Abstract
BACKGROUND
Donor behaviors in STRIDE (Strategies to Reduce Iron Deficiency), a trial to reduce iron deficiency, were examined.
STUDY DESIGN AND METHODS
Six hundred ninety-two frequent donors were randomized to receive either 19 or 38 mg iron for 60 days or an educational letter based on their predonation ferritin. Compliance with assigned pills, response to written recommendations, change in donation frequency, and future willingness to take iron supplements were examined.
RESULTS
Donors who were randomized to receive iron pills had increased red blood cell donations and decreased hemoglobin deferrals compared with controls or with pre-STRIDE donations. Donors who were randomized to receive educational letters had fewer hemoglobin deferrals compared with controls. Of those who received a letter advising of low ferritin levels with recommendations to take iron supplements or delay future donations, 57% reported that they initiated iron supplementation, which was five times as many as those who received letters lacking a specific recommendation. The proportion reporting delayed donation was not statistically different (32% vs. 20%). Of donors who were assigned pills, 58% reported taking them “frequently,” and forgetting was the primary reason for non-compliance. Approximately 80% of participants indicated that they would take iron supplements if provided by the center.
CONCLUSIONS
Donors who were assigned iron pills had acceptable compliance, producing increased red blood cell donations and decreased low hemoglobin deferrals compared with controls or with pre-STRIDE rates. The majority of donors assigned to an educational letter took action after receiving a low ferritin result, with more donors choosing to take iron than delay donation. Providing donors with information on iron status with personalized recommendations was an effective alternative to directly providing iron supplements.
Iron depletion is common among individuals who donate blood, particularly frequent donors, who have ferritin levels consistent with iron-deficient erythropoiesis in 66% of females and 49% of males.1,2 Providing information about dietary sources of iron is not an effective approach for mitigating donor iron depletion.3 Options with an empirical basis of efficacy include reducing the frequency of blood donation or taking supplemental iron.2,4 Education after donation may recommend one or both of these approaches, or the blood center can directly implement them by prolonging the required interdonation interval or by directly providing iron supplements. To date, efforts to implement donor iron supplementation have met with only limited success,5 although providing donors with additional information on their personal iron status has been suggested as a means to motivate change.6 In the absence of iron supplementation, however, recovery of iron stores after a blood donation requires 6 months or longer.1,4 Lengthening interdonation intervals to this extent may substantially reduce the ability of blood centers to collect an adequate blood supply.7 Thus, the education message for blood donors needs to be sharpened to encourage iron supplementation.
The Strategies to Reduce Iron Deficiency (STRIDE) study was a multicenter, randomized, blinded, and placebo-controlled study investigating the relative efficacy of two approaches for mitigating donor iron deficiency in frequent blood donors (clinicaltrials.gov identifier NCT02245321).8,9 Randomization to one of five groups, representing an educational or iron supplementation intervention, allowed for comparison of alternate strategies that are operationally feasible for most blood centers. Here, we have analyzed donor behaviors before, during, and after the STRIDE study period to assess the potential operational impact of various options to mitigate iron depletion in blood donors.
MATERIALS AND METHODS
Study design
STRIDE was a 2-year study of 692 frequent blood donors. The study was designed to evaluate an educational intervention and an iron-supplementation intervention, each with appropriate controls. As previously described,8 eligible frequent whole blood or double red blood cell (DRC) donors who were not taking iron supplements were recruited at an enrollment donation. Donors agreed to continue to donate whole blood or DRCs at their previous frequency and, if assigned to the blinded pill arms, not to take any self-administered iron supplements.
Enrolled donors were randomized into one of five groups. Three groups were provided 60 pills on a blinded basis to take daily at bedtime after each successful donation (120 days for DRCs). One group received 38 mg elemental iron as ferrous gluconate, a second group received 19 mg elemental iron as ferrous gluconate, and the third group received a placebo. Two other groups were sent a letter after each donation. One group received their ferritin value, which was measured at their most recent donation, with associated recommendations (referred to as the iron status letter group), and the other group received a thank you letter (referred to as the control letter group). Plasma ferritin, soluble transferrin receptor, and hemoglobin levels were measured at enrollment and each visit.
Letters sent to donors in two educational intervention groups
Letters sent to donors in the control letter group after each donation stated the following:
“Thank you for your recent blood donation visit at [BLOOD CENTER NAME]. We value your continued participation in the STRIDE research study! Please continue to donate following the recommended interval (56 days for a single donation; 112 days for a double donation) so that you give at least 2 times per year (for women) or 3 times per year (for men). Your participation in this study does not change your blood donor eligibility.”
Letters sent to donors in the iron status letter group after each blood donation included a thank you, their plasma ferritin concentration, and the following information about iron depletion:
“Regular blood donors, like you, are at risk of becoming iron deficient. Iron is important in helping your body transport oxygen. As a member of this research study, one of the tests performed during your last donation can help us predict your body’s ability to store iron. This is called a plasma ferritin test.”
Additional information provided in the letter depended upon their ferritin concentration. Letters for donors with ferritin levels 26 ng/mL or greater read:
“Your plasma ferritin level indicates that your body iron stores are adequate. Please continue to donate soon after the recommended interval (56 days for a single donation; 112 days for a double donation) so that you give at least 2 times a year (for women) or 3 times a year (for men). Your participation in this study does not change your blood donor eligibility.”
Finally, letters for donors with ferritin levels below 26 ng/mL advised:
“Your plasma ferritin level indicates that you are iron depleted or deficient. We suggest that you take an oral iron supplement daily for the next 60 days, OR refrain from donating for the next 6 months. Iron tablets can be found in the pharmacy section of your local grocery store or at any pharmacy. Iron comes in several formulations with and without multivitamins. We recommend taking an iron tablet containing 17 to 38 mg of elemental iron. Please speak with your local pharmacist if you need assistance in choosing an appropriate iron supplement.”
Pill distribution to donors in three pill groups
Donors in the pill groups were sent a bottle of pills and instructions after each donation. Details of the blinding method and management of side effects have been previously reported.8 To allow for compliance assessment, donors were asked to return unused pills or empty bottles from the previous mailing in a postage-paid envelope.
Questionnaires at study enrollment and completion
Donors completed self-administered questionnaires at baseline and study completion, including demographic characteristics, potential pica behaviors, and multivitamin usage. These data will be reported separately.10 The final questionnaire collected information concerning self-initiation of iron supplements during the study, delaying donation during the study period, and their likelihood of taking supplemental iron in the future if recommended or provided by the blood center.
Statistical methodology
Frequencies and percentages were calculated for questionnaire items. Statistical differences across groups at the final visit were assessed using chi-square analysis, and differences in means of continuous variables were assessed using a t test or an analysis of variance. We tested whether donors in the iron status letter group who had at least one plasma ferritin level below 26 ng/mL (low-ferritin letter donors) chose to take iron supplements more often than they chose to delay donation using McNemar’s test. A log-linear model was applied to assess whether receiving at least one low-ferritin letter resulted in an increased odds of delaying donation or taking iron supplements (compared with donors who never received a low-ferritin letter and those who received only a control letter).
Deferral rates were calculated as the number of deferrals divided by the total number of donor presentations to donate. Counts of red blood cell equivalents (RCEs) in each of these periods were calculated as whole blood donations plus twice the number of DRC donations. Data were normalized per year to account for differing length of time. The pre-STRIDE period consisted of the donation history for each donor, beginning with a successful donation closest to 24 months before enrollment up to the STRIDE enrollment visit. The post-STRIDE period consisted of the donation history for each donor, starting from the day after the final STRIDE visit (or the last visit if the donor did not have a final STRIDE visit) until the last donation visit on or before May 1, 2016. The enrollment donation was not included in either the pre-STRIDE period or the STRIDE period for deferral rate calculations, because this visit required a successful donation. The enrollment donation was also excluded from counts of RCE donations to allow for comparison across periods, because all three periods are then bounded by a donation on only one end.
Comparison of donation and deferral rates before, during, and after the STRIDE study were assessed by repeated-measures models. Donation rates used interdonation intervals as a continuous outcome variable, and deferral rates used deferral as a binary outcome variable. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Inc.).
RESULTS
Letter groups: reports of delaying donation and taking iron supplements
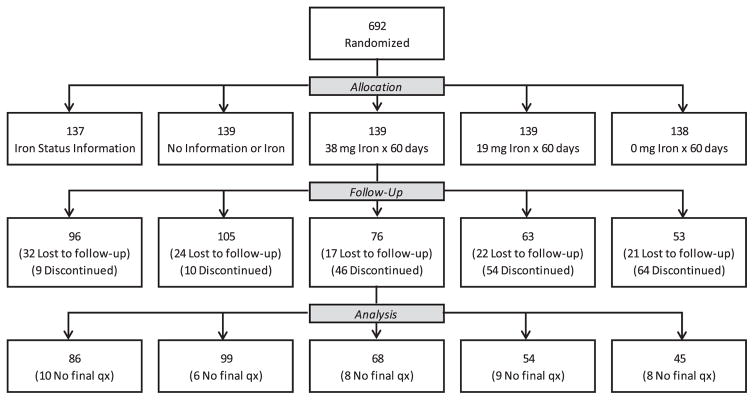
As previously reported,9 393 of the 692 randomized donors completed STRIDE. The final questionnaire was completed by 352 donors, including 86 of 96 in the iron status letter arm, 99 of 105 donors in the control letter arm, and 167 of 192 donors assigned to the pill groups. Figure 1 presents the Consolidated Standards for Reporting Trials (CONSORT) flow diagram for the study.
Fig. 1.
Enrollment and follow-up in the STRIDE study (Strategies to Reduce Iron Deficiency)9; the CONSORT flow diagram from Fig. 1 in Mast and colleagues9 is modified here with permission from the journal TRANSFUSION.
Of the 86 iron status letter donors who provided final questionnaire data, 71 had one or more ferritin values below 26 ng/mL and thus received at least one low-ferritin letter recommending iron supplements or delaying donation. The remaining 15 donors never had a ferritin value below 26 ng/mL, and thus only received the letter informing them of their acceptable ferritin value and asking them to continue frequent donations. For the purposes of analyzing the effect of the low-ferritin letter, these 15 donors were grouped with the 99 donors in the control letter arm (collectively referred to below as “receiving a no-information letter”).
Table 1 presents the numbers and percentage of donors in the letter groups who reported delaying donation or taking iron supplements over the study period stratified by whether they received a low-ferritin letter or a no-information letter. Of the donors who received low-ferritin letters, 70% took one or both of the recommended actions. Delaying donation was reported by 19.8% of those who received no-information letters compared with 31.9% of those who received one or more low-ferritin letters. The odds ratio for delaying donation during STRIDE among those who received a low-ferritin letter was 1.7, which was not significantly different (p = 0.27; 95% confidence interval, 0.78–3.8). However, it is possible that there was an effect of the low-ferritin letter causing delayed donations, but the number who received the letter was too small to achieve significance.
TABLE 1.
Reports of delaying donation and taking iron supplements in the letter group subsets*
| Variable | Control letter and iron ≥26 ng/mL in the Iron Status Group, N = 114
|
Iron <26 ng/mL in the Iron Status Group, N = 71
|
p value† | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Delayed donation | |||||
| No | 81 | 80.2 | 47 | 68.1 | 0.27 |
| Yes | 20 | 19.8 | 22 | 31.9 | |
| Total | 101 | 100.0 | 69 | 100.0 | |
| Took iron | |||||
| No | 98 | 89.1 | 30 | 42.9 | < 0.0001 |
| Yes | 12 | 10.9 | 40 | 57.1 | |
| Total | 110 | 100.0 | 70 | 100.0 | |
Note that some columns sum to less than expected because of items that were missing data.
Log-linear model test of difference in proportions between letter group subsets.
In contrast, initiation of iron supplementation was reported by 10.9% of those who received no-information letters compared with 57.1% of those who received one or more low-ferritin letters. The odds ratio for taking iron supplements during STRIDE among those who received a low-ferritin letter was 9.8 (p < 0.0001; 95% CI, 4.5–21.1). Furthermore, this odds ratio did not depend on whether or not the donor delayed donation (p = 0.79), that is, the increased odds of taking iron supplements after receiving a low-ferritin letter was statistically equivalent regardless of whether donation was delayed. When categorizing responses into one, both, or neither of the recommended actions, donors who received a low-ferritin letter were more likely to take iron than to delay donation (McNemar p value = 0.004). Different donor responses to study letters were associated with clear differences in visit frequency (i.e., attempted and successful donations) and donation productivity measured as RCE donations. Delaying donation reduced the visit frequency from a mean of 8.0 to 6.6 (p = 0.049) and reduced the RCE donations from 9.3 to 6.7 (p = 0.0004). Initiating iron supplementation had the contrary effect. Taking iron supplements was associated with increased visits (8.7 vs. 6.5 visits) and donations (9.5 vs. 7.7 RCE donations; p = 0.0004 and p = 0.009, respectively).
Pill groups: compliance
Pill compliance was assessed from two different data sources: the counts of returned pills and the self-assessment in the final questionnaire. Donors returned pills or empty bottles as requested 51.4% of the time (at 773 of 1504 visits after which pills were distributed). There was a difference by group assignment in pill return, both in the proportion of visits with returned pills and in the number of pills assumed taken from visits with returned pills. Those who received pills with 38 mg iron returned pills or empty bottles 57% of the time, those who received pills with 19 mg iron returned them 53% of the time, and the placebo group returned them 43% of the time (p < 0.0001). Similarly, although donors overall who returned pills took 75 to 100% of their assigned pills 77% of the time (Table 2), those in the placebo group were less likely to have taken more than 75% of pills, having done so 69% of the time (p = 0.02; chi-square test) (Table 2).
TABLE 2.
Evaluation of pill compliance
| Group assignment | Percentage of pills taken, from counts of pills returned*
|
Self-reports of “took pills frequently”
|
||||||
|---|---|---|---|---|---|---|---|---|
| No. | <25% of pills | 25 to <50% | 50 to <75% | 75 to 100% | No. | No, % | Yes, % | |
| Total | 773† | 5.2 | 3.9 | 14.4 | 76.6 | 164‡ | 42.1 | 57.9 |
| Iron 38 mg | 316 | 5.4 | 5.1 | 13.0 | 76.6 | 67 | 37.3 | 62.7 |
| Iron 19 mg | 272 | 2.9 | 2.2 | 12.9 | 82.0 | 53 | 35.9 | 64.2 |
| Placebo | 185 | 8.1 | 4.3 | 18.9 | 68.7 | 44 | 56.8 | 43.2 |
| Chi-square p value | 0.02 | 0.07 | ||||||
Cell values indicate the percentage of returns.
In total, 773 bottles of pills were returned of 1504 bottles of pills shipped.
Donors.
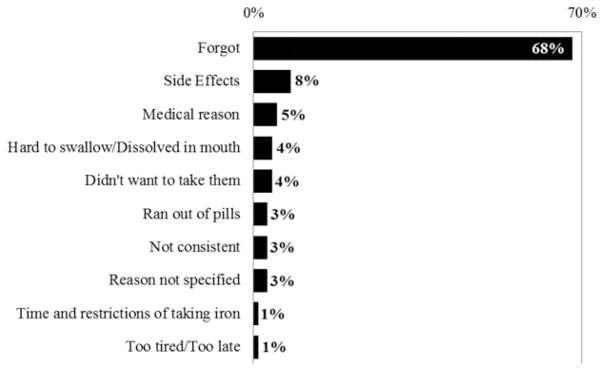
Of the 164 donors in the three pill groups who reported final questionnaire data (85% of those who had a final visit), 57.9% reported taking the iron pills provided them by the study “frequently.” A smaller proportion of donors in the placebo group reported taking the pills frequently (43 vs. >60%), but the difference did not reach significance (p = 0.07) (Table 2). The final questionnaire asked donors to provide a reason for why they did not frequently take their study pills. Two-thirds of respondents indicated that they simply “forgot” (Fig. 2).
Fig. 2.
Reasons provided by donors in the three pill groups combined (N = 416) who completed the study and provided data on pill compliance and said they did not take their supplied pills “frequently” (N = 69). Shown are the percentages of all reasons given. Donors could choose more than one reason and four did so.
Potential future use of iron supplements
In the final questionnaire, donors were asked how likely they would be to: 1) take recommended iron supplements if provided by the blood center, and 2) purchase and take recommended iron supplements. Eighty percent of donors reported that they would be likely to take recommended iron supplements provided by the blood center, and slightly fewer indicated that they would purchase and take recommended iron supplements (Table 3).
TABLE 3.
Likelihood of taking provided iron supplements or purchasing and taking recommended iron supplements by group assignment
| Group assignment | Likelihood of taking recommended/provided supplements, %
|
Likelihood of purchasing and taking recommended supplements, %
|
||||
|---|---|---|---|---|---|---|
| Likely | Neutral | Not likely | Likely | Neutral | Not likely | |
| Total | 80 | 11 | 9 | 70 | 18 | 12 |
| Iron status information | 72 | 10 | 19 | 64 | 17 | 19 |
| Control (no-information) letter | 88 | 5 | 6 | 81 | 8 | 11 |
| Iron 38 mg × 60 days | 77 | 14 | 9 | 69 | 18 | 12 |
| Iron 19 mg × 60 days | 79 | 17 | 4 | 60 | 30 | 9 |
| Placebo × 60 days | 82 | 14 | 5 | 66 | 25 | 9 |
Impact of study on blood donation behaviors
The number of RCE donations and low hemoglobin deferrals before, during, and after the STRIDE study were compared by treatment assignment to assess how study participation may have altered donor behavior and productivity. The mean interval assessed was 22.7 months before STRIDE, 17.3 months during STRIDE, and 26 months after STRIDE. There were no differences in average RCE donations per year across the study groups during the pre-STRIDE periods, reflecting successful randomization at study inception (p = 0.91) (Table 4). During STRIDE, the 19-mg and 38-mg iron groups had more RCE donations compared with the iron status letter, control letter, and placebo pill groups (p = 0.0008) (Table 4). These differences reflect decreased RCE donations in the non-iron pill groups compared with pre-STRIDE RCE donations, rather than by increased RCE donations in the two iron pill groups. During the post-STRIDE period, the five groups had modestly decreased RCE donations compared with pre-STRIDE donations (p < 0.0001) but continued to reflect high donation rates, ranging from 3.3 to 3.8 times annually. Similar to donation frequency, low hemoglobin deferral rates did not differ across the study groups during the pre-STRIDE period (p=0.73) (Table 5). During the study period, however, significant differences in deferral rates emerged across the groups (p < 0.0002), with increases from 60 to 100% in the two control groups and declines of 40% in the two groups that were assigned iron pills. With these changes, the deferral rates were 2.7 times and 3.5 times higher in the placebo pill and control letter groups, respectively, than in the 38-mg iron pill group. The iron status letter group had a statistically unchanged deferral rate from pre-STRIDE of 5.2% but significantly lower than the control deferral rate (10.7%; p = 0.004). Collectively, the post-STRIDE period deferral rates were higher than deferral rates in the pre-STRIDE period (6.3 vs. 4.9%; p = 0.02), seemingly because the 19-mg and 38-mg iron groups had increased deferral rates compared with the pre-STRIDE period (p = 0.07 and p = 0.03, respectively) (Table 5). However, there was no statistical difference across the groups in the post-STRIDE deferral rate (p = 0.59) (Table 5).
TABLE 4.
Number of red blood cell equivalent donations per year during pre-STRIDE, STRIDE, and post-STRIDE, N = 692 donors
| Group assignment* | Pre-STRIDE | STRIDE | p value† | Post-STRIDE | p value† |
|---|---|---|---|---|---|
| Iron status information only | 3.88 | 3.42 | 0.0001 | 3.28 | 0.0001 |
| No information or treatment | 3.87 | 3.48 | 0.007 | 3.69 | 0.0002 |
| Iron 38 mg × 60 days | 3.90 | 3.94 | 0.49 | 3.61 | 0.03 |
| Iron 19 mg × 60 days | 3.98 | 4.01 | 0.25 | 3.81 | 0.0009 |
| Placebo × 60 days | 3.85 | 3.62 | 0.28 | 3.39 | 0.0002 |
| p value‡ | 0.91 | 0.0008 | 0.22 |
All donors are included. Group assignments are specified in Fig. 1.
Test for change from pre-STRIDE to STRIDE and post-STRIDE using repeated measures model (see Materials and Methods).
Test for common differences among group assignments from repeated measures model (see Materials and Methods).
TABLE 5.
Low hemoglobin deferral rates during pre-STRIDE, STRIDE, and post-STRIDE, N = 692 donors
| Group assignment* | Pre-STRIDE, %† | STRIDE, %† | p value‡ | Post-STRIDE, %† | p value‡ |
|---|---|---|---|---|---|
| Iron status information only | 5.0 | 5.2 | 0.58 | 3.8 | 0.63 |
| No information or treatment | 5.1 | 10.7 | 0.0004 | 4.9 | 0.80 |
| Iron 38 mg × 60 days | 5.2 | 3.1 | 0.09 | 9.4 | 0.03 |
| Iron 19 mg × 60days | 5.1 | 3.2 | 0.06 | 7.5 | 0.07 |
| Placebo × 60 days | 3.7 | 8.3 | 0.0006 | 4.8 | 0.42 |
| Test for differences across group assignments§ | p = 0.73 | p = 0.0002 | p = 0.59 |
All donors are included. Group assignments are specified in Fig. 1.
The deferral rate was calculated as: # of hemoglobin deferrals % (# of deferrals + successful and quantity-not-sufficient donations).
Test for change from pre-STRIDE from repeated measures model (see Materials and Methods).
Test for common differences among group assignments from repeated measures model (see Materials and Methods).
DISCUSSION
Significant attention has been focused on iron depletion in blood donors, with blood centers and regulators in the United States investing considerable effort to elucidate the prevalence, determinants, and potential for successful mitigation.11–14 Major findings from the recently completed STRIDE study were that taking either low-dose or intermediate-dose iron pills (19 and 38 mg) was equivalent in improving iron stores and that providing donors with information on their iron status using personalized recommendations was almost equally effective.9 The results for hemoglobin were similar, with improvements in the 19-mg and 38-mg iron groups, stable hemoglobin levels in the iron status letter group, and declining levels in the two control groups.
Here, we have analyzed the STRIDE data set along with pre-STRIDE and post-STRIDE donation information to assess donors’ responses to the iron-deficiency mitigation strategies evaluated as well as possible attendant implications for blood center operations. We found that frequent donors who are informed of their low iron stores will take action to improve their iron status, and they are inclined to take supplemental iron over delaying their next donation by a large margin. Participants demonstrated acceptable compliance with taking assigned pills during the study. Perhaps most important, a large majority expressed willingness to take iron supplements if recommended to do so in the future. We observed that the use of supplemental iron was associated with higher donation productivity and a lower hemoglobin deferral rate compared with delaying donation or the current standard of care for blood collectors. Overall, despite donors’ lack of iron supplementation practiced before enrollment, the current results indicate the viability of recommending or providing iron to blood donors, with health benefits that accrue to the donor and efficiency gains that accrue to the blood center.
Although demonstration of the efficacy of iron supplements in reducing the risk of iron depletion is not a novel finding,15 few studies have rigorously assessed blood donor iron supplementation practices. Only one large blood center in the United States is known to directly distribute iron pills to its donors,16 and that effort is limited to female donors who are deferred for low hemoglobin levels. The Donor Iron Status Evaluation (RISE) study found that 39% of enrolled donors reported use of supplemental iron ranging from 25% of first-time male donors to 52% of frequent female donors17; the findings in frequent donors were substantiated by a large survey conducted by the American Red Cross.18 Another large survey conducted at one blood center indicated that a smaller share of donors reported iron usage (in aggregate, 21%).19 However, there were clear differences by age, sex, and donation frequency, with only 8% of first-time donors reporting use of supplemental iron compared with 32% of donors who made seven or more donations in the prior 2 years. Of those who reported iron usage, the majority (65%) of respondents cited “general health and wellness” as a motivation, compared with roughly one-third who specifically cited blood donation.
The feasibility and benefits of blood center efforts to promote iron supplementation in donors were demonstrated well during STRIDE. Donors who took iron after receipt of iron status letters demonstrated a higher rate of donation productivity compared with donors who decided to delay their donation, with a difference of roughly two donations over their 17-month average study participation. Similarly, donors in the two groups that were assigned supplemental iron benefitted from improved iron status and hemoglobin levels, whereas the blood center benefitted from higher donation productivity and fewer deferrals for low hemoglobin. Comparing the 2-year pre-STRIDE period with the STRIDE period (average, 17 months of observation), donors who received 19 or 38 mg iron pills maintained a stable, high donation rate of four RCE donations per year (p = 0.20), with a hemoglobin deferral rate that dropped from 5 to 3%, for a 40% decrease (p = 0.01). In contrast, the other three groups experienced either a drop in donation frequency (iron status letter group, not all of whom took iron; p = 0.0001), an increase in hemoglobin deferrals (placebo pill group; p = 0.0006), or both outcomes (control letter groups, p = 0.007 and p = 0.0004). Whether similar results obtained in STRIDE from a select group of high-frequency donors might be replicated in occasional or casual donors remains for future evaluation.
We observed a clear willingness of frequent donors who agreed to participate in the research protocol to take iron supplements when the donors had not been doing so before STRIDE. This finding supports the potential gains from strengthening blood center efforts to encourage iron supplementation in their blood donors. A majority of donors (57%) who received an iron status letter self-initiated iron supplementation. Similarly, a majority (58%) of those in the pill groups reported at study conclusion that they took their assigned pills “frequently,” and directly measured compliance using pill counts was acceptable. Only 10% of respondents reported they were unlikely to take supplements if they were recommended or provided by the blood center in the future, with 70 to 80% reporting that they would be likely to do so. In general, lack of compliance with assigned pills did not appear to reflect disinterest in or discomfort with the pills. The large majority simply forgot. Another 8% cited side effects as a reason for not taking iron pills, consistent with previously reported STRIDE results that 9% of donors assigned to one of three pill groups withdrew because of side effects.9 Although the completion rate of STRIDE was much lower in the three pill groups than in the two groups that received letters, no difference in adverse events was documented in the two groups that received iron compared with those who took placebo pills.8,9 Rather, many donors who discontinued study participation expressed a desire on their or their clinician’s part to be taking iron. Future iron supplementation programs should address issues of compliance, which were not the primary focus of STRIDE.
There are several limitations to the current study. First, we only assessed frequent red blood cell donors who had not recently been taking iron. Less frequent donors may not be as willing to take recommended iron, and they may not show the same benefits from doing so. The need for iron supplementation in donors donating less than our study definition of frequent donors has not been clearly shown, so this limitation may not be important. However, to the extent that iron supplement recommendations extend to regular but less frequent donors or to all or nearly all donors, these issues would need to be assessed in the target donor populations. Second, the questionnaire data were only provided at the end of the study by donors who completed the study. Since there was differential drop out among groups, caution needs to be used in interpreting the data from the questionnaire (compliance self-assessment, willingness to take iron in the future). Because return of unused pills only occurred one-half of the time, overall compliance might have been better or worse than we report.
Evaluation of donation data before and subsequent to donors’ participation in STRIDE provides valuable context to the data accrued during study follow-up. Comparisons across treatment groups indicate that donation productivity was higher in those who were assigned iron pills, and comparisons with each prior 2-year period confirm this represents a true change from groups that were statistically equivalent at randomization. In similar fashion, comparison with the pre-STRIDE period for hemoglobin deferrals complements and confirms the results directly observed during STRIDE, that hemoglobin deferrals improved in those who were assigned iron treatment and were stable or worse in those who were not. The continuing high donation frequency in the post-STRIDE period confirms that the donors who did not complete STRIDE mostly remained committed donors. The increase in hemoglobin deferrals observed post-STRIDE in the two iron pill groups is unsurprising, although it is not apparent why the deferral rate increased to levels higher than pre-STRIDE. Because no iron recommendation was made to these donors at study completion, several donors may not have continued iron supplements after STRIDE and could have experienced a drop in hemoglobin. This observation suggests that continued iron supplementation will be necessary for frequent donors.
There have been two recent operational assessments of small-scale20 and large-scale21 ferritin testing in unselected Canadian blood donors. In addition, there has been extensive operational experience with donor ferritin testing, iron deficiency, and hemoglobin deferral counseling at a blood center in Denmark.22 The Danish experience is not comparable to the Canadian studies or to the current analysis, because their approach is resource-intensive and essentially “clinical,” making their program difficult to implement. In the Canadian trials, all donors with low ferritin levels were advised to see their physician, stop donating for 6 months, and not return to donate until after their physician had established that their ferritin levels had returned to normal. Not surprisingly given the recommendations, in the Canadian studies, the donor return rate was depressed in those who were advised of a low ferritin level. In contrast, donors randomized to the STRIDE iron status information letter group who had low ferritin levels received a significantly different message: either take iron for 60 days or delay donation for 6 months, at the donor’s discretion. The decision made by STRIDE donors influenced subsequent donation behavior, with those who chose to delay donations giving fewer RCEs and those taking iron supplements giving more RCEs over the study period. The operational lesson to be learned in this comparison with the Canadian approach is that the nature and tone of recommended actions for low ferritin will have a significant effect on donors’ subsequent donation behaviors.
Many blood centers recommend iron supplementation to their regular blood donors, but they almost uniformly do not directly provide iron pills or collect information on donor supplementation practices. This report suggests a high degree of willingness on the part of blood donors to take iron and indicates that the benefits may include both donor health and well being and operational efficiencies at the blood center. At least in this select cohort of frequent donors, providing information about their iron status through ferritin testing, along with recommendations for iron supplementation, appears to be an effective alternative to directly providing iron.
Acknowledgments
This work was supported by grant 1R01HL105809 from the National Heart, Lung, and Blood Institute.
ABBREVIATIONS
- DRC(s)
double red blood cell(s)
- RCE(s)
red blood cell equivalent(s)
- STRIDE
Strategies to Reduce Iron Deficiency
Footnotes
CONFLICT OF INTEREST
Alan E. Mast reports honoraria from Siemens and research grant funding from Novo Nordisk. The remaining authors have no conflicts of interest to declare.
References
- 1.Cable RG, Glynn SA, Kiss JE, et al. Iron deficiency in blood donors: the REDS-II Donor Iron Status Evaluation (RISE) study. Transfusion. 2012;52:702–11. doi: 10.1111/j.1537-2995.2011.03401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cable RG, Glynn SA, Kiss JE, et al. Iron deficiency in blood donors: analysis of enrollment data from the REDS-II Donor Iron Status Evaluation (RISE) study. Transfusion. 2011;51:511–22. doi: 10.1111/j.1537-2995.2010.02865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delaney M, Schellhase KG, Young S, Geiger S, Fink A, Mast AE. Blood center practice and education for blood donors with anemia. Transfusion. 2011;51:929–36. doi: 10.1111/j.1537-2995.2010.02919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kiss JE, Brambilla D, Glynn SA, et al. Oral iron supplementation after blood donation: a randomized clinical trial. JAMA. 2015;6:575–83. doi: 10.1001/jama.2015.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Brien SF. Donor iron policy: from research to practice. Transfusion. 2016;56:1496–8. doi: 10.1111/trf.13555. [DOI] [PubMed] [Google Scholar]
- 6.O’Meara A, Infanti L, Stebler C, et al. The value of routine ferritin measurement in blood donors. Transfusion. 2011;51:2183–8. doi: 10.1111/j.1537-2995.2011.03148.x. [DOI] [PubMed] [Google Scholar]
- 7.Spencer BR, Johnson B, Wright DJ, et al. Potential impact on blood availability and donor iron status of changes to donor hemoglobin cutoff and interdonation intervals. Transfusion. 2016;56:1994–2004. doi: 10.1111/trf.13663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bialkowski W, Bryant BJ, Schlumpf KS, et al. The strategies to reduce iron deficiency in blood donors randomized trial: design, enrolment and early retention. Vox Sang. 2015;108:178–85. doi: 10.1111/vox.12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mast AE, Bialkowski W, Bryant BJ, et al. A randomized, blinded, placebo-controlled trial of education and iron supplementation for mitigation of iron deficiency in regular blood donors. Transfusion. 2016;56:1588–97. doi: 10.1111/trf.13469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chansky MC, King MR, Bialkowski W, et al. Qualitative assessment of pica experienced by frequent blood donors. Transfusion. 2017;57:946–51. doi: 10.1111/trf.13981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Center for Biologics Evaluation and Research, US Food and Drug Administration. Blood Products Advisory Committee; September 10, 2008; [cited 2017 Jun 20]. Available from: https://www.fda.gov/ohrms/dockets/ac/08/transcripts/2008-4379T1_3.htm. [Google Scholar]
- 12.Center for Biologics Evaluation and Research, US Food and Drug Administration. Blood Products Advisory Committee; July 27, 2010; [cited 2017 Jun 20]. Available from: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/BloodVaccinesandOtherBiologics/BloodProductsAdvisoryCommittee/UCM225389.pdf. [Google Scholar]
- 13.Center for Biologics Evaluation and Research, US Food and Drug Administration. Blood Products Advisory Committee; November 17, 2016; [cited 2017 Jun 20]. Available from: http://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/BloodVaccinesandOtherBiologics/BloodProductsAdvisoryCommittee/ucm501664.htm. [Google Scholar]
- 14.US Food and Drug Administration. Hemoglobin standards and maintaining adequate iron stores in blood donors. Public workshop; November 9, 2011; [cited 2017 Jun 20]. Available from: https://wayback.archive-it.org/7993/20170112100421/http://www.fda.gov/BiologicsBloodVaccines/NewsEvents/WorkshopsMeetingsConferences/ucm268757.htm. [Google Scholar]
- 15.Smith GA, Fisher SA, Doree C, Di Angelantonio E, Roberts DJ. Oral or parenteral iron supplementation to reduce deferral, iron deficiency and/or anaemia in blood donors. Cochrane Database Syst Rev. 2014;7:CD009532. doi: 10.1002/14651858.CD009532.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.White LK, Harris VJ, Cruz JL, Waxman DA. How do we design, implement, and manage an ongoing program to provide iron supplements to women blood donors? Transfusion. 2014;54:2795–801. doi: 10.1111/trf.12803. [DOI] [PubMed] [Google Scholar]
- 17.Cable RG, Spencer BR, Steele WR, 3rd, Mast AE. Self-reported iron supplement use in blood donors. Transfusion. 2011;51(suppl):96A–7A. [Google Scholar]
- 18.Steele WR, Notari EP, Eder AF. Iron supplement use and iron deficiency awareness among active donors: baseline survey results. Transfusion. 2013;53(suppl):105A. [Google Scholar]
- 19.Cable RG, Papallo CT, Spencer BR. Self-initiated iron supplementation (IS) in blood donors: demographics, patterns of use, and motivation. Transfusion. 2016;56(suppl):98A. doi: 10.1111/trf.15407. [DOI] [PubMed] [Google Scholar]
- 20.Goldman M, Uzicanin S, Scalia J, Scalia V, O’Brien SF. Impact of informing donors of low ferritin results. Transfusion. 2016;56:2193–8. doi: 10.1111/trf.13691. [DOI] [PubMed] [Google Scholar]
- 21.Goldman M, Uzicanin S, Osmond L, Scalia V, O’Brien SF. A large national study of ferritin testing in Canadian blood donors. Transfusion. 2017;57:564–70. doi: 10.1111/trf.13956. [DOI] [PubMed] [Google Scholar]
- 22.Magnussen K, Ladelund S. Handling low hemoglobin and iron deficiency in a blood donor population: 2 years’ experience. Transfusion. 2015;55:2473–8. doi: 10.1111/trf.13152. [DOI] [PubMed] [Google Scholar]