Abstract
Alglucosidase alfa (rhGAA) has altered the course of an otherwise fatal outcome in classic infantile Pompe disease (IPD), which presents with cardiomyopathy and severe musculoskeletal involvement. However, the response to therapy is determined by several factors including the development of high and sustained antibody titers (HSAT) to rhGAA. Cross-reactive immunologic material (CRIM) negative patients are at the highest risk for development of HSAT. Immune tolerance induction (ITI) with methotrexate, rituximab, and intravenous immunoglobulin (IVIG) has been largely successful in preventing the immune response and in achieving tolerance when done in conjunction with enzyme replacement therapy (ERT) initiation. Reducing antibody titers in cases with an entrenched immune response remains a challenge in the field despite the use of multiple immunomodulatory agents. Success has been shown with addition of bortezomib to the ITI regimen, yet the prolonged course and potential risks with the use of such agents’ demands caution.
We present here a 7-year-old CRIM-negative IPD patient who was not successfully tolerized by an ITI regimen with rituximab, methotrexate, and IVIG due to intolerability to the regimen and recurrent infections. She went on to develop HSAT, with significant clinical decline, loss of all motor abilities, and a fragile medical state, which made it challenging to institute the bortezomib based regimen to reduce HSAT. She had severe developmental delay, respiratory failure with invasive ventilation and tracheostomy, persistent hypotonia, ptosis of eyelids, diffuse severe osteopenia, contractures, and was completely G-tube fed. As a rescue mechanism, we treated her with high dose and high frequency IVIG in an attempt to reduce rhGAA IgG antibody titers (antibody titers; titers). Her titers saw a steady decline on weekly IVIG doses at 1 g/kg for 20 weeks. Subsequently when the IVIG regimen was altered to 1 g/kg every month, rising titers were detected and therefore the regimen was changed to a biweekly regimen. High dose IVIG resulted in an eightfold decrease in antibody titers. Clinically, she showed improvement with partial recovery of previously lost motor abilities, especially hand movements and better head and neck control than before. The regimen was safely tolerated with no hospitalizations. The effectiveness of IVIG as a single agent, in this case with multiple comorbidities and fragile clinical status, was lifesaving and may represent an effective, perhaps lifesaving rescue approach to reduce antibody titers.
Keywords: Pompe disease, Immunogenicity, Anti-rhGAA IgG antibodies, Immunomodulation, High dose IVIG
1. Introduction
Pompe disease is an autosomal recessive lysosomal storage disorder caused by a deficiency of acid alpha-glucosidase (GAA), leading to accumulation of glycogen in cardiac, skeletal and smooth muscle tissue [1,2]. It has been broadly classified into classic infantile Pompe disease (IPD) and late onset Pompe disease (LOPD). Classic IPD is the most severe form with cardiomyopathy presenting at birth, severe musculoskeletal involvement, and death usually by two years of age without treatment [2,3]. LOPD, which encompasses childhood, juvenile, and adult-onset disease, represents a clinical spectrum with variable presentation and severity of limb girdle muscle weakness and respiratory insufficiency from infancy to as late as the sixth decade [4,5]. The otherwise fatal course of IPD has been altered with improved survival since the availability of enzyme replacement therapy (ERT) using alglucosidase alfa (rhGAA) [6]. However, the response to ERT and thereby the clinical outcome is largely influenced by many factors including the cross-reactive immunologic material (CRIM) status and antibody response to rhGAA [7,8]. CRIM-negative patients and a subset of CRIM-positive patients develop high sustained antibody titers (HSAT) and sustained intermediate titers (SIT) neutralizing the efficacy of the therapeutic protein, thereby leading to a progressive course of illness in spite of ongoing ERT [8–10]. HSAT is defined as titers ≥1:51,200 on two or more separate occasions after ≥6 months on ERT [11], yet it needs to be recognized that titers ≥1:12, 800 over a period of time (SIT) also reduce enzyme efficacy. Pharmacokinetic studies have shown that patients with antibody titers ≥1:12,800 by Week 12 on ERT had an average increase in clearance of infused ERT by 50% from Week 1 to Week 12 [12]. Immunomodulation using rituximab, methotrexate, and IVIG, or rituximab and sirolimus/mycophenolate have been used in the ERT-naïve settings [13–15]. There is an increasing body of evidence of success of the immune tolerance induction (ITI) regimen with rituximab, methotrexate, and IVIG when used at inception of ERT. However, in rare instances there is a challenge with the regimen if a patient cannot tolerate these therapies, and/or develops HSAT or an entrenched immune response, despite these therapies. There is also a potential risk of infection with use of such agents. A combination of bortezomib, rituximab, methotrexate, and IVIG has been used in the entrenched immune response setting of IPD. This regimen was successful in inducing tolerance in cases with HSAT and thereby resulted in better clinical outcome with muscle function restoration and symptom relief [7,16]. However, in the setting of an entrenched immune response, long term use of these agents is often needed before tolerance is achieved. Overall, when the risk-benefit profile permits, it is worthwhile to use a regimen that would result in immune tolerance. However, in some instances this may not be as feasible, given the higher probability of infections in some patients. This is further complicated by their overall significant medical fragility and/or intolerance to the drugs in the regimen.
We present here a 7-year-old CRIM-negative IPD case where use of high dose IVIG regimen was implemented as a rescue protocol in an attempt to reduce HSAT. This patient was unable to tolerate agents such as rituximab and methotrexate, due to the development of repeated multiple infections after institution of therapy with these agents in the ERT-naïve setting. This patient developed HSAT to rhGAA with an ongoing progressive clinical decline. High dose IVIG was successful at reducing antibody titers and was lifesaving for this patient. It also resulted in preserving and rescuing the very limited remaining motor abilities which were critical to maintain an acceptable quality of life in this patient.
2. Case report
2.1. Initial presentation
The patient is a 7-year-old Hispanic female. She was diagnosed with hypertrophic cardiomyopathy as a newborn when she presented with supraventricular tachycardia (SVT) and had marked ventricular hypertrophy. She presented with significant motor involvement including early respiratory and cardiac failure in the first months of life. Repeated episodes of cardio-respiratory distress and infections, inability to feed orally, and failure to thrive along with severe cardiomyopathy raised the suspicion for Pompe disease. A diagnosis of CRIM-negative IPD was made at age 5 months by muscle biopsy followed by GAA gene mutation analysis. She was homozygous for c.1195-18_ 2190-20del. (deletion of exons 8 through 15), a variant that abolishes the enzyme catalytic site on exons 10 and 11 (as reported earlier) [17,18]. At the time of her diagnosis she was already on BiPAP. Urinary Glc4 levels and CK levels were elevated in line with her diagnosis of Pompe disease and she was enrolled in a clinical trial (clinicaltrials.gov # NCT00701129) to be treated with rhGAA and an ITI regimen using rituximab, methotrexate, and IVIG [7] at age 6 months. At baseline, she was antibody negative to rhGAA. She tolerated the initial cycle of ITI without any significant adverse events, and seroconverted at week 6 post-ERT with IgG antibody titer of 1:800, which increased to 1:1600 at week 10 post-ERT. In view of her enrollment in a clinical trial and the ability to use a second round of ITI only in the first 6 months of ERT, she was initiated on a second cycle of ITI on week 20 post-ERT. However, midway through the second round of ITI and a titer of 1:200, she developed complications. She was hospitalized for severe diarrhea, Clostridium difficile colitis, respiratory failure, and sepsis. ITI was stopped and she was treated for her acute complications. Following this episode of acute decompensation during the second round of ITI, she continued to receive ERT without ITI. Her antibody titers began to rise. As regards to cardiac hypertrophy, the patient had an almost complete resolution and normal ventricular systolic function, documented on serial echocardiograms, and her cardiac function remained stable on ERT. Her antibody titers increased and remained moderately high since week 44 post-ERT (1:6400 to 1:12,800; peak titers of 12,800), age 15 months. Her clinical course otherwise was marked by recurrent and prolonged hospitalizations for multiple infections. Immunodeficiency disorders were ruled out through immune assays. The patient experienced a steady and significant clinical decline with severe functional motor deficits over the years. Motor deficits worsened with titers reaching the HSAT levels as outlined below.
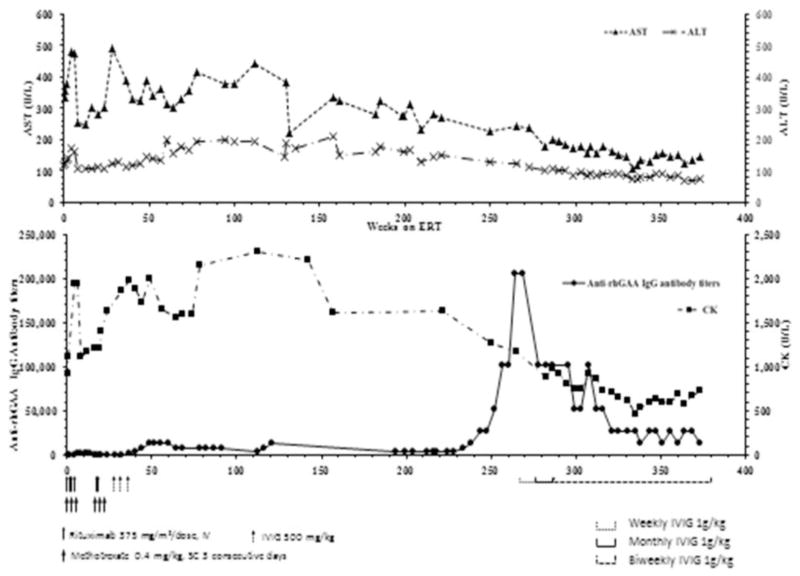
2.2. Course of illness on ERT and rising rhGAA IgG antibody titers (week 234 to week 296) (Fig. 1)
Fig. 1.
Antibody titers, CK, AST and ALT titers over time and in response to initial ITI and long term use of high dose IVIG.
As this severely affected patient survived through early childhood, she developed several long term sequelae of IPD including the following: severe developmental delay; respiratory failure with invasive ventilation and tracheostomy since age 7 months; persistent hypotonia and ptosis; diffuse severe osteopenia; and contractures with a long standing posterior dislocation of left radial head; requirement for G-tube feeding; and multiple hospitalizations for infections. All of these sequelae were managed by comprehensive multidisciplinary approach including pharmacological, dietary, respiratory, speech, language, and physical therapy alongside ERT as and when they were detected. By about age 5 years, she communicated primarily via nonverbal means, gesturing with her right hand, and to a minimal extent, with closure of her eyes and movements of mouth. She could use her right hand to operate assistive devices. She had moderately high antibody titers throughout her course with a peak of 1:12,800 (1:6400–1:12,800, Fig. 1) until week 239 post-ERT, age 5 years. Her titers thereafter showed a rapid rise peaking to 1:204,000 in week 265 through week 270 post-ERT. These HSAT titers correlated clinically with a life threatening course, and decreasing motor function with complete loss of movement of her right hand. There was also an increase in ptosis thus significantly affecting her two most important modes of communication and rendering her totally isolated and non-communicative. She developed a seizure disorder with recurrent episodes of staring and eye rolling, facial pallor, and unresponsiveness to stimuli lasting between 10 and 30 min representing partial seizures, first episode at age 5 years 5 months. Electroencephalography (EEG) was unrevealing without frank seizure activity but showed a generalized diffuse slowing. After an evaluation by a pediatric neurologist she was started on prophylactic anti-epileptic therapy with low dose levetiracetam which was increased due to breakthrough seizures. She had no seizures after instituting the higher dose of levetiracetam. Therefore, an intervention with an alternative immune modulatory treatment was warranted to prevent any further clinical decline.
2.3. Treatment with high dose and high frequency IVIG (week 265 to week 356) (Fig. 1)
In view of HSAT coupled with clinical deterioration and inability to tolerate rituximab and methotrexate, immunomodulation with an alternative approach that would be safely tolerated was needed. High dose IVIG (1 g/kg) therapy alongside ERT as a rescue protocol was initiated at week 265 post-ERT, age 5 years 6 months. IVIG was initially given as a weekly dose for 20 weeks and was changed to monthly dose of 1 g/kg at week 272 post-ERT. However, the antibody titers which had dropped to 1:51,200 on weekly dose increased to 1:102,400 on the monthly regimen. Therefore, IVIG was switched to biweekly doses of 1 g/kg and has been maintained as such. Her antibody titers after initiation of IVIG showed a steady decline thereafter, with the most recent titer of 1:12,800 at week 374 post-ERT, age 7 years 6 months (Fig. 1). Partial improvement in her right hand movements, improved ability to move her eyes, and better neck and head movements have been reported concurrent with this consistent fall in titers. However, continued evaluation of her clinical course will reveal whether there are further developments, how and when to taper IVIG and the consideration for a more definitive, possibly a limited ITI regimen to induce tolerance as her clinical fragility improves. She has had no hospitalizations or infections since start of IVIG and no drug related adverse events were noted.
3. Discussion
ERT with rhGAA has improved the survival of both CRIM-positive and CRIM-negative IPD and attempts are being made to circumvent the challenges of an entrenched immune response (HSAT and SIT) neutralizing the efficacy of ERT [19,20]. A successful ITI regimen of rituximab, methotrexate, and IVIG in the treatment naïve setting [7,15] and a multipronged approach with bortezomib and ITI regimen targeting T-cells, B-cells, and plasma cells has been the only regimen successful in achieving immune tolerance and reducing HSAT [16]. The case presented here, in which intolerance to the recommended ITI was observed, developed SIT for a prolonged period of time with ongoing clinical decline. After 239 weeks on ERT, titers started to rise peaking to 1: 204,000 at week 265 with concurrent clinical decline and loss of all residual motor function. In-vitro assays to detect neutralizing activity of antibodies were not performed on our patient, as only a subset of the CRIM-negative IPD patients who have HSAT and a poor clinical outcome demonstrated the presence of neutralizing antibodies in in-vitro assays. Even among those who had neutralizing antibodies, some demonstrated antibodies to the catalytic domain, but not the uptake domain and it does not always reflect the true in-vivo situation [20]. Given this patient’s history of earlier severe propensity for infection, using the previously successful approach of bortezomib, rituximab, and methotrexate was not possible and warranted another approach. High dose IVIG was attempted as a rescue protocol for this terminally ill patient.
A progressive course of clinical decline in a CRIM-negative IPD patient on ERT without immunomodulation and SIT (≥1:12,800) has been reported earlier and compares to the initial antibody response of our patient described in this report [21]. Similar to the reported patient who died at 3 years 9 months, this patient has had a steady clinical decline over the years and then complete loss of all motor activities as titers reached HSAT with a peak of 1: 204,000 at week 265 post-ERT. This combination of HSAT with further clinical decline presented a need for a safe intervention, in this case achieved by high dose IVIG therapy, 1 g/kg to reduce antibody titers. IVIG is known for its anti-inflammatory and immunomodulatory effects with a wide clinical use in numerous autoimmune and inflammatory conditions like antiphospholipid-antibody syndrome, chronic inflammatory demyelinating polyneuropathy, Kawasaki disease, and Crohn’s disease [22–24]. Our rationale for using high dose IVIG in this patient was the potential binding of IVIG to the neonatal Fc receptors (FcRn) [23,25] which are responsible for recycling of antibodies, including pathological antibodies such as anti-rhGAA thus blocking rhGAA antibodies from being recycled and leading to their destruction. Saturating the FcRn receptor with high dose IVIG may prevent such recycling so that anti-rhGAA IgG antibody titers decrease over time as they are directed towards the degradation pathway [26]. IVIG in lower doses, used as part of the ITI protocol with rituximab and methotrexate in CRIM-negative cases, is primarily added as a source of passive immunity as the pooled IVIG has disease specific IgG antibodies to prevent inter-current infections [7,16,22,17]. The effectiveness of high dose IVIG in this case with significant morbidity represents an alternative approach to reducing antibody titers. Clearly, it was lifesaving and prevented further increase in titers and progression of disease. Although it likely prevented infections and hospitalization in this case, it has not resulted in immune tolerance. This case represents one of the oldest surviving patients with CRIM-negative IPD.
The development of seizures in this patient raises the question of whether it is or is not related to the disease, amidst the rising queries of central nervous system involvement in long term survivors of Pompe disease. White matter involvement on brain MRI, and cognitive and behavioral impairment in these patients, has been reported in literature [28,29], but no seizures have been reported. Long term studies and more careful assessments are needed to better understand how imaging changes relate to cognition. Given the fragility of this patient with dependence on ventilator and risk of sedation, it was decided best to avert the risk by not putting this CRIM negative infantile case through an MRI. The exact cause of seizures in this case cannot be determined, yet most probably could be idiopathic. This is the first report of afebrile seizures in Pompe disease which demands further research and follow up to determine if this is an evolving phenotype of long term survivors of CRIM negative Pompe disease. Seizures in this case, initially resistant to a lower dose single drug regimen stabilized after the dosage was increased to a higher dose of levetiracetam.
This case highlights the need for an individualized approach to abrogate the immune response in cases with rare disorders such as Pompe disease. Our group has previously reported success with use of a combination of agents that act at the level of B cells, T cells, and plasma cells, yet through this case highlight the need for tailoring treatment based on patient needs. This case also emphasizes the importance of monitoring antibody titers systematically over time in cases who have initially low antibody titers (1:<6400) as the titers may continue to increase and peak several years after ERT, as seen here after 4.5 years post-ERT. In this clinical setting we carefully considered the medical fragility of this case when planning the therapeutic options to reduce the immune response. As the patient is responding better we are considering the addition of rituximab and bortezomib to the regimen. While progress has been made in the field of therapeutic proteins, with a growing understanding of the negative impact of antibodies to the clinical response and the ability to prevent an immune response, gaps still remain in the field. There is a need for agents that are safe, cheap, and result in antigen specific tolerance.
Acknowledgments
This study was partly funded by the Lysosomal Disease Network, a part of the NIH Rare Diseases Clinical Research Network (RDCRN). The Lysosomal Disease Network (5U54NS065768-05) (subaward number is N004689101) is a part of the RDCRN, an initiative of the Office of Rare Diseases Research (ORDR), and the National Center for Advancing Translational Science (NCATS). This consortium is funded through collaboration between the NCATS, the National Institute of Neurological Disorders and Stroke (NINDS), and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK).
References
- 1.Lim JA, Li L, Raben N. Pompe disease: from pathophysiology to therapy and back again. Front Aging Neurosci. 2014;6:177. doi: 10.3389/fnagi.2014.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kishnani PS, Hwu WL, Mandel H, Nicolino M, Yong F, Corzo D. A retrospective, multinational, multicenter study on the natural history of infantile-onset Pompe disease. J Pediatr. 2006;148:671–676.e672. doi: 10.1016/j.jpeds.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 3.van den Hout HMP, Hop W, van Diggelen OP, Smeitink JAM, Smit GPA, Poll-The BTT, Bakker HD, Loonen MCB, de Klerk JBC, Reuser AJJ, van der Ploeg AT. The natural course of infantile Pompe’s disease: 20 original cases compared with 133 cases from the literature. Pediatrics. 2003;112:332–340. doi: 10.1542/peds.112.2.332. [DOI] [PubMed] [Google Scholar]
- 4.Chien YH, Lee NC, Huang HJ, Thurberg BL, Tsai FJ, Hwu WL. Later-onset Pompe disease: early detection and early treatment initiation enabled by newborn screening. J Pediatr. 2011;158:1023–1027.e1021. doi: 10.1016/j.jpeds.2010.11.053. [DOI] [PubMed] [Google Scholar]
- 5.Kishnani PS, Beckemeyer AA, Mendelsohn NJ. The new era of Pompe disease: advances in the detection, understanding of the phenotypic spectrum, pathophysiology, and management. Am J Med Genet C: Semin Med Genet. 2012;160C:1–7. doi: 10.1002/ajmg.c.31324. [DOI] [PubMed] [Google Scholar]
- 6.Young SP, Zhang H, Corzo D, Thurberg BL, Bali D, Kishnani PS. Long-term monitoring of patients with infantile-onset Pompe disease on enzyme replacement therapy using a urinary glucose tetrasaccharide biomarker. Genet Med. 2009;11 doi: 10.1097/GIM.0b013e3181a87867. [DOI] [PubMed] [Google Scholar]
- 7.Banugaria SG, Prater SN, Patel TT, Dearmey SM, Milleson C, Sheets KB, Bali DS, Rehder CW, Raiman JA, Wang RA, Labarthe F, Charrow J, Harmatz P, Chakraborty P, Rosenberg AS, Kishnani PS. Algorithm for the early diagnosis and treatment of patients with cross reactive immunologic material-negative classic infantile Pompe disease: a step towards improving the efficacy of ERT. PLoS One. 2013;8:e67052. doi: 10.1371/journal.pone.0067052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Broomfield A, Fletcher J, Davison J, Finnegan N, Fenton M, Chikermane A, Beesley C, Harvey K, Cullen E, Stewart C, Santra S, Vijay S, Champion M, Abulhoul L, Grunewald S, Chakrapani A, Cleary MA, Jones SA, Vellodi A. Response of 33 UK patients with infantile-onset Pompe disease to enzyme replacement therapy. J Inherit Metab Dis. 2016;39:261–271. doi: 10.1007/s10545-015-9898-5. [DOI] [PubMed] [Google Scholar]
- 9.Kishnani PS, Goldenberg PC, DeArmey SL, Heller J, Benjamin D, Young S, Bali D, Smith SA, Li JS, Mandel H, Koeberl D, Rosenberg A, Chen YT. Cross-reactive immunologic material status affects treatment outcomes in Pompe disease infants. Mol Genet Metab. 2010;99:26–33. doi: 10.1016/j.ymgme.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Gelder CM, Hoogeveen-Westerveld M, Kroos MA, Plug I, van der Ploeg AT, Reuser AJJ. Enzyme therapy and immune response in relation to CRIM status: the Dutch experience in classic infantile Pompe disease. J Inherit Metab Dis. 2015;38:305–314. doi: 10.1007/s10545-014-9707-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Banugaria Suhrad G, Prater Sean N, Ng Yiu-Ki, Kobori Joyce A, Finkel Richard S, Ladda Roger L, Chen Yuan-Tsong, Rosenberg Amy S, Kishnani Priya S. The impact of antibodies on clinical outcomes in diseases treated with therapeutic protein: lessons learned from infantile Pompe disease. Genet Med. 2011;13:8. doi: 10.1097/GIM.0b013e3182174703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lumizyme R [Package Insert] Genzyme Corporation; Cambridge, MA: Oct, 2016. [Google Scholar]
- 13.Elder ME, Nayak S, Collins SW, Lawson LA, Kelley JS, Herzog RW, Modica RF, Lew J, Lawrence RM, Byrne BJ. B-cell depletion and immunomodulation before initiation of enzyme replacement therapy blocks the immune response to acid alpha-glucosidase in infantile-onset Pompe disease. J Pediatr. 2013;163:847–854.e841. doi: 10.1016/j.jpeds.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prater SN, Patel TT, Buckley AF, Mandel H, Vlodavski E, Banugaria SG, Feeney EJ, Raben N, Kishnani PS. Skeletal muscle pathology of infantile Pompe disease during long-term enzyme replacement therapy. Orphanet J Rare Dis. 2013;8:90. doi: 10.1186/1750-1172-8-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banugaria SG, Patel TT, Kishnani PS. Immune modulation in Pompe disease treated with enzyme replacement therapy. Expert Rev Clin Immunol. 2012;8:497–499. doi: 10.1586/eci.12.40. [DOI] [PubMed] [Google Scholar]
- 16.Kazi ZB, Prater SN, Kobori JA, Viskochil D, Bailey C, Gera R, Stockton DW, McIntosh P, Rosenberg AS, Kishnani PS. Durable and sustained immune tolerance to ERT in Pompe disease with entrenched immune responses. JCI Insight. 2016;1:e86821. doi: 10.1172/jci.insight.86821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bali DS, Goldstein JL, Banugaria S, Dai J, Mackey J, Rehder C, Kishnani PS. Predicting cross-reactive immunological material (CRIM) status in Pompe disease using GAA mutations: lessons learned from 10 years of clinical laboratory testing experience. Am J Med Genet C: Semin Med Genet. 2012;160C:40–49. doi: 10.1002/ajmg.c.31319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huie ML, Anyane-Yeboa K, Guzman E, Hirschhorn R. Homozygosity for multiple contiguous single-nucleotide polymorphisms as an indicator of large heterozygous deletions: identification of a novel heterozygous 8-kb intragenic deletion (IVS7–19 to IVS15–17) in a patient with glycogen storage disease type II. Am J Hum Genet. 2002;70:1054–1057. doi: 10.1086/339691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mendelsohn NJ, Messinger YH, Rosenberg AS, Kishnani PS. Elimination of antibodies to recombinant enzyme in Pompe’s disease. N Engl J Med. 2009;360:194–195. doi: 10.1056/NEJMc0806809. [DOI] [PubMed] [Google Scholar]
- 20.Banugaria SG, Prater SN, Ng YK, Kobori JA, Finkel RS, Ladda RL, Chen YT, Rosenberg AS, Kishnani PS. The impact of antibodies on clinical outcomes in diseases treated with therapeutic protein: lessons learned from infantile Pompe disease. Genet Med. 2011;13:729–736. doi: 10.1097/GIM.0b013e3182174703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abbott MA, Prater SN, Banugaria SG, Richards SM, Young SP, Rosenberg AS, Kishnani PS. Atypical immunologic response in a patient with CRIM-negative Pompe disease. Mol Genet Metab. 2011;104:583–586. doi: 10.1016/j.ymgme.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Späth PJ, Schneider C, von Gunten S. Clinical use and therapeutic potential of IVIG/SCIG, plasma-derived IgA or IgM, and other alternative immunoglobulin preparations. Arch Immunol Ther Exp. 2016:1–17. doi: 10.1007/s00005-016-0422-x. [DOI] [PubMed] [Google Scholar]
- 23.Perez EE, Orange JS, Bonilla F, Chinen J, Chinn IK, Dorsey M, El-Gamal Y, Harville TO, Hossny E, Mazer B, Nelson R, Secord E, Jordan SC, Stiehm ER, Vo AA, Ballow M. Update on the use of immunoglobulin in human disease: a review of evidence. J Allergy Clin Immunol. 2017 doi: 10.1016/j.jaci.2016.09.023. [DOI] [PubMed] [Google Scholar]
- 24.Kazatchkine MD, Kaveri SV. Immunomodulation of autoimmune and inflammatory diseases with intravenous immune globulin. N Engl J Med. 2001;345:747–755. doi: 10.1056/NEJMra993360. [DOI] [PubMed] [Google Scholar]
- 25.Anthony RM, Kobayashi T, Wermeling F, Ravetch JV. Intravenous gammaglobulin suppresses inflammation through a novel TH2 pathway. Nature. 2011;475:110–113. doi: 10.1038/nature10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwab I, Nimmerjahn F. Intravenous immunoglobulin therapy: how does IgG modulate the immune system? Nat Rev Immunol. 2013;13:176–189. doi: 10.1038/nri3401. [DOI] [PubMed] [Google Scholar]
- 28.Ebbink BJ, Aarsen FK, van Gelder CM, van den Hout JMP, Weisglas-Kuperus N, Jaeken J, Lequin MH, Arts WFM, van der Ploeg AT. Cognitive outcome of patients with classic infantile Pompe disease receiving enzyme therapy. Neurology. 2012;78(19):1512–1518. doi: 10.1212/WNL.0b013e3182553c11. [DOI] [PubMed] [Google Scholar]
- 29.Spiridigliozzi GA, Heller JH, Kishnani PS. Cognitive and adaptive functioning of children with infantile Pompe disease treated with enzyme replacement therapy: long-term follow-up. Am J Med Genet C Semin Med Genet. 2012;160C:22–29. doi: 10.1002/ajmg.c.31323. [DOI] [PubMed] [Google Scholar]