Abstract
We discuss the generation of primary soft tissue sarcomas in mice using the Cre-loxP system to activate conditional mutations in oncogenic Kras and the tumor suppressor p53 (LSL-KrasG12D/+; p53flox/flox). Sarcomas can be generated either by adenoviral delivery of Cre recombinase, activation of transgenic Cre recombinase with tamoxifen, or through transplantation of isolated satellite cells with Cre activation in vitro. Various applications of these models are discussed, including anticancer therapies, metastasis, in vivo imaging, and genetic requirements for tumorigenesis.
Keywords: Sarcoma, Mouse models, Cancer, Transgenic mice, Satellite cells, Genetically engineered mouse models
1 Introduction
Soft tissue sarcomas (STS) are malignant tumors of the connective tissue that affect muscle, fibrous tissue, fat, blood vessels, and nerves [1]. In humans, these tumors are often characterized by inactivating mutations in the p53 tumor-suppressor pathway and activating mutations in the oncogenic Kras pathway [2]. To examine sarcoma pathogenesis, we have utilized the Cre-LoxP system to develop mouse models of soft tissue sarcoma [3]. Cre is a site-specific recombinase that recognizes loxP sequences, resulting in excision of DNA that is flanked by two loxP sites. Sarcomas are generated by activation of Cre in mice or cells with conditional mutations in both oncogenic Kras (LSL-KrasG12D/+) and mutant p53 (p53flox/flox). In the absence of Cre recombinase, p53 is expressed at wild-type levels, whereas oncogenic Kras is not expressed. After Cre activation, the floxed p53 exons 2–10 are deleted, resulting in inactivation of both p53 alleles. At the same time, Cre activates oncogenic Kras by deleting an upstream floxed transcription/translation STOP cassette (termed “loxP-STOP-loxP,” or LSL cassette). The resulting tumor expresses KrasG12D and is p53-null.
Cre recombinase can be activated by a variety of techniques. First, Cre can be delivered directly to the animal via intramuscular injection of an adenovirus that expresses Cre (Ad-Cre) [3]. Following intramuscular injection of Ad-Cre into the compound mutant mice (LSL-KrasG12D/+; p53flox/flox), high-grade sarcomas develop at the site of injection within 2–4 months. Additionally, approximately 40 % of these mice develop lung metastases, which are common in human soft tissue sarcoma patients. A second method for deleting the loxP alleles is to use CreER technology [4]. This method uses a Cre recombinase that is fused to the hormone-binding domain of the estrogen receptor (ER) [5], generating a CreER allele that can be activated by the estrogen analog 4-hydroxy-tamoxifen (4OHT). Following 4OHT exposure, CreER translocates to the nucleus, where Cre recombinase excises DNA flanked by loxP sites. In contrast to the Ad-Cre method, this approach can restrict Cre activity to specific tissues and cell types by utilizing transgenic mice that express CreER from cell type-specific promoters. Using a Pax7-CreER allele that is specific to the Pax7+ muscle satellite cells [6], we recently generated rhabdomyosarcomas and undifferentiated pleomorphic sarcomas following intraperitoneal injection of tamoxifen [4], which is converted to 4OHT in the liver. A third option for tumor generation involves isolating tumor-initiating cells by fluorescence activated cell sorting (FACS) via expression of specific cell surface markers. These cells can be transformed in vitro by either Ad-Cre or 4-OHT (for CreER-containing cells) and then transplanted into an animal. This method allows direct isolation of putative tumor-initiating cells, facilitating the study of tumorigenesis by specific gene mutations. After isolating [7] and transforming satellite cells in vitro by deleting p53 and expressing oncogenic Kras, we have generated rhabdomyosarcomas following transplantation into recipient mice (Blum JM, Li Z, and Kirsch DG, unpublished data).
All of these techniques can be used to functionally test the role of novel therapies or genes that regulate sarcoma development in a genetically tractable system. These mouse models provide distinct advantages for examining sarcoma pathogenesis that are not achievable by cell transplant or xenograft studies [8]. First, the mutant genes that initiate sarcoma growth are expressed from their endogenous promoters at physiological levels. Second, by spatially and temporally restricting tumor initiation, sarcomas develop in adult mice at a defined anatomic site. This allows close monitoring of tumor size in response to experimental treatments. Third, tumors develop within the native tissue microenvironment, surrounded by tissue that is not transformed. This facilitates studies that examine the relationship between the tumor stroma and therapeutic response. Finally, the host has an intact immune system, in contrast to xenograft model systems, which permits further examination of the tumor stroma on sarcoma biology.
These genetically engineered mouse models of STS may be used for multiple translational applications. Several specific examples are discussed below to provide the reader with examples that may stimulate their own experiments.
First, the models initiated by intramuscular injection of Adeno-Cre or 4OHT have been used to investigate various anticancer treatments. Because the tumors are generated in the leg, monitoring changes for tumor size in response to therapy is simple using calipers. We have successfully used these models to test sarcoma response to radiation therapy [9], molecularly targeted agents against MEK [10], and combination treatments, including sunitinib with radiotherapy [9] and PI3K inhibitors with conventional doxorubicin chemotherapy [11]. Second, these tumor models metastasize to the lungs, as seen in sarcoma patients, and thus the model initiated by Adeno-Cre injection is adaptable for in vivo metastasis assays. Approximately 20 % of the mice will develop pulmonary metastases at the time of primary tumor development, but the primary tumors grow at such a rapid rate that the animals must be sacrificed before the true metastatic potential of each tumor can be determined. To circumvent this limitation, the tumor-bearing limb can be amputated, which extends the survival of the mice while allowing for potential lung metastases to grow. Following amputation, approximately 40 % of mice develop pulmonary metastases. We have used this amputation-based protocol to make several observations about sarcoma metastasis, including the role of hypoxia inducible factor-1alpha (HIF-1α) [12] and miRNAs [13]. Third, these models can be used for in vivo imaging or surgical studies because the primary tumors develop specifically at the site of intramuscular injection. We have used these models to test several novel in vivo tumor imaging systems, including intraoperative imaging with cathepsin-activatable fluorescent imaging agents [14, 15] and dual-energy microcomputed tomography [16] with gold nanoparticles [17]. Finally, the models described above can be extended to test novel gene mutations that may be relevant to distinct subtypes of sarcoma. For example, genomic analyses of patient sarcomas revealed that mutations in the neurofibromin 1 (NF1) gene are common in a number of STS. To test this observation in a genetically engineered mouse model, we crossed mice with floxed alleles for NF1 and the tumor suppressor Ink4a/Arf. Injection of Ad-Cre into either the nerve sheath or muscle of NF1flox/flox; Ink4a/Arfflox/flox mice generated sarcomas that resembled malignant peripheral nerve sheath tumors (MPNST) or high-grade myogenic sarcomas, respectively [10]. In addition to demonstrating the utility of the model for examining different genetic mutations, this study also highlights the possibility of examining alternative anatomic sites for sarcomagenesis.
2 Materials
2.1 Ad-Cre Tumor Generation
Adenovirus expressing Cre recombinase: University of Iowa Gene Transfer Vector Core. Keep frozen at −20 °C. Can order or aliquot into 25 µL aliquots to minimize effect of freeze-thaw cycles on viral titer.
2-M CaCl2.
MEM media.
Insulin syringes: ½ cc.
10 % bleach solution.
2.2 Tamoxifen-Generated Tumors
20 mg/mL tamoxifen solution in corn oil (see Note 1).
10-mM tamoxifen, 4-hydroxy stock in DMSO.
1-mL syringe, Luer-Lok tip.
25-gauge needle, 5/8 in.
2.3 Satellite Cell-Generated Tumors
Muscle digest 1: 0.2 % collagenase type II, 1× Glutamax, and 1× Pen/Strep in DMEM. Must make fresh the day of procedure.
Muscle digest 2: 0.0125 % collagenase type II, 0.2 % dispase, 1× Glutamax, and 1× Pen/Strep in F-10 media. Must make fresh the day of procedure.
20 % FBS in DMEM.
100 % FBS.
HSC buffer: 1-L HBSS with 50-mL FBS and 4 mL of 0.5-M EDTA. Filter with 0.22-µm filter and keep at 4 °C.
Sterile glass pipette with rubber bulb.
40-µm filters.
Laminin/collagen mix: 1 µg/mL collagen and 10 µg/mL laminin.
Satellite cell proliferation media: 20 % horse serum, 1 % Glutamax, 1 % pen/strep in Ham’s F-10.
bFGF for supplementing isolated satellite cells in culture.
10-mM tamoxifen, 4-hydroxy stock in DMSO.
2.4 Antibodies for FACS Sorting of Satellite Cells (See Table 1)
Table 1.
Primary antibodies
| Antibody | Concentration | Secondary |
|---|---|---|
| Ter-119 | 1:200 | Directly conjugated to PE, PE-Cy5, or FITC |
| CD45 | 1:200 | Directly conjugated to PE, PE-Cy5, or FITC |
| Cd11b/Mac1 | 1:200 | Directly conjugated to PE, PE-Cy5, or FITC |
| Sca-1 | 1:200 | Directly conjugated to APC |
| CD29/β1-integrin | 1:200 | Anti-Armenian hamster 1:200 FITC and PE |
| CXCR4 biotin/CD184 | 1:100 | Streptavidin 1:200 PE-Cy7 |
| Calcein Blue, AM | 1:1,000 | n/a |
| Propidium iodide | 1:1,000 | n/a |
| CD31 | 1:100 | Directly conjugated to FITC |
3 Methods
These techniques allow for many modifications. Injection of Ad-Cre into other anatomical sites can generate tumors at other sites in addition to the lower limb. Using other tissue-specific CreER drivers can potentially be used to generate sarcomas from different tumor-initiating cells following delivery of tamoxifen. When deciding between using an Ad-Cre-driven tumor model system or a tamoxifen-inducible CreER system, certain factors should be considered. Advantages of Ad-Cre delivery include generation of sarcomas even when the cell of origin is not known. In contrast, the CreER system enables the generation of sarcomas from specific cell types. Investigators must also consider the availability of mice in which CreER is expressed from cell type-specific promoters and the amount of time required to breed the animals with the CreER allele. Note that lung tumors may be generated in the LSL-Kras G12D/+; p53flox/flox mice using a similar protocol if Ad-Cre is delivered to the lungs by intranasal inhalation [18].
3.1 Adenoviral-Cre-Initiated Tumors in LSL-KrasG12D/+; p53flox/flox (KP) Mice (See Note 2)
We typically induce tumors in mice over 6 weeks of age, as they are large enough to achieve consistently reproducible injections.
Be certain all viral work is performed in a BSL-2 hood. Prepare the workstation with a disposable absorbent lab pad, Eppendorf rack, insulin syringes, and a beaker of 10 % bleach.
Mix up the viral injection cocktail. First, allow the virus to completely thaw. In a fresh Eppendorf tube, add 600-µL MEM, 25-µL virus, and 2-µL 2-M CaCl2. Flick the tube to mix, noting the slight color gradation from the CaCl2 density. Allow the mix to sit for at least 15 min, but not more than 60 min before injection. This time window is critical to allow the viral precipitates to grow large enough to traverse the plasma membrane of the cells, but not too large to inhibit uptake.
Anesthetize mice in a box containing isoflurane gas per your approved IACUC protocol.
Remove one mouse. Place the animal on stomach and extend one lower left leg with forceps. Position the needle over the muscle that is below the knee joint. Insert the needle into the muscle, being careful not to inject the bone. Inject 50 µL of viral mix into the leg muscle. Sometimes, it is helpful to have a lab mate assist with anesthesia and extending the leg, which may result in more consistent injections if one investigator focuses on injecting the Adeno-Cre rather than other tasks.
Place mice into a new, fresh cage. Virus is potentially infectious for the next 48 h, so if the animals are housed in a ventilated rack, then the rack should be placed on negative pressure. The cages should not be changed for the next 48 h.
Continue injections of mice with the rest of the viral cocktail (see Note 3).
Clean up the workstation using 10 % bleach to clean all tubes and syringes. Wipe down the work surface.
3.2 Tamoxifen-Initiated Tumors with CreER Technology in Pax7CreER/+; p53flox/flox; LSL-KRasG12D/+ (P7KP) Mice (See Note 4)
CreER is activated by exposure to tamoxifen and its analogs, the most potent of which is 4-hydroxy-tamoxifen (4OHT). Two delivery methods for 4OHT are available. For localized administration, 4OHT is injected directly into the muscle (intramuscular—IM). For systemic administration, tamoxifen is injected into the peritoneum (intraperitoneal—IP). The injected tamoxifen is then metabolized by the liver to yield 4OHT, which can recombine loxP sites throughout the body.
3.2.1. IM injection of 4OHT allows for localized delivery of the drug to a discrete site in the animal, resulting in a localized tumor
IM delivery of 4OHT is identical to IM injection of adenovirus as described in Subheading 1.
Anesthetize the mice in a single cage using isoflurane.
Remove one mouse at a time. Place the animal on its stomach and extend the lower left leg with forceps. Insert the needle into the gastrocnemius muscle, being careful not to insert into the bone or through the other side of the leg. Inject 50 µL of 4OHT into the leg. Sometimes, it may be helpful to have a lab mate assist with anesthesia and extending the leg, which may result in more consistent injections if one investigator focuses on injecting the 4OHT rather than other tasks.
Place the mouse into a new, fresh cage.
3.2.2. IP delivery of tamoxifen allows for systemic activation of CreER in Pax7-expressing cells throughout the animal. As mentioned above, tamoxifen is converted to its active metabolite, 4OHT, in the liver
Draw up tamoxifen slowly into a 1-mL syringe with a Luer-Lok tip. The corn oil is quite thick, so this is best done with the needle off to facilitate the process. Once air bubbles have been removed, place a 25-guage, 5/8-in.-long needle onto the syringe.
Remove a mouse from the cage and weigh it to determine tamoxifen dose.
Scruff the mouse and turn so the stomach is facing up. With your dominant hand, hold the needle parallel to the mouse with the bevel pointing upward. Insert the needle into the abdomen, to the left of the midline. Inject 10 µL of the 20 mg/mL tamoxifen stock per 1 g mouse. Be careful with needle placement, and you should place the mouse head down so the abdominal contents will move away from the needle.
Return the mouse to the cage. Be aware that the tamoxifen can be excreted in the waste products of the animal, so tamoxifen-injected mice should not be housed with animals containing CreER alleles that you do not wish to activate.
3.3 Satellite Cell Isolation, Transformation, and Transplantation (See Note 5)
After euthanasia, isolate both the upper and lower hind limb muscles of the mouse. Snip away the surrounding skin and carefully strip the muscle away from the bone using tweezers and scissors. It is important not to include other limbs or trunk muscular tissue, because the immunophenotype of satellite cells within this tissue may differ (see Note 6).
Place tissue from both hind limbs into a 50-mL conical tube containing 20 mL of the muscle digest 1 solution. Place in a shaking water bath at 37 °C for 1.5 h.
Following digestion, spin the suspension at 400 × g for 5 min at 4 °C. Discard the supernatant, resuspend the pellet in 5–7-mL 20 % FBS in DMEM to stop the reaction, and transfer this suspension into a Petri dish.
Break apart the intact myofibers from the tissue by vigorous pipetting with a sterile glass pipette. This should take approximately 5 min (see Note 7). As the mixture becomes cloudy and thick, transfer to a new 50-mL conical tube and add PBS to the remaining tissue. Continue breaking apart with pipette and repeat this process until there are no remaining muscle pieces. Fill conical tube up to 30 mL of PBS.
Spin the homogenate at 400 × g for 5 min at 4 °C.
Discard supernatant and resuspend the pellet in 30 mL of PBS and spin at 400 × g for 5 min at 4 °C to wash off all remaining FBS.
Discard the supernatant and resuspend the pellet in 10 mL of muscle digest buffer 2 in a 50-mL conical tube. Incubate for 30 min at 37 °C in a shaking water bath. This step will liberate the final myofiber-associated cells.
Add 1 mL of 100 % FBS to stop collagenase activity. Using an automatic pipettor, pipette 15–20 times to finish the dissociation process.
Filter the entire homogenate through a 40-µm filter into a 50-mL conical tube.
Spin at 400 × g for 5 min at 4 °C. The resulting pellet will contain both satellite cells and the interstitial cells, which are associated with or between the muscle fibers. Myofibers are no longer present in the solution at this time.
Begin the immunostaining protocol that will be used to isolate satellite cells by fluorescent-activated cell sorting (FACS). Bring the pellet up in 200 µL of HSC buffer. Add the antibody cocktail outlined in the accompanying antibody table to isolate cells with the immunophenotype CD45−, Ter119−, Sca1−, Cd11b−, CXCR4+, and beta1-integrin+.
Incubate on ice for 20 min protected from light. Wash in 2-mL HSC buffer and spin at 400 × g for 5 min at 4 °C.
Resuspend the pellet in 200-µL HSC buffer and add secondary antibodies. These include 1:200 FITC anti-Armenian hamster (for beta1-integrin) and 1:200 PECy7 streptavidin (for CXCR4).
Incubate on ice for 20 min protected from light. Wash in 2-mL HSC buffer and spin at 400 × g for 5 min at 4 °C.
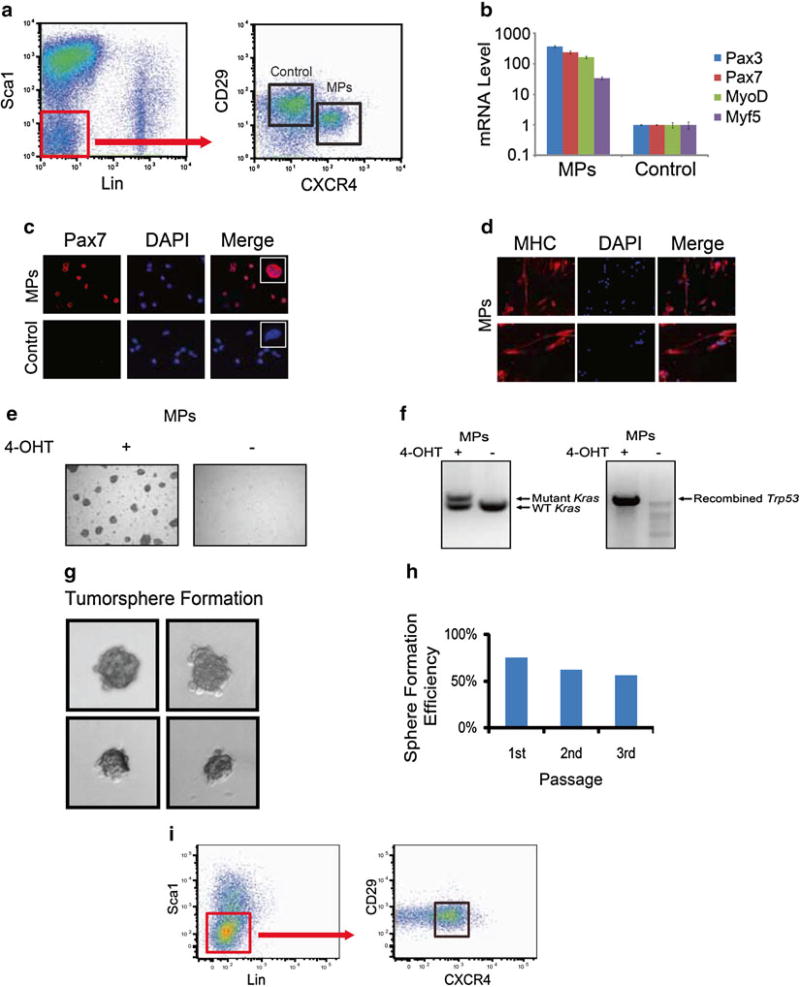
Resuspend the pellet in 300-µL HSC buffer and sort the population that is CXCR4, beta1-integrin double positive by FACS. These are the satellite cells (see Fig. 1).
Sort cells into a 96-well dish previously coated with a laminin/ collagen solution, air dried for 30 min. The dish should contain satellite cell proliferation media. Supplement the culture daily with bFGF (25 ng/mL).
After 7 days in culture, add 250 nM of 4-hydroxy-tamoxifen (4OHT) in satellite cell proliferation media to the culture.
Seven days after tamoxifen addition, the cells are ready to trypsinize and replate. Expand the culture through 2–4 additional passages until the desired number of cells is grown.
Inject between 6,500 and 300,000 cells into the gastrocnemius of a nude mouse using a ½-cc insulin syringe in a volume of 50 µL.
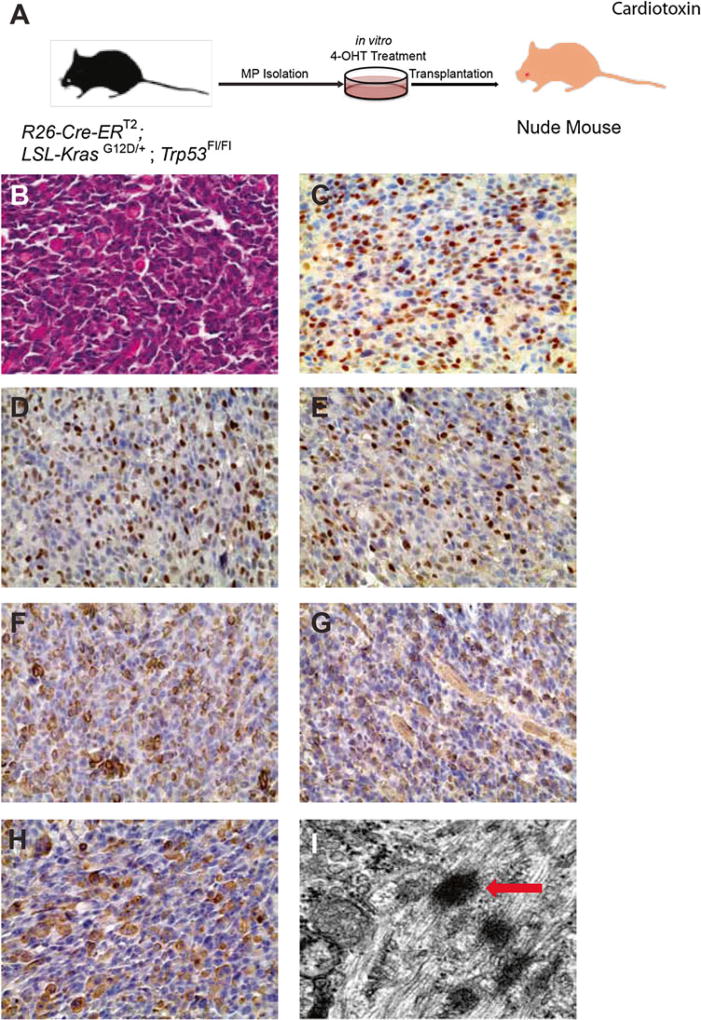
To promote engraftment, myoablation may be performed 24 h prior to transplantation by injecting 25 µL of 0.3 mg/mL cardiotoxin in the hind limb. This procedure is not necessary, but may improve engraftment (see Fig. 2).
Fig. 1.
Isolation and transformation of Pax7+ myogenic progenitors in vitro. (a) Representative FACS plot of the isolation of Pax7+ myogenic progenitors (MPs) by the immunophenotype CD45−, Ter119−, Sca1−, Mac1−, CXCR4+, CD29+, in R26CE/+; LSL-KrasG12D/+; Trp53Fl/Fl mice. (b) Sorted MPs express high levels of myogenic transcripts in comparison to non-myogenic control cells. (c) Pax7 immunofluorescence demonstrates Pax7 expression in the nucleus of sorted MPs, but not control cells (scale bar = 50 µm). Inset shows magnification of a single cell. (d) Sorted MPs cultured in differentiation media express myosin heavy chain (MHC). (e) Myogenic progenitors (MPs) from R26-Cre-ERT2; LSL-KrasG12D/+; Trp53Fl/Fl mice treated with 4-OHT are more proliferative and capable of forming colonies compared to untreated MPs, indicating transformation in vitro. (f) For MPs treated with 4-OHT, genomic DNA was isolated from myogenic progenitors with or without 4-OHT treatment for 14 days. PCR demonstrates the recombination of the mutant Kras allele as well as recombination of Trp53. (g) Single transformed myoblasts were sorted into 96-well plates with proliferation medium and formed tumorspheres. Fourteen days later, about 75 % of the wells contained tumorspheres. (h) Single cells from primary tumorspheres were capable of generating new tumorspheres over three passages. (i) Transformed cells maintain satellite cell immunophenotype after multiple passages
Fig. 2.
Allografts from a cell population transformed in vitro with mutant Kras and loss of Trp53 are UPS. (a) Myogenic progenitors were isolated and cultured, transformed in vitro, and were injected into the hind limb muscle of nude mice after pretreatment with cardiotoxin for 24 h. Characterization of allograft-derived tumors by H&E staining (b), and immunohistochemistry for Pax7 (c), MyoD (d), myogenin (e), desmin (f), actin (sarcomeric) (g), and myosin heavy chain (h). (i) Electron microscopy of allograft-derived tumors. Arrow indicates z-band and associated myofilaments. Results are representative of allografts derived from myogenic progenitors transformed in three independent experiments (n = 3)
Acknowledgments
We thank members of the Kirsch lab for helpful discussions and for contributing to the development of these techniques. We acknowledge members of Amy Wagers lab and Christoph Lepper for developing the satellite cell isolation technique and for graciously sharing their protocols.
Footnotes
Tamoxifen is poorly soluble in sterile corn oil. To aid in dissolution, a 20 mg/mL solution is heated at 50 °C for 2–5 h. 1-mL aliquots can be frozen and stored at −80 °C, though they require heating to redissolve upon thawing. Alternatively, tamoxifen can be more readily dissolved in a 5 % ethanol/95 % corn oil mixture. First, the tamoxifen is mixed in 100 % ethanol. The concentration of tamoxifen is too high for it to fully dissolve, and it generates a white suspension. Corn oil is then added to the appropriate volume. For example, we generally purchase 1 g of tamoxifen and mix with 2.5 mL of 100 % ethanol, creating a slurry. We then add 47.5 mL of corn oil to bring the mixture to the final concentration of 20 mg/mL. The solution is heated at 50 °C on a shaker for approximately 1 h to aid in dissolution of the tamoxifen. It is then frozen in 1-mL aliquots at −80 °C. In contrast to when tamoxifen is in frozen in 100 % corn oil, tamoxifen frozen in 5 % ethanol/95 % corn oil remains in solution and requires no heating prior to use.
For Ad-Cre-generated tumors, large masses develop at the site of injection within 2–4 months in ~90 % of all animals. Once detected, tumors will grow at a rapid rate, resulting in the animal needing to be sacrificed within approximately 2 weeks. Light microscopy demonstrates that these tumors are high-grade sarcomas. Gene expression analysis shows that these tumors most closely resemble the subtype of soft tissue sarcoma referred to as undifferentiated polymorphic sarcoma (UPS) [19].
Critical steps in the Ad-Cre tumor generation process include complete mixing of the viral cocktail and adherence to the 15–60-min time window for generating viral particles. Injection of the cocktail outside of this window could result in the viral precipitates being too large or too small to enter the cells. Consistency in the site of injection, depth of needle penetration, and volume injected will increase the likelihood that tumor will form at the same site on different mice. If experimental mice are scarce, then we recommend practicing Ad-Cre injections on littermates without useful genotypes to maximize reproducibility of results.
Tumor development with the Pax7-CreER system is highly dependent on the delivery method of tamoxifen. Intraperitoneal injection of tamoxifen results in tumors at multiple sites throughout the animal by 40–60 days. Gene expression analyses show these tumors are most similar to human embryonal rhabdomyosarcoma (ERMS) or UPS [4]. Tumors generated with intramuscular injection of 4OHT contain a mixed histology that includes rhabdomyoblasts that stain for RMS markers, including MyoD and myogenin, and other areas of UPS.
Isolated satellite cells appear as semi-fused fibers with fibroblastic appearance by 7 days post-isolation. The tumors generated from satellite cells transformed in vitro generally appear in nude mice within 30 days (Figs. 1 and 2). These tumors contain rhabdomyoblasts and stain for myogenin.
For satellite cell isolation, it is important to not completely mince the fresh muscle at the beginning of the protocol. Although it may appear that complete mincing will promote digestion of the tissue, this may kill the satellite cells you are attempting to isolate.
Another key step in this protocol is to thoroughly homogenize the mixture with the glass pipette and rubber bulb. Skimping on the 250 required strokes will dramatically decrease the number of cells isolated.
Disclosures : None.
References
- 1.Borden EC, Baker LH, Bell RS, Bramwell V, Demetri GD, Eisenberg BL, Fletcher CD, Fletcher JA, Ladanyi M, Meltzer P, O’Sullivan B, Parkinson DR, Pisters PW, Saxman S, Singer S, Sundaram M, van Oosterom AT, Verweij J, Waalen J, Weiss SW, Brennan MF. Soft tissue sarcomas of adults: state of the translational science. Clin Cancer Res. 2003;9(6):1941–1956. [PubMed] [Google Scholar]
- 2.Helman LJ, Meltzer P. Mechanisms of sarcoma development. Nat Rev Cancer. 2003;3(9):685–694. doi: 10.1038/nrc1168. [DOI] [PubMed] [Google Scholar]
- 3.Kirsch DG, Dinulescu DM, Miller JB, Grimm J, Santiago PM, Young NP, Nielsen GP, Quade BJ, Chaber CJ, Schultz CP, Takeuchi O, Bronson RT, Crowley D, Korsmeyer SJ, Yoon SS, Hornicek FJ, Weissleder R, Jacks T. A spatially and temporally restricted mouse model of soft tissue sarcoma. Nat Med. 2007;13(8):992–997. doi: 10.1038/nm1602. [DOI] [PubMed] [Google Scholar]
- 4.Blum JM, Li Z, Zhang M, Mito JK, Dodd LG, Van Mater D, Bennett BD, Dodd RD, Lepper C, Ma Y, Rodrigues RC, Jeffords LB, Linardic CM, Mukherjee S, Fan CM, Kirsch DG. Distinct and overlapping sarcoma subtypes initiated from muscle stem and progenitor cells. Cell Rep. 2013;5(4):933–940. doi: 10.1016/j.celrep.2013.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feil R, Wagner J, Metzger D, Chambon P. Regulation of Cre recombinase activity by mutated estrogen receptor ligand-binding domains. Biochem Biophys Res Commun. 1997;237(3):752–757. doi: 10.1006/bbrc.1997.7124. [DOI] [PubMed] [Google Scholar]
- 6.Lepper C, Conway SJ, Fan CM. Adult satellite cells and embryonic muscle progenitors have distinct genetic requirements. Nature. 2009;460(7255):627–631. doi: 10.1038/nature08209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sherwood RI, Christensen JL, Conboy IM, Conboy MJ, Rando TA, Weissman IL, Wagers AJ. Isolation of adult mouse myogenic progenitors: functional heterogeneity of cells within and engrafting skeletal muscle. Cell. 2004;119(4):543–554. doi: 10.1016/j.cell.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 8.Dodd RD, Mito J, Kirsch DG. Animal models of soft-tissue sarcoma. Dis Models Mech. 2010;3(9–10):557–566. doi: 10.1242/dmm.005223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoon SS, Stangenberg L, Lee YJ, Rothrock C, Dreyfuss JM, Baek KH, Waterman PR, Nielsen GP, Weissleder R, Mahmood U, Park PJ, Jacks T, Dodd RD, Fisher CJ, Ryeom S, Kirsch DG. Efficacy of sunitinib and radiotherapy in genetically engineered mouse model of soft-tissue sarcoma. Int J Radiat Oncol Biol Phys. 2009;74(4):1207–1216. doi: 10.1016/j.ijrobp.2009.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dodd RD, Mito JK, Eward WC, Chitalia R, Sachdeva M, Ma Y, Barretina J, Dodd L, Kirsch DG. NF1 deletion generates multiple subtypes of soft-tissue sarcoma that respond to MEK inhibition. Mol Cancer Ther. 2013;12(9):1906–1917. doi: 10.1158/1535-7163.MCT-13-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim S, Dodd RD, Mito JK, Ma Y, Kim Y, Riedel RF, Kirsch DG. Efficacy of phosphatidylinositol-3 kinase (PI3K) inhibitors in a primary mouse model of undifferentiated pleomorphic sarcoma (UPS) Sarcoma. 2012;2012:680708. doi: 10.1155/2012/680708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eisinger-Mathason TS, Zhang M, Qiu Q, Skuli N, Nakazawa MS, Karakasheva T, Mucaj V, Shay JE, Stangenberg L, Sadri N, Pure E, Yoon SS, Kirsch DG, Simon MC. Hypoxia-dependent modification of collagen networks promotes sarcoma metastasis. Cancer Discov. 2013;3(10):1190–1205. doi: 10.1158/2159-8290.CD-13-0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mito JK, Min HD, Ma Y, Carter JE, Brigman BE, Dodd L, Dankort D, McMahon M, Kirsch DG. Oncogene-dependent control of miRNA biogenesis and metastatic progression in a model of undifferentiated pleomorphic sarcoma. J Pathol. 2013;229(1):132–140. doi: 10.1002/path.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mito JK, Ferrer JM, Brigman BE, Lee CL, Dodd RD, Eward WC, Marshall LF, Cuneo KC, Carter JE, Ramasunder S, Kim Y, Lee WD, Griffith LG, Bawendi MG, Kirsch DG. Intraoperative detection and removal of microscopic residual sarcoma using wide-field imaging. Cancer. 2012;118(21):5320–5330. doi: 10.1002/cncr.27458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cuneo KC, Mito JK, Javid MP, Ferrer JM, Kim Y, Lee WD, Bawendi MG, Brigman BE, Kirsch DG. Imaging primary mouse sarcomas after radiation therapy using cathepsin-activatable fluorescent imaging agents. Int J Radiat Oncol Biol Phys. 2013;86(1):136–142. doi: 10.1016/j.ijrobp.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moding EJ, Clark DP, Qi Y, Li Y, Ma Y, Ghaghada K, Johnson GA, Kirsch DG, Badea CT. Dual-energy micro-computed tomography imaging of radiation-induced vascular changes in primary mouse sarcomas. Int J Radiat Oncol Biol Phys. 2013;85(5):1353–1359. doi: 10.1016/j.ijrobp.2012.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clark DP, Ghaghada K, Moding EJ, Kirsch DG, Badea CT. In vivo characterization of tumor vasculature using iodine and gold nanoparticles and dual energy micro-CT. Phys Med Biol. 2013;58(6):1683–1704. doi: 10.1088/0031-9155/58/6/1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DuPage M, Dooley AL, Jacks T. Conditional mouse lung cancer models using adenoviral or lentiviral delivery of Cre recombinase. Nat Protoc. 2009;4(7):1064–1072. doi: 10.1038/nprot.2009.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mito JK, Riedel RF, Dodd L, Lahat G, Lazar AJ, Dodd RD, Stangenberg L, Eward WC, Hornicek FJ, Yoon SS, Brigman BE, Jacks T, Lev D, Mukherjee S, Kirsch DG. Cross species genomic analysis identifies a mouse model as undifferentiated pleomorphic sarcoma/malignant fibrous histiocytoma. PloS One. 2009;4(11):e8075. doi: 10.1371/journal.pone.0008075. [DOI] [PMC free article] [PubMed] [Google Scholar]