Abstract
Purpose
To evaluate differences in overall survival in patients with hepatocellular carcinoma (HCC) after the establishment of a multidisciplinary clinic (MDC) for HCC.
Methods
Patient demographic and tumor characteristics of 355 patients diagnosed with HCC were collected between October 2006 and September 2011. Patients diagnosed after the initiation of the HCC MDC on October 1, 2010, were compared to patients diagnosed in the 4 years before. Patient demographics, tumor characteristics, treatment regimens, and overall survival were analyzed between the groups.
Results
A total of 105 patients were diagnosed in the time period after HCC MDC initiation compared to 250 patients in the previous 4 years. Patients diagnosed with HCC after the HCC MDC had fewer symptoms at presentation (64 vs. 78 %, p = 0.01) and earlier stage of tumor presentation [Barcelona Clinic for Liver Cancer (BCLC) A stage, 44 vs. 26 %, p = 0.0003; tumor, node, metastasis classification system stage 1, 44 vs. 30 %, p = 0.003) compared with patients diagnosed before MDC formation. The median time to treatment after diagnosis in the later period was significantly shorter than in the earlier time period (2.3 vs. 5.3 months, p = 0.002). On multivariate analysis, being seen in the HCC MDC remained independently associated with better overall survival (hazard ratio 2.5, 95 % confidence interval 2–3), after adjusting for BCLC stage and recipient of curative treatment. Patients diagnosed after HCC MDC initiation had a median survival of 13.2 months compared to the 4.8 months observed in patients diagnosed before MDC formation (p = 0.005).
Conclusions
The implementation of a MDC for the evaluation and treatment of patients with HCC is associated with improved overall survival.
Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide and is responsible for more than 500,000 deaths annually.1 The incidence and mortality of HCC have increased threefold over the past two decades, and it is the fastest-growing cause of cancer-related deaths in the United States.2 Patients diagnosed with HCC are a heterogenous group as a result of their underlying chronic liver dysfunction and presence of a concomitant malignancy. This heterogeneity requires a multifaceted treatment approach including surgical, locoregional, systemic, and supportive treatment options encompassing the specialities of surgery, radiology, oncology, hepatology, and palliative care.
The creation of multidisciplinary disease teams is considered the optimal mechanism to provide care to patients with cancer.3 A component of this approach, especially in cancer subtypes where treatment is multifaceted, encompassing locoregional and systemic options, is the multidisciplinary clinic (MDC). These clinics often utilize a same-day, single-visit format where patients are seen by physicians from multiple specialities who discuss pertinent findings in real time, resulting in a consensus treatment plan.
Despite the widespread endorsement of multidisciplinary care and MDC formation, little tangible evidence exists demonstrating a quantitative or even qualitative benefit in clinical outcomes. Previously published studies have failed to quantify changes in improved overall survival rates in other cancers and instead have focused on changes seen in clinical care recommendations, consensual team decision making, good teamwork, and increased adherence to clinical practice guidelines after either the implementation of a MDC or MDC tumor board conference.4–7 Thus, it is unclear whether the implementation of a speciality cancer MDC improves clinical care as assessed by traditional outcome measures or provides only a format to generate consensus treatment decisions.
The complex treatment decisions inherent in HCC management coupled with increasing incidence provided the impetus to forming a comprehensive HCC MDC at UT Southwestern Medical Center. The objective of this study was to evaluate the clinical impact in terms of tangible patient outcomes (i.e. overall survival) after the establishment of this clinic.
METHODS AND MATERIALS
HCC MDC Format
On October 1, 2010, a HCC MDC was implemented at Parkland Memorial Health and Hospital System (PHHS), the safety-net hospital system for Dallas County, Texas, and a sponsoring institution of UT Southwestern Medical Center. The purpose of this clinic was to provide same-site, single-visit care to patients with suspected, newly diagnosed, or established HCC. The weekly HCC MDC consists of a dedicated clinic nurse navigator who triages patient referrals and is staffed by attending physicians, fellows, and residents from surgical oncology, transplant hepatology, interventional radiology, and medical oncology. On an as-needed basis, physicians from radiation oncology and palliative care are available for consultation.
Before a clinic visit, the nurse navigator ensures that all current cross-sectional imaging is available for review. On the morning of the clinic, patients undergo routine laboratory tests to assess tumor markers and underlying liver dysfunction. In conjunction with the clinic visit, a conference is held where physicians from surgical oncology, transplant hepatology, interventional radiology, diagnostic radiology, radiation oncology, medical oncology, and palliative care discuss pertinent patient findings and formulate a consensus treatment plan implemented during afternoon patient care visits. The conference is typically attended by 20 attending physicians from these specialities as well as additional nursing and house staff. All patients with HCC diagnosed after October 1, 2010, were seen in the MDC unless they were lost to follow-up or died before the clinic visit.
Study Population
Using a prospectively maintained HCC database at UT Southwestern Medical Center, all patients with known HCC diagnosed at PHHS between October 1, 2006, and September 30, 2011, were identified. As the safety-net hospital system for Dallas County, Texas, PHHS serves a large population of patients with cirrhosis and cares for approximately 50 % of HCC patients in the area.
HCC diagnosis was based on American Association for the Study of Liver Disease (AASLD) criteria.8 Any patients without imaging studies were excluded because their tumor characteristics could not be adequately determined. We also excluded patients who had a liver mass without characteristic imaging (arterial enhancement with delayed washout) or histologic confirmation. The institutional review board of the UT Southwestern Medical Center approved this study.
Data Collection
Two cohorts of patients were classified: those diagnosed before the initiation of the HCC MDC (October 1, 2006–September 30, 2010; and those diagnosed in the year after MDC initiation (October 1, 2010–September 30, 2011). A retrospective review of each patient’s medical record was performed to obtain patient demographics, clinical history, laboratory data, and imaging results. From October 1, 2010, the database was maintained prospectively. Clinical history of interest included hepatitis B and C serostatus, α-fetoprotein (AFP), alcohol abuse (defined as intake >60 g/day for men and >40 g/day for women), treatment delivery, and long-term outcome. Laboratory data included total bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), albumin, platelet count, and international normalized ratio. Tumor characteristics of interest included the number of lesions, maximum tumor diameter, lymph node involvement, portal vein invasion, presence of extrahepatic metastases, and tumor stage at diagnosis. The Barcelona Clinic for Liver Cancer (BCLC) and tumor, node, metastasis classification system (TNM) systems were used for tumor staging.8
Screening for HCC was based on the AASLD guidelines that pertained to the time period of the study.8 A patient was considered to have received HCC screening if an ultrasound of the abdomen had been performed in the 12-month period before HCC diagnosis. Treatment for HCC was considered curative if it consisted of local ablative techniques [radiofrequency ablation (RFA) or ethanol injection], surgical resection, or liver transplantation. Treatment was considered locoregional if it consisted of any embolization techniques, including bland embolization, transarterial chemoembolization (TACE) or drug-eluting bead embolization, or stereotactic body radiation. Treatment was considered systemic if chemotherapy was provided. Patients provided with no treatment were treated with best supportive care. If patients received multiple treatments, they were classified as having received the most curative therapy. For example, if a patient received TACE before liver transplantation, he or she was classified as having liver transplantation, i.e. a curative treatment.
Statistical Analysis
Fisher’s exact and chi-square tests were used to analyze differences between the two groups of patients. Survival analysis was performed by the Kaplan–Meier method with log rank univariate analysis. All variables significant in the univariate analysis (log rank p < 0.05) were placed in the multivariate model. The hazard ratios and p values for the multivariate model were obtained by Cox proportional hazard regression. All tests were two-sided and performed at the 5 % significance level. Statistical analysis was performed using the SPSS statistical software package (version 21.0 for Macintosh; IBM, Armonk, NY, USA).
RESULTS
Patient Characteristics
A total of 355 consecutive patients diagnosed with HCC between October 2006 and September 2011 were identified (Table 1). Two hundred fifty patients were diagnosed in the 4 years before and 105 patients were diagnosed in the year after the implementation of the HCC MDC. The number of patients diagnosed between October 2006 and September 2007, October 2007 and September 2008, October 2008 and September 2009, and October 2009 and September 2010 was 42, 57, 75, and 76, respectively.
TABLE 1.
Differences in patient characteristics by HCC MDC formation
| Characteristic | After HCC MDC initiation (n = 105) | Before HCC MDC initiation (n = 250) | p value |
|---|---|---|---|
| Gender | 0.06 | ||
| Male | 75 (71 %) | 203 (81 %) | |
| Female | 30 (19) | 47 (19) | |
| Age at diagnosis, year, mean ± SD | 58.4 ± 8.2 | 56.5 ± 8.9 | 0.07 |
| Race/ethnicity | 0.78 | ||
| White | 29 (29) | 70 (28) | |
| Black | 32 (31) | 88 (35) | |
| Hispanic | 34 (32) | 69 (28) | |
| Asian | 10 (9) | 23 (9) | |
| Etiology of chronic liver disease | 0.10 | ||
| Chronic hepatitis C | 63 (60) | 167 (67) | |
| Chronic hepatitis B | 6 (6) | 28 (11) | |
| Alcohol | 13 (12) | 28 (11) | |
| Nonalcoholic steatohepatitis | 10 (10) | 9 (4) | |
| Other/unknown | 13 (12) | 18 (17) | |
| Symptoms at diagnosis | 0.01 | ||
| Yes | 67 (64) | 193 (78) | |
| No | 38 (36) | 57 (22) | |
| ECOG functional status | 0.03 | ||
| 0–1 | 97 (92) | 206 (83) | |
| > 2 | 8 (8) | 43 (17) | |
| Total serum bilirubin (mg/dl), median (range) | 1.1 (0.2–37.0) | 1.4 (0.2–35.2) | 0.21 |
| Serum albumin (g/dl), median (range) | 3.0 (1.5–4.7) | 3.1 (1.2–4.6) | 0.83 |
| Serum platelets (k/μl), median (range) | 125 (30–518) | 126 (6–842) | 0.12 |
| Serum AST (U/l), median (range) | 94 (22–3,742) | 136 (6–1,089) | 0.81 |
| Serum ALT (U/l), median (range) | 56 (12–451) | 47 (9–601) | 0.70 |
| MELD, median (range) | 11 (6–43) | 11 (6–29) | 0.12 |
| Child-Pugh-Turcotte | |||
| A | 42 (40) | 96 (39) | 0.89 |
| B | 41 (39) | 95 (38) | |
| C | 22 (21) | 58 (23) | |
| Screening ultrasound in year before HCC | diagnosis | ||
| Completed | 19 (18) | 53 (21) | 0.51 |
| Not completed | 86 (82) | 197 (79) |
MDC multidisciplinary clinic, HCC hepatocellular carcinoma, ECOG Eastern Cooperative Oncology Group, AST aspartate aminotransferase, ALT alanine aminotransferase, MELD Model for End Stage Liver Disease
Eighty-eight of the 105 patients diagnosed with HCC after October 1, 2010, were seen in the HCC MDC, while the remaining 17 patients were either lost to follow-up (n = 3) or died before their scheduled clinic visit (n = 14). Of the 250 patients diagnosed before October 1, 2010, 46 patients were subsequently seen in the HCC MDC.
Patients diagnosed in the year after the initiation of the HCC MDC presented with less symptoms including clinically relevant ascites, abdominal pain, or hepatic encephalopathy than those diagnosed in the earlier time period (64 vs. 78 %, p = 0.01) as well as better functional status as measured by Eastern Cooperative Oncology Group performance score of 0 or 1 (p = 0.03).
In both time periods, the race and/or ethnicity of the patients were evenly distributed between white, black, and Hispanic, and the etiology of chronic liver disease was predominantly chronic hepatitis C infection. There was no discernible difference in chronic liver dysfunction as measured by either the Model for End Stage Liver Disease (p = 0.12) or Child-Pugh-Turcotte (p = 0.89) scoring systems between the two groups. Patients underwent screening abdominal ultrasonography approximately 20 % of the time in the year before HCC diagnosis in both time periods.
Tumor Characteristics
Table 2 demonstrates the differences in HCC tumor presentation associated with year of HCC diagnosis. Patients diagnosed with HCC after the establishment of the HCC MDC presented more frequently with earlier stage tumors, with 43 % presenting with BCLC A stage and 44 % presenting with TNM I stage compared to 26 and 30 % of the patients diagnosed in the earlier time period (p = 0.003 and p = 0.003, respectively). Patients diagnosed in the later cohort also presented with fewer infiltrative tumors (20 vs. 31 %, p = 0.04) and a significantly lower level of AFP at diagnosis (median 30 vs. 237 ng/ml, p = 0.02) compared to the patients diagnosed in the earlier time period. Patients in the earlier time period also presented with increased incidence of portal venous tumor thrombus (40 vs. 20 %, p < 0.0001) and increased evidence of extrahepatic metastases (34 vs. 19 %, p = 0.005) compared to patients diagnosed in the later time period.
TABLE 2.
Differences in tumor characteristics by year of HCC diagnosis in relation to HCC MDC formation
| Characteristic | After HCC MDC initiation (n = 105) | Before HCC MDC initiation (n = 250) | p value |
|---|---|---|---|
| No. of tumors | 0.09 | ||
| Solitary | 56 (53 %) | 141 (56 %) | |
| Multiple | 49 (47) | 109 (44) | |
| Portal vein thrombus | < 0.0001 | ||
| Present | 21 (20) | 100 (40) | |
| Absent | 84 (80) | 150 (60) | |
| Distant metastases | |||
| Present | 20 (19) | 85 (34) | 0.005 |
| Absent | 85 (81) | 165 (66) | |
| Infiltrative HCC | 0.04 | ||
| Present | 21 (20) | 77 (31) | |
| Absent | 84 (80) | 172 (69) | |
| BCLC staging | 0.003 | ||
| A | 45 (43) | 65 (26) | |
| B | 13 (12) | 18 (7) | |
| C | 18 (17) | 79 (32) | |
| D | 29 (28) | 87 (35) | |
| AJCC TNM staging | 0.003 | ||
| I | 46 (44) | 76 (30) | |
| II | 15 (14) | 23 (9) | |
| III | 28 (26) | 82 (33) | |
| IV | 16 (15) | 69 (28) | |
| Okuda staging | |||
| I | 37 (35) | 75 (30) | 0.10 |
| II | 55 (52) | 120 (48) | |
| III | 13 (12) | 55 (22) | |
| Milan criteria for liver transplantation met | 40 (38) | 75 (30) | 0.14 |
| AFP at diagnosis, ng/ml, median (range) | 30 (1–29,100) | 237 (1–1,912,900) | 0.02 |
HCC hepatocellular carcinoma, MDC multidisciplinary clinic, BCLC Barcelona Clinic for Liver Cancer, AJCC American Joint Committee on Cancer, TNM tumor, node, metastasis classification system, AFP α-fetoprotein
Treatment Modalities
In patients diagnosed both before and after the initiation of the HCC MDC, the most common treatment modality was best supportive care (59 vs. 43 %, p = 0.03, respectively). However, patients diagnosed in the later time period were more likely to undergo curative treatment compared to patients diagnosed in the earlier time period (21 vs. 10 %, p = 0.006). Surgical resection was completed in ten patients in the later time period versus 14 patients in the earlier time period. Orthotopic liver transplantation was completed in six patients, five in the earlier time period and one in the later time period. RFA was provided to 11 patients in the later time period versus six patients in the earlier time period. Patients diagnosed after initiation of the HCC MDC underwent a similar rate of locoregional therapies (31 vs. 24 %, p = NS) and systemic therapy (6 vs. 8 %, p = NS) compared to patients diagnosed in the earlier time period. The mean time to treatment from diagnosis was significantly shorter in patients diagnosed after initiation of the HCC MDC (2.3 vs. 5.3 months, p = 0.002).
Clinical Outcomes and Predictors of Survival
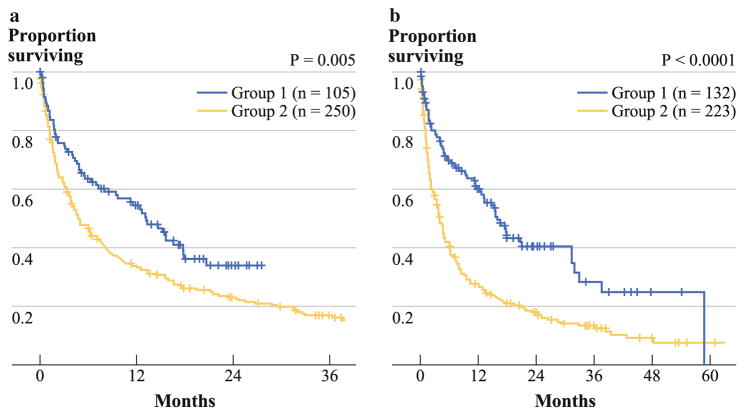
Table 3 demonstrates the clinicopathological variables associated with outcome after HCC presentation of the entire cohort of patients diagnosed after October 1, 2006. The estimated 1-year overall survival for patients diagnosed before and after MDC formation was 34 and 55 %, respectively. The median overall survival was 13.2 and 4.8 months (p = 0.005), respectively (Fig. 1a). The median follow-up was 7.9 and 4.2 months, respectively. On multivariate analysis, being seen in the HCC MDC remained independently associated with better overall survival, after adjusting for presence of early BCLC stage (A or B) and recipient of curative treatment. Patients included in the group being seen in the HCC MDC included the 88 patients diagnosed with HCC after MDC initiation and the 46 patients who were diagnosed before MDC initiation but who survived long enough to subsequently be seen in the HCC MDC. HCC diagnosed after the initiation of the HCC MDC was significant on univariate but not multivariate analysis.
TABLE 3.
Results of multivariate analysis for significant predictors of overall survival
| Characteristic | n | Median survival, mo (95 % confidence interval) | 1-year overall survival, % | Univariate p | Multivariate p | Hazard ratio (95 % confidence interval) |
|---|---|---|---|---|---|---|
| Gender | ||||||
| Male | 278 | 6.3 (4–8) | 39 | 0.97 | ||
| Female | 77 | 6.3 (0.9–12) | 44 | |||
| Age (years) | ||||||
| ≤60 | 242 | 7.2 (5–10) | 42 | 0.17 | ||
| > 60 | 113 | 4.3 (2–6) | 36 | |||
| Race/ethnicity | ||||||
| White | 99 | 7.3 (3–11) | 43 | 0.52 | ||
| Black | 122 | 5.0 (3–7) | 34 | |||
| Hispanic | 103 | 6.4 (2–11) | 43 | |||
| Asian | 33 | 7.7 (5–11) | 42 | |||
| Cause of chronic liver disease | ||||||
| HCV alone | 230 | 7.2 (5–10) | 41 | 0.13 | ||
| HBV alone | 34 | 5.9 (2–10) | 35 | |||
| Alcohol alone | 41 | 6.2 (2–10) | 38 | |||
| NASH | 19 | 13.3 (7–20) | 63 | |||
| Other | 31 | 3.1 (0.2–6) | 26 | |||
| Symptoms | ||||||
| Present | 260 | 3.8 (3–5) | 26 | < 0.001 | ||
| Absent | 95 | 24.6 (17–32)) | 76 | |||
| Child-Pugh-Turcotte | ||||||
| A | 139 | 16.5 (13–20) | 59 | < 0.001 | ||
| B | 136 | 4.3 (3–5) | 32 | |||
| C | 80 | 2.0 (1–3) | 18 | |||
| BCLC | ||||||
| A | 110 | 31.5 (25–38) | 79 | < 0.001 | < 0.001 | 2.6 (2–4) |
| B | 31 | 11.5 (7–16) | 49 | |||
| C | 97 | 3.8 (3–5) | 20 | |||
| D | 116 | 1.9 (2–2) | 16 | |||
| AJCC TNM stage | ||||||
| 1 | 122 | 24.6 (17–32) | 69 | < 0.001 | ||
| 2 | 38 | 17.8 (13–23) | 68 | |||
| 3 | 110 | 3.3 (1–5) | 24 | |||
| 4 | 85 | 2.3 (1–3) | 5 | |||
| Treatment | ||||||
| Curative | 46 | NR | 89 | < 0.001 | < 0.001 | 2.5 (2–3) |
| Locoregional | 90 | 17.8 (13–23) | 69 | |||
| Systemic | 25 | 4.6 (3–6) | 14 | |||
| None | 194 | 2 (2–3) | 15 | |||
| HCC diagnosed after MDC initiated | ||||||
| Yes | 105 | 13.2 (9–17) | 54 | 0.005 | ||
| No | 250 | 4.8 (3–6) | 34 | |||
| Patient seen in HCC MDC | ||||||
| Yes | 134 | 25.6 (12–39) | 70 | < 0.001 | < 0.001 | 2.5 (2–3) |
| No | 221 | 3.1 (2–4) | 21 | |||
HCV hepatitis C virus, HBV hepatitis B virus, NASH nonalcoholic steatohepatitis, BCLC Barcelona Clinic for Liver Cancer, AJCC American Joint Committee on Cancer, TNM tumor, node, metastasis classification system, NR not reached, HCC hepatocellular carcinoma, MDC multidisciplinary clinic
FIG. 1.
Kaplan–Meier estimated overall survival curves of patients (a) diagnosed after (blue line) or before (yellow line) the initiation of the HCC MDC and (b) either diagnosed after the initiation of the HCC MDC or who were diagnosed before MDC initiation but whose decision for treatment was made within the confines of the clinic (group 1) (blue line) versus patients who were diagnosed before MDC formation and either received no treatment or initial treatment before MDC formation (group 2) (yellow line)
In an attempt to correct for a lead-time bias by comparing patients either seen in the HCC MDC or who were diagnosed after the initiation of the HCC MDC, patients who were either diagnosed after the initiation of the HCC MDC or who were diagnosed before MDC initiation but whose decision for treatment was made within the confines of the clinic (group 1, n = 132 patients) were combined and compared to patients who were diagnosed before MDC formation and either received no treatment or initial treatment before MDC formation (group 2, n = 223 patients) (Fig. 2). Patients in group 1 had a longer median survival than patients in group 2 (15.9 vs. 3.9 months, p < 0.0001) (Fig. 1b).
FIG. 2.
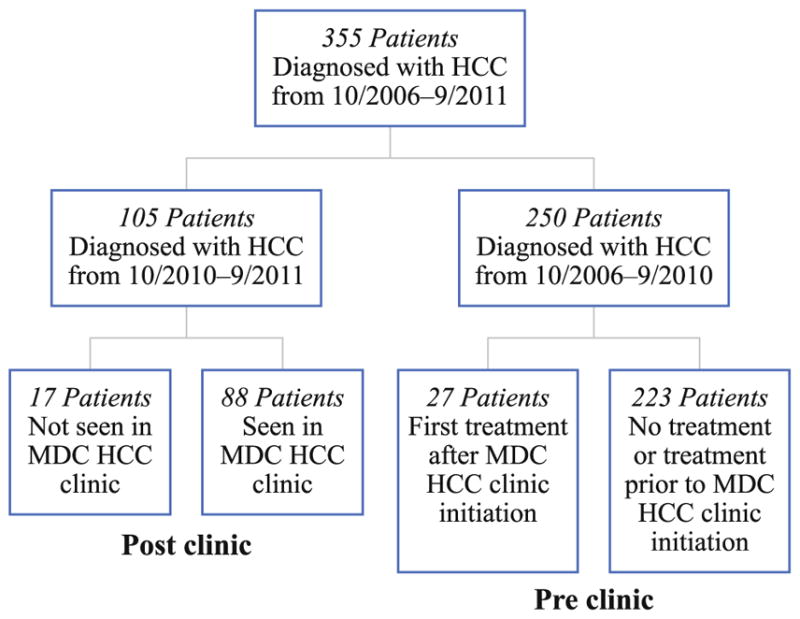
Flow diagram of patients diagnosed with HCC before and after initiation of the HCC MDC
Table 4 demonstrates the outcome of groups 1 and 2 when stratified by BCLC stage at HCC diagnosis. Median survival was not different between the groups when presenting with BCLC stage A disease. However, patients in group 1 had a significantly increased median survival compared to patients in group 2 when presenting with BCLC B, C, and D disease (respectively, 12.5 vs. 9.0 months, p = 0.05; 9.7 vs. 3.1 months, p < 0.0001; and 4.4 vs. 1.6 months, p = 0.01).
TABLE 4.
Overall survival by BCLC stage and era of HCC diagnosis and/or first treatment
| BCLC stage/era of clinic visit/treatment | n | Median survival, mo | p value |
|---|---|---|---|
| A | 0.76 | ||
| Group 1 | 60 | 31.5 | |
| Group 2 | 50 | 29.5 | |
| B | 0.05 | ||
| Group 1 | 18 | 12.5 | |
| Group 2 | 13 | 9.0 | |
| C | < 0.0001 | ||
| Group 1 | 24 | 9.7 | |
| Group 2 | 73 | 3.1 | |
| D | 0.01 | ||
| Group 1 | 30 | 4.4 | |
| Group 2 | 86 | 1.6 |
Group 1 (n = 132) comprised patients diagnosed with or first treatment of HCC after the initiation of HCC MDC; group 2 (n = 223) comprised diagnosed with or first treatment of HCC before the initiation of the HCC MDC
BCLC Barcelona Clinic for Liver Cancer, HCC hepatocellular carcinoma, MDC multidisciplinary clinic
DISCUSSION
Because of the increasing incidence of HCC seen in patients at UT Southwestern Medical Center and specifically at PHHS, a county safety-net hospital providing care for the indigent local population, a HCC MDC with an accompanying conference was formed. Despite widespread acceptance of multidisciplinary care in patients with HCC, the current study represents what is to our knowledge one of the first studies to demonstrate tangible survival benefits of a HCC MDC. Patients diagnosed with HCC in the year after formation of the HCC MDC had an overall survival nearly four times that observed in patients diagnosed in the years before MDC initiation. Undoubtedly the patients diagnosed in the later time frame had prolonged survival as a result of a more favorable stage of disease at presentation, as nearly 60 % of patients presented with either TNM stage I or II and BCLC stage A or B disease. However, after adjusting for BCLC stage in patients where treatment decisions were made within the HCC MDC, the survival benefit persisted in BCLC B, C, and D stages. Given that patients with BCLC A–stage disease are most likely referred for curative options at an earlier time period with less delay in treatment, this is not surprising.
The earlier stage of diagnosis cannot be explained by improved screening rates in the later time period: in both eras, the rate of screening approaches 20 %, consistent with previously published rates elsewhere.9,10 However, we anticipate that with greater awareness of HCC within our hospital system, HCC screening rates should increase as previously described barriers to screening are identified and eliminated.11 As part of establishing a MDC, participating physicians were encouraged to refer in all patients with a concerning finding on axial imaging or an elevated AFP level, whether being done as part of a dedicated surveillance program or as an isolated finding. This aspect of the HCC MDC likely improved time to diagnosis and may in part explain the improved tumor stage presentation after the HCC MDC was initiated. This practice undoubtedly increased the volume of patients seen in the clinic and slightly increased the false-negative rate of HCC diagnosed in the clinic. Over the year after the establishment of the clinic, 91 % of all clinic visits (average of 31 per week) were by patients with a diagnosis of HCC, so resources spent in managing non-HCC patients were minimal.
In addition to patients being referred in at an earlier stage of disease, overall survival was likely affected by the reduction in time to treatment in patients needing treatment in the later time period. Multiple publications predominantly pertaining to the treatment of breast cancer have well documented the correlation between treatment delays (exceeding 90 days) and decreased patient survival.12–14 Similarly, we have recently demonstrated that treatment delays exceeding 90 days are associated with significantly worse survival in patients with HCC.15 The reduction of time to treatment was likely the result of having all providers, including surgeons, interventional radiologists, and medical oncologists, who provide the vast majority of treatment options, present in the clinic setting, thus eliminating untimely delays from scheduling multiple physician visits on different days in varying clinic settings. In the safety-net population that this clinic primarily served, this is of the utmost importance because these patients often do not have the means to travel on multiple days for clinic appointments.
Providing quality cancer care has become exceedingly more complex in the face of the advent of multiple treatment regimens offered by a host of different providers. Over time, the decision-making progress to determine which treatment best serves a particular patient has evolved from tumor board discussions to the formation of multi-disciplinary disease teams. Recognized as the standard of care in the treatment of cancer patients, this team-based approach, which historically included only a conference-type format, has slowly led to the formation of MDCs providing single-visit, same-day consultation with multiple providers encompassing a variety of speciality fields. Although these clinics demonstrate improved patient satisfaction by reducing the often fragmented care seen with multiple physician visits, there are few empiric data to demonstrate improvement in patient outcomes in terms of survival benefits.
Previously published studies have attempted to use surrogate markers of survival, including adherence to recognized treatment guidelines, enrollment onto clinical trials, review of pathology and radiology findings, and change in treatment plans to demonstrate improvement in patient outcomes after the establishment of a multidisciplinary cancer clinic.4–7 Hong et al. in a systematic review illustrated the difficulties in correlating the establishment of multidisciplinary care and improvement of overall survival. In their series, they identified 21 studies examining the relationship between multidisciplinary care of the cancer patient and survival.16 However, the vast majority of these studies had vague definitions of what was considered multidisciplinary care, they relied on large administrative databases with inherent biases, or they utilized surrogate survival measures and not actual overall or disease-specific survival.
Two recent studies not included in the systemic review have demonstrated improved overall survival after initiation of multidisciplinary care.17,18 Both of these studies carefully defined the characteristics of a multidisciplinary care team and intervention as well as utilized actual survival data in place of surrogate markers. Kesson et al.17 demonstrated after the rearrangement of cancer care in Scotland that the formation of the MDC breast clinics decreased breast cancer–specific mortality by 18 % and led to uniformity of breast cancer care within Scotland. In a small study with a homogenous patient population, Chang et al. demonstrated the formation of a HCC MDC in a Veterans Affairs (VA) setting improved stage-for-stage overall survival. The limitation of this study was the homogenous population in the VA setting, which mainly comprised chronic hepatitis C virus infection and male patients.18
The current study had several limitations. Although the database from which the study variables were obtained was collected in a prospective fashion after initiation of the clinic in 2010, retrospective collection of data was used before this time point. This study was conducted in a safety-net hospital system where the majority of patients did not have insurance other than that provided from the county government (58 % in the later time period, 56 % in the earlier time period). This insurance, Parkland Health Plus, covers most HCC treatments including resection, RFA, TACE, radiotherapy, and systemic therapy, but it does not cover liver transplantation. Therefore, patients without other forms of insurance were not evaluated for liver transplantation as a treatment option. On the basis of the Milan criteria, 38 % of patients in the later time period and 29 % of patients in the earlier time period were eligible for evaluation of liver transplantation, although prior studies report high rates of other social and medical barriers in this population. Because the majority of patients with HCC have a low socioeconomic status, often getting their care from safety-hospital systems, we believe our results are applicable to other hospital settings. The use of a pre-post design strategy over a large time period is a recognized limitation of the current study. However, during the time period 2006–2011, treatment regimens were similar, other than the advent of systemic sorafenib therapy, which was available before 2008 in clinical trial use. The modes and timing of surveillance imaging studies and the access to liver transplantation were similar in both time periods.
In conclusion, the establishment of a multidisciplinary HCC clinic is associated with improved diagnoses at an early disease stage and shorter time to treatment. Most importantly, the implementation of a HCC MDC improved overall survival independent of tumor stage at presentation. These compelling data should provide impetus for increased adoption of multidisciplinary care for HCC patients nationally.
Acknowledgments
Supported in part by grants KL2 RR024983-04 and UL1 RR024982. The content is solely the responsibility of the authors and does not necessarily represent the official views of UT-STAR, the University of Texas Southwestern Medical Center and its affiliated academic and health care centers, the National Center for Advancing Translational Sciences, or the National Institutes of Health.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153–6. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745–50. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- 3.Lamb BW, Brown KF, Nagpal K, Vincent C, Green JS, Sevdalis N. Quality of care management decisions by multidisciplinary cancer teams: a systematic review. Ann Surg Oncol. 2011;18:2116–25. doi: 10.1245/s10434-011-1675-6. [DOI] [PubMed] [Google Scholar]
- 4.Pawlik TM, Laheru D, Hruban RH, Coleman J, Wolfgang CL, Campbell K, et al. Evaluating the impact of a single-day multi-disciplinary clinic on the management of pancreatic cancer. Ann Surg Oncol. 2008;15:2081–8. doi: 10.1245/s10434-008-9929-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang JH, Vines E, Bertsch H, Fraker DL, Czerniecki BJ, Rosato EF, et al. The impact of a multidisciplinary breast cancer center on recommendations for patient management: the University of Pennsylvania experience. Cancer. 2001;91:1231–7. doi: 10.1002/1097-0142(20010401)91:7<1231::aid-cncr1123>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 6.Fleissig A, Jenkins V, Catt S, Fallowfield L. Multidisciplinary teams in cancer care: are they effective in the UK? Lancet Oncol. 2006;7:935–43. doi: 10.1016/S1470-2045(06)70940-8. [DOI] [PubMed] [Google Scholar]
- 7.Newman EA, Guest AB, Helvie MA, Roubidoux MA, Chang AE, Kleer CG, et al. Changes in surgical management resulting from case review at a breast cancer multidisciplinary tumor board. Cancer. 2006;107:2346–51. doi: 10.1002/cncr.22266. [DOI] [PubMed] [Google Scholar]
- 8.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2012;53:1020–2. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davila JA, Morgan RO, Richardson PA, Du XL, McGlynn KA, El-Serag HB. Use of surveillance for hepatocellular carcinoma among patients with cirrhosis in the United States. Hepatology. 2010;52:132–41. doi: 10.1002/hep.23615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singal AG, Yopp A, Skinner CS, Packer M, Lee WM, Tiro JA. Utilization of hepatocellular carcinoma surveillance among American patients: a systemic revew. J Gen Intern Med. 2012;27:861–7. doi: 10.1007/s11606-011-1952-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singal AG, Yopp AC, Gupta S, Skinner CS, Halm EA, Okolo E, et al. Failure rates in the hepatocellular carcinoma surveillance process. Cancer Prev Res (Phila) 2012;5:1124–30. doi: 10.1158/1940-6207.CAPR-12-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richards MA, Westcombe AM, Love SB, Littlejohns P, Ramirez AJ. Influence of delay on survival in patients with breast cancer: a systematic review. Lancet. 1999;353:1119–26. doi: 10.1016/s0140-6736(99)02143-1. [DOI] [PubMed] [Google Scholar]
- 13.Smith EC, Ziogas A, Anton-Culver H. Delay in surgical treatment and survival after breast cancer diagnosis in young women by race/ethnicity. JAMA Surg. 2013;148:516–23. doi: 10.1001/jamasurg.2013.1680. [DOI] [PubMed] [Google Scholar]
- 14.Jung SY, Sereika SM, Linkov F, Brufsky A, Weissfeld JL, Rosenzweig M. The effect of delays in treatment for breast cancer metastasis on survival. Breast Cancer Res Treat. 2010;130:953–64. doi: 10.1007/s10549-011-1662-4. [DOI] [PubMed] [Google Scholar]
- 15.Singal AG, Waljee AK, Patel N, Tiro JA, Marrero JA, Yopp AC. Therapeutic delays lead to worse survival among patients with hepatocellular carcinoma. J Natl Compr Canc Netw. 2013;11:1101–8. doi: 10.6004/jnccn.2013.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hong NJ, Wright FC, Gagliardi AR, Paszat LF. Examining the potential relationship between multidisciplinary cancer care and patient survival: an international literature review. J Surg Oncol. 2012;102:125–34. doi: 10.1002/jso.21589. [DOI] [PubMed] [Google Scholar]
- 17.Kesson EM, Allardice GM, George WD, Burns HJ, Morrison DS. Effects of multidisciplinary team working on breast cancer survival: retrospective, comparative, interventional cohort study of 13,722 women. BMJ. 2012;344:e2718. doi: 10.1136/bmj.e2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang TT, Sawhney R, Monto A, Davoren JB, Kirkland JG, Stewart L, et al. Implementation of a multidisciplinary treatment team for hepatocellular cancer at a Veterans Affairs Medical Center improves survival. HPB (Oxford) 2008;10:405–11. doi: 10.1080/13651820802356572. [DOI] [PMC free article] [PubMed] [Google Scholar]