Abstract
Breast cancer is the type of cancer that develops from breast tissue; it is mostly common in women and it is one of the most studied diseases, largely because of its high mortality (second to lung cancer). However, it occurs in males also. This article presents a statistical study of the distribution of age, gender, length of stay, mode of diagnosis, status (dead or alive) after treatment and the location of breast cancer among 300 patients admitted in the University of Ilorin teaching hospital, Ilorin, Nigeria. The study covers a period of five (5) years; from 2011 to 2016 and logistic regression was used to perform the basic analysis in this study. It was discovered that the age of patients and the location of the breast cancer (right or left) contributes significantly to the survival of the patients. However, early detection and treatment of the disease is highly encouraged. This study also recommends that awareness should be taken to the grassroots and males should not be excluded from this discussion.
Keywords: Breast cancer, Logistic regression, Mortality, Oncology
Specifications Table
| Subject area | Medicine |
| More specific subject area | Biostatistics, Oncology |
| Type of data | Table and text file |
| How data was acquired | Unprocessed secondary data |
| Data format | Raw, analyzed |
| Experimental factors | Records of Breast cancer patients obtained from University of Ilorin Teaching Hospital (UITH), Nigeria. |
| Experimental features | Computational Analysis: Histogram, Bar-chart, Contingency tables, Logistic regression analysis. |
| Data source location | University of Ilorin Teaching Hospital (UITH), Nigeria |
| Data accessibility | All the data are available in this data article as supplementary materials |
Value of the data
-
•
The data on breast cancer could be useful for government and health workers to make decisions that would reduce the risk of breast cancer among the populace.
-
•
The data provides the analysis of the age, gender, location of the breast cancer, mode of diagnosis, length of stay (LOS), outcome of treatment of breast cancer patients for the population studied.
-
•
The data can further be analyzed using other statistical tools like chi square test, multiple linear regression and Poisson regression analysis.
-
•
The result from the analysis can be compared with other oncologic studies.
-
•
The interpretation of the data could be helpful in educational studies, epidemiologic oncology, molecular pathologic epidemiology, and breast cancer awareness, screening and so on.
-
•
The study can be replicated or extended to longitudinal studies.
-
•
The article provides insight on the impact and consequence of age and location of breast cancer on the survivability of breast cancer patients.
1. Data
The data set used in this article was collected as a secondary data and it contains information on 300 breast cancer patients. The data set was obtained from the Cancer Registry Department under the Department of Admission and Discharge Unit, University of Ilorin Teaching Hospital (UITH) Ilorin, Nigeria. It involves information on 275 females and 25 males and it covers a period of five (5) years; from 2011 to 2016. The patients were all treated as in-patients and were later discharged, of these, 97 patients were discharged dead while 203 patients were discharged alive. The raw data is available and can be assessed as Supplementary data.
Descriptive analyses were performed and logistic regression analysis was also used to describe and analyze the data set.
The data is summarized under different classifications: gender (sex), location of the breast cancer, mode of diagnosis, survival after treatment, age and length of stay in the hospital during treatment.
1.1. Analysis of age of the patients
The frequency table showing the analysis of the age of all the 300 patients is shown in Table 1.
Table 1.
Analysis of age.
| Statistics | ||
|---|---|---|
| Age | ||
| N | Valid | 300 |
| Missing | 0 | |
| Mean | 49.71 | |
| Median | 50.00 | |
| Mode | 60 | |
| Std. Deviation | 13.884 | |
| Variance | 192.768 | |
| Skewness | .572 | |
| Std. Error of Skewness | .141 | |
| Kurtosis | .479 | |
| Std. Error of Kurtosis | .281 | |
| Minimum | 20 | |
| Maximum | 96 | |
| Percentiles | 25 | 40.00 |
| 50 | 50.00 | |
| 75 | 60.00 | |
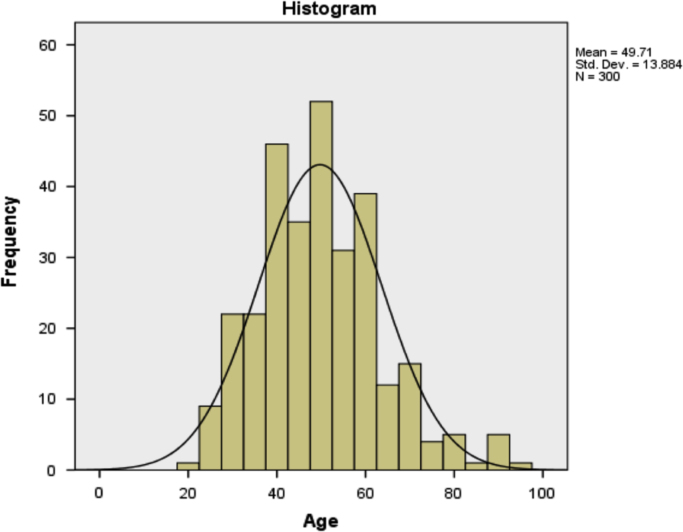
In Table 1, it can be seen that the mean age of the patients is 49.71 years, the minimum and maximum ages are 20 years and 96 years respectively. The data set is slightly positively skewed with a coefficient of skewness of 0.572.
A diagrammatic representation of the age of the patients is as shown in Fig. 1.
Fig. 1.
The distribution of age using histogram.
The age of the patients were classified into three different groups (or classes) and the respective frequencies are as shown in Table 2.
Table 2.
Classification of age of the patients.
|
Agecode | |||||
|---|---|---|---|---|---|
| Frequency | Percent | Valid Percent | Cumulative Percent | ||
| Valid | <41years | 88 | 29.3 | 29.3 | 29.3 |
| 41–55years | 115 | 38.3 | 38.3 | 67.7 | |
| > 55years | 97 | 32.3 | 32.3 | 100.0 | |
| Total | 300 | 100.0 | 100.0 | ||
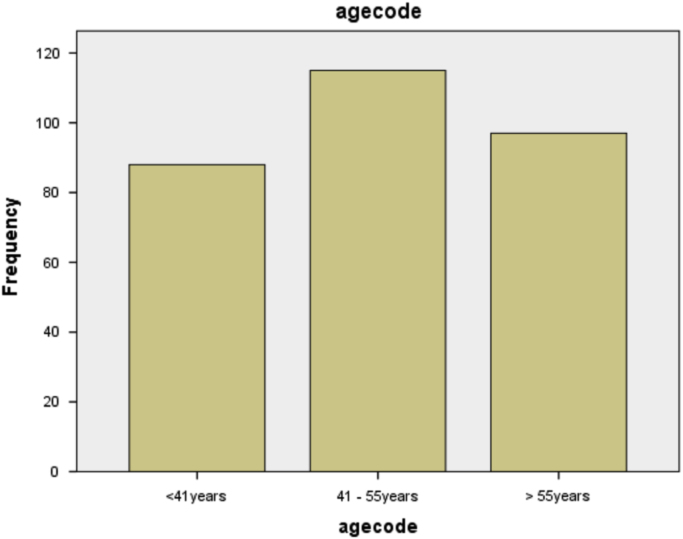
It can be seen from Table 2 that majority (115) of the patients are in the age group 41–55 years which accounts for 38.3% of the total population under study.
The diagrammatic representation of the information in Table 2 is as shown in Fig. 2.
Fig. 2.
Bar chart showing the classification of age.
1.2. Analysis on length of stay of the patients at the hospital
Information on the length of stay of the patients in the hospital before discharge is as shown in Table 3 and the respective frequencies are also displayed.
Table 3.
Classification of length of stay.
|
Loscode | |||||
|---|---|---|---|---|---|
| Frequency | Percent | Valid Percent | Cumulative Percent | ||
| Valid | < 11days | 106 | 35.3 | 35.3 | 35.3 |
| 11–21days | 101 | 33.7 | 33.7 | 69.0 | |
| > 21days | 93 | 31.0 | 31.0 | 100.0 | |
| Total | 300 | 100.0 | 100.0 | ||
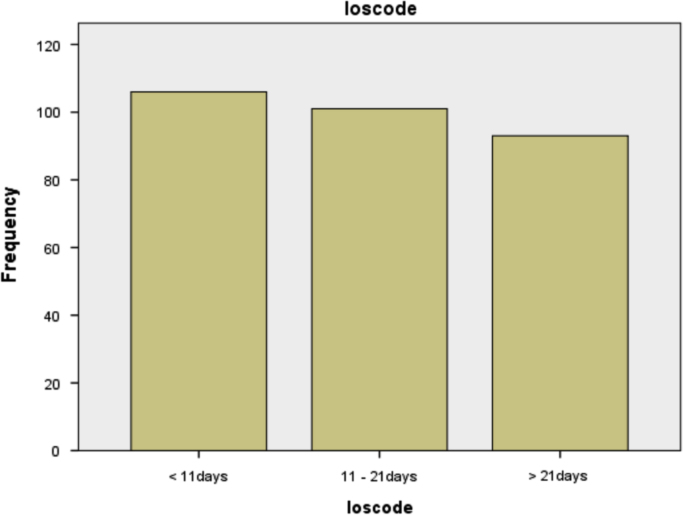
From Table 3, it can be seen that most (106) of the patients were discharged early and particularly in less than 11 days.
The diagrammatic representation is as shown in Fig. 3.
Fig. 3.
Bar chart showing the classification of length of stay.
1.3. Analysis on the gender of the patients
The information on the gender of the patients is as shown in Table 4.
Table 4.
Distribution of gender of the patients.
| Gender/sex | Frequency | Percent | Cumulative Percent | |
|---|---|---|---|---|
| Female | 275 | 91.7 | 91.7 | |
| Male | 25 | 8.3 | 100.0 | |
| Total | 300 | 100.0 | ||
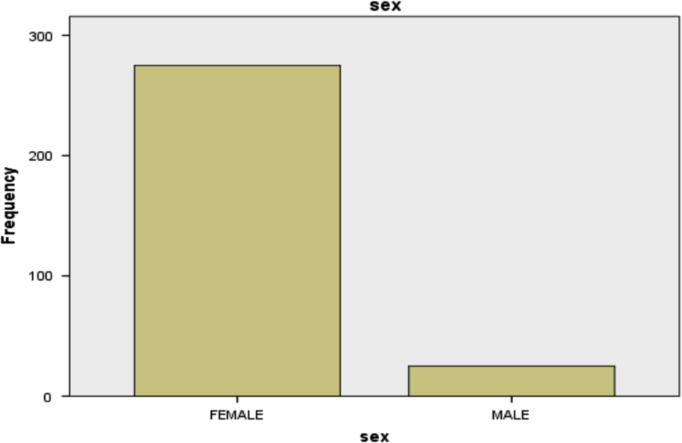
It can be seen in Table 4 that majority (275) of the patients are females. Also, the table revealed the incidence of breast cancer among male patients.
The information in Table 4 is represented diagrammatically in Fig. 4.
Fig. 4.
Bar chart showing the distribution of gender.
2. Experimental design, materials and methods
Research on breast cancer and other form of cancer are intense because of the high fatality rate of the disease if not properly managed. Several aspects of breast cancer has been studied, some of which have generated data sets. The analysis on those data sets is based on the various experimental designs, research materials and referred scientific methods. Some of such areas are: CT images, growth factor levels in incident breast cancer, hormone receptor status, cytokine circulation, secretagogue users in breast cancer treatments, chemokine levels, breast cancer and diabetes mellitus co-infection and treatment, breast cancer and HIV treatment, breast cancer and pregnancy. Others are: proteome analysis, risk factors analysis, breast examination, screening, management and breast cancer awareness, epidemiology, risk assessment tools, treatment options: radiotherapy treatment versus chemotherapy, survival analysis, breast cancer subtypes, biomarkers, socio-cultural barriers to treatment, socio-demographic factors and alternative medicine approach, genetic risk, dietary patterns, early diagnostics and treatment and others [1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26].
Chi-square test of independence can be used to analyze the data collected, for instance, a cross-tabulation of gender and outcome of the patients at the point of discharge can be classified into a r x c contigency table as shown in Table 5. In this research however, logistic regression analysis was used to analyze the data set. See similar analysis in [27], [28], [29], [30]
Table 5.
Crosstabulation for gender and outcome of patients.
|
sex * Outcome Crosstabulation | ||||
|---|---|---|---|---|
| Count | ||||
| Outcome |
Total | |||
| Alive | Dead | |||
| Sex | female | 188 | 87 | 275 |
| male | 15 | 10 | 25 | |
| Total | 203 | 97 | 300 | |
Table 6 represents the coding for variables length of stay, age, location of cancer, mode of diagnosis and gender of the patients.
Table 6.
Categorical variable coding.
| Frequency | Parameter coding |
|||
|---|---|---|---|---|
| (1) | (2) | |||
| Loscode | < 11days | 106 | 1.00 | 0.00 |
| 11–21days | 101 | 0.00 | 1.00 | |
| > 21days | 93 | 0.00 | 0.00 | |
| Agecode | <41years | 88 | 1.00 | 0.00 |
| 41–55years | 115 | 0.00 | 1.00 | |
| > 55years | 97 | 0.00 | 0.00 | |
| Location of Cancer | Both breasts | 25 | 1.00 | 0.00 |
| Left breast | 140 | 0.00 | 1.00 | |
| Right breast | 135 | 0.00 | 0.00 | |
| Mode of Diagnosis | Cytological | 166 | 1.00 | |
| Histological | 134 | 0.00 | ||
| sex | Female | 275 | 1.00 | |
| Male | 25 | 0.00 | ||
Table 7 shows the classification table at step 0.
Table 7.
Classification Table.
| Classification Tablea,b | |||||
|---|---|---|---|---|---|
| Observed | Predicted |
||||
| Outcome |
Percentage Correct | ||||
| Alive | Dead | ||||
| Step 0 | Outcome | Alive | 203 | 0 | 100.0 |
| Dead | 97 | 0 | .0 | ||
| Overall Percentage | 67.7 | ||||
Table 8 shows the variables in the equation at Step 0.
Table 8.
Variables in the equation.
| B | S.E. | Wald | df | Sig. | Exp(B) | ||
|---|---|---|---|---|---|---|---|
| Step 0 | Constant | −.738 | .123 | 35.797 | 1 | .000 | .478 |
Block 1: Method = Backward Stepwise (Conditional).
Table 9 shows the omnibus tests of model coefficients.
Table 9.
Tests of model coefficients.
|
Omnibus Tests of Model Coefficients | ||||
|---|---|---|---|---|
| Chi-square | df | Sig. | ||
| Step 1 | Step | 20.742 | 8 | .008 |
| Block | 20.742 | 8 | .008 | |
| Model | 20.742 | 8 | .008 | |
| Step 2a | Step | −.892 | 2 | .640 |
| Block | 19.850 | 6 | .003 | |
| Model | 19.850 | 6 | .003 | |
| Step 3a | Step | −.235 | 1 | .628 |
| Block | 19.616 | 5 | .001 | |
| Model | 19.616 | 5 | .001 | |
| Step 4a | Step | −.461 | 1 | .497 |
| Block | 19.155 | 4 | .001 | |
| Model | 19.155 | 4 | .001 | |
A negative Chi-squares value indicates that the Chi-squares value has decreased from the previous step.
Table 10 shows the model summary using the log-likelihood, Cox & Snell R square and Negelkerke R square.
Table 10.
Model summary.
|
Model Summary | |||
|---|---|---|---|
| Step | -2 Log likelihood | Cox & Snell R Square | Nagelkerke R Square |
| 1 | 356.872a | .067 | .093 |
| 2 | 357.764a | .064 | .089 |
| 3 | 357.998a | .063 | .088 |
| 4 | 358.459a | .062 | .086 |
Estimation terminated at iteration number 4 because parameter estimates changed by less than .001.
Table 11 shows the variables in the equation from Step 1 to Step 4:
Table 11.
Variables in the equation.
| B | S.E. | Wald | df | Sig. | Exp(B) | 95% C.I.for EXP(B) |
|||
|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||||
| Step 1a | sex(1) | −.232 | .454 | .261 | 1 | .609 | .793 | .325 | 1.932 |
| agecode | 9.641 | 2 | .008 | ||||||
| agecode(1) | −.827 | .332 | 6.194 | 1 | .013 | .437 | .228 | .839 | |
| agecode(2) | −.875 | .309 | 7.996 | 1 | .005 | .417 | .227 | .765 | |
| Location of Cancer | 9.209 | 2 | .010 | ||||||
| Location of Cancer(1) | 1.092 | .470 | 5.407 | 1 | .020 | 2.981 | 1.187 | 7.485 | |
| Location of Cancer(2) | .721 | .276 | 6.847 | 1 | .009 | 2.057 | 1.198 | 3.531 | |
| Mode of Diagnosis(1) | −.156 | .263 | .353 | 1 | .552 | .855 | .511 | 1.432 | |
| loscode | .883 | 2 | .643 | ||||||
| loscode(1) | −.238 | .319 | .559 | 1 | .455 | .788 | .422 | 1.471 | |
| loscode(2) | .031 | .316 | .010 | 1 | .921 | 1.032 | .555 | 1.918 | |
| Constant | −.271 | .503 | .289 | 1 | .591 | .763 | |||
| Step 2a | sex(1) | −.220 | .453 | .237 | 1 | .626 | .802 | .330 | 1.948 |
| agecode | 9.669 | 2 | .008 | ||||||
| agecode(1) | −.827 | .331 | 6.253 | 1 | .012 | .437 | .229 | .836 | |
| agecode(2) | −.871 | .309 | 7.964 | 1 | .005 | .419 | .229 | .766 | |
| Location of Cancer | 9.573 | 2 | .008 | ||||||
| Location of Cancer(1) | 1.093 | .468 | 5.460 | 1 | .019 | 2.983 | 1.193 | 7.462 | |
| Location of Cancer(2) | .742 | .274 | 7.323 | 1 | .007 | 2.100 | 1.227 | 3.593 | |
| Mode of Diagnosis(1) | −.166 | .263 | .397 | 1 | .529 | .847 | .506 | 1.418 | |
| Constant | −.359 | .459 | .613 | 1 | .434 | .698 | |||
| Step 3a | agecode | 10.684 | 2 | .005 | |||||
| agecode(1) | −.852 | .326 | 6.814 | 1 | .009 | .427 | .225 | .809 | |
| agecode(2) | −.898 | .304 | 8.743 | 1 | .003 | .407 | .225 | .739 | |
| Location of Cancer | 9.389 | 2 | .009 | ||||||
| Location of Cancer(1) | 1.076 | .466 | 5.325 | 1 | .021 | 2.933 | 1.176 | 7.318 | |
| Location of Cancer(2) | .728 | .272 | 7.154 | 1 | .007 | 2.072 | 1.215 | 3.533 | |
| Mode of Diagnosis(1) | −.178 | .261 | .461 | 1 | .497 | .837 | .502 | 1.398 | |
| Constant | −.528 | .303 | 3.033 | 1 | .082 | .590 | |||
| Step 4a | agecode | 10.359 | 2 | .006 | |||||
| agecode(1) | −.832 | .324 | 6.581 | 1 | .010 | .435 | .230 | .822 | |
| agecode(2) | −.877 | .302 | 8.446 | 1 | .004 | .416 | .230 | .752 | |
| Location of Cancer | 9.581 | 2 | .008 | ||||||
| Location of Cancer(1) | 1.114 | .463 | 5.784 | 1 | .016 | 3.047 | 1.229 | 7.554 | |
| Location of Cancer(2) | .722 | .272 | 7.055 | 1 | .008 | 2.059 | 1.208 | 3.509 | |
| Constant | −.640 | .256 | 6.256 | 1 | .012 | .528 | |||
Variable(s) entered on step 1: sex, agecode, LocationofCancer, ModeofDiagnosis, loscode.
Table 12 shows the Hosmer and Lemeshow Test.
Table 12.
Hosmer and Lemeshow Test.
|
Hosmer and Lemeshow Test | |||
|---|---|---|---|
| Step | Chi-square | df | Sig. |
| 1 | 8.566 | 8 | .380 |
| 2 | 1.502 | 8 | .993 |
| 3 | 1.380 | 8 | .995 |
| 4 | 1.193 | 5 | .946 |
Table 13 shows the classification table for all the steps; steps 1–4.
Table 13.
Classification Table.
| Observed | Predicted |
||||
|---|---|---|---|---|---|
| Outcome |
Percentage Correct | ||||
| Alive | Dead | ||||
| Step 1 | Outcome | Alive | 187 | 16 | 92.1 |
| Dead | 74 | 23 | 23.7 | ||
| Overall Percentage | 70.0 | ||||
| Step 2 | Outcome | Alive | 193 | 10 | 95.1 |
| Dead | 81 | 16 | 16.5 | ||
| Overall Percentage | 69.7 | ||||
| Step 3 | Outcome | Alive | 180 | 23 | 88.7 |
| Dead | 68 | 29 | 29.9 | ||
| Overall Percentage | 69.7 | ||||
| Step 4 | Outcome | Alive | 180 | 23 | 88.7 |
| Dead | 68 | 29 | 29.9 | ||
| Overall Percentage | 69.7 | ||||
a. The cut value is .500
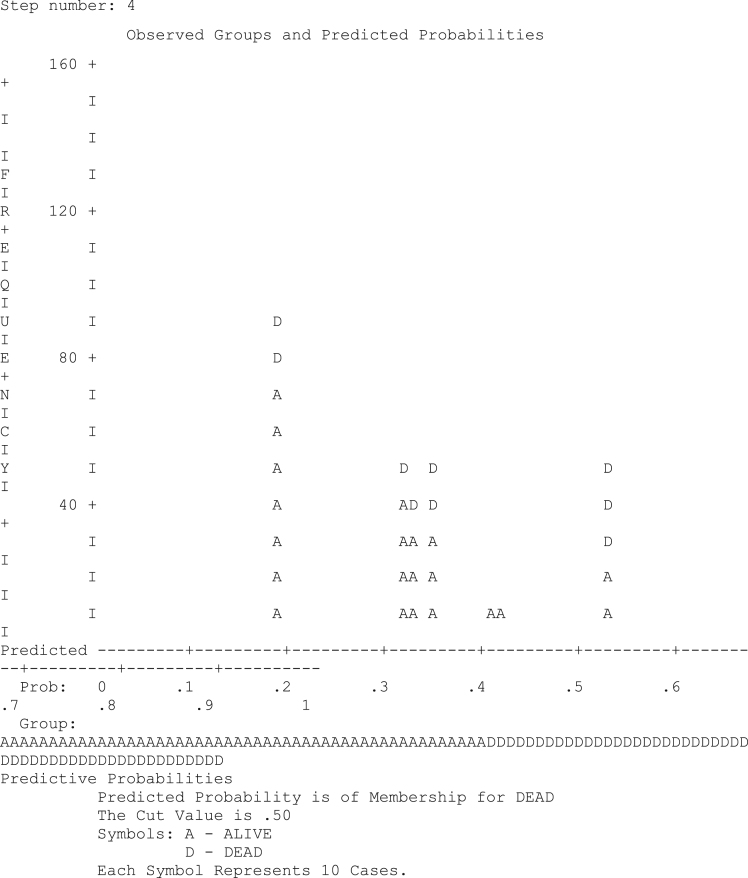
The predictive probability is as shown in Fig. 5.
Fig. 5.
Diagram of predictive probabilities.
Breast cancer is one of the dangerous diseases. It occurs in both males and females but the incidence is more in females. Based on this present study, the age of the patient and the location of the breast cancer (right breast or left breast) both contribute significantly to whether a patient would survive the breast cancer disease or not.
Acknowledgement
The authors are grateful to Covenant University for funding this research and UITH, Ilorin for making the data available.
Footnotes
Supplementary data associated with this article can be found in the online version at 10.1016/j.dib.2017.08.038.
Supplementary data associated with this article can be found in the online version at 10.1016/j.dib.2017.08.038.
Transparency document. Supplementary material
Supplementary material
.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.Ekanem I.A., Parkin D.M. Five year cancer incidence in Calabar, Nigeria (2009–2013) Cancer Epidemiol. 2016;42:167–172. doi: 10.1016/j.canep.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 2.Ogumkorode A., Holtslander L., Anonson J., Maree J. An integrative review of the literature on the determinants of health outcomes of women living with breast cancer in Canada and Nigeria from 1990 to 2014: a comparative study. Int. J. Afr. Nurs. Sci. 2017;6:52–73. [Google Scholar]
- 3.Anyanwu S.N.C., Egwuonwu O.A., Ihekwoba E.C. Acceptance and adherence to treatment among breast cancer patients in Eastern Nigeria. Breast. 2011;20(2):S51–S53. doi: 10.1016/j.breast.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 4.Oluwatosin O. Abimbola. Assessment of women's risk factors for breast cancer and predictors of the practice of breast examination in two rural areas near Ibadan, Nigeria. Cancer Epidemiol. 2010;34(4):425–428. doi: 10.1016/j.canep.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Makanjuola S.B.L., Popoola A.O., Oludara M.A. Radiation therapy: a major factor in the five-year survival analysis of women with breast cancer in Lagos, Nigeria. Radio. Oncol. 2014;111(2):321–326. doi: 10.1016/j.radonc.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 6.Ishola F., Omole O. A vision for improved cancer screening in Nigeria. Lancet Glob. Health. 2016;4(6):e359–e360. doi: 10.1016/S2214-109X(16)30062-6. [DOI] [PubMed] [Google Scholar]
- 7.Sule E.A., Ewemade F. Management of pregnancy associated breast cancer with chemotherapy in a developing country. Int. J. Surg. Case Rep. 2015;17:117–120. doi: 10.1016/j.ijscr.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Odiase E. The beliefs, knowledge, understanding, attitudes and treatment access to breast cancer amongst rural women in Northern Nigeria. Eur. J. Cancer. 2012;48:S77. [Google Scholar]
- 9.Jeddy-Agba E., McCormavk V., Adebamowo C., dos-Santos-Silva I. Stage of diagnosis of breast cancer in sub-Saharan Africa: a systematic review and meta-analysis. Lancet Glob. Health. 2016;4(12):e923–e935. doi: 10.1016/S2214-109X(16)30259-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alabi A., Olukiran G., Adejumo F. Breast cancer subtypes in an oncology centre in Nigeria: a retrospective review of 240 cases from Lagos. Eur. J. Cancer. 2017;72:S18–S19. [Google Scholar]
- 11.Dodo A.M. Sociocultural barriers to breast and cervical cancer screening in Northern Nigeria, Euro. J. Surg. Oncol. 2016;42(11):S242. [Google Scholar]
- 12.Fatokun O. Cancer control reform in Nigeria. Lancet Oncol. 2017;18(1):19–20. doi: 10.1016/S1470-2045(16)30650-7. [DOI] [PubMed] [Google Scholar]
- 13.Solola J., Olopade O. Assessing perceptions of genetic risk and breast cancer of women diagnosed and undiagnosed with breast cancer in Ibadan, Nigeria. Ann. Glob. Health. 2016;82(3):559–560. [Google Scholar]
- 14.Adesunkanmi A.R.K., Lawal O.O., Adelusola K.A., Durosimi M.A. The severity, outcome and challenges of breast cancer in Nigeria. Breast. 2006;15(3):399–409. doi: 10.1016/j.breast.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 15.Lawal O., Murphy F.J., Hogg P., Irurhe N., Nightingale J. Mammography screening in Nigeria-A Critical comparison to other countries. Radiography. 2015;21(4):348–351. [Google Scholar]
- 16.Rezaei M., Muhammadbeigi A., Khoshgard K., Haghparast A. CT images and radiotherapy treatment planning of patients with breast cancer: a dataset. Data Brief. 2017 doi: 10.1016/j.dib.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wintrob Z.A.P., Hammel J.P., Nimako G.K., Gaile D.P., Forrest A., Ceacareanu A.C. Circulating growth factors data associated with insulin secretagogue use in women with incident breast cancer. Data Brief. 2017;11:459–468. doi: 10.1016/j.dib.2017.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wintrob Z.A.P., Hammel J.P., Nimako G.K., Gaile D.P., Forrest A., Ceacareanu A.C. Dataset on growth factor levels and insulin use in patients with diabetes mellitus and incident breast cancer. Data Brief. 2017;11:183–191. doi: 10.1016/j.dib.2017.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.(a) Wintrob Z.A.P., Hammel J.P., Nimako G.K., Gaile D.P., Forrest A., Ceacareanu A.C. TH1 and TH2 cytokine data in insulin secretagogues users newly diagnosed with breast cancer. Data Brief. 2017;11:413–427. doi: 10.1016/j.dib.2017.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Wintrob Z.A.P., Hammel J.P., Nimako G.K., Gaile D.P., Forrest A., Ceacareanu A.C. TH1 and TH2 cytokines dataset in insulin users with diabetes mellitus and newly diagnosed breast cancer. Data Brief. 2017;11:331–348. doi: 10.1016/j.dib.2017.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wintrob Z.A.P., Hammel J.P., Nimako G.K., Gaile D.P., Forrest A., Ceacareanu A.C. Insulin use, hormone receptor status and hematopoietic cytokines' circulation in women with diabetes mellitus and breast cancer. Data Brief. 2017;11:382–390. doi: 10.1016/j.dib.2017.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wintrob Z.A.P., Hammel J.P., Nimako G.K., Gaile D.P., Forrest A., Ceacareanu A.C. Dataset on granulopoiesis and lymphopoiesis-stimulating cytokine levels in insulin secretagogue users with iincident breast cancer. Data Brief. 2017;11:277–283. doi: 10.1016/j.dib.2017.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wintrob Z.A.P., Hammel J.P., Nimako G.K., Fayazi Z.S., Gaile D.P., Forrest A., Ceacareanu A.C. Insulin secretagogue use and circulating inflammatory C-C chemokine levels in breast cancer patients. Data Brief. 2017;11:391–402. doi: 10.1016/j.dib.2017.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wintrob Z.A.P., Hammel J.P., Nimako G.K., Fayazi Z.S., Gaile D.P., Forrest A., Ceacareanu A.C. Circulating adipokines data associated with insulin secretagogue use in breast cancer patients. Data Brief. 2017;10:238–247. doi: 10.1016/j.dib.2016.11.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wintrob Z.A.P., Hammel J.P., Nimako G.K., Fayazi Z.S., Gaile D.P., Davis E.E., Forrest A., Ceacareanu A.C. Data report on inflammatory C-C chemokines among insulin-using women with diabetes mellitus and breast cancer. Data Brief. 2017;11:446–458. doi: 10.1016/j.dib.2017.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarvaiya H.A., Lazar L.M. Insulin simulated MCF 7 breast cancer cells: proteome dataset. Data Brief. 2016;9:579–584. doi: 10.1016/j.dib.2016.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park E. Data on cell cycle in breast cancer line, MDA-MB-231 with ferulic acid treatment. Data Brief. 2016;7:107–110. doi: 10.1016/j.dib.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jedy-Agba E., McCormack V., Olaomi O., Badejo W., Yilkudi M., Yawe T., Adebamowo S.N. Determinants of stage at diagnosis of breast cancer in Nigerian women: sociodemographic, breast cancer awareness, health care access and clinical factors. Cancer Causes Control. 2017;28(7):685–697. doi: 10.1007/s10552-017-0894-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hou N., Ndom P., Jombwe J., Ogundiran T., Ademola A., Morhason-Bello I., Huo D. An epidemiologic investigation of physical activity and breast cancer risk in Africa. Cancer Epidemiol. Prev. Biomar. 2014;23(12):2748–2756. doi: 10.1158/1055-9965.EPI-14-0675. [DOI] [PubMed] [Google Scholar]
- 29.Olajide T.O., Ugburo A.O., Habeebur M.O., Lawal A.O., Afolayan M.O., Mofikoya M.O. Awareness and practice of breast screening and its impact on early detection and presentation among breast cancer patients attending a clinic in Lagos, Nigeria. Nig. J. Clin. Pract. 2014;17(6):802–807. doi: 10.4103/1119-3077.144404. [DOI] [PubMed] [Google Scholar]
- 30.Obajimi M.O., Ajayi I.O., Oluwasola A.O., Adedokun B.O., Adeniji-Sofoluwe A.T., Mosuro O.A., Adegoke, F F. Level of awareness of mammography among women attending outpatient clinics in a teaching hospital in Ibadan. South-West Niger. BMC public health. 2013;13(1):2013. doi: 10.1186/1471-2458-13-40. (Art. 40) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material