosis of relevant parent artery (mainly M1 or basilar artery), and was considered as LAA [16]. Metabolic syndrome was defined as the presence of any 3 of 5 risk factors: elevated waist circumference (≥90 cm in men, ≥80 cm in women), triglycerides ≥150 mg/dL, reduced high-density lipoprotein cholesterol (<40 mg/dL in men, <50 mg/dL in women), elevated blood pressure (systolic ≥130 and/or diastolic ≥85 mmHg), and fasting glucose ≥100 mg/dL, with drug treatment for each risk factor being an alternate indicator [17]. END was defined as an increase of 2 points or more in the National Institutes of Health Stroke Scale (NIHSS) score, an increase of 1 point or more on the level of consciousness or motor items of the NIHSS, or the development of any new neurological deficits during the first 72 hour of hospitalization [5, 6]. The causes of END were classified as new non-border zone lesion separate in space from a previous lesion (new lesion), lesion growth, lesion progression in border zone (hypoperfusion), intracerebral hemorrhage (ICH), brain edema or herniation, medical condition (infection, cardiac disease, etc), seizure, and undetermined.
Risk factor assessment and brain imaging
Clinical information included age, gender, history of hypertension, diabetes mellitus and hyperlipidemia (defined as a total cholesterol level > 200 mg/dL or a low-density lipoprotein cholesterol > 130 mg/dL at the time of presentation or a history of treatment), current cigarette smoking, previous history of stroke and ischemic heart disease (defined as a known history or clinical demonstration of myocardial infarction or angina pectoris), atrial fibrillation, valvular heart disease, heavy alcohol consumption (>26 Soju drinks/month; about 20% alcohol), congestive heart failure (ejection fraction <40% on admission echocardiography with a previous episode of symptomatic heart failure characterized by dyspnea, cardiomegaly, and pulmonary edema), medication use (anthrombotics and statin) for ≥ 3 months at stroke onset, and NIHSS score at admission. All 516 patients underwent routine 12-lead electrocardiography, echocardiography, and 24-hour Holter monitoring. All patients underwent 1.5-T magnetic resonance imaging (MRI) on admission. The MRI consisted of the diffusion-weighted image, gradient echo image, fluid-attenuated inversion recovery image, three-dimensional time-of-flight intracranial MR angiography (MRA), and contrast-enhanced MRA including extracranial carotid and vertebral arteries. More than 50% signal loss on MRA was considered significant to the categorization of a stenosis pattern. Complete occlusion relevant to the infarct area was not counted as an atherosclerotic steno-occlusion if the patient had a high-risk cardioembolic source, and was thus classified as cardioembolism. Stenoses of brain vessels on MRA were classified as IAS or EAS, based on the location of the arterial stenosis, which included intracranial [intracranial ICA, MCA, anterior cerebral artery (ACA), intracranial vertebral artery (VA), basilar artery (BA), posterior cerebral artery (PCA)] and extracranial (extracranial ICA and VA).
Data analyses
Statistical analyses were performed with SPSS software, version 18.0 (SPSS Inc., Chicago, IL). The independent t-test or Chi-square test was used to compare the difference between patients with and without END or IAS. Logistic regression analysis was performed to analyze variables associated with END and 6-month mRS ≥ 3. Kaplan-Meier survival curves were plotted for death or further major vascular events in patients with and without END. Differences in the outcomes were evaluated using the log-rank test. A Cox proportional hazards model was used to analyze variables associated with risk of mortality. Variables with P <0.1 in the univariate analysis were used in multivariate analysis. Unadjusted and adjusted odds ratios (ORs), hazards ratios, and 95% confidence intervals (CIs) were obtained. P-values < 0.05 were considered to be statistically significant.
Results
The mean age at admission of the 516 patients (270 men and 246 women) was 67.5 years (range 18–94). Of these patients, 98 (19.0%) had an END. Table 1 presents the comparison data of patients with and without END. Patients with END were more often older, women, hypertensive, and had a higher level of blood glucose (initial and fasting), higher leukocyte count, and higher NIHSS score at admission compared to patients without END. In addition, they were more likely to have atherosclerotic stenosis and a poor functional outcome (6-month mRS ≥3). Furthermore, the association of END with poor functional outcome was significant in unadjusted and adjusted logistic regression analyses (S1 Table). There was a significant difference in the proportion of stroke subtypes between patients with and without END, with 39.8% of patients with END versus 18.4% of patients without END having LAA. In particular, BAD was closely associated with END (13 of 21 patients (61.9%) with BAD versus 85 of 495 patients (17.2%) without, P < 0.001).
Table 1. Basic characteristics of patients with and without early neurologic deterioration (END): n (%) or mean±SD.
| END (+) | END (-) | P | |
|---|---|---|---|
| N = 98 | N = 418 | ||
| Age ≥65 years | 72 (73.5) | 248 (59.3) | 0.009 |
| Women | 58 (59.2) | 188 (45.0) | 0.011 |
| Hypertension | 74 (75.5) | 272 (65.1) | 0.048 |
| Diabetes | 37 (37.8) | 120 (28.7) | 0.080 |
| Hyperlipidemia | 49 (50.0) | 216 (51.7) | 0.765 |
| Current smoking | 22 (22.4) | 116 (27.8) | 0.286 |
| Previous stroke | 18 (18.4) | 68 (16.3) | 0.616 |
| Ischemic heart disease | 22 (22.4) | 91 (21.8) | 0.884 |
| Atrial fibrillation | 31 (31.6) | 153 (36.6) | 0.355 |
| Valvular heat disease | 17 (17.3) | 91 (21.8) | 0.333 |
| Heavy alcohol consumption | 11 (11.2) | 76 (18.2) | 0.098 |
| Congestive heart failure | 15 (15.3) | 54 (12.9) | 0.532 |
| Previous medication | |||
| Antiplatelet | 44 (44.9) | 161 (38.5) | 0.245 |
| Anticoagulant | 12 (12.2) | 67 (16.0) | 0.349 |
| Statin | 29 (29.6) | 109 (26.1) | 0.479 |
| Initial systolic blood pressure (mmHg) | 140.1±28.8 | 136.3±25.8 | 0.197 |
| Initial diastolic blood pressure (mmHg) | 78.5±16.9 | 77.4±15.4 | 0.529 |
| Metabolic syndrome | 53 (54.1) | 190 (45.5) | 0.124 |
| Waist circumference (cm) | 84.5±10.1 | 84.7±9.9 | 0.878 |
| Triglycerides (mg/dL) | 101.9±49.6 | 118.8±85.9 | 0.065 |
| HDL-C (mg/dL) | 48.2±15.5 | 47.7±14.5 | 0.751 |
| Blood sugar, fasting (mg/dL) | 130.1±40.9 | 116.3±40.5 | 0.003 |
| Total cholesterol (mg/dL) | 162.6±43.2 | 167.7±42.6 | 0.292 |
| LDL-C (mg/dL) | 102.3±37.3 | 106.5±38.3 | 0.331 |
| Leukocyte count (x109/L) | 9.1±3.7 | 8.1±3.3 | 0.016 |
| Hemoglobin (g/dL) | 13.0±2.2 | 13.5±2.4 | 0.056 |
| Platelet count (x109/L) | 214.3±71.4 | 213.7±70.0 | 0.698 |
| Blood glucose at admission (mg/dL) | 156.0±66.9 | 140.8±66.6 | 0.043 |
| Initial NIHSS | 11.8±10.0 | 5.7±7.1 | <0.001 |
| Atherosclerotic stenosis | 69 (70.4) | 191 (45.7) | <0.001 |
| Stroke classification | <0.001 | ||
| Large artery atherosclerosis | 39 (39.8) | 77 (18.4) | <0.001 |
| Cardioembolism | 29 (29.6) | 170 (40.7) | 0.043 |
| Lacune | 9 (9.2) | 71 (17.0) | 0.055 |
| Two or more | 18 (18.4) | 64 (15.3) | 0.456 |
| Cryptogenic | 1 (1.0) | 33 (7.9) | 0.014 |
| Other causes | 2 (2.0) | 3 (0.7) | 0.229 |
| Reperfusion therapy (use of tPA or thrombectomy) | 5 (5.1) | 27 (6.5) | 0.616 |
| Poor functional outcome | 73 (74.5) | 105 (25.1) | <0.001 |
HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; NIHSS, National Institutes of Health Stroke Scale; tPA, tissue plasminogen activator
P was calculated by Chi-square test or independent t-test. Poor functional outcome indicates scores ≥ 3 on 6-month modified Rankin scale
In univariate logistic regression, LAA (versus all the other stroke subtypes) was significantly associated with END (P < 0.001, OR 2.927, 95% CI 1.822–4.703). The association remained significant in adjusted analysis (Table 2). In addition, IAS and NIHSS score were significant variables associated with END in the adjusted analyses even where variable for stroke subtype was changed.
Table 2. Multiple logistic regression analysis for early neurologic deterioration.
| OR (95% CI) | P | OR (95% CI) | P | |
|---|---|---|---|---|
| Age ≥ 65 years | 1.205 (0.682–2.130) | 0.521 | 1.197 (0.677–2.116) | 0.537 |
| Female | 1.170 (0.684–2.000) | 0.567 | 1.196 (0.700–2.044) | 0.512 |
| Hypertension | 1.388 (0.786–2.451) | 0.259 | 1.281 (0.726–2.262) | 0.393 |
| Heavy alcohol consumption | 0.838 (0.385–1.823) | 0.656 | 0.743 (0.341–1.620) | 0.456 |
| Blood glucose at admission (mg/dL) | 1.001 (0.997–1.004) | 0.673 | 1.001 (0.997–1.004) | 0.598 |
| Hemoglobin (g/dL) | 0.988 (0.886–1.102) | 0.828 | 0.997 (0.894–1.112) | 0.957 |
| Leukocyte count (x109/L) | 1.048 (0.982–1.118) | 0.162 | 1.043 (0.977–1.114) | 0.209 |
| Initial NIHSS score | 1.080 (1.051–1.111) | <0.001 | 1.084 (1.052–1.116) | <0.001 |
| Intracranial atherosclerotic stenosis | 1.872 (1.037–3.381) | 0.037 | 2.137 (1.202–3.799) | 0.010 |
| Large artery atherosclerosis† | 2.227 (1.229–4.035) | 0.008 | ||
| Cardioembolism† | 0.574 (0.302–1.092) | 0.091 |
OR, odds ratio; CI, confidence interval; NIHSS, National Institutes of Health Stroke Scale
†stroke subtype versus all other subtypes
The distribution of atherosclerotic stenosis was different between patients with and without END. Patients with END were more likely to have IAS, and more often had stenotic lesions in ACA, intracranial VA, BA, PCA and extracranial ICA compared with patients without END (Table 3).
Table 3. Distribution of atherosclerotic stenosis according to the presence of early neurologic deterioration (END): n (%).
| END (+) | END (-) | P | |
|---|---|---|---|
| N = 98 | N = 418 | ||
| Distribution of stenosis | <0.001 | ||
| Intracranial and extracranial | 23 (23.5) | 55 (13.2) | |
| Intracranial only | 41 (41.8) | 102 (24.4) | |
| Extracranial only | 5 (5.1) | 34 (8.1) | |
| No stenosis | 29 (29.6) | 227 (54.3) | |
| EAS | 28 (28.6) | 89 (21.3) | 0.121 |
| Extracranial ICA | 18 (18.4) | 46 (11.0) | 0.047 |
| Extracranial VA | 17 (17.3) | 62 (14.8) | 0.534 |
| IAS | 64 (65.3) | 157 (37.6) | <0.001 |
| Intracranial ICA | 19 (19.4) | 63 (15.1) | 0.293 |
| MCA | 31 (31.6) | 95 (22.7) | 0.065 |
| ACA | 21 (21.4) | 47 (11.2) | 0.007 |
| Intracranial VA | 21 (21.4) | 42 (10.0) | 0.002 |
| BA | 19 (19.4) | 28 (6.7) | <0.001 |
| PCA | 37 (37.8) | 72 (17.2) | <0.001 |
EAS, extracranial atherosclerotic stenosis; IAS, intracranial atherosclerotic stenosis; ICA, internal carotid artery; VA, vertebral artery; MCA, middle cerebral artery; ACA, anterior cerebral artery; BA, basilar artery; PCA, posterior cerebral artery
P calculated by Chi-square test
We investigated the locations of stenosis associated with END in a multivariate logistic analysis (Table 4). Stenotic lesion in BA or PCA was independently associated with END.
Table 4. Multivariate logistic regression analysis to determine the location of stenosis associated with early neurologic deterioration†.
| Location of stenosis | OR (95% CI) | P |
|---|---|---|
| Extracranial ICA | 1.412 (0.709–2.812) | 0.327 |
| Intracranial ICA | 0.725 (0.380–1.382) | 0.328 |
| MCA | 0.894 (0.503–1.590) | 0.703 |
| ACA | 1.254 (0.657–2.391) | 0.493 |
| Intracranial VA | 1.546 (0.803–2.976) | 0.192 |
| BA | 2.343 (1.152–4.764) | 0.019 |
| PCA | 2.267 (1.327–3.872) | 0.003 |
ICA, internal carotid artery; MCA, middle cerebral artery; ACA, anterior cerebral artery; VA, vertebral artery; BA, basilar artery; PCA, posterior cerebral artery
†age group (≥ 65 years), gender, hypertension, heavy alcohol consumption, blood glucose at admission, hemoglobin, leukocyte count, initial score of the National institutes of Health Stroke Scale, and stroke subtype (cardioembolism versus other subtypes) were adjusted
The most frequent cause of END was lesion growth (32.7%), followed by edema/herniation (24.5%), new lesion (12.2%), hypoperfusion (9.2%), ICH (7.1%), undetermined (6.1%), medical condition (5.1%), and seizure (3.1%). There was a significant difference of the proportion of the cause of END between patients with and without IAS (Table 5).
Table 5. Comparison of the causes of early neurologic deterioration between patients with and without intracranial atherosclerotic stenosis (IAS): n (%).
| Total | IAS (+) | IAS (-) | P = 0.028 | |
|---|---|---|---|---|
| N = 98 | N = 64 | N = 34 | ||
| New lesion | 12 (12.2) | 7 (10.9) | 5 (14.7) | |
| Lesion growth | 32 (32.7) | 23 (35.9) | 9 (26.5) | |
| Hypoperfusion | 9 (9.2) | 9 (14.1) | 0 (0) | |
| Intracerebral hemorrhage | 7 (7.1) | 2 (3.1) | 5 (14.7) | |
| Edema/hernation | 24 (24.5) | 13 (20.3) | 11 (32.4) | |
| Medical condition | 5 (5.1) | 2 (3.1) | 3 (8.8) | |
| Seizure | 3 (3.1) | 2 (3.1) | 1 (2.9) | |
| Undetermined | 6 (6.1) | 6 (9.4) | 0 (0) |
P calculated by Chi-square test
Among 98 patients with END, lesion growth or hypoperfusion was more frequently observed in those with IAS than in those without IAS (50% versus 26.5%, P = 0.025). In contrast, ICH or edema/herniation was more frequent in patient without IAS than with IAS (47.1% versus 23.4%, P = 0.017). The patients without IAS were more likely than patients with IAS to have a cardioembolic stroke type (58.8% versus 14.1%, P < 0.001) which had a higher initial score of NIHSS compared with non-cardioembolic subtypes among the 98 patients (19.0±10.4 versus 8.8±8.2, P < 0.001).
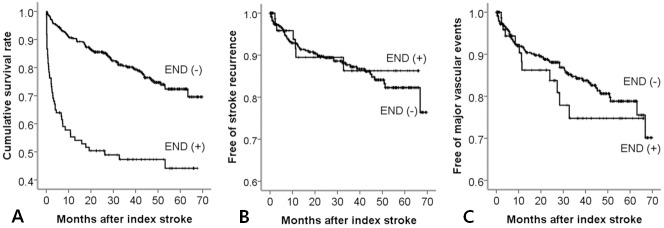
The median follow-up period was 31.7 months (mean 31.6; range 0.1–69.8). Long-term clinical outcomes (mortality, recurrence of stroke and occurrence of major vascular events) were compared between patients with and without END (Fig 1). Patients with END had a significantly higher rate of mortality (P < 0.001), but there was no significant difference in the incidence of stroke recurrence and major vascular events between the two groups. When Cox proportional hazards analysis was performed to determine variables associated with mortality, END was associated with a high risk of mortality in adjusted analysis (Table 6).
Fig 1. Kaplan-Meier curves for mortality, stroke recurrence and major vascular events.
The patients with early neurologic deterioration had a higher rate of mortality (A: P < 0.001 by log-rank test), but there was no significant difference in the rate of stroke recurrence (B: P = 0.789 by log-rank test) and major vascular events (C: P = 0.411 by log-rank test) between patients with and without early neurologic deterioration.
Table 6. Cox proportional hazards models for mortality.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Age ≥65 years | 2.874 (1.872–4.412) | <0.001 | 2.432 (1.384–4.271) | 0.002 |
| Female | 1.780 (1.260–2.514) | 0.001 | 0.863 (0.539–1.383) | 0.541 |
| Hypertension | 1.097 (0.763–1.577) | 0.617 | ||
| Diabetes | 1.000 (0.691–1.448) | 1.000 | ||
| Hyperlipidemia | 0.794 (0.566–1.115) | 0.183 | ||
| Previous stroke | 1.271 (0.828–1.950) | 0.273 | ||
| Ischemic heart disease | 1.337 (0.906–1.974) | 0.144 | ||
| Congestive heart failure | 2.329 (1.550–3.499) | <0.001 | 1.251 (0.768–2.037) | 0.368 |
| Valvular heart disease | 1.113 (0.741–1.671) | 0.606 | ||
| Atrial fibrillation | 1.506 (1.071–2.118) | 0.019 | 0.794 (0.482–1.308) | 0.365 |
| Current smoking | 0.406 (0.253–0.654) | <0.001 | 0.615 (0.304–1.242) | 0.175 |
| Heavy alcohol consumption | 0.300 (0.152–0.590) | <0.001 | 0.506 (0.174–1.466) | 0.209 |
| Initial NIHSS score | 1.094 (1.077–1.112) | <0.001 | 1.076 (1.053–1.099) | <0.001 |
| Intracranial atherosclerotic stenosis | 1.952 (1.387–2.746) | <0.001 | 1.005 (0.595–1.697) | 0.984 |
| Stroke subtype (cardioembolism) | 1.189 (0.844–1.676) | 0.321 | ||
| Early neurologic deterioration | 3.599 (2.529–5.123) | <0.001 | 3.314 (2.041–5.383) | <0.001 |
HR, Hazard ratio; CI, confidence interval; NIHSS, National institutes of Health Stroke Scale
Discussion
There are four key findings. Patients with LAA displayed a higher rate of END compared with patients with the other stroke subtypes. The intracranial distribution of atherosclerotic stenosis also had the same impact as LAA and, even without regard to the stroke symptoms, was associated with END. Of the atherosclerotic stenoses, stenotic lesion in BA or PCA was independently associated with END even after adjusting covariates. Finally, among the causes of END, lesion growth or hypoperfusion was associated with IAS.
Although LAA stroke subtype, particularly BAD, has been associated with END [4, 6, 7, 18, 19], the present data describe for the first time that the distribution of atherosclerotic stenosis (IAS) is related to END. This could be partially explained by the fact that IAS had a significant relationship to a higher rate of atherosclerotic vascular risk factors in our cohort (S2 Table) and in other studies [11, 20]. Advanced age and other risk factors may have contribution to thrombotic progression leading to END in patients with IAS. In addition, when we analyzed patients who had only IAS or EAS (i.e., excluding patients having IAS and EAS simultaneously or no stenosis), patients with IAS had more arterial stenotic lesions in brain vessels than patients with EAS only (2.7±1.8 versus 1.6±0.9, P < 0.001). Furthermore, brain region affected by IAS is more likely to have a limited collateral blood flow than a region affected by EAS [12, 21]. In other words, IAS may interrupt effective intracranial collateral channels (e. g. anterior or posterior communicating artery), thus causing only pial or meningeal to pial collateral available, whereas in EAS, collaterals across the circle of Willis may be preserved allowing the perfusion into a brain region distal to the occlusion.
In agreement with the above explanation, lesion growth or hypoperfusion was more accountable for END in patients with IAS than in patients without IAS. In contrast, ICH or edema/herniation was more frequently observed in END patients without IAS, who had a higher prevalence of cardioembolism associated with a higher initial stroke severity based on NIHSS score.
Among each lesion of IAS, stenotic lesion in BA or PCA remained significant in the association with END even after adjusting for covariates. This echoes prior results showing an association between brain stem infarct and END [6, 7]. Also, in our patients with END, 15 of 37 patients (40.5%) with PCA lesion and 11 of 19 patients (57.9%) with BA lesion had a brain stem infarct. Moreover, BA lesion in our total cohort was associated with BAD (12 of 47 patients with BA lesion, 25.5% versus 9 of 469 patient without, 1.9%, P < 0.001), which had a significant relationship to END presently and in prior studies [7, 18, 19].
The incidence of END in our patients was 19.0%, which was within the range of the reported incidence of 6.8–35% [3, 22]. This wide variation is presumed to be attributable to the difference in the definition of END, onset-visit time of included patients, and the proportion of stroke types in each study.
Similar to prior studies, patients with END had a higher mortality and poor functional recovery [1, 2]. However, there was no difference in the occurrence of further vascular events between patients with and without END, although END was associated with IAS and thus a higher atherosclerotic burden, as shown previously. This might be due to aggressive medical treatment and risk factor management for post-stroke survivors with IAS among our patients. In particular, dual antiplatelet therapy (aspirin and clopidogrel) with high-intensity statin was prescribed in those patients for several months according to a guideline from the American Heart Association and American Stroke Association [23]. This practice is further supported by the results of a recent study showing that a short course (a month) of dual antiplatelet therapy reduced END and stroke recurrence in patients with acute LAA stroke [24]. Noticeably, the effect of dual antiplatelet therapy was higher in patients with posterior circulation stroke and basilar stenosis in that study. Accordingly, dual antiplatelet therapy can be positively considered for patients with symptomatic basilar artery stenosis likely to have END.
Our study has several limitations. First, it was based on a single center data with a small number of patients, potentially leading to selection bias. Thus, our results might not be generalizable to other stroke populations. Second, we used 1.5 T MRA to determine arterial stenosis. Because this modality cannot discern an atherosclerotic plaque or subtle stenotic segment, some cases of BAD may have been misclassified as other stroke types, especially lacune [25]. Lastly, we could not completely distinguish atherosclerotic lesion from cardiac embolus in patients with cardioembolic stroke. In this study, complete occlusion relevant to cardioembolic infarct was excluded in determining distribution of stenosis because higher prevalence of cardiac embolus than atherosclerosis has been reported in such lesions [26, 27]. Even so, the completely occluded lesions might possibly include underlying atherosclerotic lesion. In addition, embolus within a cerebral artery may also cause vessel narrowing if not completely occlusive, which may mimic a stenosis on MRA. Thus, susceptibility-weighted image may be of help (which was not analyzed in this study) to determine a thrombus signal [28].
Conclusions
Pre-stroke distribution of atherosclerotic stenosis (IAS) as well as stroke subtypes of LAA (or BAD) may determine neurologic worsening during the early stage of ischemic stroke. IAS may limit collateral channels, leading to stroke progression in border zone, and interrupt the recovery of blood flow in the penumbra zone, resulting in lesion growth. More research is needed to confirm these results.
Supporting information
(XLS)
(DOCX)
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the Soonchunhyang University Research Fund. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Thanvi B, Treadwell S, Robinson T. Early neurological deterioration in acute ischaemic stroke: predictors, mechanisms and management. Postgrad Med J 2008;84:412–7. doi: 10.1136/pgmj.2007.066118 [DOI] [PubMed] [Google Scholar]
- 2.Kwan J, Hand P. Early neurological deterioration in acute stroke: clinical characteristics and impact on outcome. QJM 2006;99:625–33. doi: 10.1093/qjmed/hcl082 [DOI] [PubMed] [Google Scholar]
- 3.Siegler JE, Boehme AK, Kumar AD, Gillette MA, Albright KC, Beasley TM, et al. Identification of modifiable and nonmodifiable risk factors for neurologic deterioration after acute ischemic stroke. J Stroke Cerebrovasc Dis 2013;22:e207–13. doi: 10.1016/j.jstrokecerebrovasdis.2012.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogata T, Yasaka M, Wakugawa Y, Ibayashi S, Okada Y. Predisposing factors for acute deterioration of minor ischemic stroke. J Neurol Sci 2009;287:147–50. doi: 10.1016/j.jns.2009.08.006 [DOI] [PubMed] [Google Scholar]
- 5.Chung JW, Kim N, Kang J, Park SH, Kim WJ, Ko Y, et al. Blood pressure variability and the development of early neurological deterioration following acute ischemic stroke. J Hypertens 2015;33:2099–106. doi: 10.1097/HJH.0000000000000675 [DOI] [PubMed] [Google Scholar]
- 6.Weimar C, Mieck T, Buchthal J, Ehrenfeld CE, Schmid E, Diener HC; German Stroke Study Collaboration. Neurologic worsening during the acute phase of ischemic stroke. Arch Neurol 2005;62:393–7. doi: 10.1001/archneur.62.3.393 [DOI] [PubMed] [Google Scholar]
- 7.Miyamoto N, Tanaka Y, Ueno Y, Kawamura M, Shimada Y, Tanaka R, et al. Demographic, clinical, and radiologic predictors of neurologic deterioration in patients with acute ischemic stroke. J Stroke Cerebrovasc Dis 2013;22:205–10. doi: 10.1016/j.jstrokecerebrovasdis.2011.07.018 [DOI] [PubMed] [Google Scholar]
- 8.Ois A, Martinez-Rodriguez JE, Munteis E, Gomis M, Rodríguez-Campello A, Jimenez-Conde J, et al. Steno-occlusive arterial disease and early neurological deterioration in acute ischemic stroke. Cerebrovasc Dis 2008;25:151–6. doi: 10.1159/000113732 [DOI] [PubMed] [Google Scholar]
- 9.Siegler JE, Samai A, Semmes E, Martin-Schild S. Early Neurologic Deterioration after Stroke Depends on Vascular Territory and Stroke Etiology. J Stroke 2016;18:203–10. doi: 10.5853/jos.2016.00073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong LK. Global burden of intracranial atherosclerosis. Int J Stroke 2006;1:158–159. doi: 10.1111/j.1747-4949.2006.00045.x [DOI] [PubMed] [Google Scholar]
- 11.Pu Y, Dou X, Liu L. Natural history of intracranial atherosclerotic disease. Front Neurol 2014;5:125 doi: 10.3389/fneur.2014.00125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee SJ, Kim JS, Chung SW, Kim BS, Ahn KJ, Lee KS. White matter hyperintensities (WMH) are associated with intracranial atherosclerosis rather than extracranial atherosclerosis. Arch Gerontol Geriatr 2011;53:e129–e132. doi: 10.1016/j.archger.2010.07.008 [DOI] [PubMed] [Google Scholar]
- 13.Lee SJ, Lee DG, Lim DS, Hong S. Impact of Intracranial Atherosclerotic Stenosis on the Prognosis in Acute Ischemic Stroke Patients with Cardioembolic Source. Eur Neurol 2015;73:271–7. doi: 10.1159/000381336 [DOI] [PubMed] [Google Scholar]
- 14.Kasner SE. Clinical interpretation and use of stroke scales. Lancet Neurol. 2006; 5:603–612. doi: 10.1016/S1474-4422(06)70495-1 [DOI] [PubMed] [Google Scholar]
- 15.Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- 16.Bang OY. Intracranial atherosclerosis: current understanding and perspectives. J Stroke 2014;16:27–35. doi: 10.5853/jos.2014.16.1.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009;120:1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644 [DOI] [PubMed] [Google Scholar]
- 18.Jeong HG, Kim BJ, Yang MH, Han MK, Bae HJ. Neuroimaging markers for early neurologic deterioration in single small subcortical infarction. Stroke 2015;46:687–91. doi: 10.1161/STROKEAHA.114.007466 [DOI] [PubMed] [Google Scholar]
- 19.Petrone L, Nannoni S, Del Bene A, Palumbo V, Inzitari D. Branch Atheromatous Disease: A Clinically Meaningful, Yet Unproven Concept. Cerebrovasc Dis 2016;41:87–95. doi: 10.1159/000442577 [DOI] [PubMed] [Google Scholar]
- 20.Park JH, Kwon HM, Roh JK. Metabolic syndrome is more associated with intracranial atherosclerosis than extracranial atherosclerosis. Eur J Neurol. 2007;14:379–86 doi: 10.1111/j.1468-1331.2007.01682.x [DOI] [PubMed] [Google Scholar]
- 21.Ogasawara K., Ogawa A., Yoshimoto T. Cerebrovascular reactivity to acetazolamide and outcome in patients with symptomatic internal carotid or middle cerebral artery occlusion: a xenon-133 single-photon emission computed tomography study. Stroke 2002;33:1857–62. [DOI] [PubMed] [Google Scholar]
- 22.Kim YD, Choi HY, Jung YH, Yoo J, Nam HS, Song D, et al. The Ischemic Stroke Predictive Risk Score Predicts Early Neurological Deterioration. J Stroke Cerebrovasc Dis 2016;25:819–24. doi: 10.1016/j.jstrokecerebrovasdis.2015.12.003 [DOI] [PubMed] [Google Scholar]
- 23.Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, et al. ; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Peripheral Vascular Disease. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014;45:2160–236. doi: 10.1161/STR.0000000000000024 [DOI] [PubMed] [Google Scholar]
- 24.Wang C, Yi X, Zhang B, Liao D, Lin J, Chi L. Clopidogrel plus aspirin prevents early neurologic deterioration and improves 6-month outcome in patients with acute large artery atherosclerosis stroke. Clin Appl Thromb Hemost 2015;21:453–61. doi: 10.1177/1076029614551823 [DOI] [PubMed] [Google Scholar]
- 25.Ryoo S, Park JH, Kim SJ, Kim GM, Chung CS, Lee KH, et al. Branch occlusive disease: clinical and magnetic resonance angiography findings. Neurology 2012;78:888–96. doi: 10.1212/WNL.0b013e31824c4699 [DOI] [PubMed] [Google Scholar]
- 26.Molina CA, Montaner J, Abilleira S, Ibarra B, Romero F, Arenillas JF, et al. Timing of spontaneous recanalization and risk of hemorrhagic transformation in acute cardioembolic stroke. Stroke 2001;32:1079–84. [DOI] [PubMed] [Google Scholar]
- 27.Cho AH, Kwon SU, Kim JS, Kang DW. Evaluation of early dynamic changes of intracranial arterial occlusion is useful for stroke etiology diagnosis. J Neurol Sci 2012;312:127–30. doi: 10.1016/j.jns.2011.08.004 [DOI] [PubMed] [Google Scholar]
- 28.Park MG, Oh SJ, Baik SK, Jung DS, Park KP. Susceptibility-Weighted Imaging for Detection of Thrombus in Acute Cardioembolic Stroke. J Stroke 2016;18:73–9. doi: 10.5853/jos.2015.01417 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLS)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.