Abstract
Purpose
In this work, we investigated the relative effects of static magnetic field exposure (10.5 T) on two physiological parameters; blood pressure (BP) and heart rate (HR).
Methods
In vivo we recorded both blood pressure and heart rate in 4 swine (3 female, 1 male) while they were positioned within a 10.5 T magnet. All measurements were performed invasively within these anesthetized animals by the placement of pressure catheters into their carotid arteries.
Results
We measured average increases of 2.0 mmHg (SD, 6.9 mmHg) in systolic BP and an increase of 4.5 mmHg (SD, 13.7 mmHg) in the diastolic BPs: We also noted average increase of 1.2 bpm (SD, 2.5 bpm) in the heart rates during such.
Conclusion
Data regarding changes in blood pressure and heart rate in anesthetized swine due to whole body 10.5 T exposure is reported.
Keywords: 10.5 T, static field exposure, physiological effects, heart rate, blood pressure
Introduction
Ultra-high field magnetic resonance imaging (MRI) studies yield higher signal to noise ratio (SNR), increased sensitivity, improved susceptibility contrast and increased chemical shift dispersions. In addition, greater spectral separation can be obtained with magnetic resonance spectroscopy (MRS) at ultra-high fields.
The first 10.5T human whole body MRI scanner was installed at the Center for Magnetic Resonance Research (CMRR) in 2014. The system is passively shielded and has a bore diameter of 88cm, length of 4.1 m, width of 3.2 m, and achieves a static magnetic field (B0) homogeneity of <0.07ppm/25 cm. Importantly, as more studies are daily performed worldwide at higher field strengths, safety aspects of exposing patients to such high fields should continue to be critically investigated. Hence, from a safety aspect we need to identify the relative effects of these ultra-high static magnetic fields on large mammalian and human physiology. Early on in the 1930’s, such studies investigated the possible effects of high magnetic field on flowing conductive liquids. Hartman predicted (1) and Lazarus verified (2), that conducting fluids flowing transversely to a magnetic field develop induced currents resulting from the Lorentz force. The induced currents interacting with the transverse magnetic field produce body forces on the fluid resulting in increased pressure gradients in the direction of fluid flow. Human blood is an electrically conductive liquid (3) and it is therefore reasonable to assume the existence of such forces acting on it as it flows throughout the human vascular system, especially when exposed to magnetic field strengths as high as 10.5 T.
A previous study estimated a 10% decrease in blood velocity due to exposure of conductive blood tissue to a magnetic field of 5 T (4). In that work, pressure calculations were made using an approximate model for conducting liquids flowing in non-conducting, circular tubes with flow transverse to a magnetic field and by assuming that no magnetic fields were induced in the fluid. In a later study, an exact solution was presented (5) for the same problem. Then the calculated changes in the hydrostatic pressures across vessels in the human vasculature, in the presence of static magnetic fields, were mathematically modeled. As a validation of the model, the flow and pressure in a straight circular tube transverse to a magnetic field (4.7 T) was solved and compared to experimental measurements. In addition, an upper bound for the magneto-hydrodynamic vascular pressure of a human in a 10-T magnetic field was estimated. The predicted change between the total hydrodynamic vascular pressure and the total magneto-hydrodynamic vascular pressure was less than 0.2%. Although experimental data was provided in that work, it was limited to 4.7 T and no in-vivo experiments were performed. Another group investigated the effects of static magnetic field exposures (up to 8 T) on vital signs of normal human volunteers (6). Systolic and diastolic blood pressures were measured non-invasively using pressure cuffs. It was noted that a statistically significant induced increase of 3.6mmHg was observed in the systolic blood pressures in response to the 8.0 T field.
Performing non-invasive, reproducible, blood pressure (BP) assessments can be a difficult task, especially in an ultra-high field MR environment. For examples, various MR studies have been conducted using non-invasive techniques but these reports have noted their own drawbacks in terms of data acquisition and accuracy (7–9). In other words, invasive blood pressure measurements are considered as more accurate technique and have been performed during in-vivo non-human, animal studies (9).
In the present study, we invasively measure blood pressures and heart rates in anesthetized swine as an accurate means to investigate short term physiological effects of 10.5T static magnetic field exposure.
Methods
Animal Preparation
We measured invasively blood pressures and heart rates from 4 swine (3 female, 1 male) in-vivo, while they were in the 10.5 T magnet: located within the Center for Magnetic Resonance Research (CMRR) at the University of Minnesota. The weights of the animals varied between 32 kg-73 kg.
Animals were acclimated within the Research Animal Resources (RAR) facility at the CMRR for 2–3 days prior to study so to minimize anxiety. They were fasted for 12 h prior to the induction of the anesthesia to avoid complications (10): water was available for these animals.
Anesthesia was initially induced in all animals with a Telazol (5–10 mg/kg) + Xylazine (1–3 mg/kg) mixture, administered in the RAR facility via intramuscular injections. Animals were then transported to preparation room where we immediately started to monitor physiological parameters (SpO2, EtCO2, heart rates, and temperatures) using a patient monitor (Invivo 3150 MRI, Gainsville, FL). The initial monitoring and recording these physiological parameters in the prep room, enabled us to assess the animals given anesthetic depth, so to decide when to intubate and initiate the surgery required for probe placement. Once a given animal was intubated, we then administered isoflurane (~1.5%) via a vaporizor, in 50% air / 50% oxygen mixture. A ventilator (Ohmeda 7000) was used to provide positive pressure ventilation at a respiratory rate of 11–12 cycles/min. In each animal, an ear vein was catheterized so to administer saline solution (0.9 % NaCl) as needed to prevent animal dehydration.
After all the physiological parameters were stable, we made an incision in the neck to expose the right carotid artery. The distal end of this artery was ligated in order to prevent blood flow. A small hole was then made in the artery and a pressure catheter was immediately inserted into the artery, with a length of 20–30 cm. The catheter was then fixed in place and the surrounding tissue was sutured shut. The animal was left lying on the operation table for approximately 30 minutes to stabilize. After ensuring that all of the physiological parameters were unchanging, we started recording the pressure waveform in the preparation room. All surgeries and experiments were conducted in accordance with an approved IACUC protocol (ID: 1408-31748A) at the University of Minnesota.
We used a single pressure sensor system for all of our experiments (AD Instruments, Sydney, Australia) which consisted of a Blood Pressure Transducer, a quad Bridge Amplifier and data acquisition hardware (Power Lab). The system was calibrated prior to each study outside of the scanner in a low magnetic field environment: a calibrated sphygmomanometer was used to manually provide high and low reference pressure values required for the calibration. The electronic components of the system remained outside of the shield during the whole study to minimize any interaction with the static magnetic field. A single channel was used to record the pressure data: a sampling rate of 4 kHz was used. No digital filtering or data processing was performed during acquisition. Data were collected at three conditions in the following order: 1) in the prep room, 2) in the 10.5 T magnet room on the table outside the bore, 3) inside the bore (landmarked on the head). For two animals, we recorded measurements with one additional landmark location on their chests. All measurements were performed with each animal fully anesthetized and lying in a supine position. Durations of data acquisition ranged from 10 – 30 minutes per location. Average systolic/diastolic blood pressures and average heart rates were calculated after the data collections using an in-house Matlab (Natick, MA, USA) script: it performed peak detection and basic statistical analysis. Data are presented as means and standard deviations and 95% confidence intervals for both the mean blood pressures and the mean heart rates were noted.
Results
Figure 1 shows the raw waveforms obtained by the employed data acquisition system. The high frequency content corresponds to pressure transition between systole and diastole phases of the functional swine heart (the waveform was scaled to clearly display a few cycles). The low frequency content was due in part to ventilation of the animal (~ 11–12 cycles/min). Such waveforms were later analyzed to calculate the mean systolic and diastolic blood pressures as well as heart rates. Figure 2 shows the mean value of these parameters for each animal studied, in each of the aforementioned experimental conditions: i.e., in the prep room, in the magnet room on the table outside the bore, and inside the bore). The mean values of the same parameters (averaged across swine) and their SD are shown in Table 1. The comparisons between the prep room and the magnet room (outside the bore) data shows that the cardiovascular parameters of these animals were stable during the transport. Considering the two conditions within the 10.5 T magnet room (on the table outside of the bore and inside the bore), the observed increases in the mean values of systolic BPs, diastolic BPs and the heart rates were 2 mmHg, 4.5 mmHg, and 1.2 bpm, respectively. Note that examination of the field patterns of the magnet (made available from the manufacturer) showed that animals experienced approximately 1 T when they were on the patient table outside the bore.
Figure 1.
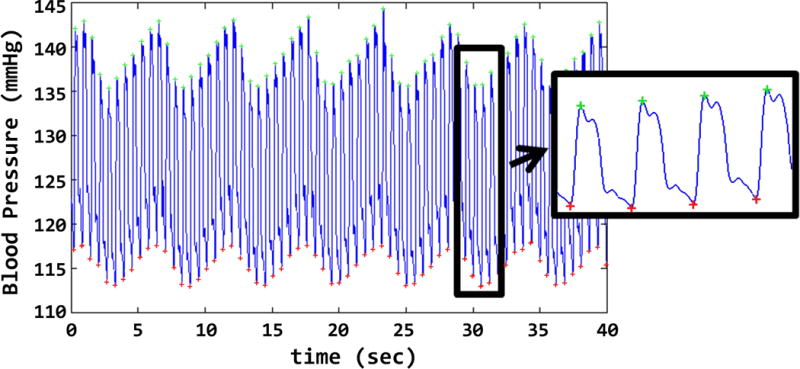
The waveform as obtained by the data acquisition system is shown. Analysis was made after the experiment to calculate the systolic /diastolic pressures and the heart rate.
Figure 2.
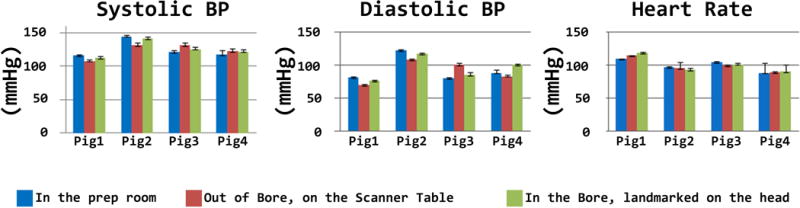
Mean and standard deviation value of blood pressure and heart rate obtained from 4 swine are shown for 3 different measurement sets. Error bars denote the within-condition within-swine standard deviation.
Table 1.
The mean values of measurements (across swine) obtained during three different conditions are shown. Considering the two conditions within the 10.5 T magnet room (on the table outside of the bore and inside the bore), the observed increases in the mean values of systolic BPs, diastolic BPs and the heart rates were 2 mmHg, 4.5 mmHg, and 1.2 bpm, respectively.
| Mean/STD | Prep Room | Outside Bore | In the Bore |
|---|---|---|---|
| Systolic BP(mmHg) | 125/13.4 | 123.7/11.3 | 125.7/12.1 |
| Diastolic BP(mmHg) | 92.7/19.8 | 90/17.5 | 94.5/17.9 |
| Heart Rate(bpm) | 99.2/9.2 | 99.3/10.65 | 100.5/12.5 |
Finally, Figure 4 shows the mean and SD of the blood pressure in two swine when they were landmarked at head vs chest inside the bore. The SBP and diastolic BP were both reduced by 2.5 mmHg respectively when the landmark positions of the animals were changed from head to chest. The analysis of the field maps of the magnet shows that moving the animal between two landmark positions corresponded to a change in the field from 10.5 T to 10.0 T.
Figure 4.
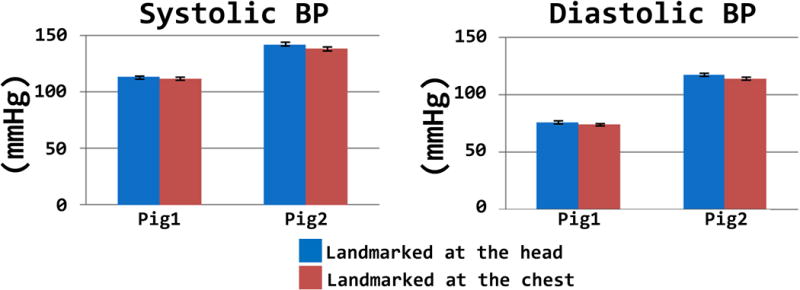
The mean value of the blood pressure due to different land-marking conditions are shown. Error bars denote the within condition within-swine standard deviation.
Discussion and Conclusions
Here we describe in vivo animal studies performed to investigate the potential physiological effects of being scanned within a 10.5 T magnetic field exposure. The changes in blood pressures and heart rates in healthy swine due to whole body 10.5 T exposure is reported. Further, the changes in blood pressures and heart rates due to land marking the animals at different anatomical locations(head vs chest) is also reported(i.e., this shift within the 10.5 T bore of 50–60 cm, corresponded to 0.5 T change in field strength).
We utilized four healthy swine in our experiments, which had body masses similar to adult humans. We calculated the confidence interval curves assuming a T-distribution which is appropriate for small sample size (n<30). The means and the standard deviations obtained for the studied parameters from this sample population were used in our confidence interval calculations.
It should be noted that using our experimental approach for monitoring that if blood leaked into the pressure catheter, it could clot and disrupt the resultant data acquisition. Thus, in order to prevent such an issue, we injected heparin into the IV tube (as needed): note that in a patient scan they would likely not be on heparin.
Investigation of the convergence of the confidence interval curve was useful to determine the minimal number of animals required for this work. In addition, we compared our confidence interval (for n=4) to the report by Nakao et. al. (11) who monitored blood pressure changes in swine caused by moving the animals to various anatomical positions (rolling from supine to left lateral and supine to right lateral). Relative to our data, in their work the changes in the left and right ventricular pressure were measured as swine were shifted from supine to left lateral and supine to right lateral positions. Our data compared well with theirs for the left ventricular systolic pressures. This can be justified easily because the pressure measured in carotid artery is approximately equal to the left ventricular pressure. Through this comparison, we related the possible effects of 10.5T exposure to any effects observed due to a normal and presumably safe behavior in swine. It should also be noted, that our confidence interval was smaller than, or comparable to, the blood pressure changes associated with normal animal activities. Nevertheless, this confidence interval may be made even smaller by performing more experiments, but we were convinced that this was not necessary since more animal sacrifice would not add critical data to our study.
In order to assess the confidence levels of our measurements we calculated confidence intervals for the changes in the mean systolic BP (ΔSBP) in the magnet room (on the table out of the bore vs inside the bore). A confidence interval was calculated based on the standard deviations obtained from two experimental conditions as a function of sample size. After calculating this for our sample size of 4 swine, we repeated the calculation as a function of sample size to examine how much narrower the interval could be with additional animals. We then compared the confidence interval of ΔSBP to the reported values in (7) for the change in the systolic blood pressures due to a change in the relative positions of the animals. Figure 3a shows the variations of ΔSBPs the with respect to number of animals studied (sample size). Figure 3b shows the variation of SBPs for swine transitioning from supine to left lateral and supine to right lateral positions. For n=4, the half-width of the 95% confidence interval for the change in SBP is 13.1 mmHg. For comparison, supine to left lateral and supine to right lateral transitions resulted in an increase in SBP of 13.3 mmHg and 5.9 mmHg which are comparable to, or smaller than, the values measured in 10.5 T exposure experiments. Doubling the number of animals (n=8) would yield a confidence interval of 7.2 mmHg. However, we believed that further animal sacrifice was not necessary.
Figure 3.
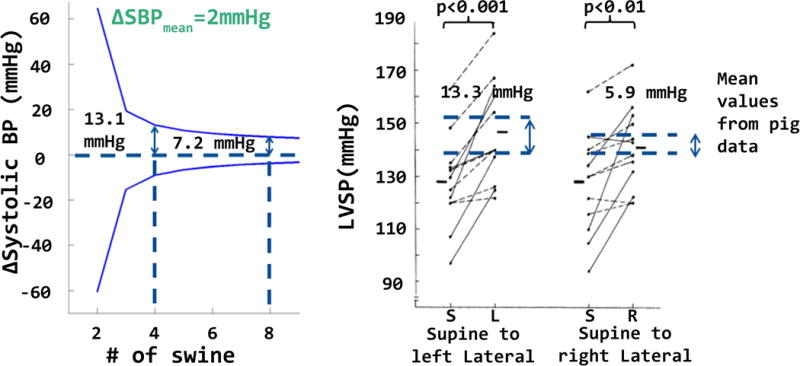
Width of the confidence intervals (for changes in the mean systolic BP, ΔSBP) based on the standard deviation is shown. The variation of the confidence interval was useful to determine the minimal number of animals required for this work. Figure also shows the comparison of our confidence interval to the report by Nakao et. al. (11) who monitored left ventricular systolic blood pressure (LVSP) changes in swine caused by moving the animals to various anatomical positions (rolling from supine to left lateral and supine to right lateral).
In our studies we measured only the heart rate and blood pressure in swine exposed to 10.5 T static magnetic field. Future safety studies may be useful to investigate changes in other physiological parameters such as vascular compliance, cardiac output, and blood flow.
We conducted our studies using a horizontal bore magnet. Our results do not directly apply to imaging subjects with open-bore / vertical magnets. It is expected that the effects of static field exposure would be maximized when the flow is transverse to main magnetic field. This scenario could be realized for a patient with a normal anatomy inside an open bore system. Although the static fields in current open bore systems are significantly lower than 10.5 T, detailed safety studies will need to be performed to assess the physiological effects of these systems as the field strength increases.
In the present study we investigated the relative cardiovascular effects of exposure to 10.5T magnetic field on healthy swine. The effects of these ultra-high magnetic fields on the monitored parameters of blood pressure and heart rate were minimal. For example, the relative increases in recorded blood pressures attributable to the magneto-hydrodynamic effects were comparable to elevations in blood pressure associated with normal animal activity (such as rolling over).
Acknowledgments
Research reported in this publication was supported by the National Institute Of Biomedical Imaging And Bioengineering of the National Institutes of Health under Award Number K99EB021173
Grant Support: P41 EB015894, S10 RR029672, K99EB021173
References
- 1.Hartmann J. Theory of the laminar flow of an electrically conductive liquid in a homogeneous magnetic field. K Dan Vidensk Selsk Mat Fys Medd. 1937;15(6):1–28. [Google Scholar]
- 2.Hartmann J, Lazarus F. Experimental investigations on the flow of mercury in a homogeneous magnetic field. K Dan Vidensk Selsk Mat Fys Medd. 1937;15(7):1–45. [Google Scholar]
- 3.HIRSCH FG, et al. “THE ELECTRICAL CONDUCTIVITY OF BLOOD” I RELATIONSHIP TO ERYTHROCYTE CONCENTRATION. 1950;5(11):1017–1035. [PubMed] [Google Scholar]
- 4.Gaffey CT, et al. A Study of the Influence of Stationary Magnetic-Fields on the Electrocardiographic Indexes and Blood-Pressure of Monkeys. Biophysical Journal. 1982;37(2):A344–A344. [Google Scholar]
- 5.Keltner, et al. Magnetohydrodynamics of Blood Flow. Magn Reson Med. 1990;16:139–149. doi: 10.1002/mrm.1910160113. [DOI] [PubMed] [Google Scholar]
- 6.Chakeres DW, et al. Effect of static magnetic field exposure of up to 8 Tesla on sequential human vital sign measurements. J Magn Reson Imaging. 2003;18(3):346–52. doi: 10.1002/jmri.10367. [DOI] [PubMed] [Google Scholar]
- 7.Roeleveld RJ, Marcus JT, Boonstra A, Postmus PE, Marques KM, Bronzwaer JGF, Vonk-Noordegraaf A. A comparison of noninvasive MRI-based methods of estimating pulmonary artery pressure in pulmonary hypertension. J Magn Reson Imaging. 2005;22:67–72. doi: 10.1002/jmri.20338. [DOI] [PubMed] [Google Scholar]
- 8.Marcus JT, Noordegraaf AV, Roeleveld RJ, Postmus PE, Heethaar RM, Van Rossum AC, Boonstra A. Impaired left ventricular filling due to right ventricular pressure overload in primary pulmonary hypertension: noninvasive monitoring using MRI. CHEST Journal. 2001;119(6):1761–1765. doi: 10.1378/chest.119.6.1761. [DOI] [PubMed] [Google Scholar]
- 9.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG, Roccella EJ. Recommendations for blood pressure measurement in humans and experimental animals part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45(1):142–161. doi: 10.1161/01.HYP.0000150859.47929.8e. [DOI] [PubMed] [Google Scholar]
- 10.Shrivastava D, Hanson T, Kulesa J, Tian J, Adriany G, Vaughan JT. Radiofrequency heating in porcine models with a “large” 32 cm internal diameter, 7 T (296 MHz) head coil. Magn Reson Med. 2011;66:255–263. doi: 10.1002/mrm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakao SH, Come PC, Miller MJ, Momomura S, Sahagian P, Ransil BJ, Grossman W. Effects of supine and lateral positions on cardiac output and intracardiac pressures: an experimental study. Circulation. 1986 Mar 1;73(3):579–85. doi: 10.1161/01.cir.73.3.579. [DOI] [PubMed] [Google Scholar]


